Abstract
The ketogenic diet is an effective treatment for the patients with intractable epilepsy, however, the diet therapy can sometimes be discontinued by complications. Protein–losing enteropathy is a rarely reported serious complication of the ketogenic diet. We present a 16-month-old Down syndrome baby with protein-losing enteropathy during the ketogenic diet as a treatment for West syndrome. He suffered from diarrhea, general edema and hypoalbuminemia which were not controlled by conservative care for over 1 month. Esophagogastroduodenoscopy and stool alpha-1 antitrypsin indicated protein-losing enteropathy. Related symptoms were relieved after cessation of the ketogenic diet. Unexplained hypoalbuminemia combined with edema and diarrhea during ketogenic suggests the possibility of protein-losing enteropathy, and proper evaluation is recommended in order to expeditiously detect it and to act accordingly.
Keywords: Ketogenic diet, protein-losing enteropathies, hypoalbuminemia
INTRODUCTION
The ketogenic diet (KD), is an effective treatment for intractable childhood epilepsy, such as West syndrome (WS), Lennox-Gastaut syndrome, and sometimes for refractory status epilepticus.1 The use of this treatment, however, is sometimes limited due to its complications, despite of the efficacy. While some side effects are benign and treatable, others are more problematic and rarely can interrupt the diet therapy itself.2,3,4 Protein-losing enteropathy (PLE) is a rarely recognized serious complication of the KD, and only two cases have been reported.5,6
We experienced one case of PLE during the KD in a 16 month old male Down syndrome baby with WS.
CASE REPORT
A 16-month-old male baby was diagnosed with Down syndrome by chromosome analysis and clinical features after birth. He presented epileptic spasms since the age of 5 months and was diagnosed with WS. He started to be treated by multiple antiepileptic drugs (AEDs), including vigabatrin, clobazam, valproic acid and oral steroids, but his epileptic seizures, developmental regression, and hypsarrhythmia on electroencephalogram persisted.
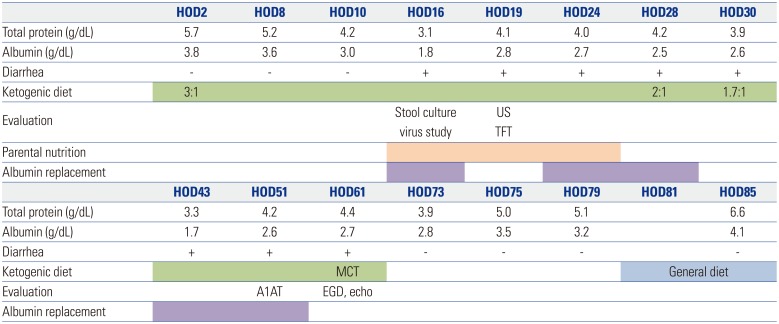
KD with 3:1 ratio of fat to non-fat was started by milk formula, and multiple AEDs (vigabatrin, clobazam, and deflazacort) were maintained at the same doses. Vomiting and diarrhea were developed after 1st week of KD. The albumin level which was 3.8 g/dL at initiation of the diet decreased to 1.8 g/dL after 16 days of KD. He was treated by intravenous albumin and intermittent parenteral nutritional support. Lipid and non-fat ratio of KD deescalated from 2:1 on the 28th days to 1.7:1 on the 30th days of the diet. Because he had not improved after 4 weeks, we changed KD to medium chain triglyceride formula on the 61st days of the diet.
Laboratory tests, abdominal ultrasonography, esophagogastroduodenoscopy (EGD), colonoscopy, and alpha-1 antitrypsin (A1AT) in stool, were done. There was no proteinuria in urine dipstick test, and blood test revealed blood urea nitrogen 9.2 mg/dL, creatinine 0.2 mg/dL, aspartate aminotransferase 16 IU/L, alanine aminotrasnferase 5 IU/L, and normal thyroid function. Echocardiography revealed normal heart function without any structural abnormalities. There were no pathogens in stool bacterial culture, but norovirus infection was detected in stool virus study. Abdominal ultrasonography revealed grossly normal liver and gallbladder, and preserved corticomedullary differentiation in both kidneys, and there was no skin lesions that could produce protein loss.
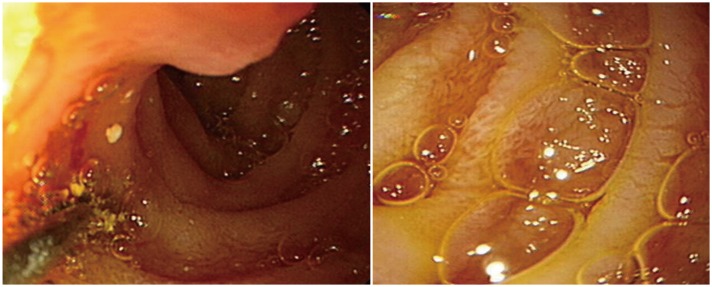
EGD revealed edematous mucosa in the duodenum, and the biopsy results revealed lymphatic ectasia in the lamina propria; thus intestinal lymphangiectasia was diagnosed (Fig. 1). A1AT of the stool was 56.60 mg/dL. These findings were concordant with interstitial lymphangiectasia as a presentation of PLE.
Fig. 1. Edematous mucosa and some whitish patches were noted on the duodenum. Slightly edematous gastric mucosa was also noted. Biopsies were acquired from the duodenum and the stomach. Pathology result: ectatic lymphatics in lamina propria, suspicious for intestinal lymphangiectasia.
We discontinued the KD after 8 weeks and supported him with balanced nutrition. His general condition improved, and hypoalbuminemia, edema, and diarrhea were resolved after cessation of KD (Fig. 2).
Fig. 2. Scheme of ketogenic diet, nutritional support, evaluation and laboratory data. On HOD2, the ketogenic diet (3:1, fat:non-fat) was started. diarrhea and hypoalbuminemia occurred. through laboratory test, we checked normal renal function, liver function, normal flora in stool culture and noroviral infection confirmed in viral study on HOD16. And parental nutrition and albumin replacement were started. abdominal ultrasonography and thyroid function test were normal on HOD19. On HOD28, the ketogenic diet was reduced from 3:1 to 1.7:1. The symptoms were persistent, and after EGD and echocardiography on HOD61, the ketogenic diet was stopped, and a MCT was started. HOD, hospitalization day; US, abdominal ultrasonography; TFT, thyroid function test; MCT, medium chain triglycerides; A1AT, alpha-1 antitrympsin; EGD, endoscopic gastroduodenoscopy; echo, echocardiography; MCT, medium chain triglyceride diet.
DISCUSSION
With increasing use of KD, more early and late complications have recently been reported. Kang, et al.2 and Suo, et al.3 showed that hypoproteinemia is commonly appeared on KD with approximately 10% of the patients: 12 of 129 patients (9.3%) and 39 of 317 patients (12.3%), respectively. Gluconeogenic consumption due to restriction of carbohydrate and reduced protein intake were suspected as mechanism of hypoproteinemia. Patients with hypoproteinemia can be improved by increase of protein intake with maintenance of lipid-to-nonlipid ratio.4
Gastrointestinal (GI) disturbance, common early and late complication of KD, is related to poor tolerance of the diet and it can disturb the maintenance and efficacy of KD. The symptoms of GI disturbance were seen in 73 (56.6%) patients, reported by Kang, et al.4 Transient GI disturbances are explained by defective absorption and intolerance of high-lipid diet, there fore, adaptation periods seem to be required.
Hypoproteinemia and diarrhea as early complications of KD are expected to be controlled by well-known conservative cares. However, patients with abnormal progression, for example persistent symptoms, or who need intravenous replacement of protein continuously, have to undergo further evaluation for other causes including PLE.
In most cases, PLE can be diagnosed by history, physical examination and clinical manifestations, nevertheless, documentation of GI losses of protein by fecal A1AT levels or with functional imaging is necessary.7,8
There are two main mechanisms of PLE-mucosal injury or lymphatic abnormalities, sometimes both of them together.8,9,10 Mucosal injury is caused by variety of conditions-inflammatory or ulcerative diseases, such as infections, Crohn's disease/ulcerative colitis or GI malignancies, and other non-ulcerative diseases. Intestinal lymphangiectasia is characterized by diffuse or local dilatation of the enteric lymphatics and leakage of lymphatic fluid rich in albumin and other proteins into the GI tract. Increased leakage of protein-rich fluids across damaged mucosa can cause loss of protein, and mucosal cell injury is related to secondary lymphangiectasia. Abnormality of the lymphatic drainage can be caused by either congenital defect as primary intestinal lymphangiectasia or by secondary causes as GI obstruction, congestive heart failure, lymphoma and other intestinal damage.
Norovirus infection is a major cause of GI disturbance in children. Fecal shedding of norovirus persists for up to 2 weeks, however, it can persist for up to 5 weeks in immune-compromised patient.11 Furthermore, norovirus infection is associated with exacerbation of inflammatory bowel disease.12 However, there is no definite relationship between noroviral infection and PLE. In this case, immune-compromised status due to taking steroid for long times could have made GI tract inflammation worse.
There have been two published case reports of PLE upon initiation of the KD-one case was explained by allergic gastroenteritis from a soy allergy,4 and the other case seemed to have been caused by the diet itself.5 Mucosal injury by viral infection and secondary lymphangiectasia which was aggravated by KD itself are highly suspected mechanism of PLE in our case.
PLE, a rarely experienced serious complication of the KD, can interrupt the maintenance of diet therapy. Early detection and active intervention are important in maintaining the health of such patients.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.DiMario FJ, Jr, Holland J. The ketogenic diet: a review of the experience at Connecticut Children's Medical Center. Pediatr Neurol. 2002;26:288–292. doi: 10.1016/s0887-8994(01)00405-2. [DOI] [PubMed] [Google Scholar]
- 2.Kang HC, Kim YJ, Kim DW, Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005;46:272–279. doi: 10.1111/j.0013-9580.2005.48504.x. [DOI] [PubMed] [Google Scholar]
- 3.Suo C, Liao J, Lu X, Fang K, Hu Y, Chen L, et al. Efficacy and safety of the ketogenic diet in Chinese children. Seizure. 2013;22:174–178. doi: 10.1016/j.seizure.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45:1116–1123. doi: 10.1111/j.0013-9580.2004.10004.x. [DOI] [PubMed] [Google Scholar]
- 5.Moriyama K, Watanabe M, Yamada Y, Shiihara T. Protein-losing enteropathy as a rare complication of the ketogenic diet. Pediatr Neurol. 2015;52:526–528. doi: 10.1016/j.pediatrneurol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Ballaban-Gil K, Callahan C, O'Dell C, Pappo M, Moshé S, Shinnar S. Complications of the ketogenic diet. Epilepsia. 1998;39:744–748. doi: 10.1111/j.1528-1157.1998.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 7.Nanjo S, Nishikawa J, Miwa S, Mihara H, Fujinami H, Yoshita H, et al. Immune-mediated protein-losing enteropathy with Down syndrome. Intern Med. 2014;53:2301–2305. doi: 10.2169/internalmedicine.53.1980. [DOI] [PubMed] [Google Scholar]
- 8.Dinari G, Rosenbach Y, Zahavi I, Sivan Y, Nitzan M. Random fecal alpha 1-antitrypsin excretion in children with intestinal disorders. Am J Dis Child. 1984;138:971–973. doi: 10.1001/archpedi.1984.02140480073022. [DOI] [PubMed] [Google Scholar]
- 9.Wang SJ, Tsai SC, Lan JL. Tc-99m albumin scintigraphy to monitor the effect of treatment in protein-losing gastroenteropathy. Clin Nucl Med. 2000;25:197–199. doi: 10.1097/00003072-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Braamskamp MJ, Dolman KM, Tabbers MM. Clinical practice. Protein-losing enteropathy in children. Eur J Pediatr. 2010;169:1179–1185. doi: 10.1007/s00431-010-1235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Ven AA, Hoytema van Konijnenburg DP, Wensing AM, van Montfrans JM. The role of prolonged viral gastrointestinal infections in the development of immunodeficiency-related enteropathy. Clin Rev Allergy Immunol. 2012;42:79–91. doi: 10.1007/s12016-011-8292-9. [DOI] [PubMed] [Google Scholar]
- 12.Khan RR, Lawson AD, Minnich LL, Martin K, Nasir A, Emmett MK, et al. Gastrointestinal norovirus infection associated with exacerbation of inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48:328–333. doi: 10.1097/mpg.0b013e31818255cc. [DOI] [PubMed] [Google Scholar]