Abstract
Long-term exposure to ambient fine particulate pollution (PM2.5) has been associated with cardiovascular diseases. Hypertension, a major risk factor for cardiovascular diseases, has also been hypothesized to be linked to PM2.5. However, epidemiological evidence has been mixed. We examined long-term association between ambient PM2.5 and hypertension and blood pressure. We interviewed 12,665 participants aged 50 years and older and measured their blood pressures. Annual average PM2.5 concentrations were estimated for each community using satellite data. We applied two-level logistic regression models to examine the associations, and estimated hypertension burden attributable to ambient PM2.5. For each 10 μg/m3 increase in ambient PM2.5, the adjusted odds ratio of hypertension was 1.14 (95% confidence interval, 1.07, 1.22). Stratified analyses found that overweight and obesity could enhance the association, and consumption of fruit was associated with lower risk. We further estimated that 11.75% (95% confidence interval: 5.82%, 18.53%) of the hypertension cases (corresponding to 914, 95% confidence interval: 453, 1442 cases) could be attributable to ambient PM2.5 in the study population. Findings suggest that long-term exposure to ambient PM2.5 might be an important risk factor of hypertension, and is responsible for significant hypertension burden in adults in China. A higher consumption of fruit may mitigate, while overweight and obesity could enhance this effect.
Keywords: Air pollution, PM2.5, hypertension, effect modification, disease attribution
Long-term exposures to ambient air pollution have been linked with cardiovascular morbidity and mortality.1, 2 Hypertension is one important risk factor for cardiovascular diseases. It has been therefore hypothesized that exposure to air pollution could chronically raise blood pressure, thereby increasing hypertension.3
Such a link has been investigated in a few studies. These studies showed that long-term exposure to ambient PM10 and NO2 was significantly associated with increased hypertension.4, 5 However, the association between ambient PM2.5 and hypertension has been inconclusive.6 One recent meta-analysis pooling five studies7 found a positive association, but the association was non-significant, indicating that more studies are warranted.
It is important to identify effect modifiers of the health effects of air pollution, which may provide important clues for targeting intervention programs.8 Previous studies have examined the effect modifiers of the association between particulate matter pollution and cardiopulmonary health, and found that body weight and dietary intake of oxidants might be the important effect modifiers.9 Few studies have explored the potential effect modification of overweight/obesity and dietary factors (consumption of fruit and vegetables) on the effects of PM2.5 on hypertension. After inhaled in the respiratory tract, the particles could trigger oxidative reactions leading to oxidative stress and damage due to the pro-oxidant-antioxidant imbalance.10, 11 Antioxidants play a critical role in defense against inflammatory oxidative stress induced by air pollutants.11 Dietary consumption of fruit and vegetables are the primary source of antioxidants and related compounds, in particular vitamin C, carotenoids, and other phytochemicals.12 Therefore, we hypothesize that higher consumption of fruit and vegetables may mitigate the adverse effects of PM2.5 on hypertension.
To address these research questions, we used a large nationwide survey among adults in China with three specific objectives: 1) to examine whether long-term exposure to ambient PM2.5 is associated with risk of hypertension; 2) to determine whether overweight/obesity and dietary factors could modify the association; 3) to estimate hypertension risk attributable to ambient PM2.5 exposure.
Methods
Population
As part of the World Health Organization’s (WHO) Study on global AGEing and adult health (SAGE), we surveyed a group of Chinese respondents during 2007–2010.13 The participants were interviewed through a face-to-face household survey. A five-stage sampling approach was applied. At the first stage, the city of Shanghai and seven provinces of Guangdong, Hubei, Jilin, Shaanxi, Shandong, Yunnan, and Zhejiang (Figure 1 shows their locations) were selected. One county from a rural area and one district from an urban area in each province were then randomly selected. In total, we selected 64 principle sample units (two urban and two rural townships/communities from each county/district), 127 secondary sample units (two villages/enumeration areas per township/community), and 254 tertiary sample units (two residential blocks per village). The participants were randomly selected to do the interview and body check-up.13 SAGE-China Wave 1 consisted of a total of 15,050 participants.13 Among them, 1,671 were aged 18–49 years and 13,379 aged 50 years and older. This study was restricted to those aged 50 years and older. Among them, 12,665 had complete information and blood pressure measurement.
Figure 1.
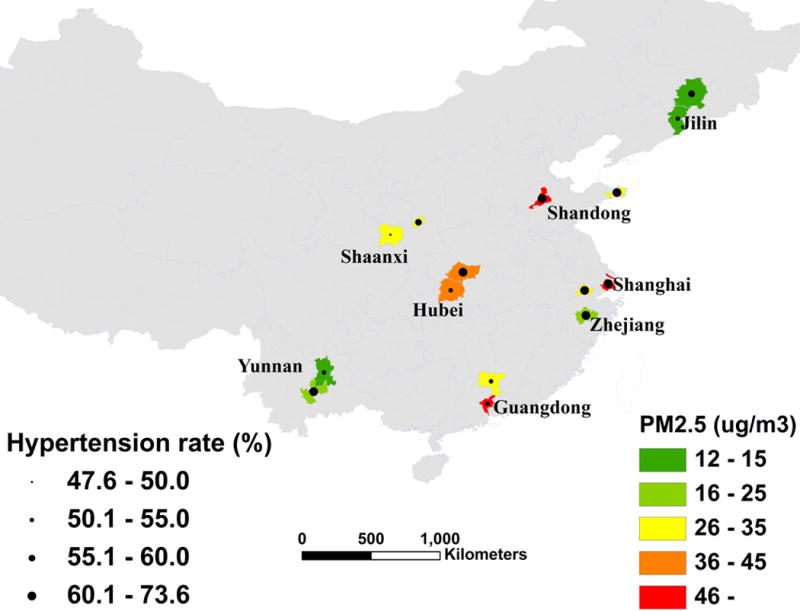
The geographic location of the study areas showing PM2.5 concentration and prevalence of hypertension.
Approval to conduct this study was granted by the Ethics Committee of the Chinese Centre for Disease Control and Prevention. Informed consent was obtained from each participant before the interview.
Hypertension
The arterial blood pressure of each participant was measured three times on the right arm/wrist of the seated participants using a wrist blood pressure monitor. The average value of the latter two was used as the blood pressure for this analysis.14 Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg,5 and/or self-reported current treatment of hypertension with antihypertensive medication within the last two weeks before the interview.
Air pollution
We applied the method developed by van Donkelaar and co-workers to estimate the outdoor PM2.5 concentrations.15 These values provided a long-term average level of exposure to PM2.5 at approximately 10 km * 10 km resolution. They are derived from a combination of observations from the Moderate Resolution Imaging Spectroradiometer and Multiangle Imaging Spectroradiometer instruments from the Terra satellite, and simulations with the GEOS-Chem chemical transport model16. The community locations were geo-coded using Google Earth. The estimated annual ambient PM2.5 concentrations were then matched to the participants in the corresponding communities. We used the average PM2.5 concentrations for the three years preceding the survey as the estimated surrogate of exposure.
Consumption of fruit and vegetables
We solicited information on the consumption of fruits (such as apple, pear, peach, banana, orange, watermelon, etc.) and vegetables (such as cabbage, spinach, leek, tomato, cucumber, celery, green pepper, eggplant, etc.).14 For the stratified analyses, we classified responses into two levels: sufficient and insufficient intake. Two or more servings were considered sufficient for fruit consumption and five or more servings of vegetables were considered sufficient. In keeping with previous research, fewer servings were considered insufficient.14
Covariates
A number of covariates were collected, including demographic, socioeconomic, and lifestyle factors, such as age, sex, marital status, body mass index (BMI), smoking status and amount, alcohol consumption, physical activity, education, annual household income, and consumption of fruit and vegetables. Marital status was divided into married (currently married or cohabiting) and unmarried (never married, separated, divorced, or widowed). Overweight was defined as BMI between 24 and 28 kg/m2, and obesity was defined as BMI of 28 kg/m2 or higher.17 Physical activity was measured in terms of intensity, duration, and frequency of physical activity, as described elsewhere.18 The total time spent in physical activity during a typical week, including the number of days and intensity, was used to generate low, moderate, and high categories of physical activity levels.18
We also collected information on indoor air pollution. Domestic fuel type and ventilation apparatus were included as the indicators. Two fuel types were mainly used: clean fuels, including electricity and natural gas, and unclean fuels, such as coal, wood, dung and agricultural residues.
Statistical analysis
Blood pressure and hypertension for participants in the same community may be correlated with each other, violating the independence assumption of regression models. We therefore considered a two-level logistic regression model to examine the effects on hypertension and linear mixed models to examine the effects on blood pressure, where participants were considered as the first-level unit, and the community as the second-level unit.5
Following the univariate analyses, multivariate models were fit to control for potential confounding factors, such as age, sex, BMI, consumption of fruit and vegetables smoking, alcohol drinking, physical activity, education, annual household income, fuel type and ventilation. The effect estimates were presented for per 10 μg/m3 increase in ambient PM2.5.
Stratified analysis
Stratified analyses were conducted to examine potential effect modification of body weight, consumption of fruit and vegetables, and region of the study. We fit separate models for weight (normal, overweight, and obesity), consumption of fruit and vegetables (sufficient and insufficient), and region of the survey (north and south region). One study from north China has reported an association between ambient PM10 and hypertension,5 but the evidence in south China is limited. There are significant differences between the north and south areas of China in characteristics, such as diet, lifestyle, climate, chemical components of PM2.5, and some unmeasured factors, which may potentially confound the associations between PM2.5 and hypertension. We thus conducted stratified analyses by dividing the survey region into south (including four provinces: Guangdong, Yunnan, Zhejiang and Shanghai) and north (including the other four provinces: Jilin, Shandong, Hubei and Shaanxi). We tested the statistical significance of differences in the effect estimates between the strata by calculating the 95% confidence interval as:
where b1 and b2 were the effect estimates for each stratum, and SE1 and SE2 were the standard errors.19
Estimating attributable hypertension risk
We further estimated the hypertension burden attributable to ambient PM2.5.20 We used two indicators, namely, attributable cases and population attributable fraction, to reflect the hypertension burden.8 The ambient PM2.5 level (25 μg/m3) set by the WHO’s Air Quality Guidelines was used as the reference concentration.
Sensitivity analyses were performed. First, we used average PM2.5 concentrations for one, two, four and five years before the survey. Second, we excluded the participants with respiratory and cardiovascular diseases.
All analyses were conducted using R version 3.2.2. P-value < 0.05 was used to determine statistically significance.
Results
The average age of the participants was 63.0 years and 5,895 (46.6%) were males. Among the participants, 7,777 participants were hypertensive, giving a prevalence rate of 61.4%. Among them, 3,538 (45.5%) were hypertensive before the survey, 4,239 (54.5%) were found to be hypertensive by this survey, 2,802 (36.0%) received antihypertensive treatment, of which 722 (25.8%) were under control (systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg). In general, areas with higher PM2.5 tended to have higher prevalence of hypertension (Figure 1).
The general characteristics of the participants are presented in supplementary Table S1. Participants with hypertension were statistically older than the normotensive participants (64.4 versus 60.8 years), had higher BMI (24.62 versus 23.21 kg/m2), and were exposed to higher ambient PM2.5 (33.70 versus 30.85 μg/m3). Hypertensive participants had lower consumption of fruit (2.40 versus 2.51 servings per day), but higher consumption of vegetables (7.14 versus 6.82 servings per day). Normotensive participants were more likely to be married, live in urban areas, have higher educational levels, to smoke, and to have higher physical activity levels. There was no significant difference in sex, household income, drinking status, fuel type and ventilation between the hypertensive and normotensive groups.
Table 1 displayed the associations between ambient PM2.5 and hypertension and blood pressures in the univariate and multivariate regression models. For each 10 μg/m3 increase in PM2.5, the odds ratio (OR) of hypertension was 1.16 (95% confidence interval (CI): 1.08, 1.24) before adjusting for any covariates, and remained similar after adjusting for various confounders (adjusted OR=1.14, 95% CI: 1.07, 1.22). Ambient PM2.5 was also associated with increased systolic and diastolic blood pressures, each 10 μg/m3 increase in ambient PM2.5 corresponded to a 1.04 mmHg (95% CI: 0.31, 1.78) increase in diastolic blood pressure and a 1.30 mmHg (95% CI: 0.04, 3.56) increase in systolic blood pressure.
Table 1.
Estimated effects of hypertension and blood pressure with long-term exposure to ambient PM2.5 in China
| Category | Crude estimate | 95% CI | Adjusted estimate* | 95% CI |
|---|---|---|---|---|
| Hypertension† | 1.16 | 1.08, 1.24 | 1.14 | 1.07, 1.22 |
| Diastolic blood pressure‡ | 0.99 | 0.23, 1.75 | 1.04 | 0.31, 1.78 |
| Systolic blood pressure‡ | 1.33 | −0.12, 2.79 | 1.30 | 0.04, 3.56 |
Adjusted for age, sex, BMI, consumption of fruit and vegetables, smoking amount, alcohol consumption, physical activity, marital status, urbanity, household income, educational level, domestic fuel type and ventilation.
the effect for hypertension was OR (odds ratio);
the effect for blood pressure was absolute change in mmHg.
The stratified association between ambient PM2.5 and hypertension (Table 2) suggested that body weight and consumption of fruit could modify the PM2.5-hypertension association. We observed higher ORs with increased body weight, suggesting that overweight or obesity may enhance this association. While higher consumption of fruit was found to alleviate the effect; a weaker association was observed among those with higher consumption of fruit (OR=1.12, 95% CI: 1.04, 1.20) than for those with lower consumption of fruit (OR=1.18, 95% CI: 1.09, 1.27). We did not observe significant differences by consumption of vegetables and region of survey, though a relatively larger effect was found in the north region (OR=1.16, 95% CI: 1.05, 1.29) than in the south region (OR=1.11, 95% CI: 1.03, 1.20).
Table 2.
Estimated odds ratio (OR) of hypertension associated with each 10 μg/m3 increase in ambient PM2.5 in stratified analyses
| Category | Adjusted OR* | 95% CI | p value † |
|---|---|---|---|
| Weight | |||
| Normal | 1.11 | 1.03, 1.20 | |
| Overweight | 1.15 | 1.07, 1.24 | |
| Obesity | 1.18 | 1.07, 1.30 | <0.05 |
| Vegetable intake | |||
| Sufficient | 1.13 | 1.04, 1.22 | |
| Insufficient | 1.12 | 1.04, 1.21 | >0.05 |
| Fruit intake | |||
| Sufficient | 1.12 | 1.04, 1.20 | |
| Insufficient | 1.18 | 1.09, 1.27 | <0.05 |
| Region | |||
| North | 1.16 | 1.05, 1.29 | |
| South | 1.11 | 1.03, 1.20 | >0.05 |
Adjusted for age, sex, BMI (only for the analyses for groups of consumption of fruit and vegetables), consumption of fruit and vegetables (only for the analyses for groups of weight), smoking amount, alcohol consumption, physical activity, marital status, urbanity, household income, educational level, domestic fuel type and ventilation.
p value is for the difference among the strata.
Estimates of the hypertension burden attributable to ambient PM2.5 were illustrated in Table 3. The population attributable risk due to ambient PM2.5 higher than 25 μg/m3 was 11.75% (95% CI: 5.82%, 18.53%), corresponding to 914 (95% CI: 453, 1442) hypertension cases. Comparable estimates were observed between males and females and between young and old age groups. For instance, the population attributable risk was 11.36% (95% CI: 4.57%, 19.26%) among males, and 12.25% (95% CI: 5.73%, 19.84%) among females. The attributable hypertension cases were 413 (95% CI: 166, 699) among males and 509 (95% CI: 238, 823) among females. We estimated that 11.11% (95% CI: 5.17%, 17.92%) and 10.63% (95% CI: 1.98%, 21.26%) of the hypertension cases could be prevented among participants younger than 65 years and 65 years and older, respectively.
Table 3.
Population attributable risks of hypertension due to ambient PM2.5 in the study population *
| Category | Odds ratio | Population attributable fraction (%, 95% CI) | Attributable cases (95% CI) |
|---|---|---|---|
| Overall | 1.14 (1.07, 1.22) | 11.75 (5.82, 18.53) | 914 (453, 1442) |
| Sex | |||
| Male | 1.13 (1.06, 1.22) | 11.36 (4.57, 19.26) | 413 (166, 699) |
| Female | 1.15 (1.07, 1.23) | 12.25 (5.73, 19.84) | 509 (238, 823) |
| Age group | |||
| <65 yrs | 1.14 (1.07, 1.21) | 11.11 (5.17, 17.92) | 461 (215, 743) |
| ≥65 yrs | 1.12(1.02, 1.23) | 10.63 (1.98, 21.26) | 386 (72, 772) |
The reference PM2.5 concentration was the WHO’s Ambient Air Quality Guidelines (25μg/m3).
The associations between PM2.5 and hypertension did not materially change in the sensitivity analyses (supplementary Table S2). When using one-year and five-year average PM2.5 concentrations as the exposure variable, the ORs for each 10 μg/m3 increase were 1.12 (95% CI: 1.04, 1.21) and 1.15 (95% CI: 1.07, 1.24), respectively. When we excluded the respiratory patients and cardiovascular cases from the data, the associations remained robust, with ORs of 1.17 (95% CI: 1.09, 1.25) and 1.16 (95% CI: 1.08, 1.23) for the one-year and five-year average PM2.5 concentrations, respectively.
Discussion
To our knowledge, this is the first study simultaneously examining the long-term effects of ambient PM2.5 on hypertension and blood pressure, and the attributable hypertension burden due to ambient PM2.5 in China. Our study found that exposure to ambient PM2.5 was associated with increased risk of hypertension and increased blood pressure. We also observed that overweight or obesity could enhance the association, and higher consumption of fruit might reduce the association. We further estimated that about 12% of the hypertension cases could be attributable to ambient PM2.5 exposure in the study population.
The observed associations between PM2.5 and hypertension and blood pressure were consistent with a few studies.21–23 The analysis of the American Cancer Society Cancer Prevention Study II cohort demonstrated a 20% increase in hypertension mortality associated with each 10 μg/m3 increase in PM2.5.23 A Canadian cohort study reported that PM2.5 was associated with incident hypertension with a magnitude comparable to ours (Hazard ratio=1.13).22 Another cross-sectional study reported a relatively smaller association (OR=1.05).21 Conversely, non-significant associations were reported in several studies. For instance, one cross-sectional study including 27,752 Taipei residents found a non-significantly negative association between PM2.5 and hypertension.24 A cross-sectional study in Germany observed that each 2.4 μg/m3 increase in PM2.5 was associated with a 1.4 mmHg increase in systolic blood pressure and a 0.9 mmHg increase in diastolic blood pressure, which was consistent with our estimates.25 Similar results have been reported in a Taiwan study.26 Whereas, non-significant effects were reported in a cohort of black women living in Los Angeles, US4 and in one cross-sectional study from Germany.27 These inconsistent findings may be partly due to the differences in chemical constituents of the airborne particles, exposure assessment, population activity patterns, and susceptibility to the air pollution exposures.7
The association between PM2.5 exposure and hypertension has some biological plausibility. Several pathways, including systemic inflammation, oxidative responses, and endothelial dysfunction, have been supported by human experiments6 and epidemiological studies.1, 28 The systemic inflammatory and oxidative stress may result in increased sympathetic tone, potentially cause arterial remodeling,29 and increase the circulation of activated immune cells and inflammatory cytokines.30 Subsequently, endothelial dysfunction, an imbalance in vascular homeostatic responses, may be induced.31 Other possible mechanisms included the imbalance in the autonomic nervous system and direct vasoconstriction.4
This study found that overweight/obesity could enhance the effects of ambient PM2.5 on hypertension, which has not been reported previously. However, this finding was consistent with a few studies on the association between other air pollutants and health outcomes. For instance, a Chinese study found that overweight/obesity could enhance the respiratory effects of PM10 and NO2 among children.32 One US cohort study observed that obesity could modify the short-term effect of ozone (O3) on lung function.33 A clinical trial examining the association between BMI and acute spirometric response to ozone exposure found that the decreased lung function due to O3 exposure was significantly correlated with BMI.34
The underlying mechanism for the enhanced effects of overweight/obesity remained largely unclear. It was possible that systematic inflammation has played a role as PM2.5-induced inflammation has been reported to be higher among participants with higher BMI.35 This finding has also been supported by animal experimental studies,36 which showed that obese mice presented higher airway inflammation than normal-weighted mice.
One important finding of this study was that a higher consumption of fruit could mitigate the adverse effects of ambient PM2.5 on hypertension. This finding was in line with the hypothesized biological mechanism of the health effects of ambient PM2.5, which was mainly related to inflammation and peroxidation through oxidative stress.37 The rich content of vitamin C, carotenoids, and flavonoids in dietary fruit may play an important role in this effect, given the effects of antioxidants against endogenous and exogenous oxidative damage to the airways.38 This finding has been supported by experimental studies. One study compared the oxidative stress in animals that were exposed to the air pollution in Boston and then moved into filtered clean air, resulting in a continuous reduction in oxidative stress in the lungs.39 Similarly, a randomized controlled trial showed that antioxidant supplementation (vitamins C and E) could reduce the adverse effects of air pollution and inflammatory response.40
The modification effect of fruit was convergent with other studies. One study from Detroit also reported that dietary antioxidant intake may protect against the adverse cardiovascular effects of ambient PM2.5.41 A cohort study in Mexico reported that a high intake of fruit could modulate the adverse effect of O3 on lung function among children.42 Studies from Spain and Denmark also suggested that intake of fruit was associated with decreased mental health effects of air pollution.43, 44 Similarly, two recent literature reviews supported that dietary antioxidants may play a modulating role on the adverse health effect of air pollution.10, 45
This study did not find significant effect modification by consumption of vegetables, which might be due to that we did not have sufficient exposure assessment of vegetable consumption, such as the consumption duration, ways of cooking vegetables, and types of vegetables, leading to exposure misclassification. Among the Chinese population, people usually intake uncooked fruit, but cooked vegetables, it was possible that some antioxidant components in the vegetables have been reduced during the cooking process. More studies are warranted to further examine the protective effects of fruit and vegetables consumption for those exposed to ambient PM2.5.
We have estimated the hypertension burden attributable to ambient PM2.5. Previous studies have mainly examined the association between air pollution and health outcomes using relative risk or odds ratios. Compared with those indicators, the attributable risk may provide additional information by showing the proportion of cases due to ambient PM2.5 exposure.20 This approach has been applied in a few previous studies. For example, one study estimated that 1.2% of premature deaths could be prevented if the ambient PM2.5 standard was attained.46 One of our recent studies also showed that about 3.79% of the all-cause mortality could be prevented if the WHO’s annual PM2.5 concentration guideline was attained in six Chinese cities.8
Our study had several strengths. It was a large population-based study with representative adults to examine the association betweenPM2.5 and hypertension in China. We collected extensive individual-level information, allowing us to have a better adjustment for important risk factors; for example, compared with previous studies, we controlled for indoor air pollution. In addition, the use of satellite-based estimates of PM2.5 exposure enabled us to have a virtually complete spatial coverage of PM2.5 among the study participants in the absence of air monitoring data.
This study was also subject to several limitations. This was a cross-sectional study, limiting our ability to establish a causal relationship. We used a three-year average of satellite-based PM2.5 concentrations as the exposure indicator, which may not directly reflect the actual PM2.5 exposure, and exposure misclassification was possible. We estimated yearly mean PM2.5 concentrations during 2013–2015 at about 10 communities within the SAGE project, and compared the estimated concentrations with the measured PM2.5 concentrations, and found that the estimated PM2.5 concentrations could well represent the actual measurements (with a R2 of 0.81, Figure S1). Additionally, we used a wrist device to measure the blood pressure, which might not accurately assess the blood pressure. However one previous validation study indicated that the wrist device could provide acceptable blood pressure measurements.47 Furthermore, some important risk factors of hypertension were not considered, such as noise exposure, migration status, genetic background, and different food styles among different geographic areas. However, in the models, we have included the community as one variable at the second level, which can serve as a proxy for the genetic background and dietary style among different areas. Additionally, the consumption of fruit and vegetables was a complex factor, which might be correlated with other dietary and cardiovascular disease risk factors, such as sodium salt intake, glucose, or lipid metabolism. Lastly, we did not have access to other air pollutants and meteorological factors, and were therefore unable to control for them in our statistical models.
Perspectives
This study suggests that long-term exposure to ambient PM2.5 is associated with increased risk of hypertension and increased blood pressure, and responsible for a remarkable hypertension burden in adults in China. Overweight and obese status could enhance this effect, whereas higher consumption of fruit may mitigate it.
Supplementary Material
Novelty and Significance.
What Is New?
This is a large scale population-based study among Chinese adults aged 50 years and older. The annual PM2.5 concentrations were estimated using satellite data, and a two-level logistic regression model was used to examine the long-term effects of ambient PM2.5 on hypertension and blood pressure.
What Is Relevant?
This study suggests that long-term exposure to ambient PM2.5 is significantly associated with increased risk for hypertension and blood pressure, is responsible for significant hypertension burden in adults in China.
Summary
Long-term exposure to ambient PM2.5 is an important risk factor for arterial blood pressure and hypertension among Chinese adults.
Acknowledgments
We thank the participants who had been generous with their time and assistance. We would also thank the anonymous reviewers for their valuable comments.
Source of Funding
This study was funded by WHO, the US National Institute on Aging through Interagency Agreements (OGHA 04034785; YA1323-08-CN-0020; Y1-AG-1005-01) and through a research grant (R01-AG034479), and Science and Technology Commission of Shanghai Municipality (10XD1403600) and the Health Fields Specific Research Grant (201202012).
Footnotes
Disclosures
None.
Contributor Information
Hualiang Lin, Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention.
Yanfei Guo, Shanghai Municipal Centre for Disease Control and Prevention.
Yang Zheng, Shanghai Municipal Centre for Disease Control and Prevention.
Qian Di, Department of Environmental Health, Harvard T.H. Chan School of Public Health.
Tao Liu, Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention.
Jianpeng Xiao, Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention.
Xing Li, Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention.
Weilin Zeng, Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention.
Lenise A Cummings-Vaughn, School of Medicine, Washington University in St. Louis.
Steven W. Howard, College for Public Health & Social Justice, Saint Louis University
Michael G. Vaughn, College for Public Health & Social Justice, Saint Louis University
Zhengmin (Min) Qian, College for Public Health & Social Justice, Saint Louis University.
Wenjun Ma, Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention.
Fan Wu, Shanghai Municipal Centre for Disease Control and Prevention.
References
- 1.Liu L, Ruddy T, Dalipaj M, Poon R, Szyszkowicz M, You H, Dales RE, Wheeler AJ. Effects of indoor, outdoor, and personal exposure to particulate air pollution on cardiovascular physiology and systemic mediators in seniors. J Occup Environ Med. 2009;51:1088–1098. doi: 10.1097/JOM.0b013e3181b35144. [DOI] [PubMed] [Google Scholar]
- 2.Maheswaran R, Pearson T, Smeeton NC, Beevers SD, Campbell MJ, Wolfe CD. Outdoor air pollution and incidence of ischemic and hemorrhagic stroke. Stroke. 2011;43:22–27. doi: 10.1161/STROKEAHA.110.610238. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz J, Alexeeff SE, Mordukhovich I, Gryparis A, Vokonas P, Suh H, Coull BA. Association between long-term exposure to traffic particles and blood pressure in the veterans administration normative aging study. Occup Environ Med. 2012;69:422–427. doi: 10.1136/oemed-2011-100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR, Rosenberg L. Air pollution and incidence of hypertension and diabetes mellitus in black women living in los angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong GH, Qian ZM, Xaverius PK, Trevathan E, Maalouf S, Parker J, Yang L, Liu MM, Wang D, Ren WH. Association between long-term air pollution and increased blood pressure and hypertension in china. Hypertension. 2013;61:578–584. doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 6.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai Y, Zhang B, Ke W, Feng B, Lin H, Xiao J, Zeng W, Li X, Tao J, Yang Z, Ma W, Liu T. Associations of short-term and long-term exposure to ambient air pollutants with hypertension a systematic review and meta-analysis. Hypertension. 2016;116:07218. doi: 10.1161/HYPERTENSIONAHA.116.07218. [DOI] [PubMed] [Google Scholar]
- 8.Lin H, Liu T, Xiao J, Zeng W, Li X, Guo L, Zhang Y, Xu Y, Tao J, Xian H, Syberg KM, Qian Z, Ma W. Mortality burden of ambient fine particulate air pollution in six chinese cities: Results from the pearl river delta study. Environ Int. 2016;96:91–97. doi: 10.1016/j.envint.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Hart JE, Puett RC, Rexrode KM, Albert CM, Laden F. Effect modification of long‐term air pollution exposures and the risk of incident cardiovascular disease in us women. Journal of the American Heart Association. 2015;4:e002301. doi: 10.1161/JAHA.115.002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romieu I, Castro-Giner F, Kunzli N, Sunyer J. Air pollution, oxidative stress and dietary supplementation: A review. Eur Respir J. 2008;31:179–197. doi: 10.1183/09031936.00128106. [DOI] [PubMed] [Google Scholar]
- 11.Kelly F, Dunster C, Mudway I. Air pollution and the elderly: Oxidant/antioxidant issues worth consideration. Eur Respir J. 2003;21:70s–75s. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
- 12.Balsano C, Alisi A. Antioxidant effects of natural bioactive compounds. Curr Pharm Des. 2009;15:3063–3073. doi: 10.2174/138161209789058084. [DOI] [PubMed] [Google Scholar]
- 13.Wu F, Guo Y, Kowal P, Jiang Y, Yu M, Li X, Zheng Y, Xu J. Prevalence of major chronic conditions among older chinese adults: The study on global ageing and adult health (sage) wave 1. PLoS One. 2013;8:e74176. doi: 10.1371/journal.pone.0074176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Guo Y, Chatterji S, Zheng Y, Naidoo N, Jiang Y, Biritwum R, Yawson A, Minicuci N, Rodriguez A. Common risk factors for chronic non-communicable diseases among older adults in china, ghana, mexico, india, russia and south africa: The study on global ageing and adult health (sage) wave 1. BMC Public Health. 2015;15:88. doi: 10.1186/s12889-015-1407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, Villeneuve PJ. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: Development and application. Environ Health Perspect. 2010;118:847. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy RC, Remer LA, Dubovik O. Global aerosol optical properties and application to moderate resolution imaging spectroradiometer aerosol retrieval over land. Journal of Geophysical Research: Atmospheres. 2007;112:D13210. [Google Scholar]
- 17.He W, Li Q, Yang M, Jiao J, Ma X, Zhou Y, Song A, Heymsfield SB, Zhang S, Zhu S. Lower bmi cutoffs to define overweight and obesity in china. Obesity. 2015;23:684–691. doi: 10.1002/oby.20995. [DOI] [PubMed] [Google Scholar]
- 18.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (gpaq): Nine country reliability and validity study. Journal of Physical Activity & Health. 2009;6:790. doi: 10.1123/jpah.6.6.790. [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Wang H, Wang Q, Xin Q, Lin H. The effect of meteorological factors on adolescent hand, foot, and mouth disease and associated effect modifiers. Global Health Action. 2014;7:24664. doi: 10.3402/gha.v7.24664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H, Liu T, Xiao J, Zeng W, Li X, Guo L, Xu Y, Zhang Y, Vaughn MG, Nelson EJ, Qian Z, Ma W. Quantifying short-term and long-term health benefits of attaining ambient fine particulate pollution standards in guangzhou, china. Atmos Environ. 2016;137:38–44. [Google Scholar]
- 21.Johnson D, Parker JD. Air pollution exposure and self-reported cardiovascular disease. Environ Res. 2009;109:582–589. doi: 10.1016/j.envres.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Kopp A. Spatial association between ambient fine particulate matter and incident hypertension. Circulation. 2014;129:562–569. doi: 10.1161/CIRCULATIONAHA.113.003532. [DOI] [PubMed] [Google Scholar]
- 23.Pope CA, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–115. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 24.Chen SY, Wu CF, Lee JH, Hoffmann B, Peters A, Brunekreef B, Chu DC, Chan CC. Associations between long-term air pollutant exposures and blood pressure in elderly residents of taipei city: A cross-sectional study. Environ Health Perspect. 2015;123:779–784. doi: 10.1289/ehp.1408771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuks K, Moebus S, Hertel S, Viehmann A, Nonnemacher M, Dragano N, Möhlenkamp S, Jakobs H, Kessler C, Erbel R. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ Health Perspect. 2011;119:1706. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chuang KJ, Yan YH, Chiu SY, Cheng TJ. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in taiwan. Occup Environ Med. 2010;68:64–68. doi: 10.1136/oem.2009.052704. [DOI] [PubMed] [Google Scholar]
- 27.Babisch W, Wolf K, Petz M, Heinrich J, Cyrys J, Peters A. Associations between traffic noise, particulate air pollution, hypertension, and isolated systolic hypertension in adults: The kora study. Environ Health Perspect. 2014;122:492. doi: 10.1289/ehp.1306981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brook RD, Bard RL, Burnett RT, Shin HH, Vette A, Croghan C, Phillips M, Rodes C, Thornburg J, Williams R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup Environ Med. 2011;68:224–230. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- 29.Huang W, Wang G, Lu SE, Kipen H, Wang Y, Hu M, Lin W, Rich D, Ohman Strickland P, Diehl SR. Inflammatory and oxidative stress responses of healthy young adults to changes in air quality during the beijing olympics. American Journal ofRespiratory and Critical Care Medicine. 2012;186:1150–1159. doi: 10.1164/rccm.201205-0850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JGS, Fayad ZA, Fuster V, Lippmann M. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 31.Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Dong G, Qian Z, Liu M, Wang D, Ren W, Fu Q, Wang J, Simckes M, Ferguson T, Trevathan E. Obesity enhanced respiratory health effects of ambient air pollution in chinese children: The seven northeastern cities study. Int J Obes. 2013;37:94–100. doi: 10.1038/ijo.2012.125. [DOI] [PubMed] [Google Scholar]
- 33.Alexeeff SE, Litonjua AA, Suh H, Sparrow D, Vokonas PS, Schwartz J. Ozone exposure and lung function: Effect modified by obesity and airways hyperresponsiveness in the va normative aging study. Chest. 2007;132:1890–1897. doi: 10.1378/chest.07-1126. [DOI] [PubMed] [Google Scholar]
- 34.Bennett WD, Hazucha MJ, Folinsbee LJ, Bromberg PA, Kissling GE, London SJ. Acute pulmonary function response to ozone in young adults as a function of body mass index. Inhal Toxicol. 2007;19:1147–1154. doi: 10.1080/08958370701665475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Margretardottir OB, Thorleifsson SJ, Gudmundsson G, Olafsson I, Benediktsdottir B, Janson C, Buist AS, Gislason T. Hypertension, systemic inflammation and body weight in relation to lung function impairment—an epidemiological study. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2009;6:250–255. doi: 10.1080/15412550903049157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shore SA, Rivera-Sanchez YM, Schwartzman I, Johnston RA. Responses to ozone are increased in obese mice. J Appl Physiol. 2003;95:938–945. doi: 10.1152/japplphysiol.00336.2003. [DOI] [PubMed] [Google Scholar]
- 37.Götz AA, Rozman J, Rödel HG, Fuchs H, Gailus-Durner V, de Angelis MH, Klingenspor M, Stoeger T. Comparison of particle-exposure triggered pulmonary and systemic inflammation in mice fed with three different diets. Part Fibre Toxicol. 2011;8:30. doi: 10.1186/1743-8977-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowler RP, Crapo JD. Oxidative stress in airways: Is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med. 2002;166:S38–43. doi: 10.1164/rccm.2206014. [DOI] [PubMed] [Google Scholar]
- 39.Evelson P, González-Flecha B. Time course and quantitative analysis of the adaptive responses to 85% oxygen in the rat lung and heart. Biochimica et Biophysica Acta (BBA)-General Subjects. 2000;1523:209–216. doi: 10.1016/s0304-4165(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 40.Romieu I, Meneses F, Ramirez M, Ruiz S, PADILLA RP, Sienra JJ, Gerber M, Grievink L, Dekker R, Walda I. Antioxidant supplementation and respiratory functions among workers exposed to high levels of ozone. Am J Respir Crit Care Med. 1998;158:226–232. doi: 10.1164/ajrccm.158.1.9712053. [DOI] [PubMed] [Google Scholar]
- 41.Schulz AJ, Mentz GB, Sampson NR, Dvonch JT, Reyes AG, Izumi B. Effects of particulate matter and antioxidant dietary intake on blood pressure. Am J Public Health. 2015;105:1254–1261. doi: 10.2105/AJPH.2014.302176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romieu I, Barraza-Villarreal A, Escamilla-Núñez C, Texcalac-Sangrador JL, Hernandez-Cadena L, Díaz-Sánchez D, De Batlle J, Del Rio-Navarro BE. Dietary intake, lung function and airway inflammation in mexico city school children exposed to air pollutants. Respir Res. 2009;10:122. doi: 10.1186/1465-9921-10-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guxens M, Aguilera I, Ballester F, Estarlich M, Fernández-Somoano A, Lertxundi A, Lertxundi N, Mendez MA, Tardón A, Vrijheid M. Prenatal exposure to residential air pollution and infant mental development: Modulation by antioxidants and detoxification factors. Environ Health Perspect. 2012;120:144. doi: 10.1289/ehp.1103469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raaschou-Nielsen O, Andersen ZJ, Jensen SS, Ketzel M, Sørensen M, Hansen J, Loft S, Tjønneland A, Overvad K. Traffic air pollution and mortality from cardiovascular disease and all causes: A danish cohort study. Environmental Health. 2012;11:60. doi: 10.1186/1476-069X-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tong H. Dietary and pharmacological intervention to mitigate the cardiopulmonary effects of air pollution toxicity. Biochim Biophys Acta. 2016;1860:2891–2898. doi: 10.1016/j.bbagen.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Cohen AJ, Ross Anderson H, Ostro B, Pandey KD, Krzyzanowski M, Künzli N, Gutschmidt K, Pope A, Romieu I, Samet JM. The global burden of disease due to outdoor air pollution. Journal of Toxicology and Environmental Health, Part A. 2005;68:1301–1307. doi: 10.1080/15287390590936166. [DOI] [PubMed] [Google Scholar]
- 47.Tian HY, Liu WJ, Li SG, Song Z, Gong W. Validation of the transtek tmb-988 wrist blood pressure monitor for home blood pressure monitoring according to the international protocol. Blood Press Monit. 2010;15:326–328. doi: 10.1097/MBP.0b013e32833f56fb. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


