Abstract
Farming sturgeons is an economically important practice in a number of Asian and European countries. However, since it is not widely implementedin Turkey, fertilized eggs necessary for research and industrial activities are imported from Germany. Due to the interest of several fish farms in culturing sturgeon in Turkey and the lack of relevant data, this study investigated bacteria related health problems of two different sturgeon species, the diamond sturgeon (Acipenser gueldenstaedtii) and the Siberian sturgeon (Acipenser baerii). The fungal, parasitic and bacterial pathogens found in these fish were investigated until the fish reached about 3 kg of weight (3+ years). A number of bacterial disease pathogens (Acinetobacter radioresistens, some Aeromonas and Pseudomonas species and Bacillus mycoides) and parasite Trichodina sp. and fungus Saprolegnia sp. were identified in the fish. Both phenotypic and molecular characterizations of the isolated bacteria were performed. Furthermore, swim bladder and spinal problems, cannibalism, tumor growth and mechanical injuries on the external surface of the fish were observed during the study period.
Key Words: Bacteria, Health problems, Sturgeons, Turkey
Introduction
Sturgeons are valuable because of their meat and caviar. However, they take longer to mature and are more difficult to culture compared to other fish species (Chebanov and Galich, 2013 ▶). The production of feed for this species may also be considered as another limiting factor in its culture. It is believed that sturgeons are comparatively more resistant to fish diseases; neverthe-less, many studies have shown that their diseases are idiopathic and involve different pathogens.
The most common bacterial pathogen associated with sturgeons (Huso huso and Acipenser gueldenstaedtii) held in a marine environment is Vibrio anguillarum (Austin and Austin, 2007 ▶). The authors of this study believe that V. anguillarum infections increase fish intestinal and peritoneal bleeding. Other reports state that V. alginolyticus and Pasteurella sp. cause mortality in Acipenser baerii (Costinar et al., 2010 ▶). Apart from the mobile Aeromonas sp., Aeromonas salmonicida has been reported to cause a disease that manifests itself as furuncles on the skin of Acipenser oxyrinchus oxyrinchus (Mohler, 2003 ▶). Yersiniosis, or enteric redmouth disease, is frequently seen in aquaculture units and caused by Yersinia ruckeri, which has been isolated from A. baerii in rare cases (Noga, 2010 ▶). Streptococcus dysgalactiae, a Gram-positive bacterium, has been isolated from Acipenser schrenckii in China. Hemorrhages, abdominal swelling and ascites have also been reported in cultured sturgeons (Yang and Li, 2009 ▶). Flavobacterium johnsonae was isolated from cultured sturgeons (Acipenser gueldenstaedtii) (Bauer et al., 2002 ▶) in Russia, and Aeromonas hydrophila and Pseudomonas fluorescence were isolated from adult A. gueldenstaedtii. These bacteria are known to cause serious conditions such as hemorrhage, exophthalmus and ulcers, ultimately leading to the death of the cultured fish (Prearo et al., 2009 ▶; Noga, 2010 ▶).
Studies on parasites in sturgeons show that Trichodina reticulate, a ciliated parasite, and Diplostomum spathaceum, a digenean parasite, are present in reared Acipenser persicus larvae (Bazari et al., 2010 ▶). The authors of this report mention that these parasites were isolated from fish specially reared and released in their habitat. They also found out that these parasites were isolated from various sturgeon species in their natural habitat in Russia (Bauer et al., 2002 ▶).
Fungal diseases of sturgeons listed in a handbook on white sturgeon aquaculture (Acipenser transmontanus) include Saprolegnia sp. as infectious agents, especially in fingerling and spawning adults (Conte, 1988 ▶). Jalilpoor et al. (2006) ▶ also isolated Penicillium spp., Fusarium spp., Mucor spp. and Saprolegnia sp. from the eggs of A. persicus, and found that the mortality rate was highest (7-22%) during Saprolegnia sp. infestation. Three distinct viral diseases, white sturgeon iridovirus (WSIV), white sturgeon herpesvirus-1,2 (WSHV-1, 2) and shovelnose sturgeon iridovirus (SSIV) (Yu-Ping and Di, 2005 ▶), are known to occur in sturgeons.
Sturgeon aquaculture is underdeveloped in Turkey, therefore, limited information is available on their pathogen profile, especially when compared to rainbow trout (Oncorhynchus mykiss), European sea bass (Dicentrarchus labrax) and seabream (Sparus aurata) aquaculture. The pathogens isolated from sturgeons in Turkey are, in fact, isolated from eggs imported from other countries for scientific studies, and individual fish raised from these imported eggs. So far, only bacterial pathogens have been isolated from sturgeons in Turkey and include F. johnsonae (Karataş et al., 2010 ▶), A. hydrophila, and Flavobacterium hydatis, all isolated from A. gueldenstaedtii (Timur et al., 2010 ▶). Another study investigated deformations during larval develop-ment and early feeding stages of the Siberian sturgeon (Acipenser baerii) (Kurtoglu et al., 2015 ▶).
Despite the live natural stock of sturgeons of the Black Sea coastal line, sturgeon culture in Turkey is underdeveloped and scientific data is insufficient. However, in the last few decades, several studies on sturgeon culture have been conducted and aquaculture enterprises have shown more interest in farming this fish. The present study focused on the bacteriological, fungal and parasitological pathogens associated with two different species of sturgeons, namely, the diamond sturgeon (Acipenser gueldenstaedtii) and the Siberian sturgeon (Acipenser baerii), reared in the North Eastern part of Turkey.
Materials and Methods
Fertilized eggs of the diamond sturgeon (Acipenser gueldenstaedtii) and the Siberian sturgeon (Acipenser baerii) were imported from Germany in 2011 as part of the Recep Tayyip Erdoğan University (RTEU) aqua-culture research activity. These eggs were incubated in the RTEU Aquaculture Research and Application Center and were periodically inspected. Five fish were sampled from each species monthly for health problems until they reached an average weight of 3 kg ± 0.25. The study was conducted between July 2011 and May 2014 and all health problems observed during this period were carefully recorded. Fish were fed with appropriate commercial trout feed for different sizes. Quality parameters of the water used in the present study are presented in Table 1.
Table 1.
Water quality parameters
| Parameters | Spring water for incubation | Rearing water |
|---|---|---|
| Total hardness (mg/L as CaCO3) | 25 | 35 |
| Temperature (°C) | 11 | 4-17 |
| pH | 7.2 | 6.5-7.5 |
| Dissolved oxygen (mg/L) | 11.4 | 7-11.1 |
| Ammonia (un-ionized) (mg/L) | >0.01 | >0.01 |
| Nitrite (mg/L) | >0.01 | >0.01 |
| Alkalinity (mg/L as CaCO3) | 13 | 50 |
Fungal and parasitic examinations
All fungal infections observed in the eggs were examined microscopically, at a low magnification of ×4. Samples taken to identify parasitic infections were prepared using scrapings from gills and the skin and stool samples. These samples were examined micros-copically at a magnification of ×40 (Timur and Timur, 2003 ▶) and the parasites were identified based on morphological criteria (Lom and Dykova, 1992 ▶). The parasites isolated from the fish were fixed and stained by ethyl alcohol (10%) and crystal violet used in Gram-stain (one drop/10 cc).
Bacteriological examinations
In order to eliminate non-target and contaminant bacteria, the outer surfaces of normal fish and diseased fish affected by gas problems, external injuries and exophthalmia were cleared for the presence of pathogenic bacteria and cleaned with 70% alcohol. After cleaning, liver, kidney and spleen samples of the fish (5 g-3 kg) were transferred to Tryptic Soy Agar (TSA) under aseptic conditions. The bacterial colonies obtained from these samples were found to be pure colonies and were preserved in media containing 15% glycerol at -70°C for future use (Lasee, 1995 ▶).
Before subjecting the isolates to phenotypic tests, certain pre-tests, such as cytochrome oxidase, Gram-staining, catalase, and motility tests, were conducted. The Analytical Profile Index (API) test (API 20E and API 20NE) was also performed on all bacteria. Glutamate Starch Phenol Red (GSP) selective agar was used to distinguish between the Aeromonas and Pseudomonas species. Blood agar hemolysis was used to identify the Bacillus species, and the bacteria were classified as β-hemolytics or non-hemolytics. Rod-shaped Gram-stained bacteria that showed the presence of an endospore were subjected to motion tests as necessary. The susceptibility to penicillin was also taken into consideration to characterize the bacterial isolates.
For the purpose of identifying bacterial species accurately, DNA obtained from the bacteria was subjected to a universal PCR reaction and the PCR product was sequenced. Bacterial DNA was isolated as follows. Twenty-four-hour fresh bacterial cultures in Tryptic Soy Broth (TSB) medium were centrifuged in 0.5 ml tubes at 4000 × g for 5 min, the supernatant was then discarded, and 100 μL distilled water was added to the pellet. This mixture was boiled to 100°C for 10 min and centrifuged at 20000 × g for 2 min. The supernatant was then stored at -20°C. A PCR reaction for the 16S rRNA gene common to all eubacteria used universal primers (27 F 5´ AGA GTT TGA TCC TGG CTC AG-3´, 1492 R 5´ GTT TAC CTT GTT ACG ACT T-3´) to obtained of the PCR product (1465-bp). Nucleotide sequences were aligned using the BioEdit Sequence Alignment Editor (North Carolina State University, Raleigh, North Carolina) and these sequences were identified using the BLAST program (<http://www.ncbi. nlm.nih.gov>) (Altınok and Grizzle, 2001 ▶).
Results
Fungal and parasitic detections
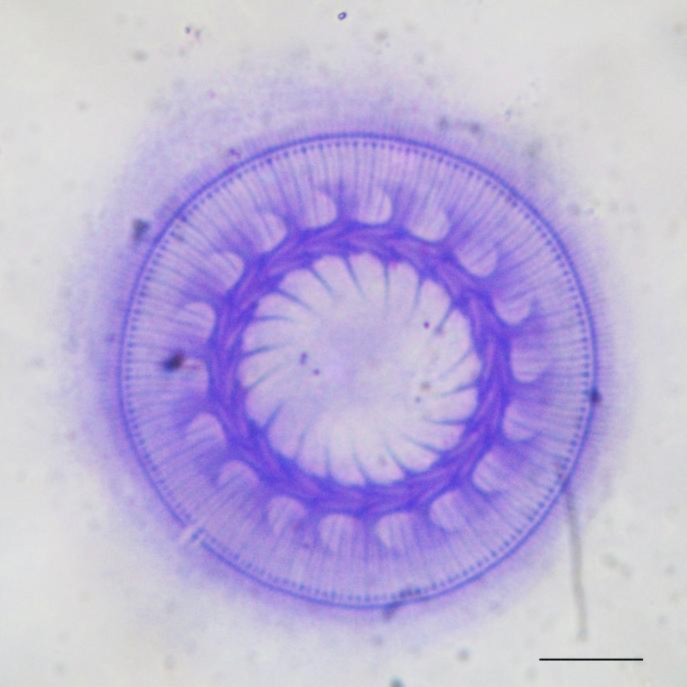
From the beginning, the most distinct health problem was found to be suberication of the dead eggs of both sturgeon species. The fungus isolated from the dead eggs was identified as Saprolegnia sp. From the hatching stage to the larval stage, the survival rate of the sturgeons was found to be 40% on day 90. Trichodina sp. was the only parasitic protozoan species found in the gills of the two species (prevalence, 0.5%) (Fig. 1). During the study period, no tumors were observed in the juveniles.
Fig. 1.
Trichodina sp. (scale bar, 10 μm
The problems of the feeding stage
As the artemia cysts were not completely removed after Artemia (Artemia salina) feeding, they were con-sumed by the larvae. Thus the leading health problem at this stage was death (30% mortality rate) caused by the deposition of A. salina cysts in the intestine. During artificial bait adaptation, a low rate of cannibalism (1%) was especially observed in A. gueldenstaedtii larvae that had completed the egg stage.
Swim bladder problem
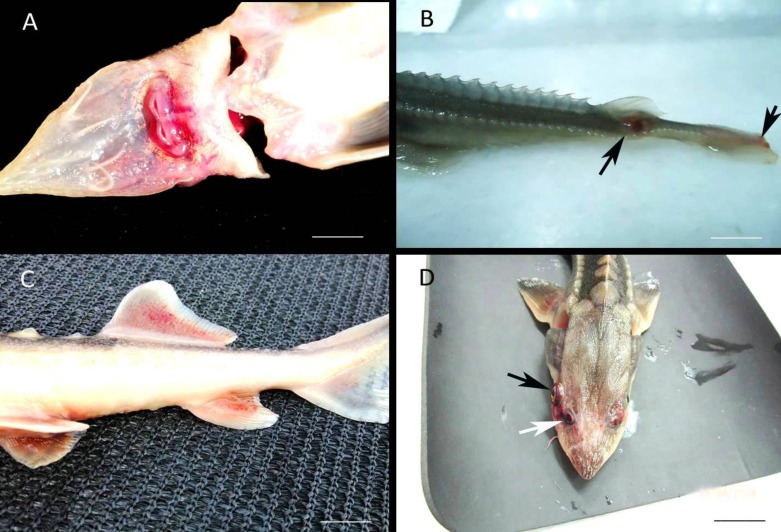
After three months, 5% of the fish were found to be affected by gas problems. Inflation of the swim bladder occurred in both species of sturgeon larvae (A. baerii and A. gueldenstaedtii) using the artificial bait (Figs. 2A, B). The fish also swam upside-down on the surface, exhibited problems in bait taking (Figs. 2C, D), and displayed permanent spinal curvature due to the diurnal gas problem. A bacterial examination of these sturgeons showed the presence of A. hydrophila and Bacillus mycoides in the pneumatic duct which connects the air bladder and the pharynx.
Fig. 2.
Inflation of swim bladder, fill with gas and spinal deformities in Acipenser baerii larvae. Gas problems in swim bladders of different size of fish (A, B), spinal deformities (C, D) (scale bars A: 3 cm, and B: 7 cm
Bacterial detections
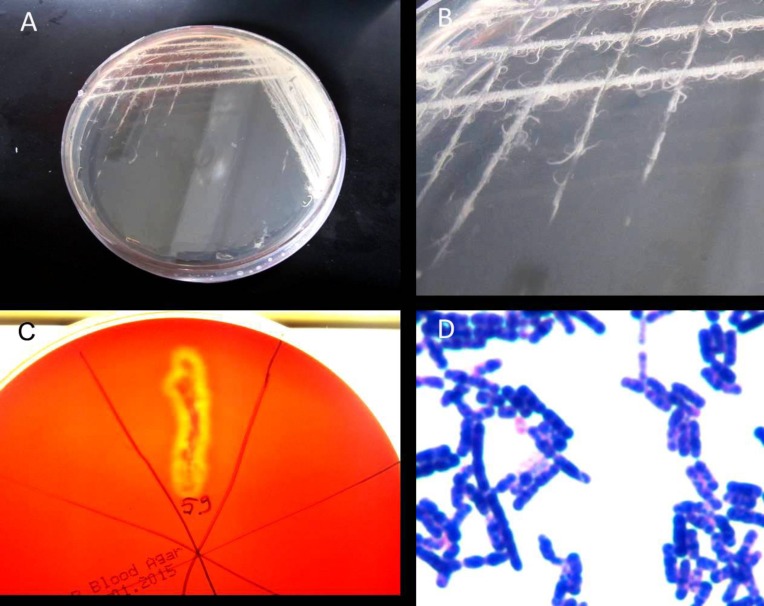
A total of 30 different bacterial strains were isolated from the external lesions and the internal organs of the sturgeons, and biochemical tests carried out after bacterial purification identified these bacteria as belonging to seven different bacterial species. Information on these bacterial species is given in Table 2. The species not subjected to the API test, Gram-positive rods (Fig. 3D) that formed a fringe shaped colony in TSA medium (Figs. 3A, B), demonstrated β hemolysis (Fig. 3C) and resistance to penicillin, were non-motile and were all classified as B. mycoides (Fig. 3C). The presence of many bacterial species implied a mixed infection of these fish, and hemorrhages were observed on the mouth of all bacteria-infected fish (Fig. 4A), apart from the presence of expanded internal organs, and unilateral exophthalmos (Fig. 4D).
Table 2.
Bacterial species and their isolation area on fish
| Bacteria | API CODES | Mole | n | Fish species | Tissues of fish |
|---|---|---|---|---|---|
| Serratia sp. | API 20E 5305363 (91%) | 93% | 3 | Acipenser gueldenstaedti | Liver |
| Acinetobacter radioresistens | API 20NE 0000032 (96.7%) | NA | 3 | Acipenser gueldenstaedti | Skin, liver |
| Aeromonas hydrophila | API 20NE 7047127 (99.7%) | 94% | 7 |
Acipenser baeri
Acipenser gueldenstaedti |
Mouth, skin, liver, pneumatic duct |
| Aeromonas sobria | API 20NE 7136755 (98.4%) | 97% | 2 | Acipenser baeri | Liver |
| Bacillus mycoides | NA | NA | 5 | Acipenser baeri | Esophagus |
| Citrobacter freundii | API 20E 1604573 (95%) | 95% | 2 |
Acipenser baeri
Acipenser gueldenstaedti |
Liver, kidney |
| Pseudomonas sp. | API 20NE 3467747 (94%) | 95% | 8 | Acipenser gueldenstaedti | Mouth, liver, kidney |
| Pseudomonas flourescens | API 20NE 0057555 (99.6%) | 96% | 4 | Acipenser baeri | Skin |
| Pseudomonas putida | API 20NE 0142457 (99.8%) | 96% | 1 | Acipenser baeri | Liver |
n: Number of bacteria, and NA: Not application
Fig. 3.
Bacillus mycoides isolated from the esophagus of Acipenser baeri. The colonies of Bacillus mycoides on TSA medium (A, B), β hemolysis on blood agar (C), and Gram-stain of the bacteria (D
Fig. 4.
External deformations on the fish caused by the bacteria. Hemorrhages on around the mouth (A), and the base of dorsal and caudal fins (B, C), unilateral exophthalmus (black arrow) on the fish Acipenser gueldenstaedtii, spiraculum (white arrow) (D) (scale bars, A: 2 cm, B: 2 cm, C: 5 cm, and D: 5 cm
Other problems
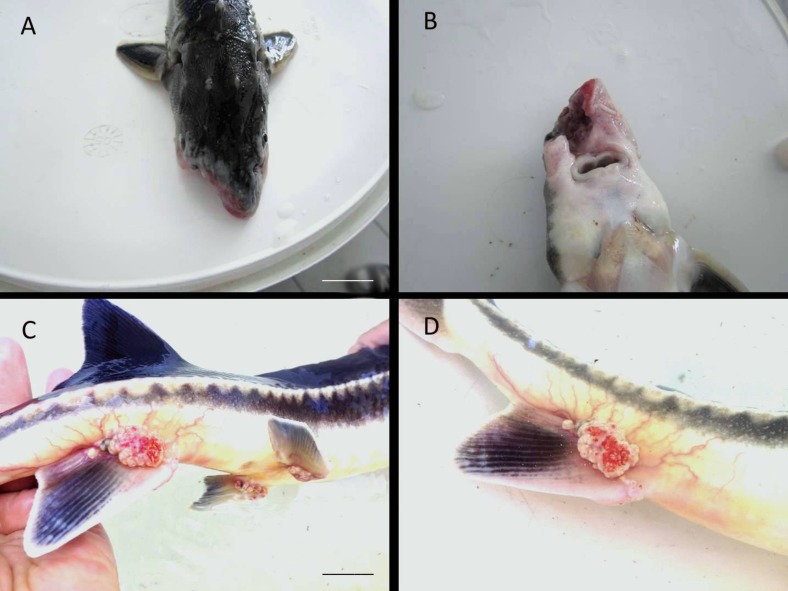
Tumors were observed occasionally at the end of the ventral fins of the Russian sturgeons (A. gueldenstaedtii) that were older than two years and had reached a weight of 3 kg (Figs. 5C, D). Such tumors were not observed in A. baerii individuals. Moreover, mechanical injuries on the mouth were observed, especially in A. baerii individuals (Figs. 5A, B).
Fig. 5.
Mechanical injuries and tumor on Acipenser gueldenstaedtii, mechanical injuries on the mouth of the fish (A, B), clear tumors on the base of the anal fins of the fish (C, D) (scale bars, A: 5 cm, and C: 4 cm
Discussion
Fungal infestation of fish eggs is a common problem in all cultured fish species, and many studies have tried to overcome this problem by treating the eggs with disinfectants and improving water quality (Timur and Timur, 2003 ▶; Lasee, 1995 ▶; Noga, 2010 ▶). Saprolegnia sp. infestations during the incubation period are frequent in rainbow trout commonly reared in Turkey, and formaldehyde applications are used to treat these infections. An intense infestation of Saprolegnia sp. species also causes heavy loss in sturgeon eggs, and the reported death rate of Acipenser persicus eggs due to Saprolegnia sp. is between 7% and 22% (Jalilpoor et al., 2006 ▶). Post-infection measures and ensuring good microbiological quality of the water and its sufficient ventilation are very important. Careful removal of the infected eggs is another technique effective in decreasing loss. Nanosil, Chloramine-T and hydrogen peroxide can also be used to prevent fungal infestation of sturgeon eggs (Ghazvini and Rudsari, 2012 ▶). Available data on cannibalism in sturgeons predominantly pertain to the giant sturgeon (Huso huso); nevertheless, reports of cannibalism in other surgeon species also exist. Cannibalism has been observed in A. gueldenstaedtii larvae at post-incubation days 18 and 36 (Memiş et al., 2009 ▶). In the present study, we observed a low rate of cannibalism which occurred only in A. gueldenstaedtii larvae. To avoid cannibalism, it is important to pay attention to bait sequence and live and artificial baits. For various reasons, the swim bladder disease is very common in fish; the supersaturation of gas, bacterial infections, and sudden changes in water temperature and pressure have all been reported as causes of air bladder diseases (Austin and Austin, 2007 ▶). In this study, the air bladder gas disease was observed in both species of sturgeon due to the supersaturation of gas after adaptation to artificial bait. This can be due to the fact that sturgeons are physostome fish and their air bladder is connected to their esophagus. A large quantity of bacteria was isolated from the esophageal samples, and A. hydrophila was found to be the predominant species. Bacteria such as Yersinia ruckeri, A. hydrophila and Vibrio spp., have an important role among pathogens that cause fish diseases (Kayis et al., 2009 ▶). Literature on bacterial pathogens of sturgeons is limited, probably because the aquaculture of sturgeons is not as common as that of other species and also because sturgeons are quite resistant to diseases after they reach a certain size. Bacterial pathogens isolated from sturgeons in recent years include Flavobacterium johnsoniae, A. hydrophila (Acipenser gueldenstaedtii) (Timur et al., 2010 ▶) and Flavobacterium columnare (Acipenser oxyrinchus desotoi) (Altınok et al., 2001 ▶). In our study, we isolated A. hydrophila, Acinetobacter radioresistens, Aeromonas sobria, A. salmonicida, B. mycoides, Citrobacter freundi, Pseudomonas fluorescens, Pseudomonas putida and Serratia sp. species from the sturgeons; this knowledgebase is important as it will help create a bacterial profile of sturgeons reared in Turkey. The existence of parasitic pathogens in sturgeons has been frequently reported, especially in those cultured in earth pools. T. reticulate, D. spathaceum, and helminths are some of the parasites that have been isolated from sturgeons (Bazari et al., 2010 ▶). In our study, Trichodina sp. was the only parasitic species isolated from sturgeons. It is possible that the use of fiberglass tanks rather than soil pools, provided more favorable conditions for sturgeon culture, especially during the incubation period. Nevertheless, reports of parasites isolated from sturgeons are available and specifically differentiate between fish reared in soil pools and those cultured in their natural environment.
In the present study, we reported on the health problems of sturgeons from the incubation period until they reached a body weight of 3 kg. The following conclusions and recommendations are based on our observations. As aquaculture of sturgeons in Turkey can be initially developed only by importing eggs, more detailed studies are necessary to investigate health problems during production stages. Sturgeons need to be treated with appropriate disinfectants during the incubation period, dead eggs should be removed immediately and the water should be properly ventilated. Baiting should be done frequently in the larval stage to avoid cannibalism. To prevent gas disease and hemorrhage-related deaths due to bacterial pathogens, microbial contamination and water quality should be carefully monitored. Using fiberglass and concrete ponds instead of earth pools may help reduce or even avoid parasite infestations.
Acknowledgment
The study was funded by Recep Tayyip Erdoğan University Research Project Fund (Project number; 2011.103.02.3).
References
- Altinok, I, Grizzle, JM. Effects of low salinities on Flavobacterium columnare infection of euryhaline and freshwater stenohaline fish. J. Fish Dis. 2001;24:361–367. [Google Scholar]
- Austin, B, Austin, DA. Bacterial fish pathogens: diseases of farmed and wild fish. 4th Edn. Chichester, UK: Praxis Publ. Ltd.; 2007. pp. 357–411. [Google Scholar]
- Bauer, ON, Pugachev, ON, Voronin, VN. Study of parasites and diseases of sturgeons in Russia: a review. J. Appl. Ichthyol. 2002;18:420–429. [Google Scholar]
- Bazari Moghaddam, S, Mokhayer, B, Shenavar Masouleh, AR, Jalilpour, J, Masoumzadeh, M, Alizadeh, M. Study on parasitic infestation on Persian sturgeon (Acipenser persicus) larvae and fingerlings in the Shahid Dr. Beheshti Hatchery. JIFRO. 2010;9:342–351. [Google Scholar]
- Chebanov, MS, Galich, EV. Sturgeon hatchery manual. FAO Fisheries and Aquaculture Technical Paper 558. FAO Fisheries and Aquaculture Technical Paper 558. 2013. pp. 1–17.
- Conte, FS. Hatchery manual for the white sturgeon (Acipenser transmontanus Richardson): with application to other North American Acipenseridae. University of California (System). Cooperative Extension, UCANR Publications; 1988. [Google Scholar]
- Costinar, L, Herman, V, Pascu, C, Marcu, AD, Marcu, A, Faur, B. Isolation and characterization of Vibrio alginolyticus and Pasteurella spp from Siberian sturgeon Acinpenser baerii. Lucrari Stiintifice Medicina Veterinara. 2010;43:125–128. [Google Scholar]
- Ghazvini, A, Rudsari, HV, Takami, GA, Masuleh, ARS, Ashourpour, A. Disinfection efficiency of three anti-fungal agents (nanosil, chloramine-T and hydrogen peroxide) on Persian sturgeon (Acipenser persicus, Borodin 1897) larvae. Int. J. Biol. 2012;4:138–145. [Google Scholar]
- Jalilpoor, J, Masouleh, AS, Masoumzadeh, M. Fungal flora in Acipenser persicus eggs with particular emphasis on Saprolegnia sp (Oomycetes) and mortality during mass incubation at the Shahid Beheshti hatchery. J. Appl. Ichthyol. 2006;22:265–268. [Google Scholar]
- Karataş, S, Ercan, D, Stenium, TM, Turgay, E, Memiş, D, Candan, A. First isolation of a Flavobacterium johnsoniae like bacteria from Cultured Russian sturgeon in Turkey. J. Anim. Vet. Adv. 2010;9:1943–1946. [Google Scholar]
- Kayis, S, Ozcelep, T, Capkin, E, Altinok, I. Protozoan and metazoan parasites of fish in the Turkey and their applied treatments. IJA. 2009;61:93–102. [Google Scholar]
- Kurtoğlu, İZ, Ak, K, Delihasan Sonay, F, Kayış, Ş, Balta, F, Köse, Ö, Şahin, T. Encountered deforma-tions, during the larvae development and early feeding stages in Siberian sturgeon, Acipenser baerii. ECJSE. 2015;2:1–11. [Google Scholar]
- Lasee, BA. Introduction to fish health management, U.S. Fish and Wildlife Service. Lester Avenue Onalaska, Wisconsin: La Crosse Fish Health Center 555; 1995. 54650 pp. [Google Scholar]
- Lom, J, Dykova, L. Protozoan parasites of fishes. Dev. Aquacult. Fish. Sci. 1992;26:250–301. [Google Scholar]
- Memiş, D, Ercan, E, Çelikkale, MS, Timur, M, Zarkua, Z. Growth and survival rate of Russian sturgeon (Acipenser gueldenstaedtii) larvae from fertilized eggs to artificial feding. Turkish J. Fish. Aquatic Sci. 2009;9:47–52. [Google Scholar]
- Mohler, JV. Culture manual for the Atlantic sturgeon. Westgate Center Drive Hadley, Massachusetts: A Region 5 U.S. Fish & Wildlife Service Publication 300; 2003. [Google Scholar]
- Noga, EJ. Fish disease – diagnosis and treatment. 2nd Edn. New Jersey: Hoboken, Wiley-Blackwell; 2010. pp. 197–350. [Google Scholar]
- Prearo, M, Squadrone, S, Fioravanti, ML, Giorgi, I, Marturano, S, Gasparri, F, Abete, MC, Zanoni, RG. Italian farmed sturgeon: mortality events during 2004-2008, WAS 2009. Veracruz, Mexico: 2009. 681 pp. [Google Scholar]
- Timur, G, Akaylı, T, Korun, J, Yardımcı, ERA. Study on bacterial haemorrhagic septicemia in farmed Young Russian sturgeon in Turkey (Acipencer gueldenstaedtii) Turk. J. Fish. Aquat. Sci. 2010;25:19–27. [Google Scholar]
- Timur M, Timur G. Fish diseases. Publications of Istanbul University. Publication No: 4426, Section of Aquatic Publication Number: 5; pp. 1–213. [Google Scholar]
- Yang, W, Li, A. Isolation and characterization of Streptococcus dysgalactiae from diseased Acipenser schrenckii. Aquaculture. 2009;294:14–17. [Google Scholar]
- Yu-Ping, H, Di, W. A review of stugeon virosis. J. Foresty Res. 2005;16:79–82. [Google Scholar]