Abstract
Objective
Ovarian needle aspiration and biopsy (ONAB) may be employed for pretreatment diagnosis of ovarian malignancies or intraoperatively to facilitate removal of ovarian masses. However, there is reluctance to utilize this procedure due to potential cyst rupture or seeding of malignant cells. The objective of this study was to examine the efficacy of ONAB over a 13-year period at our institution.
Methods
Between 2000 and 2013, all ONAB specimens were identified from the Queen's Medical Center Pathology Department database. All cytologic specimens were reviewed and correlated with histopathologic findings. A retrospective chart review was conducted to retrieve data on clinical course and treatment.
Results
This study identified 144 cases of ovarian masses sampled by aspiration or needle biopsy between 2000 and 2013. Ninety-two (64%) cases had corresponding histopathology, 84 (91%) of which were obtained concomitantly. On histology, 12 (13%) cases were malignant and 80 (87%) benign. Three false negative cases were noted; 2 serous borderline tumors and 1 mucinous cystadenocarcinoma. These were sampling errors; no diagnostic tumor cells were present in the aspirates. Sensitivity and specificity of ONAB in the detection of malignancy were 75% and 100%, respectively. The positive and negative predictive values were 100% and 96%, respectively.
Conclusion
ONAB represents a valuable tool in the diagnosis of malignancy and treatment of ovarian masses. In our study, it was highly specific, with excellent positive and negative predictive value.
Keywords: Needle Biopsy, Ovarian Neoplasms
INTRODUCTION
Ovarian cysts and tumors often represent a diagnostic challenge. Although ovarian cancer is relatively uncommon, accounting for only 3% of malignancies in women, it is the leading cause of gynecologic cancer deaths in the United States. Malignant ovarian tumors often present with identifiable imaging characteristics and an elevated CA-125 level [1]. Traditionally, however, definitive diagnosis requires removal of the entire adnexa with histopathologic evaluation. Historically, needle aspiration has been discouraged, because of the risk of spillage and possible upstaging. Needle aspiration, however, is widely used for a variety of non-ovarian tumors, with no adverse impact on prognosis [2]. Furthermore, studies on peritoneal seeding with ovarian needle biopsy are limited, and the magnitude of risk is poorly defined [3]. Needle aspiration, therefore, may be considered as a diagnostic alternative to removal of the adnexa through an open operation.
There are specific clinical scenarios when ovarian needle aspiration may represent the preferred approach. In a patient that is medically unfit for open surgery, needle biopsy represents a safer and non-invasive diagnostic tool. Needle biopsy may also be used in patients that present with a clinically advanced malignancy, when primary or neoadjuvant chemotherapy may be appropriate. Alternatively, needle aspiration of an adnexal cyst may be used to facilitate removal utilizing a minimally invasive approach. Intra-operative decompression can be used to facilitate removal of the adnexa. Finally, bedside aspiration of a benign-appearing simple cyst may be used for diagnostic reassurance and symptomatic relief. The current study is a retrospective review of the utilization of ovarian needle biopsy and aspiration at our institution. Cytologic findings are presented; and sensitivity and specificity are determined based on subsequent histopathologic diagnoses.
MATERIALS AND METHODS
Institutional Review Board approval was obtained from the Queen's Medical Center prior to reviewing patient materials and records. A Natural Language Search was undertaken in our CoPath™ (Cerner Information Systems, Tucson, AZ, USA) database to search for all women who underwent ovarian needle aspirations and/or biopsies at the Queen's Medical Center and Hawaii Pathologists' Laboratory from April 1, 2000 to September 30, 2013.
Radiologists performed image-guided biopsies of solid pelvic masses in patients with evidence of advanced stage malignancy or in cases of suspected cancer recurrence (Fig. 1). For solid tissue biopsy, radiologists performed computerized tomography-assisted biopsies, using the Quick-Core® Coaxial Biopsy Needle Set (Cook Medical, Bloomington, IN, USA) with 17G or 19G guiding needles and 18G or 20G co-axial needles. In all procedures, intraprocedural rapid on-site evaluation (ROSE) with imprint cytology was performed concurrently by a cytopathologist. After performing the ovarian core needle biopsy, the radiologist placed the tissue on a slide, which was then moved into a formalin container by cytology personnel. The slide with the imprint cytology of the core material was then stained on-site with the Diff-Quick stain and immediately evaluated by a cytopathologist, in order to ensure adequate tissue for histologic evaluation.
Fig. 1.
Ovarian needle biopsy and cyst aspiration (2000–2013) with histologic follow-up.
Gynecologists, surgeons, and other clinicians performed cyst aspirations (Fig. 1) in a number of ways. In most cases, the cyst was aspirated intraoperatively at the time of laparoscopy or laparotomy to decompress the cyst and facilitate cyst removal. Laparoscopically, the ovarian cyst was aspirated using a laparoscopic suction device with attached needle, or cyst fluid was collected during decompression of the mass after the cyst or ovary was removed and placed in a bag. At the time of laparotomy, the cyst cavity would be entered using a gallbladder trocar, scalpel, or needle; and the cyst fluid collected. Cyst aspiration was also occasionally performed at the bedside for persistent simple ovarian cysts. This was done transvaginally under ultrasound guidance using a 17G needle with manual or electric suction. ROSE was not performed during any of the ovarian cyst aspirations. In cases where concurrent cytology and surgical specimens were obtained, the cytologic diagnoses were usually rendered independently of the histologic evaluations of the surgical specimens.
For ovarian cyst fluids, 2 cytospin slides were prepared by centrifuging the cyst contents for 5 minutes at 2,300 revolutions per minute in the Rotina 380 Benchtop Centrifuge (Andrews Hettich GmbH & Co. KG, Tuttlinggen, Germany). Air-dried and 95% alcohol-fixed cytospin preparations were stained with Diff-Quik and Papanicolaou stains (Thermo Fischer Scientific, Waltham, MA, USA) stains, respectively. In cases with sufficient cellularity, 95% alcohol was added to the residual material and centrifuged in order to create a cell block. The resultant cell pellets were wrapped in rice paper and submitted for routine histologic processing.
An unpaired 2-tailed t-test for continuous data statistical calculations was performed using GraphPad statistical software (GraphPad, La Jolla, CA, USA). A p-value of <0.05 was considered statistically significant.
RESULTS
This study identified 144 cases of ovarian lesions diagnosed by aspiration cytology or needle biopsy in our institution between 2000 and 2013. The cytologic diagnosis, derived from both ovarian aspiration cytology as well as imprint cytology of ovarian needle biopsies, was benign in 135 cases (94%) and malignant in 9 (6%) cases. There were a total of 12 malignancies: 3 cases that were reported as benign on cytology were subsequently found to be malignant on histopathology. The overall mean age of the patients in our study was 42 years (range 13–83). The correlation of cytologic diagnoses with patient age is shown in Table 1. The mean age of patients with benign lesions was 41 years, whereas the mean age of patients with malignant lesions was 54 years (p<0.010). Of the 9 malignant lesions diagnosed on cytology, 4 were treated with primary surgery. Two patients were treated with neoadjuvant chemotherapy due to evidence of advanced stage disease followed by interval surgery. Two patients were treated with chemotherapy alone. Clinical follow-up information was not available for the remaining case. There were no known complications resulting from the ovarian aspiration or biopsy procedure.
Table 1. Ovarian needle aspiration/biopsy diagnosis and patient age (144 cases).
| Cytologic diagnosis | No. (%) of cases | Mean age (range) | |
|---|---|---|---|
| Benign | 135 (94) | 41 (13–83) | |
| Cyst | 134 (93) | 41 (13–83) | |
| Infection | 1 (1) | 43 (43) | |
| Malignant | 9 (6) | 54 (23–74) | |
| Total | 144 (100) | 42 (13–83) | |
The type of ovarian lesion sampled differed by specialty (Fig. 1, Table 2). There were 9 solid ovarian masses; the remaining 135 specimens were cystic. Radiologists performed computed tomography (CT)-guided needle biopsies on all 9 (6%) solid ovarian masses. Eight of these solid masses were cytologically identified as malignant; the benign solid lesion was a fibroma. Gynecologists submitted the majority of cystic specimens, accounting for 122 (85%) cases overall, and general surgeons aspirated 4 (3%) cases. The “other” category refers to physicians of unknown medical specialty who contributed 9 (6%) specimens. The general surgeons and “other” clinicians were practicing in outside hospitals where they were required to perform ovarian procedures due to the shortage of gynecological medical personnel. Nearly all of the cystic specimens sampled by gynecologists, surgeons, and other clinicians were diagnosed cytologically as benign. The only cystic mass that was diagnosed by cytology as malignant was a clear cell carcinoma aspirated intraoperatively by a gynecologist.
Table 2. Ovarian cytologic diagnosis by biopsy operator/clinician (144 cases).
| Operator | Total (%) | Benign | Malignant neoplasms | |
|---|---|---|---|---|
| Cyst | Infection | |||
| Gynecologist/surgeon | 126 (88) | 124 | 1 | 1 |
| Radiologist | 9 (6) | 1 | - | 8 |
| Other | 9 (6) | 9 | - | - |
| Total | 144 (100) | 134 | 1 | 9 |
Neither tumor laterality nor size had any bearing on the sensitivity of ovarian aspiration and needle biopsy cytology. The mean size of malignant tumors detected on cytology was 11.6 cm (range 3.0–18.1 cm), whereas the mean size of the 3 false negative cases was 20.2 cm (range 10.2–40.0 cm). For masses with a cystic component, cyst volume similarly was not a predictor of malignancy. Cyst fluid volume for benign lesions ranged from 1.0 to 700.0 mL. Four of the malignancies examined cytologically were cystic with fluid volumes ranging from 12.5 to 2,000.0 mL.
The presence of clear fluid may indicate a benign mass as all malignant cystic masses contained dark brown or black fluid, and there were no instances of malignancy when clear cyst fluid was obtained. However, the presence of brown or black fluid does not reliably predict a cancerous lesion. Only 18% of all brown or black cystic fluids were malignant, and 13% of benign cases also presented with brown cystic fluid. Of the 4 cystic malignancies with cytologic fluid evaluation, 3 were false negatives. Two were borderline serous tumors with 12.5 mL of cloudy brown fluid and 20 mL of turbid brown, thick, viscous liquid, respectively. The third false negative case was a mucinous cystadenocarcinoma with 110 mL of black fluid. Cyst rupture occurred intraoperatively, which resulted in upstaging from IA to IC. The cytologically positive cystic malignancy was a clear cell carcinoma that yielded 2,000 mL of dark brown fluid. Intraoperative cyst aspiration did not result in upstaging in this case as pelvic tumor implants were noted at the time of surgery.
Ninety-two (64%) of the 144 cases had corresponding histologic specimens; 84 of these specimens were removed concurrently at the time of surgery (Table 3). For patients with corresponding histology, 83 (90%) cases were classified cytologically as benign, and 9 (10%) were classified cytologically as malignant. Three false negative cytologic diagnoses were identified when compared to the histopathologic specimen: 2 borderline serous tumors, and 1 mucinous cystadenocarcinoma. All of these masses were cystic. Gynecologists sampled the mucinous cystadenocarcinoma and one of the serous borderline tumors. A general surgeon submitted the remaining serous borderline tumor. The cytologically identified malignancies included 7 primary epithelial ovarian cancers (Figs. 2, 3, 4, 5), 1 granulosa cell tumor (Fig. 6), and 1 metastatic lung adenocarcinoma (Fig. 7). All 3 false negative specimens were sampling errors; no diagnostic tumor cells were present in the cyst aspirates. For patients without corresponding histology, 52 cases were classified as cytologically benign. There were no false positive cases. The overall sensitivity and specificity of ovarian needle aspiration and biopsy (ONAB) was 75% and 100%, respectively. The positive and negative predictive values for the detection of a malignancy were 100% and 96%, respectively.
Table 3. Cytologic-histologic correlation of ovarian lesions (92 cases with corresponding histology).
| Variables | Histologic diagnosis | Total | Cytologic diagnosis | |
|---|---|---|---|---|
| Benign | Malignant | |||
| Benign (n=80) | Benign cyst | 19 | 19 | 0 |
| Endometriotic cyst | 12 | 12 | 0 | |
| Hydatid cyst of Morgagni | 2 | 2 | 0 | |
| Hydrosalpinx | 3 | 3 | 0 | |
| Mature cystic teratoma | 10 | 10 | 0 | |
| Serous cystadenoma | 27 | 27 | 0 | |
| Mucinous cystadenoma | 7 | 7 | 0 | |
| Malignant (n=12) | Serous borderline tumor | 2 | 2 | 0 |
| Mucinous borderline tumor | 2 | 0 | 2 | |
| Serous cystadenocarcinoma | 2 | 0 | 2 | |
| Mucinous cystadenocarcinoma | 1 | 1 | 0 | |
| Clear cell carcinoma | 1 | 0 | 1 | |
| Endometrioid carcinoma | 1 | 0 | 1 | |
| Granulosa cell tumor | 1 | 0 | 1 | |
| Metastatic lung adenocarcinoma | 1 | 0 | 1 | |
| Ovarian carcinoma NOS | 1 | 0 | 1 | |
| Total | 92 | 83 | 9 | |
NOS, not otherwise specified.
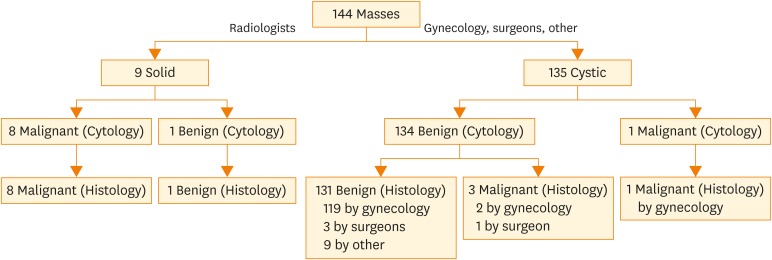
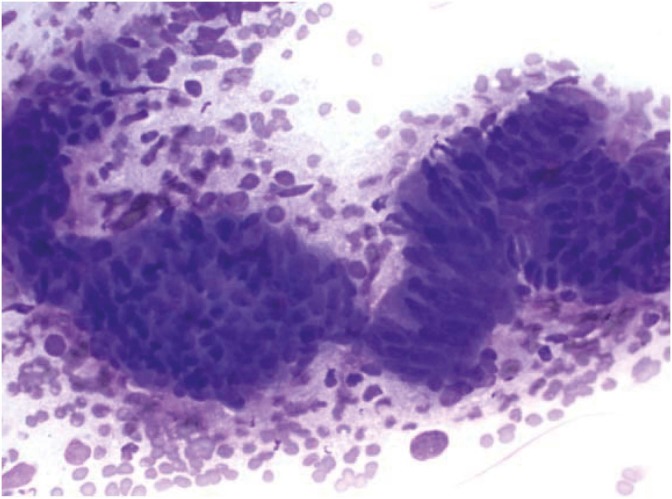
Fig. 2.
Cytology of serous ovarian carcinoma with marked nuclear pleomorphism and prominent nucleoli (Diff-Quik, ×600).
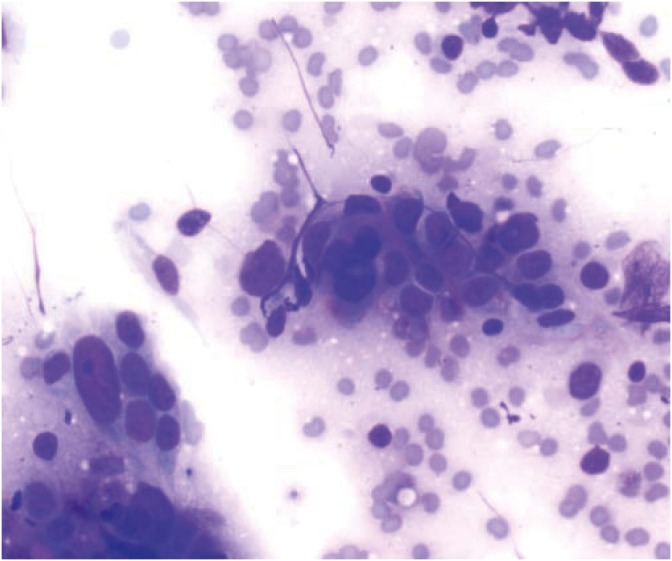
Fig. 3.
Cytology of mucinous borderline tumor showing honeycomb tumor sheets in a background of abundant mucin (Diff-Quik, ×200).
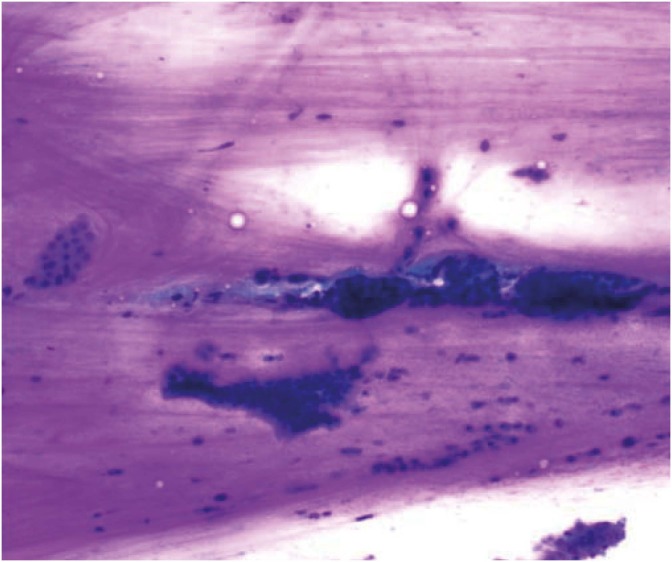
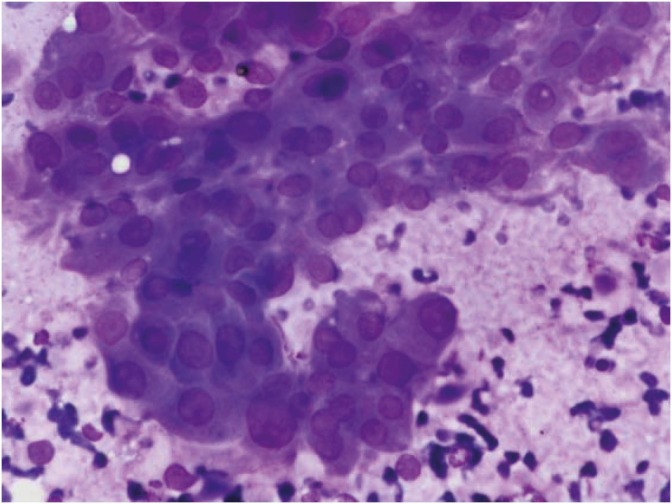
Fig. 4.
Cytology of clear cell ovarian carcinoma: 3-dimensional tumor cluster with enlarged vesicular nuclei, prominent nucleoli, and abundant clear cytoplasm (Pap, ×1,000).
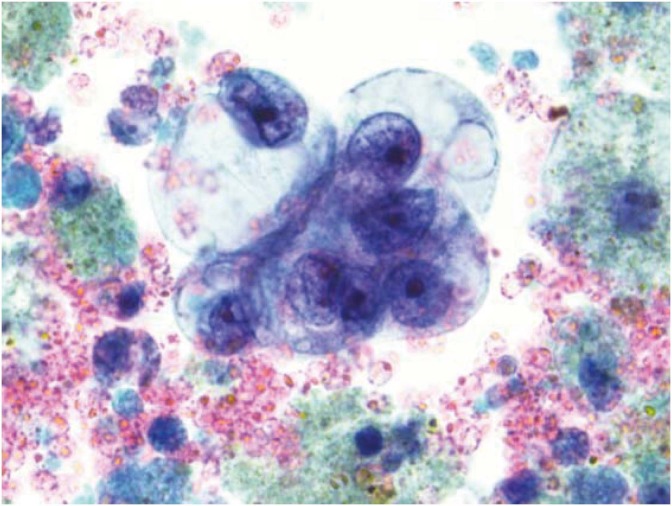
Fig. 5.
Cytology of endometrioid ovarian carcinoma with crowded overlapping clusters containing columnar tumor cells (Diff-Quik, ×400).
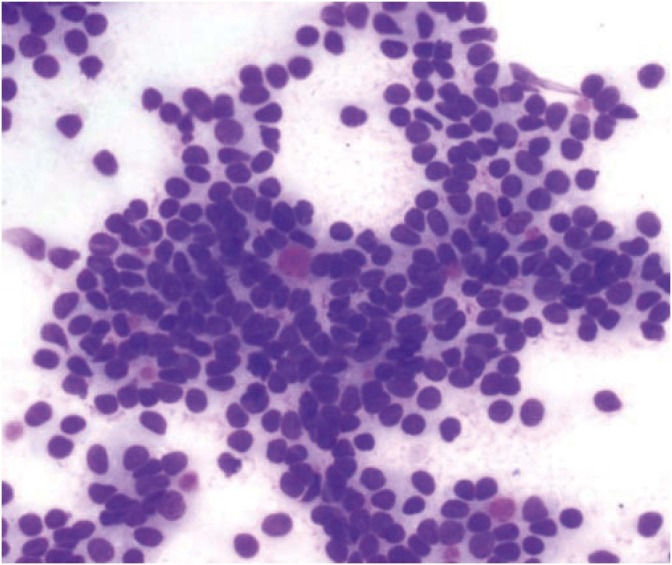
Fig. 6.
Cytology of granulosa cell tumor with Call-Exner bodies and intranuclear grooves (Diff-Quik, ×600).
Fig. 7.
Metastatic pulmonary adenocarcinoma showing pseudopapillary cluster with nuclear enlargement and cellular crowding (Diff-Quik, ×400).
All 8 of the solid non-cystic ovarian lesions biopsied by radiologists were diagnosed correctly by cytology as malignancies. All of these radiologically-guided procedures employed ROSE by the cytopathologist. In all 9 cases, including the 8 malignancies, the ROSE imprint cytology diagnosis correlated with the final histologic diagnosis. ROSE was not performed in any of the ovarian cyst aspirations by gynecologists, surgeons, or other clinicians.
DISCUSSION
Ovarian aspiration and needle biopsy can play a valuable role in the diagnosis and management of ovarian masses. However, its use has been discouraged due to low sensitivity rates and concerns about peritoneal tumor seeding. Evidence of malignant dissemination with ovarian aspiration is sparse. There are no reports of such adverse complications in the literature after 2000 [3]. Furthermore, the only prior reference citing any complications was published over 20 years ago [4]; this was a report of 2 cases. In our study, there was 1 known instance of upstaging as a result of intraoperative rupture of a malignant ovarian cyst. This is not a concern in patients who already have evidence of high-stage malignancy or who are medically unfit for surgery. Therefore, we believe that needle aspiration of ovarian masses is a viable diagnostic alternative to an invasive surgical procedure. It can be used to obtain a tissue diagnosis in patients with clinicoradiographic evidence of advanced cancer who can benefit from neoadjuvant chemotherapy or in those who are poor surgical candidiates. Aspiration and decompression of benign-appearing cystic ovarian masses may also serve a therapeutic purpose by facilitating removal during minimally invasive surgery. While needle aspiration may have limitations, our study shows that it provides excellent specificity and positive predictive value in the diagnosis of ovarian cystic masses as well as very low complication rates.
In prior studies, sensitivity rates of ovarian aspiration and needle biopsy varied widely from 25% to 100% [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. We reported sensitivity of ovarian needle biopsy to be 75%, which is within the range of prior studies. We discovered 3 false negative cases due to sampling error, including 2 serous borderline tumors and 1 mucinous cystadenocarcinoma. False negativity has been attributed to multiple factors, including insufficient or low specimen cellularity [6,7,8,10,12,15,16,17,20,21,22], borderline ovarian tumors [3,12,14,20,21,23], sampling error [5,13], cellular degeneration [15,18], interpretation error [17] and ovarian surface involvement by metastatic carcinoma [20]. Insufficient or low cellularity of cystic samples was the most commonly reported cause of false negative results, rendering the specimen non-diagnostic in 17%–43% of studies [6,7,8,10,11,12,15,16,17,20,21,22]. Borderline tumors may be difficult to diagnose on cytologic examination because the neoplastic cells show less cellular atypia than frank carcinomas [3,12,14,20,21,23]. Furthermore, stromal invasion may not be definitively assessed by aspiration cytology or needle biopsy.
Reported specificity rates ranged from 84%–100%. Specificity in our study was 100%. Misinterpretation appeared to be the major reason for false positive cases [13,16,17]. Roy et al. [13] reported a false positive case of mucinous cystadenoma read as mucinous cystadenocarcinoma on cytology due to the presence of small glandular clusters and a small cell ball giving the impression of multilayering. Uguz et al. [16] had a false positive case of a serous papillary cystadenoma that was read as malignant on cytology due to the presence of highly cellular smears and atypical cellular features. They also stated that mature teratomas and fibromas can show concerning cytologic features on smears. Kadivar et al. [17] also found that cellular atypia and hypercellularity can be found in some follicular cysts, leading to potential false positive diagnoses. There were no false positive cases in our series.
In our study, neither cyst fluid volume nor appearance was a reliable predictor of malignancy. Fluid volumes for malignant cysts ranged from 12.5–2,000.0 mL, whereas those for benign lesions ranged from 1.0 to 700.0 mL, indicating a significant overlap between the 2 diagnostic categories. The appearance of the cyst fluid in malignant cases was either brown or black; however 18 benign cysts, including 8 cases of endometriosis, also produced brown appearing cyst fluid. Benign cysts containing brown or black fluid had volumes between 4 and 700 mL (mean 78 mL), which showed significant overlap with malignant cysts (range 12.5–2,000.0 mL; mean 536 mL). Although the appearance and volume of the cyst fluid did not reliably correlate with malignancy, it is notable that all cysts with clear fluid were benign. Therefore, when clear fluid is aspirated from an ovarian cyst, cytologic evaluation can be safely omitted.
The presence of a solid mass should raise suspicion for malignancy. Eight of the 9 solid ovarian masses were diagnosed as malignant. These lesions ranged in size from 3.0–18.1 cm (mean 11.3 cm).
The addition of ROSE to ovarian needle biopsy/aspiration adds to the diagnostic accuracy of ovarian core needle biopsy. In our series, radiologists performed the majority of biopsies of malignant lesions with a high accuracy rate. All 8 of the solid, non-cystic ovarian specimens biopsied under CT-guidance by radiologists were correctly diagnosed as malignant. Image-guided biopsy allows for precise sampling of the solid tumor component. The use of ROSE with assistance from cytopathologists was provided in all image-guided biopsy cases; this can assure specimen adequacy and improve diagnostic sensitivity. Gynecologists and surgeons, conversely, did not utilize ROSE for concurrent cytologic evaluation. Therefore, the role of ROSE during intra-operative cyst drainage remains unclear.
In conclusion, ovarian aspiration and needle biopsy is a promising tool for clinicians in the evaluation and management of ovarian masses. Our study demonstrates excellent positive predictive value and good sensitivity. Evolving techniques, including the addition of ROSE to image-guided biopsy, improves diagnostic accuracy. Pretreatment diagnosis allows for an informed approach to treatment planning. The study also demonstrates that aspiration of cystic ovarian masses can be safely utilized to facilitate surgery. Concerns for peritoneal tumor seeding during ovarian aspiration or biopsy are not well-documented in the literature, and further study is needed to investigate the subsequent effect on outcome.
ACKNOWLEDGMENTS
We would like to thank the Hawaii Pathologists' Laboratory and Dr. Mikio Hashiguchi of Okinawa Chubu Hospital.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
This article has been presented as a poster presentation at the Society of American Cytopathology Annual Scientific Meeting, 2012 [1] and the 102nd Annual Meeting of the Japanese Society of Pathology, 2013 [2]. Also, it was not presented at the 2013 ASCO Annual Meeting but was published in conjunction with the meeting [3].
- Mafnas C, Kaneshiro R, Hashiguchi M, Tauchi-Nishi P. Cytologic-histologic correlation of ovarian aspiration cytology/needle biopsy: a study of 80 cases with a review of the literature. J Am Soc Cytopathol 2012;1 Suppl 1:S58-9.
- Nagamine K, Shinoda H, Terada K, Shimizu D, Tauchi-Nishi P. Cytologic-histologic correlation of ovarian aspiration cytology/needle biopsy: a study of 84 cases with a review of the literature. Poster presentation at the 102nd Annual Meeting of the Japanese Society of Pathology, Sapporo, Japan, 2013.
- Nagamine K, Terada K, Shimizu D, Tauchi-Nishi P. Ovarian needle biopsy: a study of 84 cases with cytologic-histologic correlation. J Clin Oncol 2013;31 Suppl: abstr e16501.
- Conceptualization: T.N.P.
- Data curation: N.K., K.J., K.R., T.N.P.
- Formal analysis: N.K., T.N.P., T.K.
- Funding acquisition: T.N.P.
- Investigation: N.K., K.J., K.R., T.N.P.
- Methodology: T.N.P.
- Project administration: T.N.P., T.K.
- Resources: N.K., K.J., T.N.P.
- Supervision: T.N.P., T.K.
- Validation: N.K., T.N.P., T.K.
- Visualization: N.K., K.R., T.N.P.
- Writing - original draft: N.K., K.J., T.N.P.
- Writing - review & editing: N.K., T.N.P., T.K.
References
- 1.American College of Obstetricians and Gynecologists ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol. 2007;110:201–214. doi: 10.1097/01.AOG.0000263913.92942.40. [DOI] [PubMed] [Google Scholar]
- 2.van Zante A, Ljung BM. Fine-needle aspiration versus core needle biopsy: reconsidering the evidence of superiority. Cancer. 2016;124:853–856. doi: 10.1002/cncy.21788. [DOI] [PubMed] [Google Scholar]
- 3.Cole L, Mount S, Nuzzo E, Wong C. Aspiration cytology of ovarian cystic masses: histologic correlation and review of the literature. Acta Cytol. 2011;55:19–25. doi: 10.1159/000320873. [DOI] [PubMed] [Google Scholar]
- 4.Trimbos JB, Hacker NF. The case against aspirating ovarian cysts. Cancer. 1993;72:828–831. doi: 10.1002/1097-0142(19930801)72:3<828::aid-cncr2820720331>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 5.Moran O, Menczer J, Ben-Baruch G, Lipitz S, Goor E. Cytologic examination of ovarian cyst fluid for the distinction between benign and malignant tumors. Obstet Gynecol. 1993;82:444–446. [PubMed] [Google Scholar]
- 6.Dordoni D, Zaglio S, Zucca S, Favalli G. The role of sonographically guided aspiration in the clinical management of ovarian cysts. J Ultrasound Med. 1993;12:27–31. doi: 10.7863/jum.1993.12.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Ganjei P, Dickinson B, Harrison T, Nassiri M, Lu Y. Aspiration cytology of neoplastic and non-neoplastic ovarian cysts: is it accurate? Int J Gynecol Pathol. 1996;15:94–101. doi: 10.1097/00004347-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Balat O, Sarac K, Sonmez S. Ultrasound guided aspiration of benign ovarian cysts: an alternative to surgery? Eur J Radiol. 1996;22:136–137. doi: 10.1016/0720-048x(95)00719-7. [DOI] [PubMed] [Google Scholar]
- 9.Caspi B, Goldchmit R, Zalel Y, Appelman Z, Insler V. Sonographically guided aspiration of ovarian cyst with simple appearance. J Ultrasound Med. 1996;15:297–300. doi: 10.7863/jum.1996.15.4.297. [DOI] [PubMed] [Google Scholar]
- 10.Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol. 1999;180:550–553. doi: 10.1016/s0002-9378(99)70252-8. [DOI] [PubMed] [Google Scholar]
- 11.Allias F, Chanoz J, Blache G, Thivolet-Bejui F, Vancina S. Value of ultrasound-guided fine-needle aspiration in the management of ovarian and paraovarian cysts. Diagn Cytopathol. 2000;22:70–80. doi: 10.1002/(sici)1097-0339(200002)22:2<70::aid-dc3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Onsurbe P, Ruiz Villaespesa A, Sanz Anquela JM, Valenzuela Ruiz PL. Aspiration cytology of 147 adnexal cysts with histologic correlation. Acta Cytol. 2001;45:941–947. doi: 10.1159/000328368. [DOI] [PubMed] [Google Scholar]
- 13.Roy M, Bhattacharya A, Roy A, Sanyal S, Sangal MK, Dasgupta S, et al. Fine needle aspiration cytology of ovarian neoplasms. J Cytol. 2003;20:31–35. [Google Scholar]
- 14.Papathanasiou K, Giannoulis C, Dovas D, Tolikas A, Tantanasis T, Tzafettas JM. Fine needle aspiration cytology of the ovary: is it reliable? Clin Exp Obstet Gynecol. 2004;31:191–193. [PubMed] [Google Scholar]
- 15.Hemalatha AL, Divya P, Mamatha R. Image-directed percutaneous FNAC of ovarian neoplasms. Indian J Pathol Microbiol. 2005;48:305–309. [PubMed] [Google Scholar]
- 16.Uguz A, Ersoz C, Bolat F, Gokdemir A, Vardar MA. Fine needle aspiration cytology of ovarian lesions. Acta Cytol. 2005;49:144–148. doi: 10.1159/000326122. [DOI] [PubMed] [Google Scholar]
- 17.Kadivar M, Karamvandi M. Fine needle aspiration cytology of ovarian lesions: Is it reliable? Asia Pac J Clin Oncol. 2008;4:143–148. [Google Scholar]
- 18.Khan N, Afroz N, Aqil B, Khan T, Ahmad I. Neoplastic and nonneoplastic ovarian masses: diagnosis on cytology. J Cytol. 2009;26:129–133. doi: 10.4103/0970-9371.62180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sood T, Handa U, Mohan H, Goel P. Evaluation of aspiration cytology of ovarian masses with histopathological correlation. Cytopathology. 2010;21:176–185. doi: 10.1111/j.1365-2303.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- 20.Mehdi G, Maheshwari V, Afzal S, Ansari HA, Ansari M. Image-guided fine-needle aspiration cytology of ovarian tumors: an assessment of diagnostic efficacy. J Cytol. 2010;27:91–95. doi: 10.4103/0970-9371.71872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel S, Agarwal D, Goel N, Naim M, Khan T. Ekrammulah. Ultrasound guided fine needle aspiration cytology in ovarian neoplasms: an assessment of diagnostic accuracy and efficacy and role in clinical management. Internet J Pathol. 2010;11:1–6. [Google Scholar]
- 22.Gupta N, Rajwanshi A, Dhaliwal LK, Khandelwal N, Dey P, Srinivasan R, et al. Fine needle aspiration cytology in ovarian lesions: an institutional experience of 584 cases. Cytopathology. 2012;23:300–307. doi: 10.1111/j.1365-2303.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- 23.Bandyopadhyay A, Chakraborty J, Chowdhury AR, Bhattacharya A, Bhattachrya P, Chowdhury M. Fine needle aspiration cytology of ovarian tumors with histological correlation. J Cytol. 2012;29:35–40. doi: 10.4103/0970-9371.93218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garady C, Boerner SL. Does cytological examination of ovarian cyst fluids obtained during oophorectomy add useful information? Mod Pathol. 2013;26:A369. [Google Scholar]
- 25.Ray S, Gangopadhyay M, Bandyopadhyay A, Majumdar K, Chaudhury N. USG guided FNAC of ovarian mass lesions: a cyto-histopathological correlation, with emphasis on its role in pre-operative management guidelines. J Turk Ger Gynecol Assoc. 2014;15:6–12. doi: 10.5152/jtgga.2014.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal S, Chakrabarti S, Deuoghuria D, Phukan JP, Sinha A, Mondal PK. Evaluation of ultrasound-guided fine-needle aspiration cytology of ovarian masses with histopathological correlation. Acta Cytol. 2015;59:149–155. doi: 10.1159/000380937. [DOI] [PubMed] [Google Scholar]
- 27.García-Tejedor A, Castellarnau M, Burdio F, Fernández E, Martí D, Pla MJ, et al. Ultrasound-guided aspiration of adnexal cysts with a low risk of malignancy: is it a recommendable option? J Ultrasound Med. 2015;34:985–991. doi: 10.7863/ultra.34.6.985. [DOI] [PubMed] [Google Scholar]