Abstract
Objective
To evaluate the oncologic safety of ovarian preservation (OP) in premenopausal women diagnosed with the International Federation of Gynecology and Obstetrics (FIGO) stage I uterine sarcoma.
Methods
The National Cancer Institute's Surveillance, Epidemiology, and End Results database was accessed and a cohort of women aged ≤50 diagnosed between 1988–2013 with a sarcoma limited to the uterus was drawn. Based on site-specific surgery codes, women who underwent hysterectomy with or without oophorectomy and did not receive radiation therapy were selected for further analysis. Overall (OS) and cancer-specific (CSS) survival were determined following generation of Kaplan-Meier curves; comparisons were made with the log-rank test. A Cox-proportional hazard model was constructed to control for possible confounders.
Results
A total of 1,482 women were included in the analysis; 800 (54.0%) were diagnosed with leiomyosarcoma (LMS), 520 (35.1%) with low-grade endometrial stromal sarcoma (LG-ESS), and 162 (10.9%) with adenosarcoma (AS). The OP group included 418 women (28.2%). Differences in the rate of OP were noted based on histology (p=0.014), year of diagnosis (p=0.001), patient age (p<0.001) and race (p=0.012). There was no difference in OS (p=0.220) or CSS (p=0.210) between women who had OP and those who did not. Multivariate analysis confirmed that OP was not associated with a worse mortality.
Conclusion
In this population-based cohort of women with sarcoma limited to the uterus, OP was not associated with worse oncologic outcomes. OP could be considered for women with LMS, sparing them from the morbidity associated with iatrogenic menopause. No conclusions could be made for those with LG-ESS or AS.
Keywords: Sarcoma, Leiomyosarcoma, Adenosarcoma of the Uterus, Endometrial Stromal Sarcoma, Ovariectomy
INTRODUCTION
Uterine sarcomas are a heterogenous group of tumors deriving from mesenchymal cells and accounting for approximately 3% of all uterine malignancies [1]. The most common histologic subtype is leiomyosarcoma (LMS) followed by low-grade endometrial stromal sarcoma (LG-ESS) and adenosacroma [1]. In general, uterine sarcomas exhibit an aggressive clinical behavior and are usually associated with high rates of recurrence and a poor prognosis [1,2]. Interestingly, their peak incidence occurs at a younger age compared to the more prevalent endometrial tumors [3]. Standard surgical management of uterine sarcoma includes hysterectomy and bilateral salpingo-oophorectomy (BSO) performed to remove any potential metastases to the ovaries [2,4]. Moreover, given that most histologic subtypes of uterine sarcoma frequently express estrogen and progesterone receptors, iatrogenic menopause aims in eliminating the possibility of tumor recurrence and growth due to stimulation from endogenous steroid hormones [5,6,7,8]. However, iatrogenic menopause is associated with significant short and long-term morbidity, having a significant impact on the quality of life of patients [9]. In addition, the diagnosis of LMS and LG-ESS is often established from the histopathology report following surgery for a presumed benign indication; for these women the removal of the ovaries might require a re-operation [10,11]. While the safety of ovarian preservation (OP) for early stage endometrial cancer has been studied extensively, current evidence for women with uterine sarcoma is scarce and mainly derives from small case-series [6,8,11,12,13,14,15,16,17,18,19,20,21,22,23]. In this retrospective study, we investigate the oncologic safety of OP for premenopausal women diagnosed with a sarcoma limited to the uterus (the International Federation of Gynecology and Obstetrics [FIGO] stage I), using a large multi-institutional population-based database.
MATERIALS AND METHODS
A cohort of women diagnosed between 1988 and 2013 with a uterine sarcoma was drawn from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) database [24]. Data deriving from 18 cancer registries were included in the present study (namely Detroit, Iowa, Kentucky, Louisiana, Utah, Connecticut, New Jersey, Atlanta, Rural and Greater Georgia, Alaska, California, Hawaii, Los Angeles, New Mexico, San Francisco, San Jose, and Seattle) as released in April 2016 [25]. The SEER database covers approximately 27.8% of the total United States population based on the 2010 census and incorporates high-quality data on primary malignant tumors [24]. All patient data are de-identified and available to the public for research purposes. An exemption was also granted from obtaining institutional review board approval.
The following inclusion criteria were applied: 1) patient age ≤50 years; 2) diagnosis of a primary tumor with malignant behavior located at the uterus (International Classification of Diseases for Oncology, 3rd Edition [ICD-O-3]/World Health Organization [WHO] 2008 site-specific codes C540–549, 559); 3) active follow-up (diagnosis not obtained from autopsy or death certificate); and 4) histologically confirmed sarcoma limited to the uterus (FIGO stage I disease). The following ICD-O-3 histology codes were employed to identify cases of uterine sarcoma: LMS (8,890/3, 8,891/3, 8,896/3), endometrial stromal sarcoma (8,805/3, 8,930/3, 8,931/3), and adenosarcoma (AS) (8,933/3). Based on histological and grading information, cases of endometrial stromal sarcoma were further classified into low-grade (well and moderately differentiated) and high-grade (poorly differentiated and undifferentiated) tumors. Women who did not undergo hysterectomy (assessed from site-specific surgery codes), as well as those with lymph node or distant metastases were excluded. As previously described site-specific surgical codes including oophorectomy were considered as the oophorectomy group, and surgical codes without oophorectomy were considered as the OP group [26]. Women with unknown status of oophorectomy were excluded from further analysis. Moreover, given that the administration of radiation therapy can have a negative impact ovarian function and induce premature ovarian failure women who received any form of radiotherapy were excluded. Lastly, since carcinosarcoma is currently regarded as a dedifferentiated form of endometrial carcinoma, and not as part of the pure sarcoma group, we opted not to include in the present study women diagnosed with this tumor [1].
Information available at the “EOD-10” and “Collaborative Staging” fields was employed to determine tumor size and extent and the presence of positive lymph node or distant metastases. Stage I disease was defined as tumor limited to the uterus as outlined in the revised 2008 FIGO staging system for uterine sarcomas [1]. Sub-classification into FIGO stage IA, IB, and IC disease was based on tumor size for women with LMS and endometrial stromal sarcoma (<5 cm, ≥5 cm) and on the presence and depth of myometrial invasion for those with AS [1]. Given that substage IC is only valid for AS, it was grouped with FIGO stage IB for analysis purposes [1]. Women who underwent lymphadenectomy/lymph node sampling (lymph node dissection [LND]) were identified from information available at the histopathology report. Given that treatment of uterine sarcomas did not change drastically following a specific year, year of diagnosis was mathematically classified into 3 time periods, 1988–1996, 1997–2005, and 2006–2013 to control for possible advances in surgical techniques and knowledge on the management of uterine sarcomas. Women were also categorized into 4 age groups; ≤35, 36–40, 41–45, 46–50 years.
Primary outcome was death from all causes (overall survival [OS]) while secondary outcome was death from uterine sarcoma (cancer-specific survival [CSS]). Possible “follow-up discard” cases were excluded from the survival analysis. Five-year OS and CSS rates were calculated following generation of Kaplan-Meier curves; univariate comparisons were made with the log-rank test. Women who were alive at the last follow-up were censored. For the estimation of CSS, only those with one primary tumor or the first of multiple primary tumors were included; women who died from causes other than ovarian cancer were censored. In addition, a Cox proportional hazard model was constructed to assess overall and cancer-specific mortality after controlling for variables that according to univariate analysis are associated with survival. Frequency of distribution of categorical and continuous variables was compared with the χ2 test and Mann-Whitney U test, respectively. All statistical analyses were performed with the SPSS ver. 24 statistical package (SPSS Inc., Chicago, IL, USA) and the alpha level of statistical significance was set at 0.05.
RESULTS
A total of 1,516 women with FIGO stage I uterine sarcoma who met the inclusion criteria were identified. Given the very low incidence of high-grade endometrial stromal sarcoma (HG-ESS) (34 women) in the present study these cases were excluded from further analysis. The final study population included 1,482 patients; 800 (54.0%) cases of LMS, 520 (35.1%) cases of LG-ESS, and 162 (10.9%) cases of AS. Median patient age was 44 years (range 16–50); 10.7% were ≤35 years while 16.5%, 31.4%, and 41.4% were 36–40, 41–45, and 46–50 years old, respectively. The majority were of White (57.1%) race followed by Hispanic (16.9%), Black (14.8%), and Asian/Native American (11.2%). Median tumor size was 6.5 cm (range 0.1–55.0 cm); 8 cm for LMS, 5 cm for LG-ESS, and 3.5 cm for AS (p<0.001). Based on available information on tumor extent, the majority of women with LMS presented with stage IB disease (80.3%) compared to 50.6% of those with LG-ESS (p<0.001). For cases of AS, 69 (58%) women had stage IA while 42 (35.3%) and 8 (6.7%) had stage IB and IC disease, respectively. According to the histopathology report, LND was performed in 29.4% of the patients; higher rates of LND were noted for women with AS (50.6%) and LG-ESS (29.0%) compared to LMS (25.3%) (p<0.001).
Overall, OP was documented in 418 women (28.2%). An increase in the rate of OP was noted per study period; 21.6% of women diagnosed between 1988–1996 did not undergo oophorectomy compared to 26.5% and 32.9% of those diagnosed between 1997–2005 and 2006–2013, respectively (p=0.001). Women who did not undergo oophorectomy were younger compared to those who did (median age 42 vs. 45 years, p<0.001). More specifically rate of OP was 41.8% and 38.8% for women aged ≤35 and 36–40 years compared to 33.3% and 16.6% for those aged 41–45 and 46–50 years (p<0.001). Rate of OP was also lower among Asian/Native American (23.6%) and White (26.1%) women compared to women of Black (32.6%) and Hispanic race (34.8%) (p=0.012). Following stratification by tumor histology, rate of OP was higher for women diagnosed with LMS (29.6%) and LG-ESS (29.0%) compared to those diagnosed with AS (18.5%) (p=0.014). By logistic regression, year of diagnosis (p<0.001), age (p<0.001), and histology (p=0.001) but not patient race (p=0.190) was associated with the likelihood to undergo oophorectomy. Women who did not undergo removal of the ovaries were less likely undergo LND (16.5% vs. 34.4%, p<0.001). No differences in the rate of OP were noted based on region of diagnosis (p=0.200), marital status (p=0.440), tumor size (p=0.680), or FIGO substage (p=0.770).
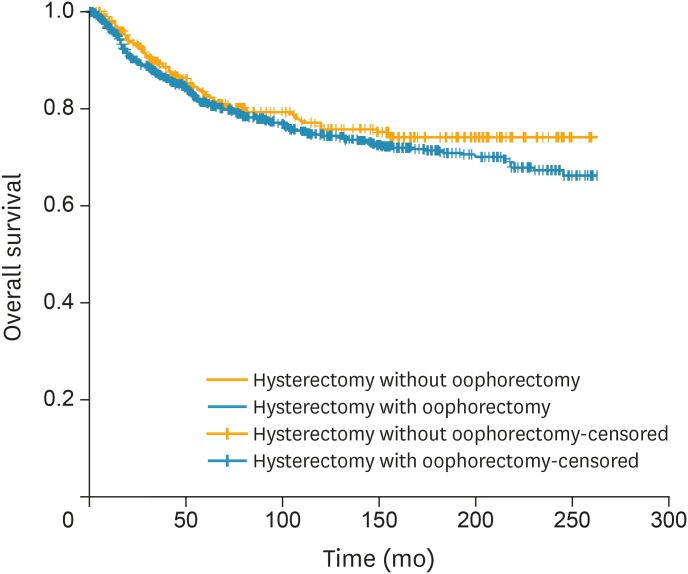
According to the reverse Kaplan-Meier method, median follow-up in the OP and oophorectomy groups was 94 and 123 months, respectively. There was no difference in overall (p=0.220) (Fig. 1) or cancer-specific (p=0.210) survival between women who underwent oophorectomy and those who did not. Similar results were noted when women who did not undergo comprehensive staging (LND in addition to hysterectomy) were excluded (OS: p=0.680; CSS: p=0.280). Following stratification by FIGO substage, no difference in survival was noted for women stage IA disease (OS: p=0.220; CSS: p=0.470). However, for those with stage IB disease OP was associated with a better survival (OS: p=0.044; CSS: p=0.040).
Fig. 1.
OS of women diagnosed with FIGO stage I uterine sarcoma stratified by performance of oophorectomy (n=1,052 in oophorectomy and n=414 in OP group, p=0.220 from log-rank test).
FIGO, the International Federation of Gynecology and Obstetrics; OP, ovarian preservation; OS, overall survival.
In the present cohort by univariate analysis, variables associated with OS were age (p=0.001), FIGO substage (p<0.001), and histology (p<0.001) but not year of diagnosis (p=0.780), marital status (p=0.690) or patient race (p=0.290), while LND approached statistical significance (p=0.068). Similarly parameters associated with CSS were histology (p<0.001), patient age (p=0.004), FIGO substage (p<0.001) but not year of diagnosis (p=0.870), patient race (p=0.770), LND (p=0.250), or marital status (p=0.420). In the multivariate model, after controlling for patient age, tumor histology, FIGO substage and performance of LND, OP was not associated with worse mortality (hazard ratio [HR]=0.86; 95% confidence interval [CI]=0.63–1.17; p=0.340). Similarly, after controlling for patient age, tumor histology and FIGO substage, OP was not associated with worse cancer-specific mortality (HR=0.85; 95% CI=0.61–1.20; p=0.360).
Differences in OS and CSS between the OP and oophorectomy groups were also evaluated following stratification by tumor histology. In the LMS group, while women with OP had better 5-year OS (72.8% vs. 68.9%) and CSS (74.2% vs. 70.8%) rates, this difference did not reach statistical significance (OS: p=0.078; CSS: p=0.098). Variables associated with survival were age (OS: p=0.006; CSS: p=0.004) and FIGO substage (OS: p<0.001; CSS: p<0.001). After controlling for age and FIGO substage, OP was not associated with worse overall (HR=0.82; 95% CI=0.59–1.14; p=0.230) or cancer-specific mortality (HR=0.84; 95% CI=0.59–1.20; p=0.340).
For women diagnosed with LG-ESS, OP was associated with comparable OS (p=0.410) and CSS (p=0.560) rates while no variable was associated with OS or CSS. In the AS group, no difference in OS (p=0.350) or CSS (p=0.690) was observed based on the performance of oophorectomy. Only FIGO substage was associated with OS (p=0.021) and CSS (p=0.016). After controlling for FIGO substage, OP was not associated with a worse mortality (HR=0.49; 95% CI=0.06–3.87; p=0.500). Figs. 2, 3, 4 depict OS in OP and oophorectomy groups for each histologic subtype of uterine sarcoma. Table 1 summarizes demographic, clinicopathological characteristics, and survival of women aged ≤50 years with uterine sarcoma limited to the uterus stratified by performance of oophorectomy.
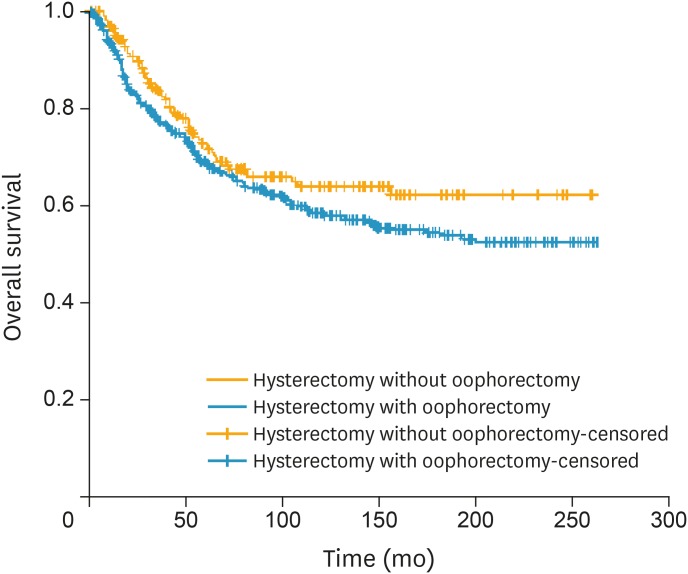
Fig. 2.
OS of women with FIGO stage I LMS of the uterus stratified by performance of oophorectomy (n=557 in oophorectomy and n=233 in OP group, p=0.078 from log-rank test).
FIGO, the International Federation of Gynecology and Obstetrics; LMS, leiomyosarcoma; OP, ovarian preservation; OS, overall survival.
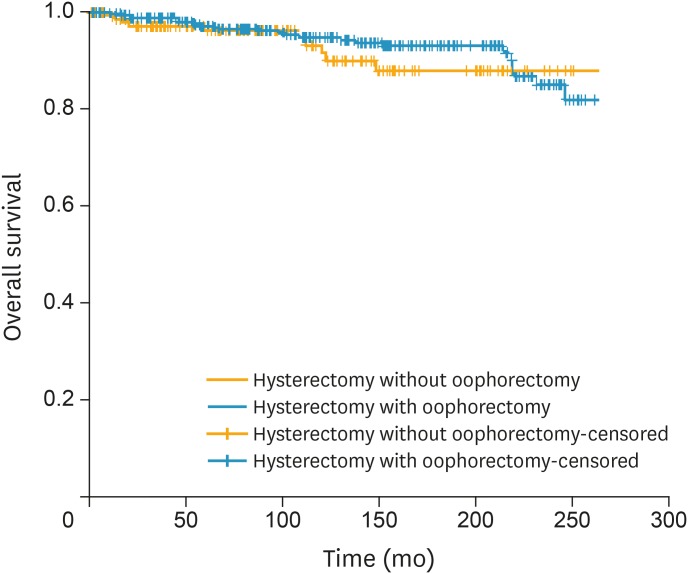
Fig. 3.
OS of women with FIGO stage I LG-ESS of the uterus stratified by performance of oophorectomy (n=366 in oophorectomy and n=151 in OP group, p=0.410 from log-rank test).
FIGO, the International Federation of Gynecology and Obstetrics; LG-ESS, low-grade endometrial stromal sarcoma; OP, ovarian preservation; OS, overall survival.
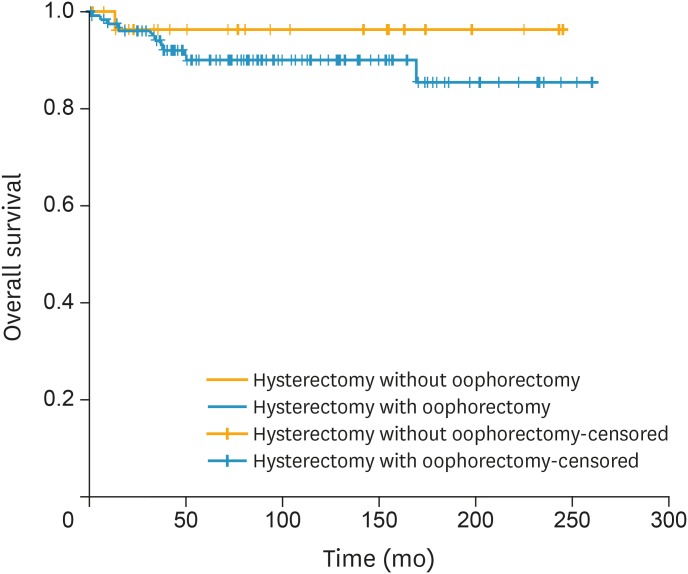
Fig. 4.
OS of women with FIGO stage I AS of the uterus stratified by performance of oophorectomy (n=129 in oophorectomy and n=30 in OP group, p=0.350 from log-rank test).
AS, adenosarcoma; FIGO, the International Federation of Gynecology and Obstetrics; OP, ovarian preservation; OS, overall survival.
Table 1. Demographic, clinicopathological characteristics, and survival of premenopausal women aged ≤50 years diagnosed with uterine sarcoma limited to the uterus stratified by performance of oophorectomy.
| Characteristics | OP | Oophorectomy | p-value | Overall | |
|---|---|---|---|---|---|
| Age (yr) | 42 (18–50) | 45 (16–50) | <0.001† | 44 (16–50) | |
| Age (yr) | <0.001‡ | ||||
| ≤35 | 66 (41.8) | 92 (58.2) | 158 (10.7) | ||
| 36–40 | 95 (38.8) | 150 (61.2) | 245 (16.5) | ||
| 41–45 | 155 (33.3) | 311 (66.7) | 466 (31.4) | ||
| 46–50 | 102 (16.6) | 511 (83.4) | 613 (41.4) | ||
| Race* | 0.012‡ | ||||
| White | 220 (26.1) | 624 (73.9) | 844 (57.1) | ||
| Hispanic | 87 (34.8) | 163 (65.2) | 250 (16.9) | ||
| Black | 71 (32.6) | 147 (67.4) | 218 (14.8) | ||
| Asian/native American | 39 (23.6) | 126 (76.4) | 165 (11.2) | ||
| Region | 0.201‡ | ||||
| West | 219 (27.7) | 572 (72.3) | 791 (53.4) | ||
| East | 109 (31.8) | 234 (68.2) | 343 (23.1) | ||
| Central | 90 (25.9) | 258 (74.1) | 348 (23.5) | ||
| Year of diagnosis | 0.001‡ | ||||
| 1988–1996 | 66 (21.6) | 240 (78.4) | 306 (20.6) | ||
| 1997–2005 | 145 (26.5) | 402 (73.5) | 547 (36.9) | ||
| 2006–2013 | 207 (32.9) | 422 (67.1) | 629 (42.4) | ||
| Marital status* | 0.440‡ | ||||
| Married | 244 (27.1) | 655 (72.9) | 899 (63.2) | ||
| Single/separated/widowed | 152 (29.1) | 371 (70.9) | 523 (36.8) | ||
| Histology | 0.014‡ | ||||
| LMS | 238 (29.6) | 563 (70.4) | 800 (54.0) | ||
| LG-ESS | 151 (29.0) | 369 (71.0) | 520 (35.1) | ||
| AS | 30 (18.5) | 132 (81.5) | 162 (10.9) | ||
| FIGO substage* | 0.770‡ | ||||
| IA | 107 (28.2) | 272 (71.8) | 379 (33.4) | ||
| IB/IC | 220 (29.1) | 537 (70.9) | 757 (66.6) | ||
| Tumor size*, median (cm) | 6.5 (n=315) | 6.9 (n=761) | 0.680† | 6.5 (n=1,076) | |
| LND | <0.001‡ | ||||
| No/unknown | 349 (33.3) | 698 (66.7) | 1,047 (70.6) | ||
| Yes | 69 (15.9) | 366 (84.1) | 435 (29.4) | ||
| 5-yr OS (%) | 83.5 | 81.2 | 0.220§ | 81.9 | |
| 5-yr CSS (%) | 84.8 | 82.9 | 0.210§ | 83.4 | |
Data shown are median (range) or number (%) not otherwise specified.
AS, adenosarcoma; CSS, cancer-specific survival; FIGO, the International Federation of Gynecology and Obstetrics; HG-ESS, high-grade endometrial stromal sarcoma; LG-ESS, low-grade endometrial stromal sarcoma; LMS, leiomyosarcoma; LND, lymph node dissection; OP, ovarian preservation; OS, overall survival.
*Based on available information; †p-value from Mann-Whitney U test; ‡p-value from χ2 test; §p-value from log-rank test.
DISCUSSION
To our knowledge this is the largest population-based study evaluating the oncologic safety of OP for patients with uterine sarcoma while focusing solely on premenopausal women diagnosed with stage I disease, according to the revised FIGO staging system. After controlling for major confounders such as FIGO substage, tumor histology and patient age no mortality benefit was found for those who had oophorectomy.
LG-ESS is a tumor characterized by an indolent course and a very favorable prognosis when limited to the uterus [1]. Since LG-ESS is regarded as a hormone responsive tumor due to the consistent expression of estrogen and progesterone receptors [6,8,27,28], several retrospective studies have evaluated the safety of OP reaching conflicting conclusions [6,8,11,12,13,14,15,16,29,30]. In a multi-institutional case-control study, Li et al. [11] compared 12 premenopausal women who did not undergo BSO with 24 matched controls and did not find any differences in relapse rate, progression free, or OS. Moreover, in another retrospective study, Amant et al. [15] reported similar relapse rates for premenopausal women with stage I LG-ESS regardless of the performance of BSO (3/12, 25% and 1/6, 17% in BSO and OP groups, respectively). However, other studies suggest women with preserved ovarian function may indeed face a higher risk of tumor recurrence [6,14,16,30]. A recurrence rate of 100% (9/9) was observed for patients with ESS and preserved ovarian function compared to 22.7% (10/44) for those without [16]. Similarly, Yoon et al. [6] reported a lower recurrence rate for premenopausal women who underwent BSO (n=34) compared to those who did not (n=54). Nevertheless, BSO was not associated with better OS [6]. In a similar study, ovarian-sparing procedures were independently associated with relapse but had no impact on OS [13]. Discrepancy in the reported tumor recurrence rates might be explained by variations in the histopathological criteria employed for the diagnosis of LG-ESS or the small number of patients enrolled which does not permit to control for confounders such as age and stage [31]. It should be noted that the majority of recurrences can be successfully salvaged with early secondary cytoreductive surgery [6,14]. While in our analysis we did not observe any difference in OS or CSS between the 2 groups, given that most high quality studies point to a higher relapse rate in women with retained ovarian function, OP should be discouraged until further multi-institutional studies shed light on this matter. Since, LG-ESS has a good prognosis, a large cohort with long-term follow-up and an adequate number of events would be required to minimize type II error. Currently, the National Comprehensive Cancer Network (NCCN) clinical practice guidelines suggest providers to consider re-resection of the ovaries for patients with LG-ESS [32]. On the other hand, the Gynecologic Cancer InterGroup (GCIG) group recommendations, state that OP could be considered in young women with small tumors [33] while the recent ESMO guidelines conclude that “the added value of BSO is not established, particularly in pre-menopausal women [34].”
In accordance to literature most women in our cohort were diagnosed with LMS [1]. While this tumor exhibits an aggressive behavior even when diagnosed at an early stage [1], in our study almost a third of all women did not undergo oophorectomy and OP was not associated with a worse OS or CSS. The safety of OP in this group has been evaluated in several retrospective studies, concluding to a very low rate of adnexal involvement and similar relapse rates and survival compared to women who underwent BSO [17,18,19,35,36,37], however these results are biased by heterogeneity and small sample size. Giuntoli et al. [17] performed a subgroup analysis of 25 premenopausal women who did not undergo BSO and 25 matched controls, and did not note any differences in the risk of recurrence or CSS; nevertheless, it should be noted that the control group included women who had radiotherapy. Likewise, Wu et al. [19] did not find any difference in recurrence risk between women who underwent BSO and those who did not. Interestingly, similar to our results, the OP group had a decreased risk of death that approached statistical significance (p=0.052), however results were not controlled for patient age and stage [19]. In our study, we performed a sub-analysis of women with LMS and no difference was found in mortality even after controlling for age and FIGO substage. Major advantages of our analysis are the exclusion of women who received radiotherapy and the large number of events observed in each group that permitted an adequate power.
AS is a rare mixed tumor composed of a benign epithelium and a homogenous low-grade sarcoma [1]. For women diagnosed with uterine AS the reported rate of ovarian involvement is relative low. Clement et al [38] examined 100 patients who underwent BSO and found only 2 cases of ovarian metastases, both with grossly abnormal adnexa. In another retrospective study, no ovarian metastases were observed in 16 patients who had BSO [20]. However, Carrol et al. [21] reported a rate of 8% (5/60) for women without evidence of extrauterine tumor spread (2 of these patients had grossly normal ovaries) while no isolated ovarian recurrence was observed. In our analysis, we did not find any difference in OS or CSS between the 2 groups. Given though the low number of events observed in this histologic group, conclusions cannot be made. Nevertheless, since the reported low rate of ovarian involvement, OP could be considered for women with stage I disease, if intraoperative inspection of the ovaries does not reveal any gross abnormalities [21].
Several limitations of the present study should be noted. Firstly, information on tumor recurrence was not available thus we could not investigate differences in the rate and patterns of recurrence or progression free survival. In addition, we were not able to assess if any women included in the oophorectomy group were administered hormonal replacement therapy. Also, information on the intraoperative gross appearance of the ovaries which may have influenced the surgeon's decision to perform oophorectomy was not available. As previous reports have pointed, patient's surgical history and exact details on the surgical procedure performed are not collected in the SEER database as such we were not able to exclude from our analysis a small number of women who might have undergone BSO/unilateral salpingo-oophorectomy (USO) during a previous gynecologic procedure or those with partially retained hormonal function [26,39]. Lastly, due to the lack of central pathology review we cannot exclude possible tumor misclassifications.
In this population-based cohort OP was not associated with an increased risk of mortality for premenopausal women diagnosed with a sarcoma limited to the uterus. However, careful evaluation of our data, following stratification by histologic subtype, support existing evidence that OP can be considered only for women with LMS sparing them from the morbidity associated with iatrogenic menopause. This decision should be individualized based on patient preferences and follow extensive counseling. If the ovaries are preserved, a long-term vigorous follow-up is imperative and any tumor recurrences should be managed aggressively, if possible, with secondary cytoreductive surgery. Given the low incidence of uterine sarcoma multi-institutional prospective collaborations are greatly warranted to further clarify the oncologic safety of OP for women with LG-ESS or AS.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: N.D.
- Data curation: N.D.
- Formal analysis: N.D.
- Funding acquisition: C.D.E., F.M., H.K.
- Investigation: N.D., C.D.E., F.M., H.K.
- Methodology: N.D., C.D.E., F.M., H.K.
- Project administration: N.D.
- Resources: N.D.
- Software: N.D.
- Supervision: N.D., C.D.E., F.M., H.K.
- Validation: N.D., C.D.E., F.M., H.K.
- Visualization: N.D., C.D.E., F.M., H.K.
- Writing - original draft: N.D., C.D.E., F.M., H.K.
- Writing - review & editing: N.D., C.D.E., F.M., H.K.
References
- 1.D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Tropé CG, Abeler VM, Kristensen GB. Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncol. 2012;51:694–705. doi: 10.3109/0284186X.2012.689111. [DOI] [PubMed] [Google Scholar]
- 3.Felix AS, Cook LS, Gaudet MM, Rohan TE, Schouten LJ, Setiawan VW, et al. The etiology of uterine sarcomas: a pooled analysis of the epidemiology of endometrial cancer consortium. Br J Cancer. 2013;108:727–734. doi: 10.1038/bjc.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amant F, Coosemans A, Debiec-Rychter M, Timmerman D, Vergote I. Clinical management of uterine sarcomas. Lancet Oncol. 2009;10:1188–1198. doi: 10.1016/S1470-2045(09)70226-8. [DOI] [PubMed] [Google Scholar]
- 5.Leitao MM, Jr, Hensley ML, Barakat RR, Aghajanian C, Gardner GJ, Jewell EL, et al. Immunohistochemical expression of estrogen and progesterone receptors and outcomes in patients with newly diagnosed uterine leiomyosarcoma. Gynecol Oncol. 2012;124:558–562. doi: 10.1016/j.ygyno.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Yoon A, Park JY, Park JY, Lee YY, Kim TJ, Choi CH, et al. Prognostic factors and outcomes in endometrial stromal sarcoma with the 2009 FIGO staging system: a multicenter review of 114 cases. Gynecol Oncol. 2014;132:70–75. doi: 10.1016/j.ygyno.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 7.Amant F, Schurmans K, Steenkiste E, Verbist L, Abeler VM, Tulunay G, et al. Immunohistochemical determination of estrogen and progesterone receptor positivity in uterine adenosarcoma. Gynecol Oncol. 2004;93:680–685. doi: 10.1016/j.ygyno.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Chu MC, Mor G, Lim C, Zheng W, Parkash V, Schwartz PE. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol. 2003;90:170–176. doi: 10.1016/s0090-8258(03)00258-0. [DOI] [PubMed] [Google Scholar]
- 9.Wright JD. Take ‘em or leave ’em: management of the ovaries in young women with endometrial cancer. Gynecol Oncol. 2013;131:287–288. doi: 10.1016/j.ygyno.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Pritts EA, Vanness DJ, Berek JS, Parker W, Feinberg R, Feinberg J, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12:165–177. doi: 10.1007/s10397-015-0894-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li AJ, Giuntoli RL, 2nd, Drake R, Byun SY, Rojas F, Barbuto D, et al. Ovarian preservation in stage I low-grade endometrial stromal sarcomas. Obstet Gynecol. 2005;106:1304–1308. doi: 10.1097/01.AOG.0000185511.91694.1e. [DOI] [PubMed] [Google Scholar]
- 12.Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Urgesi A, et al. Endometrial stromal sarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;63:247–253. doi: 10.1006/gyno.1996.0314. [DOI] [PubMed] [Google Scholar]
- 13.Bai H, Yang J, Cao D, Huang H, Xiang Y, Wu M, et al. Ovary and uterus-sparing procedures for low-grade endometrial stromal sarcoma: a retrospective study of 153 cases. Gynecol Oncol. 2014;132:654–660. doi: 10.1016/j.ygyno.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Feng W, Hua K, Malpica A, Zhou X, Baak JP. Stages I to II WHO 2003-defined low-grade endometrial stromal sarcoma: how much primary therapy is needed and how little is enough? Int J Gynecol Cancer. 2013;23:488–493. doi: 10.1097/IGC.0b013e318247aa14. [DOI] [PubMed] [Google Scholar]
- 15.Amant F, De Knijf A, Van Calster B, Leunen K, Neven P, Berteloot P, et al. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br J Cancer. 2007;97:1194–1199. doi: 10.1038/sj.bjc.6603986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li N, Wu LY, Zhang HT, An JS, Li XG, Ma SK. Treatment options in stage I endometrial stromal sarcoma: a retrospective analysis of 53 cases. Gynecol Oncol. 2008;108:306–311. doi: 10.1016/j.ygyno.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Giuntoli RL, 2nd, Metzinger DS, DiMarco CS, Cha SS, Sloan JA, Keeney GL, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89:460–469. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 18.Gadducci A, Landoni F, Sartori E, Zola P, Maggino T, Lissoni A, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;62:25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]
- 19.Wu TI, Chang TC, Hsueh S, Hsu KH, Chou HH, Huang HJ, et al. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol. 2006;100:166–172. doi: 10.1016/j.ygyno.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Tanner EJ, Toussaint T, Leitao MM, Jr, Hensley ML, Soslow RA, Gardner GJ, et al. Management of uterine adenosarcomas with and without sarcomatous overgrowth. Gynecol Oncol. 2013;129:140–144. doi: 10.1016/j.ygyno.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 21.Carroll A, Ramirez PT, Westin SN, Soliman PT, Munsell MF, Nick AM, et al. Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol. 2014;135:455–461. doi: 10.1016/j.ygyno.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TS, Lee JY, Kim JW, Oh S, Seong SJ, Lee JM, et al. Outcomes of ovarian preservation in a cohort of premenopausal women with early-stage endometrial cancer: a Korean Gynecologic Oncology Group study. Gynecol Oncol. 2013;131:289–293. doi: 10.1016/j.ygyno.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Sun C, Chen G, Yang Z, Jiang J, Yang X, Li N, et al. Safety of ovarian preservation in young patients with early-stage endometrial cancer: a retrospective study and meta-analysis. Fertil Steril. 2013;100:782–787. doi: 10.1016/j.fertnstert.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute (US) Data & software for researchers [Internet] Bethesda, MD: National Cancer Institute; [cited 2017 Feb 1]. Available from: https://seer.cancer.gov/resources/ [Google Scholar]
- 25.National Cancer Institute (US) SEER research data 1973–2013 when using SEER*Stat [Internet] Bethesda, MD: National Cancer Institute; 2016. [cited 2017 Feb 1]. Available from: https://seer.cancer.gov/data/citation.html. [Google Scholar]
- 26.Matsuo K, Machida H, Shoupe D, Melamed A, Muderspach LI, Roman LD, et al. Ovarian conservation and overall survival in young women with early-stage low-grade endometrial cancer. Obstet Gynecol. 2016;128:761–770. doi: 10.1097/AOG.0000000000001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz L, Merino MJ, Sakamoto H, Schwartz PE. Endometrial stromal sarcoma: a clinicopathologic study of 11 cases with determination of estrogen and progestin receptor levels in three tumors. Gynecol Oncol. 1987;26:87–97. doi: 10.1016/0090-8258(87)90074-6. [DOI] [PubMed] [Google Scholar]
- 28.Ioffe YJ, Li AJ, Walsh CS, Karlan BY, Leuchter R, Forscher C, et al. Hormone receptor expression in uterine sarcomas: prognostic and therapeutic roles. Gynecol Oncol. 2009;115:466–471. doi: 10.1016/j.ygyno.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 29.Kim WY, Lee JW, Choi CH, Kang H, Kim TJ, Kim BG, et al. Low-grade endometrial stromal sarcoma: a single center's experience with 22 cases. Int J Gynecol Cancer. 2008;18:1084–1089. doi: 10.1111/j.1525-1438.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- 30.Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL., Jr Treatment of endometrial stromal tumors. Gynecol Oncol. 1990;36:60–65. doi: 10.1016/0090-8258(90)90109-x. [DOI] [PubMed] [Google Scholar]
- 31.Feng W, Hua K, Gudlaugsson E, Yu Y, Zhou X, Baak JP. Prognostic indicators in WHO 2003 low-grade endometrial stromal sarcoma. Histopathology. 2013;62:675–687. doi: 10.1111/j.1365-2559.2011.04115.x. [DOI] [PubMed] [Google Scholar]
- 32.Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, et al. Uterine sarcoma, version 1.2016: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2015;13:1321–1331. doi: 10.6004/jnccn.2015.0162. [DOI] [PubMed] [Google Scholar]
- 33.Amant F, Floquet A, Friedlander M, Kristensen G, Mahner S, Nam EJ, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for endometrial stromal sarcoma. Int J Gynecol Cancer. 2014;24:S67–S72. doi: 10.1097/IGC.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 34.ESMO/European Sarcoma Network Working Group Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii102–iii112. doi: 10.1093/annonc/mdu254. [DOI] [PubMed] [Google Scholar]
- 35.Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL., Jr Treatment of uterine leiomyosarcoma. Obstet Gynecol. 1988;71:845–850. [PubMed] [Google Scholar]
- 36.Larson B, Silfverswärd C, Nilsson B, Pettersson F. Prognostic factors in uterine leiomyosarcoma. A clinical and histopathological study of 143 cases. The Radiumhemmet series 1936–1981. Acta Oncol. 1990;29:185–191. doi: 10.3109/02841869009126543. [DOI] [PubMed] [Google Scholar]
- 37.Leitao MM, Sonoda Y, Brennan MF, Barakat RR, Chi DS. Incidence of lymph node and ovarian metastases in leiomyosarcoma of the uterus. Gynecol Oncol. 2003;91:209–212. doi: 10.1016/s0090-8258(03)00478-5. [DOI] [PubMed] [Google Scholar]
- 38.Clement PB, Scully RE. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum Pathol. 1990;21:363–381. doi: 10.1016/0046-8177(90)90198-e. [DOI] [PubMed] [Google Scholar]
- 39.Wright JD, Buck AM, Shah M, Burke WM, Schiff PB, Herzog TJ. Safety of ovarian preservation in premenopausal women with endometrial cancer. J Clin Oncol. 2009;27:1214–1219. doi: 10.1200/JCO.2008.19.8150. [DOI] [PubMed] [Google Scholar]