Abstract
Objectives:
To investigate the diagnostic accuracy of ultrasound for evaluation of inflammatory activity in patients with Crohn’s disease (CD).
Methods:
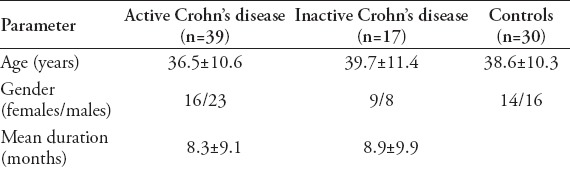
Fifty-six patients with histologically proven CD (39 with active, 17 with inactive disease) and 30 healthy volunteers as a control group were enrolled in the study at WeiFang People’s Hospital, Weifang Province, China from October 2012 to December 2014. Bowel wall thickness, and vascularity pattern were measured by Doppler ultrasound.
Results:
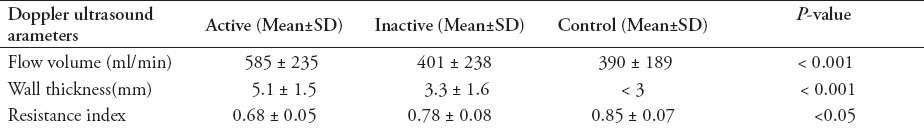
There was a significant difference in flow volume of the superior mesenteric artery (585 ± 235 ml/min) in the patients with active disease, compared with those with inactive disease (401 ± 238 ml/min) and the control group (390 ± 189 ml/min, p<0.001). Wall thickness was 5.1 ± 1.5 mm in the active CD group, 3.3 ± 1.6 mm in the inactive disease group (p<0.001) and <3 mm in the control group. Resistance index in the thickened bowel wall showed some differences: 0.68 ± 0.05 in the active disease group, 0.78 ± 0.08 in the inactive disease group, and 0.85 ± 0.07 in the control group (p<0.05).
Conclusion:
Doppler ultrasound is a useful diagnostic tool in detecting CD and assessing inflammatory activity.
Periodic assessment of the inflammatory activity and extent of Crohn’s disease (CD) is important to plan the proper therapeutic schedule. The small bowel is involved in 30-40% of patients with CD and the ileocecal segment in 50%. Endoscopic evaluation of the terminal ileum is feasible, and a rate of 95% has been reported.1,2 However, it is an invasive method with poor tolerance. In recent years, wireless capsule endoscopy, computed tomography (CT), magnetic resonance imaging (MRI), and biomarkers have been used as effective tools for diagnose of inflammatory bowel disease.3-7 Each method comes with its own advantages and disadvantages.8,9 Doppler ultrasound is a noninvasive, safe, well tolerated, cheap, and reproducible technique. Doppler ultrasound might play a significant role for investigation of CD.The purpose of this prospective study was to test the diagnostic accuracy of ultrasound in the evaluation of inflammatory activity in patients CD.
Methods
We enrolled 56 patients (25 female, 31 male; mean age 37.5 ± 10.8 years; range 20-62 years) with histologically proven CD (39 with active, 17 with inactive disease) and 30 healthy volunteers as a control group between 2012 and 2014 (Table 1). The mean post-diagnosis time was 8.5 ± 9.3 years. Diagnosis was performed according to the second European Evidence-Based Consensus on the diagnosis and management of CD. Patients who underwent abdominal surgery were excluded. Ethics approval was given by the Research Ethics Committee of WeiFang People’s Hospital. All patients provided written informed consent. Endoscopy with biopsy was performed in all cases. Patients with any surgical procedures on the small bowel or colon were excluded. Disease activity was assessed using the CD Activity Index (CDAI). Clinical response was a decrease in CDAI of 100 points. Disease was classified as inactive if CDAI was <150 and active with CDAI ≥150. CD with moderate activity has a CDAI of 150-450 and strong activity has CDAI >450.10 Sonography was performed using a GE LOGIQ S6 scanner (GE, Fairfield, USA.). The examinations were performed after the patient had fasted for at least 8 hours. A graded compression technique was used. Ultrasound was performed by the same sonographer with 16 years working experience in the patient cohort, who was blind for the patients. Sonography included evaluation of bowel wall thickness and spectral analysis of the thickened bowel wall. The gastrointestinal tract was divided into 5 segments: ileum and cecum, ascending colon, transverse colon, descending colon, and the rectosigmoid. The entire abdomen was examined with more attention to the terminal ileum. Maximum bowel wall thickness was assessed. Bowel wall thickness >3.5 mm was considered abnormal.11,12 The probe was placed 1-2 cm from the origin of the superior mesenteric artery in all patients. The Doppler parameters of the mesenteric artery were: peak systolic velocity (PSV), end-diastolic velocity (EDV), resistance index (RI), mean velocity flow (MV), and flow volume (ml/min) was automatically calculated by the Doppler instrument (MV × cross-sectional area). Each measurement was picked-up 3 times and the average was recorded.
Table 1.
Characteristics of 56 patients with history of chronic disease and 30 healthy volunteers.
The results were analyzed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 18. Kruskal-Wallis and Mann-Whitney were used to test the differences in the 3 groups and p<0.05 was considered to be statistically significant.
Results
The parameters of the superior mesenteric artery showed no significant difference in the 3 groups, except the flow volume. There was a significant difference in flow volume of the superior mesenteric artery in the active CD group (585 ± 235 ml/min), compared with the inactive disease (401 ± 238 ml/min) and control (390 ± 189 ml/min, p<0.001, Figure 1) groups. Median wall thickness of the affected bowel loop showed a significant difference between the active (5.1 ± 1.5 mm) and inactive CD (3.3 ± 1.6 mm) groups and the control group (<3 mm, p<0.001, Figure 2). Resistance index and flow volume of the mural artery showed significant differences in the three groups. Median RI in the inactive CD group was 0.78 ± 0.08, compared with 0.85 ± 0.07 in the control group, and low resistance arterial spectrum was found in the active disease group (0.68 ± 0.05, p<0.05, Figure 3, Table 2).
Figure 1.
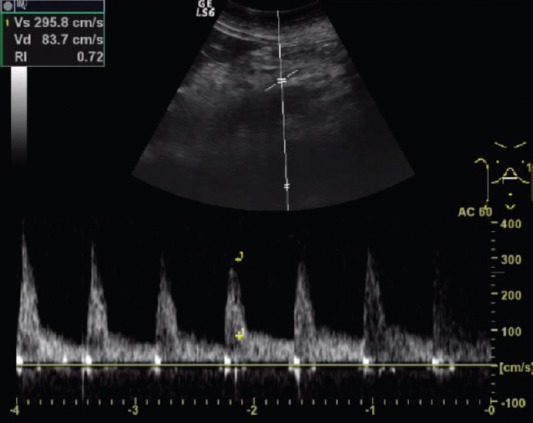
Flow volume of the superior mesenteric artery of patient with active Crohn disease.
Figure 2.
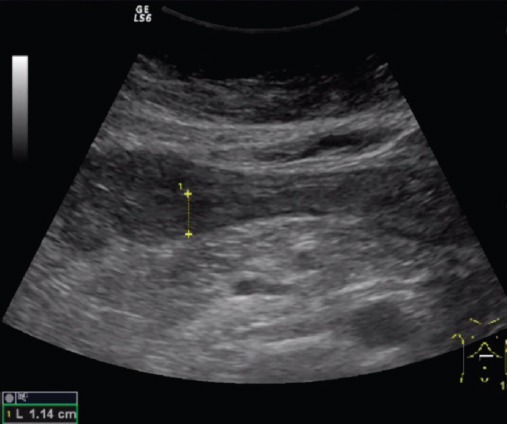
Wall thickness of the affected bowel loop of patient with active Crohn disease.
Figure 3.
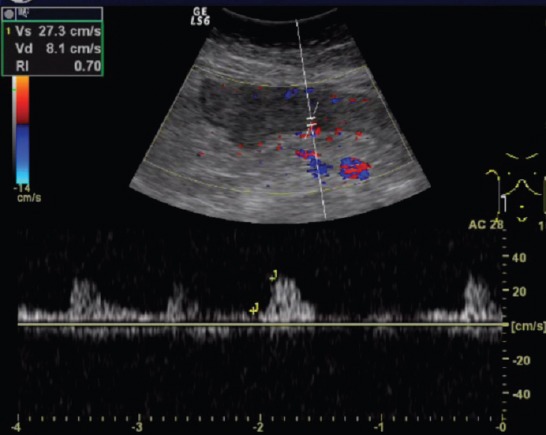
Resistance index in affected bowel wall of patient with active Crohn disease.
Table 2.
Doppler parameter values of patients from the 3 groups.
Discussion
Periodic assessment is important for CD, which has a chronic relapsing course.13 The CDAI is the gold standard for evaluating disease activity, but this index has a low sensitivity as it is based on subjective symptoms. Barium enema, small bowel follow-through, and colonoscopy remain important tests for diagnosing CD. The CT and MRI are techniques of choice in some cases. More than 20% of CD patients present before the age of 18 years.14 Colonoscopy and barium studies, and CT have important risks of perforation and cumulative ionizing radiation, therefore, their use is limited in patients with CD, especially for young patients and those with multiple relapses.15 Ultrasound has the significant advantage of being noninvasive, widely available, cheap, radiation free, and user friendly.16-18 Inflamed bowel segments with a thickened, hypoechoic wall are easily differentiated from normal bowel segments by ultrasound. So, ultrasound is well positioned for assessment of CD.14,19 Evaluation of thickened bowel wall is important for assessing CD activity.
A correlation between bowel wall thickness and clinical activity has already been reported.18 The threshold for a positive diagnosis is from 1.5 to 3 mm in the terminal ileum and <2 mm in the colon for children and 3 mm for adults.20-22 Patients with active CD have thicker affected bowel loops than patients with inactive disease. The reason for thickening is a combination of inflammation, edema and muscular spasm in active disease. It is difficult for Doppler ultrasound to be diagnostic in the initial stage of CD when only the mucosa is involved. In this case, endoscopy and conventional radiology are dependable. In advanced stages, pathomorphological changes affect the submucosa or muscular layer, and Doppler ultrasound is more accurate.23 In rare cases, thickened bowel wall is found in inactive CD for fibrosis. These patients would be false positive and hard to differentiate from the active group when assessing CD by ultrasound. Our study showed no significant difference in PSV and EDV values between active and inactive CD, but we found a significant difference in RI values in mural arteries. Higher values of flow volume and lower RI of mural arteries in thickened bowel wall were recorded in different studies.22,24,25 The probable reason is arterial hyperemia in the thickened gut wall of Crohn’s disease. In addition, ultrasound is useful for identifying intra-abdominal complications (abscesses, fistulae and strictures).26,27 Ultrasound has an overall sensitivity of 74% and a specificity of 78% for detection of small bowel CD lesions, especially in the terminal ileum (90–95%).28
The sonographer scanned the patients. It would be better if the results were evaluated by 2 or more radiologists for ultrasound, the operator’s subjective factors determine the outcome. More cases are needed to validate the findings of this study. In further studies, Contrast-enhanced ultrasound may provide valuable quantitative assessment of the bowel.
In conclusion, the results of this study show that ultrasound is a good technique to detect affected bowel segments in patients with CD. Sonography is a useful tool in the assessment and follow-up of patients with CD.
Footnotes
Authorship entitlement.
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
The international Committee of Medical Journal Editors has recommended the following criteria for authorship; these criteria are still appropriate for those journals that distinguish authors from other contributors.
Authorship credit should be based on 1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) intellectual content; and 3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship.
An author should be prepared to explain the order in which authors are listed.
References
- 1.Migaleddu V, Scanu AM, Quaia E, Rocca PC, Dore MP, Scanu D, et al. Contrast-enhanced ultrasonographic evaluation of inflammatory activity in Crohn’s disease. Gastroenterology. 2009;137:43–52. doi: 10.1053/j.gastro.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 2.Jun S, Hua RZ, Lu TJ, Xiang C, Dong XS. Are endoscopic grading and scoring systems in inflammatory bowel disease the same? Saudi Med J. 2008;29:1432–1437. [PubMed] [Google Scholar]
- 3.Ouahed J, Shagrani M, Sant'Anna A. Role of wireless capsule endoscopy in reclassifying inflammatory bowel disease in children. Jornal De pediatria. 2013;89:204–209. doi: 10.1016/j.jped.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Kovanlikaya A, Watson E, Hayward J, Beneck D, Sockolow R, Solomon A, et al. Magnetic resonance enterography and wireless capsule endoscopy in the evaluation of patients with inflammatory bowel disease. Clin Imaging. 2013;37:77–82. doi: 10.1016/j.clinimag.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petruzziello C, Calabrese E, Onali S, Zuzzi S, Condino G, Ascolani M, et al. Small bowel capsule endoscopy vs conventional techniques in patients with symptoms highly compatible with Crohn’s disease. J Crohns Colitis. 2011;5:139–147. doi: 10.1016/j.crohns.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Hisamatsu T, Okamoto S, Hashimoto M, Muramatsu T, Andou A, Uo M, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PloS One. 2012;7:e31131. doi: 10.1371/journal.pone.0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuksel ES, Ipek S, Topal F, Koc G, Akpinar Z, Slaughter JC, et al. Assessment of presence and grade of activity in ileal Crohn’s disease. Turk J Gastroenterol. 2014;25:264–270. doi: 10.5152/tjg.2014.3862. [DOI] [PubMed] [Google Scholar]
- 8.Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas L, Geboes K, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. Gut. 2006;55:1–15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez MJ, Ripolles T, Paredes JM, Blanc E, Marti-Bonmati L. Assessment of the extension and the inflammatory activity in Crohn’s disease: comparison of ultrasound and MRI. Abdom Imaging. 2009;34:141–148. doi: 10.1007/s00261-008-9365-y. [DOI] [PubMed] [Google Scholar]
- 10.Schirbel A, Reichert A, Roll S, Baumgart DC, Buning C, Wittig B, et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J Gastroenterol. 2010;16:3168–3177. doi: 10.3748/wjg.v16.i25.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migaleddu V, Quaia E, Scano D, Virgilio G. Inflammatory activity in Crohn disease: ultrasound findings. Abdom Imaging. 2008;33:589–597. doi: 10.1007/s00261-007-9340-z. [DOI] [PubMed] [Google Scholar]
- 12.Pallotta N, Giovannone M, Pezzotti P, Gigliozzi A, Barberani F, Piacentino D, et al. Ultrasonographic detection and assessment of the severity of Crohn’s disease recurrence after ileal resection. BMC Gastroenterol. 2010;10:69. doi: 10.1186/1471-230X-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabrese E, Zorzi F, Pallone F. Ultrasound of the small bowel in Crohn’s disease. Int J Inflam. 2012;2012:964720. doi: 10.1155/2012/964720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casciani E, De Vincentiis C, Polettini E, Masselli G, Di Nardo G, Civitelli F, et al. Imaging of the small bowel: Crohn’s disease in paediatric patients. World J Radiol. 2014;6:313–328. doi: 10.4329/wjr.v6.i6.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ripolles T, Martinez MJ, Paredes JM, Blanc E, Flors L, Delgado F. Crohn disease: correlation of findings at contrast-enhanced US with severity at endoscopy. Radiology. 2009;253:241–248. doi: 10.1148/radiol.2531082269. [DOI] [PubMed] [Google Scholar]
- 16.Ripolles T, Martinez-Perez MJ, Blanc E, Delgado F, Vizuete J, Paredes JM, et al. Contrast-enhanced ultrasound (CEUS) in Crohn’s disease: technique, image interpretation and clinical applications. Insights Imaging. 2011;2:639–652. doi: 10.1007/s13244-011-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabrese E, Zorzi F, Zuzzi S, Ooka S, Onali S, Petruzziello C, et al. Development of a numerical index quantitating small bowel damage as detected by ultrasonography in Crohn’s disease. J Crohns Colitis. 2012;6:852–860. doi: 10.1016/j.crohns.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Braden B, Ignee A, Hocke M, Palmer RM, Dietrich C. Diagnostic value and clinical utility of contrast enhanced ultrasound in intestinal diseases. Dig Liver Dis. 2010;42:667–674. doi: 10.1016/j.dld.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Thomson M, Rao P, Berger L, Rawat D. Graded compression and power Doppler ultrasonography versus endoscopy to assess paediatric Crohn disease activity pre- and posttreatment. J Pediatr Gastroenterol Nutr. 2012;54:404–408. doi: 10.1097/MPG.0b013e3181f8b55d. [DOI] [PubMed] [Google Scholar]
- 20.Alison M, Kheniche A, Azoulay R, Roche S, Sebag G, Belarbi N. Ultrasonography of Crohn disease in children. Pediatr Radiol. 2007;37:1071–1082. doi: 10.1007/s00247-007-0559-1. [DOI] [PubMed] [Google Scholar]
- 21.Darge K, Anupindi S, Keener H, Rompel O. Ultrasound of the bowel in children: how we do it. Pediatr Radiol. 2010;40:528–536. doi: 10.1007/s00247-010-1550-9. [DOI] [PubMed] [Google Scholar]
- 22.Sjekavica I, Barbaric-Babic V, Sunjara V, Kralik M, Senecic-Cala I, Dujsin M, et al. Resistance index in mural arteries of thickened bowel wall: predictive value for Crohn disease activity assessment in pediatric patients. Wien Klin Wochenschr. 2013;125:254–260. doi: 10.1007/s00508-013-0357-8. [DOI] [PubMed] [Google Scholar]
- 23.Fraquelli M, Sarno A, Girelli C, Laudi C, Buscarini E, Villa C, et al. Reproducibility of bowel ultrasonography in the evaluation of Crohn’s disease. Dig Liver Dis. 2008;40:860–866. doi: 10.1016/j.dld.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Sjekavica I, Barbaric-Babic V, Krznaric Z, Molnar M, Cukovic-Cavka S, Stern-Padovan R. Assessment of Crohn’s disease activity by doppler ultrasound of superior mesenteric artery and mural arteries in thickened bowel wall: cross-sectional study. Croat Med J. 2007;48:822–830. doi: 10.3325/cmj.2007.6.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karoui S, Nouira K, Serghini M, Ben Mustapha N, Boubaker J, Menif E, et al. Assessment of activity of Crohn’s disease by Doppler sonography of superior mesenteric artery flow. Croat Med J. 2010;4:334–340. doi: 10.1016/j.crohns.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652–1657. doi: 10.1136/gut.2004.041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castiglione F, de Sio I, Cozzolino A, Rispo A, Manguso F, Del Vecchio Blanco G, et al. Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with Crohn’s disease. Am J Gastroenterol. 2004;99:1977–1983. doi: 10.1111/j.1572-0241.2004.40267.x. [DOI] [PubMed] [Google Scholar]
- 28.Bremner AR, Griffiths M, Argent JD, Fairhurst JJ, Beattie RM. Sonographic evaluation of inflammatory bowel disease: a prospective, blinded, comparative study. Pediat Radiol. 2006;36:947–953. doi: 10.1007/s00247-006-0245-8. [DOI] [PubMed] [Google Scholar]


