Abstract
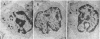
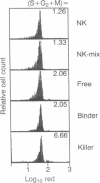
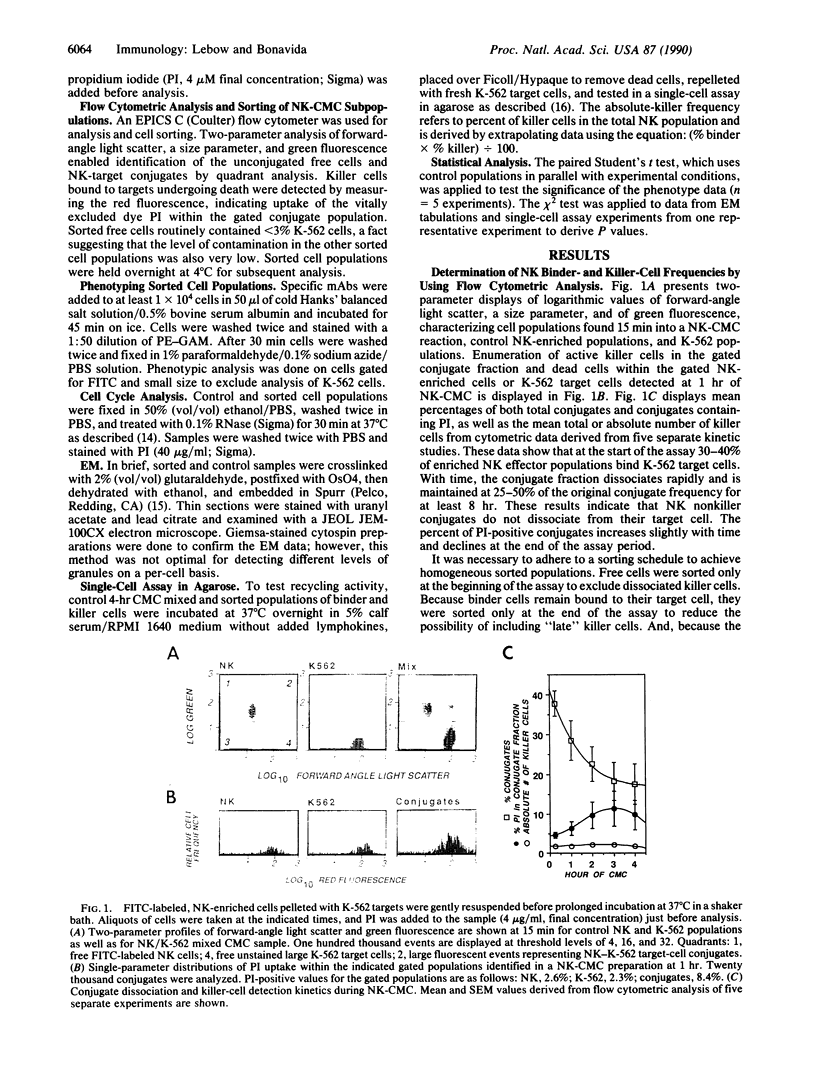
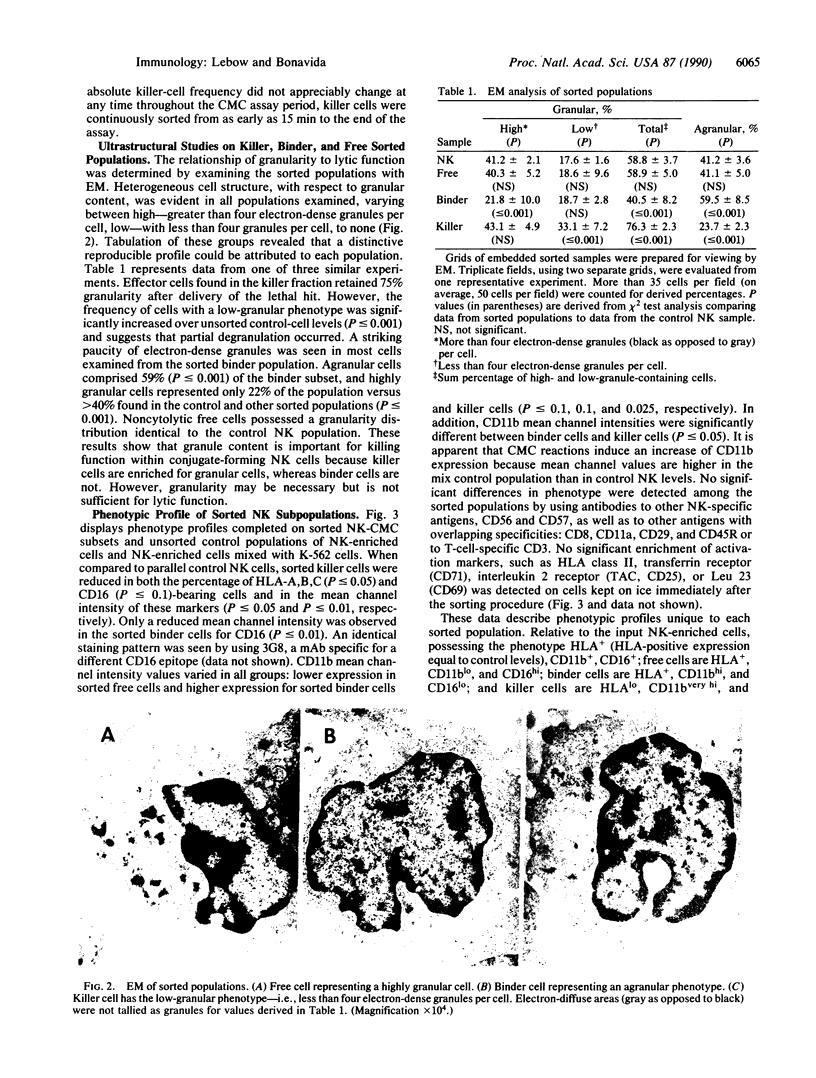
Natural killer (NK) cells form three functionally distinct populations of effectors: competent cytolytic effectors able to bind and kill target cells and two subsets of nonlytic effectors, one able and the other unable to bind target cells. A flow cytometric method was developed, based on size and two-color fluorescence of NK cell-target conjugates, for the characterization and sorting of highly purified subpopulations--killer cells, nonkiller binder cells, and free cells. Ultrastructural examination revealed that granule content was reduced in the killer cells and absent in most of the binder cells. Quantitative differences in the expression level of HLA class I, CD11b (C3bi receptor), and CD16 (receptor for the Fc portion of IgG) antigens could differentiate the subsets. The killer phenotype was HLAlo, CD11bvery hi, and CD16very lo; the binder phenotype was CD11bhi and CD16lo; and the free-cell phenotype was CD11blo and CD16hi. Cell activation was not requisite for lytic function because no difference in either expression of activation markers or cell cycle could be established among the sorted subpopulations. Although recycling function was inhibited, retention of lytic activity was enriched 4-fold in the sorted killer cell population. These results represent characterization of a successful bulk isolation of competent killer, nonkiller binder, and free cells in human NK-cell populations and should aid our understanding of NK-cell development, lineage, and function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams S. I., Brahmi Z. Target cell directed NK inactivation. Concomitant loss of NK and antibody-dependent cellular cytotoxicity activities. J Immunol. 1988 Mar 15;140(6):2090–2095. [PubMed] [Google Scholar]
- Bonavida B., Bradley T. P., Grimm E. A. Frequency determination of killer cells by a single-cell cytotoxic assay. Methods Enzymol. 1983;93:270–280. doi: 10.1016/s0076-6879(83)93049-5. [DOI] [PubMed] [Google Scholar]
- Carpen O., Virtanen I., Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982 Jun;128(6):2691–2697. [PubMed] [Google Scholar]
- Dent G. A., Leglise M. C., Pryzwansky K. B., Ross D. W. Simultaneous paired analysis by flow cytometry of surface markers, cytoplasmic antigens, or oncogene expression with DNA content. Cytometry. 1989 Mar;10(2):192–198. doi: 10.1002/cyto.990100210. [DOI] [PubMed] [Google Scholar]
- Garcia-Peñarrubia P., Koster F. T., Bankhurst A. D. Population distributions of natural killer and K562 target cell conjugates. Nat Immun Cell Growth Regul. 1989;8(2):57–65. [PubMed] [Google Scholar]
- Henkart M. P., Henkart P. A. Lymphocyte mediated cytolysis as a secretory phenomenon. Adv Exp Med Biol. 1982;146:227–247. doi: 10.1007/978-1-4684-8959-0_13. [DOI] [PubMed] [Google Scholar]
- Hercend T., Reinherz E. L., Meuer S., Schlossman S. F., Ritz J. Phenotypic and functional heterogeneity of human cloned natural killer cell lines. Nature. 1983 Jan 13;301(5896):158–160. doi: 10.1038/301158a0. [DOI] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Katz J. D., Mitsuyasu R., Gottlieb M. S., Lebow L. T., Bonavida B. Mechanism of defective NK cell activity in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. II. Normal antibody-dependent cellular cytotoxicity (ADCC) mediated by effector cells defective in natural killer (NK) cytotoxicity. J Immunol. 1987 Jul 1;139(1):55–60. [PubMed] [Google Scholar]
- Kornbluth J., Spear B., Raab S. S., Wilson D. B. Evidence for the role of class I and class II HLA antigens in the lytic function of a cloned line of human natural killer cells. J Immunol. 1985 Feb;134(2):728–735. [PubMed] [Google Scholar]
- Lanier L. L., Buck D. W., Rhodes L., Ding A., Evans E., Barney C., Phillips J. H. Interleukin 2 activation of natural killer cells rapidly induces the expression and phosphorylation of the Leu-23 activation antigen. J Exp Med. 1988 May 1;167(5):1572–1585. doi: 10.1084/jem.167.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L. L., Le A. M., Civin C. I., Loken M. R., Phillips J. H. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986 Jun 15;136(12):4480–4486. [PubMed] [Google Scholar]
- Lebow L. T., Stewart C. C., Perelson A. S., Bonavida B. Analysis of lymphocyte-target conjugates by flow cytometry. I. Discrimination between killer and non-killer lymphocytes bound to targets and sorting of conjugates containing one or multiple lymphocytes. Nat Immun Cell Growth Regul. 1986;5(5):221–237. [PubMed] [Google Scholar]
- McGinnes K., Chapman G., Marks R., Penny R. A fluorescence NK assay using flow cytometry. J Immunol Methods. 1986 Jan 22;86(1):7–15. doi: 10.1016/0022-1759(86)90258-9. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Sharrow S. O., Timonen T., Herberman R. B. Determination of surface antigens on highly purified human NK cells by flow cytometry with monoclonal antibodies. J Immunol. 1981 Dec;127(6):2401–2409. [PubMed] [Google Scholar]
- Perussia B., Acuto O., Terhorst C., Faust J., Lazarus R., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. II. Studies of B73.1 antibody-antigen interaction on the lymphocyte membrane. J Immunol. 1983 May;130(5):2142–2148. [PubMed] [Google Scholar]
- Perussia B., Starr S., Abraham S., Fanning V., Trinchieri G. Human natural killer cells analyzed by B73.1, a monoclonal antibody blocking Fc receptor functions. I. Characterization of the lymphocyte subset reactive with B73.1. J Immunol. 1983 May;130(5):2133–2141. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G. Antibody 3G8, specific for the human neutrophil Fc receptor, reacts with natural killer cells. J Immunol. 1984 Mar;132(3):1410–1415. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G. Inactivation of natural killer cell cytotoxic activity after interaction with target cells. J Immunol. 1981 Feb;126(2):754–758. [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Jackson A., Warner N. L., Faust J., Rumpold H., Kraft D., Lanier L. L. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984 Jul;133(1):180–189. [PubMed] [Google Scholar]
- Press J. L. H-2 haplotype-specific conversion of the Ir gene phenotype expressed by low-responder mice immunized with a p-azophenylarsonate conjugate of (T,G)-A--L. J Immunol. 1982 May;128(5):2236–2242. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. E., Caulfield J. P., Michon J., Hein A., Kamada M. M., MacDermott R. P., Stevens R. L., Ritz J. T11/CD2 activation of cloned human natural killer cells results in increased conjugate formation and exocytosis of cytolytic granules. J Immunol. 1988 Feb 1;140(3):991–1002. [PubMed] [Google Scholar]
- Shau H., Dawson J. R. Identification and purification of NK cells with lysosomotropic vital stains: correlation of lysosome content with NK activity. J Immunol. 1985 Jul;135(1):137–140. [PubMed] [Google Scholar]
- Shau H., Golub S. H. Depletion of NK cells with the lysosomotropic agent L-leucine methyl ester and the in vitro generation of NK activity from NK precursor cells. J Immunol. 1985 Feb;134(2):1136–1141. [PubMed] [Google Scholar]
- Shi T. X., Tong M. J., Bohman R. The application of flow cytometry in the study of natural killer cell cytotoxicity. Clin Immunol Immunopathol. 1987 Dec;45(3):356–365. doi: 10.1016/0090-1229(87)90088-2. [DOI] [PubMed] [Google Scholar]
- Slezak S. E., Horan P. K. Cell-mediated cytotoxicity. A highly sensitive and informative flow cytometric assay. J Immunol Methods. 1989 Feb 24;117(2):205–214. doi: 10.1016/0022-1759(89)90142-7. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarcone D., Tilden A. B., Cloud G., Friedman H. M., Landay A., Grossi C. E. Flow cytometry evaluation of cell-mediated cytotoxicity. J Immunol Methods. 1986 Nov 20;94(1-2):247–255. doi: 10.1016/0022-1759(86)90239-5. [DOI] [PubMed] [Google Scholar]