Abstract
Objectives:
To describe the clinical characteristics of pediatric patients colonized or infected by Stenotrophomonas maltophilia (S. maltophilia) at a Saudi children’s hospital, to identify risk factors associated with infection, and to investigate the antimicrobial resistance patterns of this emerging pathogen.
Methods:
In this cross-sectional observational study, 64 non-duplicating S. maltophilia strains were isolated in Najran Maternity and Children’s Hospital, Najran, Saudi Arabia between January 2015 to February 2016. Antimicrobial susceptibility testing was performed using the reference broth microdilution method.
Results:
In this study, 48 (75%) isolates were identified in true infections and 16 (25%) isolates were considered colonization. The main types of S. maltophilia infection were pneumonia in 22 (45.8%) patients and bloodstream infection in 14 (29.2%) patients. The significant risk factors included exposure to invasive procedure (p=0.02), and presence of acute leukemia as an underlying disease (p=0.02). The most active antimicrobials were trimethoprim/sulfamethoxazole (100% sensitivity) and tigecycline (93.7% sensitivity).
Conclusions:
Stenotrophomonas maltophilia is an emerging nosocomial pathogen among pediatric patients. Accurate identification and susceptibility testing of this emerging pathogen are crucial for the management of infected patients and prevention of spread of this nosocomial pathogen.
Stenotrophomonas maltophilia (S. maltophilia) is an aerobic, glucose non-fermentative, Gram-negative bacillus that is widely distributed in various environments and equipment, especially in hospitals.1,2 This bacterium is increasingly recognized as an emerging global opportunistic pathogen, causing hospital-acquired infections, such as bacteremia, pneumonia, endocarditis, and meningitis, as well as urinary tract, ocular, bone and joint, skin, and soft tissue, and gastrointestinal infections. It is occasionally associated with septic shock in critically ill and immunosuppressed patients; especially in intensive care units (ICUs).3-6 Infections with S. maltophilia are associated with high morbidity and mortality rates. Therapy for these infections represents a significant challenge for clinicians because of the organism’s high level of intrinsic resistance to multiple classes of antibiotics, afforded by various mechanisms such as decreased permeability, production of β-lactamases and of aminoglycoside modifying enzymes, or the presence of multidrug efflux pumps.7 Resistance can also emerge during therapy. Moreover, identification of S. maltophilia can be problematic for microbiologists. In many cases, isolation of S. maltophilia from clinical specimens may be misidentified as colonization rather than infection.8,9 Meanwhile, susceptibility testing methods of this organism are difficult. The commonly investigated antibiotics for in vitro activity against S. maltophilia include trimethoprim/sulfamethoxazole (SXT), fluoroquinolones, ticarcillin/clavulanate (TIM), minocycline (MI) and tigecycline (TGC).10 Trends of increasing resistance to these individual antimicrobials have been recently reported in many clinical studies and combined therapeutic regimens are recommended.11,12 Most clinical and epidemiological studies of S. maltophilia have focused on the adult population,5,8,11,13,14 and only a few reports have described nosocomial infections in pediatric patients.12,15-17 Moreover, the national data on the antimicrobial susceptibility of S. maltophilia in Saudi Arabia is limited. The present study aimed to describe the clinical characteristics of pediatric patients colonized or infected by S. maltophilia at a Saudi children’s hospital, and to identify risk factors associated with infection, as well as to investigate the antimicrobial resistance patterns of this emerging pathogen. Improved knowledge of these aspects could help the management and selection of empirical therapies for such infections.
Methods
This cross-sectional descriptive study was conducted at Najran Maternity and Children’s Hospital, a 250-bed, tertiary care hospital in Najran, a city in southwestern Saudi Arabia, between January 2015 and February 2016. The study was reviewed and approved by the Scientific Research Committee of the College of Medicine, Najran University. The study included children ≤5 years of age who presented with signs and symptoms of healthcare-associated infections, and were admitted to the pediatric ward, or ICUs (neonatal ICU or pediatric ICU). Written consent was obtained from their parents. The demographic and clinical data of the hospitalized children were recorded, including age, gender, type of infection, underlying disease, length of hospital stay, ICU admission, invasive procedure, and antimicrobial therapy within the last 3 months.
Health-care associated infections were defined using criteria of the standard Centers for Disease Control/National Healthcare Safety definition Network (CDC/NHSN). Decisions regarding Infection or colonization were made after the following factors were considered: the patient’s clinical history, physical findings, body temperature at the time of culture, leukocyte count, C-reactive protein level, culture results of specimens from other sites, clinical course, and response to therapy. Briefly, colonization was defined as the presence of S. maltophilia on skin, mucous membranes, in wounds, or in secretions without causing adverse clinical signs or symptoms. A bloodstream infection was considered to be associated with a central venous catheter (CVC) if the patient had a CVC in place at the time of isolation and no other source of infection was identified.19 Ventilator-associated pneumonia (VAP) was defined as an infection in a child requiring at least 48 hours of mechanical ventilation and developing new and persistent radiographic evidence of focal infiltrates 48 hours or more after the initiation of mechanical ventilation.18 Urinary tract infection was defined as the isolation of Stenotrophomonas from a catheterized urine sample (>50,000 colony count), and wound infection as the isolation of Stenotrophomonas without any mixed growth from the wound, along with clinical features of wound infection. Conjunctivitis was defined as the presence of a purulent ocular discharge, erythema, and edema of the lids. Neutropenia was defined as an absolute neutrophil count <1500 cells/mm3 documented at least once during the week before isolation.
Bacteriological methods
Stenotrophomonas maltophilia strains were isolated from various clinical specimens including blood, urine, respiratory secretions (tracheal aspirate, and bronchoalveolar lavage), pus, ocular swabs, and tips of CVCs from different patients (one isolate/patient). The isolates were identified by the standard microbiological methods, including microscopy, culture characteristics, catalase, oxidase, aesculin hydrolysis, lysine decarboxylase and DNase,20 the Analytical Profile Index (API) system (bioMérieux, Marcy l’Etoile, France), and the automated Vitek 32 system (bioMérieux, Marcy l’Etoile, France).
Antimicrobial susceptibility testing was performed using the reference broth microdilution method following the guidelines of the Clinical and Laboratory Standards Institute (CLSI).10 The minimum inhibitory concentrations of 7 antibiotics were determined. The antibiotics tested included TIM, ceftazidime (CAZ), MI, levofloxacin (LVX), TGC, chloramphenicol, and SXT. For TGC, the criteria of ≤1 mg/mL for susceptibility and ≥2.0 mg/mL for resistance were applied based on the clinical breakpoints of the European Committee on Antimicrobial Susceptibility Testing for Enterobacteriaceae.21 The CLSI interpretive criteria for S. maltophilia were used for other agents.10 Intermediately-resistant isolates were considered to be resistant. Quality control was performed using Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 29213.
Statistical analysis. Data were coded, validated, and analyzed using the Student’s t-test, the chi squared test, antecedent 95% confidence intervals (95% CI) or Fisher’s exact test, where appropriate. All comparisons were 2-tailed, and P values <0.05 were considered statistically significant. All analyses were performed with the Statistical Package for the Social Sciences (SPSS), Version 15.0 (SPSS Inc., Chicago, IL, USA).
Results
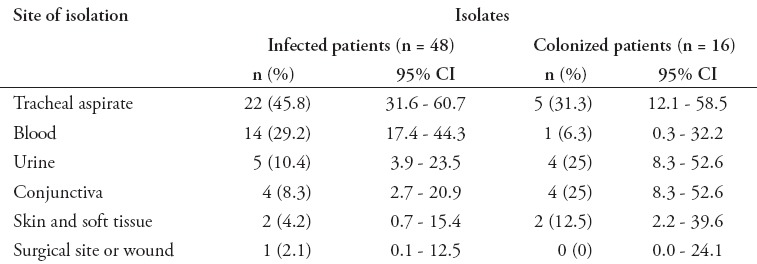
Sixty-four non-duplicating S. maltophilia strains were isolated during the study period, of which 48 (75%) were identified in true infections and 16 (25%) isolates were considered colonization, because those patients had no infection criteria. The main type of infection associated with S. maltophilia was pneumonia in 22 (45.8%) patients, of whom 5 (22.7%) cases had VAP, followed by BSI in 14 (29.2%) patients, of whom 2 (14.3%) patients had CVC-related bacteremia, urinary tract infection in 5 (10.4%) patients, ocular infection in 4 (8.3%) patients, and skin and soft tissue infection in 2 (4.2%) patients. Only one (2.1%) patient had surgical wound infection. The proportions of infecting and colonizing isolates according to the site of isolation are presented in (Table 1). Twenty-two (45.8%) S. maltophilia isolates from infected patients were obtained from tracheal aspirates, while 14 (29.2%) strains were isolated from blood, 5 (10.4%) from urine, and 4 (8.3%) from ocular discharge. There was no poly-microbial infection in the 48 patients.
Table 1.
Site of isolation of Stenotrophomonas maltophilia nosocomial isolates.
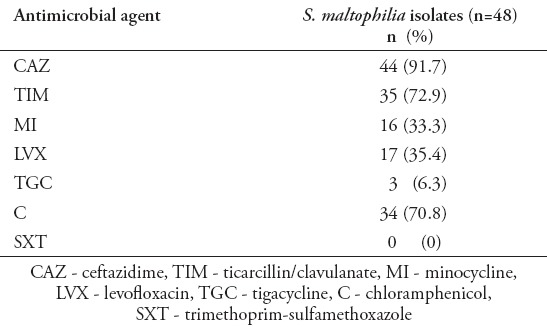
The risk factors of S. maltophilia infections are listed in Table 2. Univariate analysis indicated that patients who were subjected to CVC (p=0.04) or who had acute leukemia as an underlying disease (p=0.02) were significantly infected with S. maltophilia. The age, gender, prematurity, underlying diseases other than acute leukemia, mechanical ventilation, and prior antibiotic therapy were not significantly different in infected and colonized patients. The antimicrobials tested, and the percentages of resistant isolates are listed in Table 3. Overall, with the exception of SXT that inhibited all isolates and TGC that inhibited 94% of isolates, more than 75% of isolates was resistant to CAZ and chloramphenicol. The rate of susceptibility to MI was 66.7%, to LVX was 64.7%, and to TIM was 27.1%.
Table 2.
Risk factors for Stenotrophomonas maltophilia (S. maltophilia) infections.
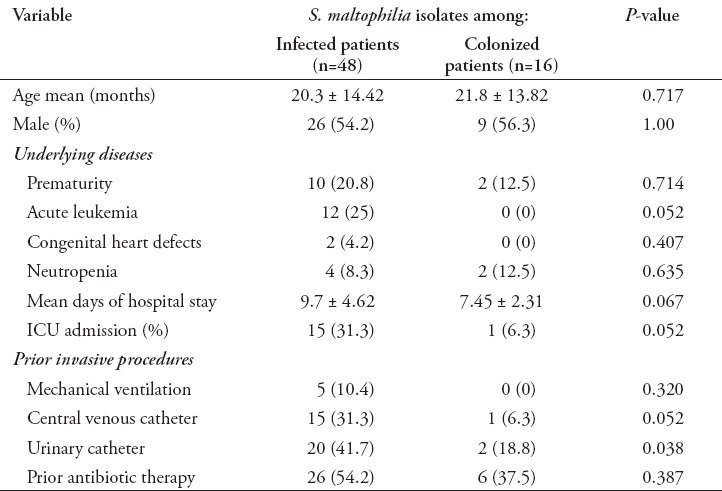
Table 3.
Number and percentages of Stenotrophomonas maltophilia (S. maltophilia) isolates resistant to selected antimicrobial agents.
Discussion
Globally, S. maltophilia has become the third most common non-fermentative Gram-negative bacilli responsible for nosocomial infections, behind P. aeruginosa and Acinetobacter species.1 Recently, S. maltophilia has emerged as an important opportunistic nosocomial pathogen among pediatric patients. The emergence of this pathogen is probably related to advances in medical care that has led to an increase in the number and survival rates of severely ill, debilitated children who are the vulnerable group at the highest risk for acquiring infection by this pathogen.2,5 S. maltophilia has a variety of clinical presentations. Respiratory tract infection, especially VAP, and BSI are the most common infections reported in many clinical and epidemiological studies. The spectrum of the clinical diseases caused by S. maltophilia in this study was similar to that observed in other relevant studies.3,5,11-13
Differentiation between S. maltophilia colonization and infection is difficult for both the clinicians and the microbiologists. Previously, S. maltophilia was considered a low-virulence pathogen. Its isolation from the respiratory tract has been frequently interpreted as colonization rather than as infection.8,9 Many previous studies have found a large proportion of colonizing S. maltophilia isolates, with rates of true infection of 13-71%.13-15,22,23 In this study, 75% of the 64 isolates were identified as true infections. The low rate of colonization in this study may be related to the definitions used. The CDC/NHSN criteria used to define whether an isolate was infecting or not were chosen to give uniformity to the data obtained.18 Moreover, identification of true S. maltophilia infection may be more problematic when S. maltophilia is not the only organism isolated. Sattler et al17 investigated episodes of infection from non-respiratory sites and reported that 70.6% of S. maltophilia isolates were from poly-microbial cultures, which yielded other obligate pathogens (Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella species and Acinetobacter species). Several factors raise doubts about the significance of S. maltophilia in the etiology of poly-microbial infections; namely, the high proportion of poly-microbial isolation in most studies, the co-isolation of pathogens that are well known to cause infection at these sites, and the high cure rate despite inappropriate therapy.24 The pathogenic role of S. maltophilia in poly-microbial infections is still debatable. It is noteworthy that all S. maltophilia isolates in this study were mono-bacterial, and more likely to be a cause of infection than colonization.2,3
In this study, the predisposing factors associated with S. maltophilia infection were similar to those reported by other studies that primarily described S. maltophilia isolations among adult patients.5,11,13,14,16,25 These risk factors included prolonged hospitalization, presence of a CVC, neutropenia, ICU admission, mechanical ventilation, or tracheotomy, prior antibiotic therapy, and underlying disease. It is of particular importance that acute leukemia was recorded as the leading underlying disease in this study associated with S. maltophilia infection. In contrast, Sattler et al17 found that prematurity, primary immunodeficiency, and congenital heart disease were unique and more common risk factors among pediatric patients in their study. In contrast with many previous studies, exposure to antibiotics in this study was found in both infected and colonized patients.13,14,22,26 Carbapenems are among the classes of antibiotics most frequently associated with S. maltophilia isolation.26 However, several studies have implicated other classes of antibiotics including ampicillin, gentamicin, vancomycin, metronidazole, piperacillin, cephalosporins, and tobramycin, as one of the significant risk factors for the development of S. maltophilia colonization and infection.15,22,23 Cephalosporins, carbapenems, and aminoglycosides are empirically used for pediatric and adult nosocomial infections in our hospital. The management of S. maltophilia-associated infection is difficult because many clinical strains of S. maltophilia display both intrinsic resistance to various classes of antibiotics such as carbapenems and aminoglycosides, and induced resistance to fluoroquinolones, which are used empirically for nosocomial sepsis. It appears that SXT is the most sensitive antibiotic for S. maltophilia-associated infections.22 In agreement with this finding, all S. maltophilia isolates in this study were completely susceptible to SXT. Many case-control studies have actually suggested that SXT protects against S. maltophilia infection or colonization.23,27 However, there is a concern with over using SXT in the neonatal period, as SXT is known to cause adverse events related to bone marrow suppression. Moreover, SXT competes with bilirubin for binding to albumin, and it can increase the level of indirect bilirubin.28 In a previous study, 23% of S. maltophilia infections occurred in the first week of life, but no hyperbilirubinemia was observed.16 Mutlu et al16 reported that no adverse side effects, including increased indirect hyperbilirubinemia, were observed with the use of SXT in the neonatal period, and the authors recommended this antibiotic for antimicrobial therapy of S. maltophilia pediatric patients including the neonates. In the last few years, several reports have shown that the prevalence of SXT-resistant S. maltophilia strains is increasing.29-32 The SENTRY Antimicrobial Surveillance Program investigated the antimicrobial susceptibility patterns of prominent Gram-negative isolates from pneumonic patients at 28 medical centers in USA, and Europe and the Mediterranean region (EMR) from 2009 to 2012.33 In this study, the resistance rate to SXT among S. maltophilia isolates was 3.7% in the USA and 2.3% in the EMR. The SXT-resistance rates have been reported to be between 8-18% in the Asia-Pacific region.34 In a recent Saudi study, 2 cases of S. maltophilia infection were SXT-resistant.32 Many clinical studies showed that patients receiving LVX and MI had clinical outcomes similar to those receiving SXT for the treatment of S. maltophilia infections,29,35 and could be alternative therapeutic options. The SENTRY report found that LVX-resistance ranged from 16.2% in the EMR, to 24.9% in the USA, whereas, all isolates from both regions were MI-susceptible.33 In contrast, less than one third of our isolates were resistant to both drugs. It is worth noting that rapid in vitro and in vivo resistance to fluoroquinolones might emerge during therapy, and clinical application of MI is still limited.1 In this study, TGC was the most active antimicrobial agent against S. maltophilia isolates (94% susceptibility) after SXT. In a recent SENTRY study in 11 Latin American countries, TGC inhibited 91.5% and 83% of 141 S. maltophilia isolates at ≤2 and ≤1 µµµg/ml.36 These findings highlight the in vitro activity of TGC against S. maltophilia and this antibiotic can be used as alternative empiric therapy for S. maltophilia infection.
This study has some potential limitations related to the small number of S. maltophilia isolates from a single center study. Therefore, further epidemiological multi-center studies of longer surveillance duration are necessary to better understand the prevalence and the distribution of S. maltophilia associated infections, and prevent the spread of these multi-drug resistant nosocomial isolates.
In conclusion, S. maltophilia is an emerging nosocomial pathogen among pediatric patients. It is capable of causing different types of healthcare associated infections. The most important risk factors for S. maltophilia infection among hospitalized children are invasive procedures, prolonged hospitalization, and ICU admission. The SXT regimen is an appropriate choice for S. maltophilia infection, and TGC could be a useful alternative treatment option specially as part of combination regimens. Accurate identification and susceptibility testing of this emerging pathogen are critical for the management of infected patients and prevention of spread of this nosocomial pathogen.
Footnotes
Authorship entitlement.
Excerpts from the Uniform Requirements for Manuscripts Submitted to Biomedical Journals updated November 2003.
Available from www.icmje.org
The international Committee of Medical Journal Editors has recommended the following criteria for authorship; these criteria are still appropriate for those journals that distinguish authors from other contributors.
Authorship credit should be based on 1) substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) intellectual content; and 3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.
Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship.
An author should be prepared to explain the order in which authors are listed.
References
- 1.Looney WJ, Narita M, Muhlemann K. Stenotrophomonas maltophilia : an emerging opportunist human pathogen. Lancet Infect Dis. 2009;9:312–323. doi: 10.1016/S1473-3099(09)70083-0. [DOI] [PubMed] [Google Scholar]
- 2.Brooke JS. Stenotrophomonas maltophilia : An emerging global opportunistic pathogen. Clin Microbiol Rev. 2012;25:2–41. doi: 10.1128/CMR.00019-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott IJ, Slavin MA, Turnidge JD, Thursky KA, Worth LJ. Stenotrophomonas maltophilia : emerging disease patterns and challenges for treatment. Expert Rev Anti Infect Ther. 2011;9:471–488. doi: 10.1586/eri.11.24. [DOI] [PubMed] [Google Scholar]
- 4.Garazi M, Singer C, Tai J, Ginocchio CC. Bloodstream infections caused by Stenotrophomonas maltophilia : a seven-year review. J Hosp Infect. 2012;81:114–118. doi: 10.1016/j.jhin.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Pien CJ, Kuo HY, Chang SW, Chen PR, Yeh HW, Liu CC, et al. Risk factors for levofloxacin resistance in Stenotrophomonas maltophilia from respiratory tract in a regional hospital. J Microbiol Immunol Infect. 2015;48:291–295. doi: 10.1016/j.jmii.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Guyot A, Turton TF, Garner D. Outbreak of Stenotrophomonas maltophilia on an intensive care unit. J Hosp Infect. 2013;85:303–307. doi: 10.1016/j.jhin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Brooke JS. New strategies against Stenotrophomonas maltophilia : a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev Anti Infect Ther. 2014;12:1–4. doi: 10.1586/14787210.2014.864553. [DOI] [PubMed] [Google Scholar]
- 8.del Toro MD, Rodriguez-Bano J, Herrero M, Rivero A, Garcia-Ordonez MA, Corzo J, et al. Clinical epidemiology of Stenotrophomonas maltophilia colonization and infection: a multicenter study. Medicine (Baltimore) 2002;81:228–239. doi: 10.1097/00005792-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Pakyz AL, Farr BM. Rates of Stenotrophomonas maltophilia colonization and infection in relation to antibiotic cycling protocols. Epidemiol Infect. 2009;137:1679–1683. doi: 10.1017/S0950268809002830. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing. 26th ed. Wayne (PA): M100S. CLSI; 2016. [Google Scholar]
- 11.Samonis G, Karageorgopoulos DE, Maraki S, Levis P, Dimopoulou D, Spernovasilis NA, et al. Stenotrophomonas maltophilia infections in a general hospital: patient characteristics, antimicrobial susceptibility, and treatment outcome. Plos One. 2012;7:e37375. doi: 10.1371/journal.pone.0037375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guzoglu N, Demirkol FN, Aliefendioglu D. Haemorrhagic pneumonia caused by Stenotrophomonas maltophilia in two newborns. J Infect Dev Ctries. 2015;9:533–535. doi: 10.3855/jidc.5463. [DOI] [PubMed] [Google Scholar]
- 13.Xun M, Zhang Y, Li B, Wu M, Zong Y, Yin Y. Clinical characteristics and risk factors of infections caused by Stenotrophomonas maltophilia in a hospital in northwest China. J Infect Dev Ctries. 2014;8:1000–1005. doi: 10.3855/jidc.4236. [DOI] [PubMed] [Google Scholar]
- 14.Asaad AM, Al-Ayed MS, Qureshi MA. Emergence of unusual nonfermenting gram-negative nosocomial pathogens in a Saudi hospital. Jpn J Infect Dis. 2013;66:507–511. doi: 10.7883/yoken.66.507. [DOI] [PubMed] [Google Scholar]
- 15.Arthur C, Tang X, Romero JR, Gossett JG, Harik N, Prodhan P. Stenotrophomonas maltophilia infection among young children in a cardiac intensive care unit: a single institution experience. Pediatr Cardiol. 2015;36:509–515. doi: 10.1007/s00246-014-1041-0. [DOI] [PubMed] [Google Scholar]
- 16.Mutlu M, Yılmaz G, Aslan Y, Bayramoglu G. Risk factors and clinical characteristics of Stenotrophomonas maltophilia infections in neonates. J Microbiol Immunol Infect. 2011;44:467–472. doi: 10.1016/j.jmii.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Sattler CA, Mason EO, Kaplan SL. Non-respiratory Stenotrophomonas maltophilia infection at a children’s hospital. Clin Infect Dis. 2000;31:1321–1330. doi: 10.1086/317473. [DOI] [PubMed] [Google Scholar]
- 18.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection:2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.In Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. Manual of Clinical Microbiology Handbook. Washington (DC): ASM Press; 2003. [Google Scholar]
- 21.European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints. [[Accessed 2011 March 1]]. Available from URL: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.3_pdf.pdf .
- 22.Fihman V, Le Monnier A, Corvec S, Jaureguy F, Tankovic J, Jacquier H, et al. Stenotrophomonas maltophilia -the most worrisome threat among unusual non-fermentative gram-negative bacilli from hospitalized patients: a prospective multicenter study. J Infect. 2012;64:391–398. doi: 10.1016/j.jinf.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Kastoris AC, Vouloumanou EK, Rafailidis PI, Kapaskelis AM, Dimopoulos G. Attributable mortality of Stenotrophomonas maltophilia infections: a systematic review of the literature. Future Microbiol. 2009;4:1103–1109. doi: 10.2217/fmb.09.84. [DOI] [PubMed] [Google Scholar]
- 24.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, et al. Bad bugs, no drugs: no ESKAPE!An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 25.Xun M, Zhang Y, Li B, Wu M, Zong Y, Yin Y. Clinical characteristics and risk factors of infections caused by Stenotrophomonas maltophilia in a hospital in northwest China. J Infect Dev Ctries. 2014;8:1000–1005. doi: 10.3855/jidc.4236. [DOI] [PubMed] [Google Scholar]
- 26.Gales AC, Jones RN, Forward KR, Linares J, Sader HS, Verhoef J. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in severely ill patients: geographic patterns, epidemiological features and trends in the SENTRY Antimicrobial Surveillance Program (1997-1999) Clin Infect Dis. 2001;32(Suppl2):104–113. doi: 10.1086/320183. [DOI] [PubMed] [Google Scholar]
- 27.Averbuch D, Cordonnier C, Livermore DM, Mikulska M, Orasch C, Viscoli C, et al. Targeted therapy against multi-resistant bacteria in leukemic and hematopoietic stem cell transplant recipients: guidelines of the 4th European Conference on Infections in Leukemia (ECIL-4 2011) Haematologica. 2013;98:1836–1847. doi: 10.3324/haematol.2013.091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Springer C, Eyal F, Michel J. Pharmacology of trimethoprim-sulfamethoxazole in newborn infants. J Pediatr. 1982;100:647–650. doi: 10.1016/s0022-3476(82)80778-6. [DOI] [PubMed] [Google Scholar]
- 29.Cho SY, Kang CI, Kim J, Ha YE, Chung DR, Lee NY, et al. Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob Agents Chemother. 2014;58:581–583. doi: 10.1128/AAC.01682-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juhasz E, Pongracz J, Ivan M, Kristof K. Antibiotic susceptibility of sulfamethoxazole-trimethoprim resistant Stenotrophomonas maltophilia strains isolated at a tertiary care center in Hungary. Acta Microbiol Immunol Hung. 2015;62:295–305. doi: 10.1556/030.62.2015.3.7. [DOI] [PubMed] [Google Scholar]
- 31.Wei C, Ni W, Cai X, Zhao J, Cui J. Evaluation of Trimethoprim/Sulfamethoxazole (SXT), Minocycline, Tigecycline, Moxifloxacin, and Ceftazidime Alone and in Combinations for SXT-Susceptible and SXT-Resistant Stenotrophomonas maltophilia by In Vitro Time-Kill Experiments. PloS One. 2016;11:e0152132. doi: 10.1371/journal.pone.0152132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Jasser AM. Stenotrophomonas maltophilia resistant to trimethoprim-sulfamethoxazole: an increasing problem. Ann Clin Microbiol Antimicrob. 2006;5:23–25. doi: 10.1186/1476-0711-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sader HS, Farrell DJ, Flamm RK, Jones RN. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized with pneumonia in US and European hospitals: Results from the SENTRY Antimicrobial Surveillance Program 2009-2012. Int J Antimicrob Agents. 2014;43:328–334. doi: 10.1016/j.ijantimicag.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Wu H, Wang JT, Shiau YR, Wang HY, Lauderdale TL, Chang SC, et al. A multicenter surveillance of antimicrobial resistance on Stenotrophomonas maltophilia in Taiwan. J Microbiol Immuno Infect. 2012;45:120–126. doi: 10.1016/j.jmii.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Wang YL, Scipione MR, Dubrovskaya Y, Papadopoulos J. Monotherapy with fluoroquinolone or trimethoprim-sulfamethoxazole for treatment of Stenotrophomonas maltophilia infections. Antimicrob Agents Chemother. 2014;58:176–182. doi: 10.1128/AAC.01324-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sader HS, Castanheira M, Farrell DJ, Flamm RK, Mendes RE, Jones RN. Tigecycline antimicrobial activity tested against clinical bacteria from Latin American medical centers: results from SENTRY Antimicrobial Surveillance Program (2011-2014) Int J Antimicrob Agents. 2016;48:144–150. doi: 10.1016/j.ijantimicag.2016.04.021. [DOI] [PubMed] [Google Scholar]


