Abstract
Cemento-ossifying fibromas are rare fibro-osseous benign neoplasms that affect the jaws. They are included in the group of mesodermal odontogenic tumors and commonly present as a progressively growing lesion that might attain enormous size with resultant deformity, if left untreated. A confusion prevails on the terminology, which can only be confirmed by histopathologic evaluation. A case of cemento-ossifying fibroma involving the right mandible is described in a 30 year-old female patient. The clinical, radiographic, histologic features are presented and the various differential diagnosis are discussed.
Cemento-ossifying fibroma (COF) is a distinct form of a benign fibro-osseous tumor, affecting predominantly the craniofacial region. Cemento-ossifying fibroma was initially classified by the World Health Organization (WHO) as a fibro-osseous neoplasm.1,2 However, they do not arise in the long bones, and occur mostly in the tooth-bearing areas of the jaws. Their resemblance to ossifying fibroma and cemento-osseous dysplasias give evidence for an odontogenic origin.3 They are derived from the mesenchymal blast cells of the periodontal ligament, and have a potential to form fibrous tissue, cement and bone or a combination of such elements.4,5 It was reported by Eversole et al6 that these cementum-like structures are associated with membranous bone, and may not be related to cementogenesis. Cemento-ossifying fibroma has always been surrounded by controversy regarding the terminology and criteria of diagnosis.7 In the decades since the conception of the terminology, much has changed regarding our understanding, imaging, histopathological categorization and treatment strategies of the various fibro-osseous lesions. Even then, the differences between the broad spectrum of ossifying fibromas remains unclear. The following case report attempts to differentiate between lesions presenting with similar clinical, radiographic and histologic presentations; as well as describe the varying manifestations of COF, with reference to previous literature.
Case Report
A 30 year old female patient with no other relevant medical history, reported to the department with a swelling on the right side of the face since the last 2 years with occasional pain (Figure 1). The swelling seemed to be gradually increasing in size, reaching its present size in the last 6 months. No history of previous similar swelling, toothache, or numbness could be elicited. Extra-oral examination revealed a well-defined, dome-shaped swelling extending over the right body of the mandible up to the lower border of the mandible inferiorly. There was no erythema or surface ulceration of the swelling. On palpation, a bony hard consistency of the swelling was elicited, with no evident tenderness or increase in temperature. Expansion of the buccal cortical plate was evident. Intraorally, the swelling was observed in the lower buccal vestibule leading to obliteration of the mucobuccal fold with respect to lower right molars and measured approximately 3cm x 4cm in size. Tooth mobility and mild tenderness on percussion was observed in the teeth associated with the lesion (namely, lower right molars and root stump). Digital panoramic radiograph taken revealed a well-defined multilocular radiolucent lesion in the right mandibular body region extending up to the ramus (Figure 2). The lesion was seen extending beyond the lower border of the mandible with expansion and thinning of the cortical plates. The internal structure of the lesion was mainly radiolucent, with diffuse scattered radiopacity. Root resorption of lower right first and second molars was evident. Occlusal radiograph showed well-defined expansion of both the buccal and lingual cortical plates arising from lower right first molar region, with evidence of ill-defined diffuse septa, suggesting a multi-locular appearance with diffuse irregular radiopacity within the largely radiolucent lesion (Figure 3).
Figure 1.
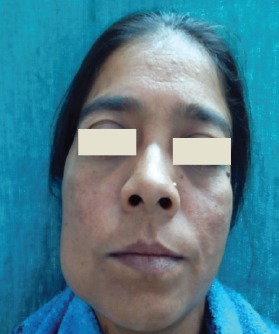
Facial asymmetry of the right side with a well-defined dome-shaped swelling over the right body of mandible.
Figure 2.
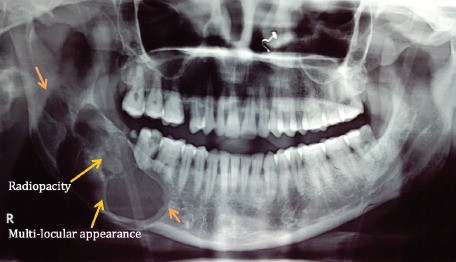
Panoramic radiograph reveals a well-defined multilocular radiolucent lesion in the right mandibular body region extending up to the ramus, with expansion of cortical plate and scattered diffuse radiopacity inside the radiolucent lesion
Figure 3.
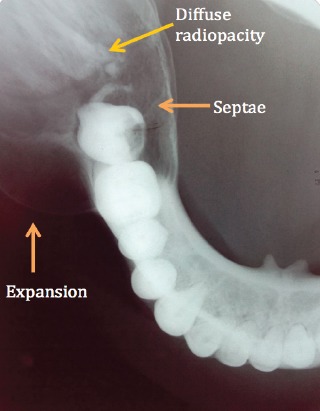
Mandibular occlusal radiograph reveals a well-defined expansion of both the buccal and lingual cortical plates arising from lower right 1st molar region, with evidence of septa suggesting a multi-locular appearance and diffuse irregular radiopacity within the largely radiolucent lesion
Figure 4.
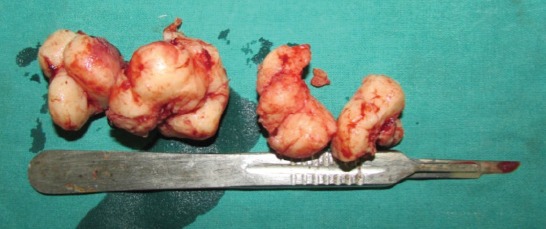
Gross specimen after surgical enucleation.
Figure 5.
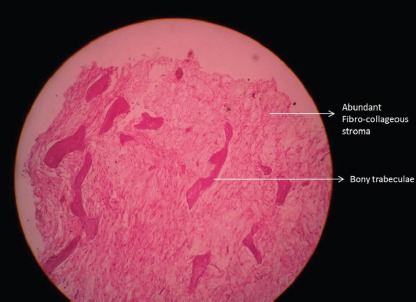
Low power histopathological picture showing a fair number of bony trabecula admixed with abundant loose fibrocollagenous stroma.
Figure 6.
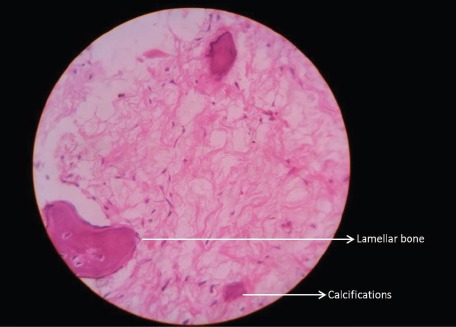
High power histopathological picture showing lamellar bone with osteoblastic rimming and psammomatous-like nodules and calcifications in the fibrous stroma (haematoxylin-eosin x400).
Discussion
In 1872, Menzel first described a variant of ossifying fibroma in the mandible, and called it as COF. In 1971, WHO classified COF under cementum forming lesions that also included fibrous dysplasia, ossifying fibroma, and COF.5,6 The hybrid name of central COF is often used because it comprised a whole spectrum of fibro-osseous lesions that arise from the periodontal ligament, which range from those with only cementum deposition to those with only bone deposition.8,9 However, according to the new WHO classification of 2005, the term “cemento-ossifying ossifying fibroma” has been reduced to “ossifying fibroma” (OF).9 Thus, OF and COFs are considered odontogenic in origin, mainly from the periodontal ligament. However, in recent years, the presentation of microscopically indistinguishable lesions in the frontal, temporal, sphenoid, and ethmoid bones disputes the above assumption. This may be explained by the fact that the lesion might develop from an ectopic periodontal membrane, and thus occur outside the jaws. Also, the periodontal membrane being a mesodermal germ layer points towards the fact that some primitive mesenchymal cells are capable of differentiating in a similar manner to produce a tumor.10 Cemento-ossifying fibromas lesions may occur in 2 forms, a central variant arising from the periodontal ligament adjacent to root apex, and a peripheral variant occurring solely in the soft tissues area of the jaw. While the central variant (described above) is a neoplastic entity, the peripheral variant is a focal, reactive non-neoplastic growth of soft tissues, which may be composed of mature collagen, cellular fibroblastic tissue, mineralized tissue and multinucleated giant cells. The latter is also known as peripheral cementifying fibroma, ossifying fibro-epithelial polyp, peripheral fibroma with calcification, calcifying fibroblastic granuloma.13 These lesions are slow-growing and are most often seen in the third and fourth decades of life. There is a female prominence in these lesions, with a ratio ranging from 2:17 to 5:111. While one half of all cases are asymptomatic, the growth of the tumor over time may lead to facial asymmetry; the lesion causing cortical expansion. It has been shown that in Asian populations, ossifying fibromas presented with considerably greater swelling.12 The mandible is more commonly affected than the maxilla. It may present in childhood as juvenile aggressive COF, which is clinically more aggressive and more vascular at a pathological level.8,14 While the etiology is yet unknown, trauma may act as a predisposing factor, which suggest a connective-tissue-reactive etiology rather than a neoplastic one.15 It has been proposed that trauma or dental extractions may leave part of the periodontal membrane attached to the wall of the alveolus, predisposing to stimulation and subsequent deposition of cementum; thus, supporting the theory of periodontal membrane as the origin of COF.5,16
Radiographically, they present typically as well-defined, solitary radiolucencies with scattered radiopaque foci. They vary in radiopacity depending on the amount of cementum and bone that have been deposited. In the early stages, COF may appear as unilocular or multilocular radiolucent lesion and as the lesion matures, they may transform into a radiopaque one, resulting in a lesion with mixed density.15,17 The radiographic characteristics of central ossifying fibroma were described by Eversole et al6 and 2 major patterns were noted- expansile unilocular radiolucencies and multilocular configuration. Relatively well-defined, there’s often a sclerotic rim evident in the host bone as a result of peripheral osteocondensation. They expand the surrounding cortical bone without cortical perforation, while maintaining a spherical shape and may result in tooth divergence and resorption.8 Infact, a centrifugal growth pattern (equal expansion in all directions) is an important diagnostic feature of a COF.14
Waldron and Giansanti15 had reported that COF showed lytic lesions in 26% of cases, 63% were lytic with radiopaque foci, and 12% were diffuse and homogenous appearance. It follows a similar radiographic appearance as ossifying fibromas. In contrast, a retrospective study caried out by Titinchi et al12 revealed almost 49.2% of the lesions of ossifying fibroma as radiopaque, 34.9% lesions as mixed radiolucent-radiopaque, and only 15.9% lesions as radiolucent. Also, 84.1% lesions were unilocular in panoramic radiographs and only 15.9% cases were multilocular. The multilocular lesions were more prominent in the mandibular posterior regions and in patients younger than 20-years. In our case, the multilocular radiolucency seen was in the mandibular posterior region, but older age group involvement was noted.12 Barberi et al19 categorized the radiographic pattern as follows: defined lesion without scelorotic rim (40%), defined lesion with sclerotic rim (45%), and lesion with ill-defined border (15%).16 Titinchi et al found almost 93.6% lesions with well-defined margins easily distinguishable from healthy bone.12 This was similar to our lesion, with well-defined margins.12
A larger tumor may involve the nasal septum, orbital floor and infraorbital foramen. Root resorption and tooth displacement may be evident, suggesting an active proliferating stage.17 According to Titinchi et al,12 root resorption was seen in only 12.7% patients, and mostly in well-defined multilocular lesions in the younger patient. In our case, root resorption was seen in a multilocular lesion but in an older age group.12
The extent to which the tumor spreads, guides surgical therapy. Not much literature is available of Cone Beam Computed Tomography (CBCT) findings of Ossifying Fibroma/COF. As per the findings of Kuta et al,8 MRI examination showed isointense to muscle on T1 signal and diffuse homogenous low signal on T2, which most likely represented the low-free water content of the calcific and fibrous tumor.
Various lesions may show clinical and radiographic resemblance to COF as mentioned above. Radiographically, depending on the amount of cementum deposition, it may resemble fibrous dysplasia, cemento-osseous dysplasia, odontogenic cysts and tumors like keratocystic odontogenic tumor, calcifying odontogenic cysts (Gorlin cysts), and calcifying epithelial odontogenic tumors (Pindborg tumors). The well-defined border of the central cementoossifying fibroma helps differentiate it from the aggressive sarcomas and carcinomas. The important diagnostic feature in COF is that it shows a centrifugal growth pattern which gives the tumor a round shape; this helps de-lineate from a typical keratocystic odontogenic tumor. Fibrous dysplasia has a characteristic “ground glass” appearance as well as a blending margin with the surrounding bone, not seen in the central cementoossifying fibroma. Also, despite the stage of development, the lesion of COF is always well circumscribed and demarcated from surrounding bone, in contrast to true fibrous dysplasia. Cemento-osseous dysplasias show wide sclerotic border of the cysts and is multifocal, which COF is not. The radiologic differentiation of central cementoossifying fibroma from Gorlin cysts and Pindborg tumors is difficult if not associated with impacted teeth, with which they have a high association; the final diagnosis is based on histologic appearance.8,17
Histopathologically, the COF shows a hypercellular fibrous connective tissue stroma within which there was a proliferation of irregularly shaped calcifications. The variable calcifications represent various stages of deposition of bone and cementum. It is difficult to differentiate histologically between osteoid and cementum. When most of the calcified fragments comprise immature cementum with basophilic coloration on hematoxylin and eosin-stained sections, they are named central COF.8 On the other hand, when the calcified fragments comprise osteoid with typical eosinophilic coloration on hematoxylin and eosin-stained sections, they are named as central ossifying fibromas. Endo et al attempted to distinguish COF from ossifying fibroma and fibrous dysplasias by immunohistochemistry. Keratan sulphate and chondroitin-4-sulfate were assessed. It was found that COFs showed significant immune-reactivity for keratan sulphate, while intense immunostaining for chondroitin-4-sulfate was observed in ossifying fibromas and fibrous dysplasias.13,20
The hybrid term central cementoossifying fibroma was thus adopted to indicate the likely presence of both types of tissue within the same lesion and because of the difficulty in differentiating reliably between immature bone and immature cementum. Thus, central COF is considered the most accurate histologic term; and it can be interchanged with either central ossifying fibroma or central COF.8 Cemento-ossifying fibroma is sharply circumscribed and demarcated from bone and thus it should be excised conservatively. The recommended treatment is enucleation of smaller ossifying fibromas, curettage of lesions where no clear radiolucency is present around the lesion and mono-bloc resection with bone reconstruction for larger tumors in close proximity to the inferior border of the mandible.12 Due to its radio-resistance, radiotherapy is complicated.1 Prognosis of this lesion is fair; however, relapse of COF is higher in case of maxillary COF compared to the mandibular ones due to the greater difficulty of their surgical removal and their larger size at the time of presentation.14 Complete surgical removal of the lesion at the earliest possible stage has been advised by numerous investigators.12 The average recurrence rate of Ossifying fibromas in general is reported as 10.1%, with an average follow-up of 25-months. Eversole et al6 reported a recurrence rate of 28% after enucleation and curettage of 22 patients affected by OF who were followed for a period of 38 months.6 Liu5 also reported a recurrence rate of 27.2%.5 Hence an average follow-up period of 10-years is advocated. It is also believed that surgical intervention can reactivate the growth of a lesion.12
In conclusion, COF should be considered in the differential diagnosis of lesions that present clinically as a slow-growing tumor, especially in females. Though there is no classic appearance that can help distinguish it from ossifying fibroma, it appears that the distinction between cemento-ossifying and ossifying variants is academic, as no behavioral differences exist. High recurrence rates call for a thorough surgical treatment approach for such cases.
Footnotes
References
- 1.White SC, Pharoah MJ. Oral radiology: Principles and interpretation. St. Louis (MO): Mosby/Elsevier; 2009. [Google Scholar]
- 2.Goncalves M, Pispico R, Alves Fde A, Lugao CE, Goncalves A. Clinical, radiographic, biochemical and histological findings of florid cemento-osseous dysplasia and report of a case. Braz Dent J. 2005;16:247–250. doi: 10.1590/s0103-64402005000300014. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Sharma NK, Raj V, Sethi T. Ossifying fibroma of maxilla in a male child: Report of a case and review of the literature. Natl J Maxillofac Surg. 2011;2:73–79. doi: 10.4103/0975-5950.85859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ram R, Singhal A, Singhal P. Cemento-ossifying fibroma. Contemp Clin Dent. 2012;3:83–85. doi: 10.4103/0976-237X.94553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Wang H, You M, Yang Z, Miao J, Shimizutani K, et al. Ossifying fibromas of the jaw bone:20 cases. Dentomaxillofac Radiol. 2010;39:57–63. doi: 10.1259/dmfr/96330046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eversole LR, Leider AS, Nelson K. Ossifying fibroma: a clinicopathologic study of sixty-four cases. Oral Surg Oral Med Oral Pathol. 1985;60:505–511. doi: 10.1016/0030-4220(85)90239-7. [DOI] [PubMed] [Google Scholar]
- 7.Sarwar HG, Jindal MK, Ahmad S. A case report of cemento-ossifying fibroma. J Maxillofac Oral Surg. 2010;9:178–181. doi: 10.1007/s12663-010-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuta AJ, Worley CM, Kaugars GE. Central Cemento-ossifying fibroma of the Maxillary sinus: A review of six cases. AJNR Am J Neuroradiol. 1995;16:1282–1286. [PMC free article] [PubMed] [Google Scholar]
- 9.Reichart PA, Philipsen HP, Sciubba JJ. The new classification of head and neck tumours (WHO)-any changes? Oral Oncol. 2006;42:757–758. doi: 10.1016/j.oraloncology.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Trijolet JP, Parmentier J, Sury F, Goga D, Mejean N, Laura B. Cemento-ossifying fibroma of the mandible. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:30–33. doi: 10.1016/j.anorl.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Akcam T, Altug HA, Karakoc O, Sencimen M, Ozkan A, Bayar GR, Gunhan O. Synchronous ossifying fibromas of the jaws: A review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(5 Suppl):S120–S125. doi: 10.1016/j.oooo.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Titinchi F, Morkel J. Ossifying Fibroma: Analaysis of treatment methods and recurrence patterns. J Oral Maxillofac Surg. 2016;24 doi: 10.1016/j.joms.2016.05.018. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Chatterjee A, Ajmera N, Singh A. Peripheral cemento-ossifying fibroma of maxilla. J Indian Soc Periodontol. 2010;14:186–89. doi: 10.4103/0972-124X.75915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertrand B, Ely P, Cornelius JP, Gosseye S, Clotuche J, Gilliard C. Juvenile aggressive cemento-ossifying fibroma: case report and review of the literature. Laryngoscope. 1994;103:1385–1390. doi: 10.1288/00005537-199312000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Galdeano Arenas M, Crespo Pinilla JI, Alvarez Otero R, Espeso Ferrero A, Verrier Hernandez A. Cemento-ossifying fibroma of mandibular gingiva: a single case report. Med Oral. 2004;9:177–179. 176-177. [PubMed] [Google Scholar]
- 16.Kristensen S, Tveteras K. Aggressive cementifying fibroma of the maxilla. Acta Otorhinolayngol. 1986;243:102–105. doi: 10.1007/BF00453758. [DOI] [PubMed] [Google Scholar]
- 17.Mithra R, Baskaran P, Sathyakumar M. Imaging in the diagnosis of cemento-ossifying fibroma: a case series. J Clin Imaging Sci. 2012;2:52. doi: 10.4103/2156-7514.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldron CA, Giansanti JS. Benign fibro-osseous lesions of the jaws: a clinical-radiologic-histologic review of sixty-five cases. Oral Surg. 1973;35:340–350. doi: 10.1016/0030-4220(73)90072-8. [DOI] [PubMed] [Google Scholar]
- 19.Barberi A, Cappabianca S, Collela G. Bilateral cement-ossifying fibroma of the maxillary sinus. Br J Radiol. 2003;76:279–280. doi: 10.1259/bjr.76.904.760279. [DOI] [PubMed] [Google Scholar]
- 20.Endo Y, Uzawa K, Mochida Y, Nakatsuru M, Shiiba M, Yokoe H, et al. Differential distribution of glycosaminoglycans in human cementifying fibroma and fibro-osseous lesions. Oral Dis. 2003;9:73–76. doi: 10.1034/j.1601-0825.2003.02889.x. [DOI] [PubMed] [Google Scholar]


