Abstract
Objectives:
To determine the occurrence of Mycoplasma genitalium (M. genitalium) infection in infertile women attending infertility clinic and its association to infertility.
Methods:
Endocervical specimens were collected from women presenting with primary and secondary infertility and from fertile women as control group. Real-time polymerase chain reaction (PCR) assay was performed to detect the presence of M. genitalium from these endocervical specimens.
Results:
Mycoplasma genitalium was detected in 3% of infertile women. There was no statistically significant difference between infertility and control group as to signs and symptoms except for signs of cervicitis were presented only among infertile group. We found no significant differences among fertile and infertile women for M. genitalium infection.
Conclusion:
An association between M. genitalium infection and infertility may exist. Screening of women for M. genitalium infection is recommended as part of investigations for infertility.
Infertility is a worldwide health problem with a current global infertility of 15%.1 In approximately 10% to 15% of couples, no direct cause of their infertility can be found, and 20% - 40% of couples may have multiple factors for infertility.2 Approximately 35% of infertile women are afflicted with post-inflammatory changes of the oviduct or surrounding peritoneum that interfere with tubal-ovarian function mostly as a result from infection and are likely to develop an ectopic (tubal) pregnancy.3 Mycoplasma genitalium (M. genitalium) is a small parasitic bacterium that lives on various cells of the body including the genital and respiratory tracts, fallopian tube cells, and spermatozoa.4 The human urogenital tract is the preferred site of colonization, which strongly indicates that M. genitalium is sexually transmitted.5 Mycoplasma genitalium was detected in mid-trimester of women delivering preterm,6 and among women with tubal factor infertility.7 Immunological assays cannot reliably detect the presence of M. genitalium.8 Currently, several tests are being used to detect Mycoplasma infections including nucleic acid amplification testing (NAATs), and a quantitative real-time Light Cycler polymerase chain reaction (PCR) assay which targets the gene gap encoding glyceraldehyde-3-phosphate dehydrogenase.9 In Saudi Arabia, discussing issues about sexually transmitted infections (STIs) is considered taboo where ethics and social factors give rise to many obstacles, thus there is a possibility that STIs might pose a significant public health threat. Data of M. genitalium infections are limited in Saudi Arabia. This study was conducted to determine the occurrence of M. genitalium among infertile women, compare it with a control of fertile women, evaluate its association with the type of infertility, infertility factors and their occurrence, and established a convenient method for rapid detection and identification of M. genitalium infection among infertile women.
Methods
Between October 2012 and July 2013, this prospective case control study was conducted in King Khalid University Hospital (KKUH) and King Abdulaziz University Hospital (KAUH), both in Riyadh, Saudi Arabia. Ethical consent was obtained from the ethical committee board of KKUH. One hundred infertile married women with primary and secondary infertility aged between 19 and 46 years who attended the outpatient infertility clinic at KKUH for infertility examination and fulfilled the inclusion criteria during the period of study and agreed to participate were enrolled and formed the case group. Another group of 100 women who attended the clinics for gynecologic purposes, either for routine or annual check-up comprised the control group. Relevant medical records were reviewed for any possible present and past medical or surgical diseases. Pregnant women and women who had taken antibiotics in the previous 30 days were excluded from this study.
Participants from both groups completed a questionnaire form on clinico-demographic information. Gynecological data including symptomatologies of gynecologic infection were obtained and recorded. Endocervical swabs by a speculum examination were collected from all participants. Swabs were stored at 4°C until transported to the laboratory, and stored at 20°C until processed. Deoxyribonucleic acid were extracted using the MagNA Pure Compact Nucleic Acid Isolation Kit (Roche Diagnostics and MagNA Pure Compact system, Mannheim, Germany), and was processed according to manufacturers protocol. Deoxyribonucleic acid amplification and detection protocol was carried out using the Light Cycler Fast Start DNA Master HybProbe according to the manufacturers protocol. PCR assays were carried out using the LightCycler PCR program, and melting curves were obtained according to the LightCycler instrument operator’s manual (TIB Molbiol, Germany).
Statistical analysis were performed using Statistical Package for Social Sciences (SPSS) version 19.0 (IBM Corp., Armonk, NY, USA). Results are presented as frequencies and percentages for categorical variables, and mean and standard deviation for numerical variables. We used chi-square test to compare between infertile group and the non-infertile group, and to compare between primary infertility and secondary infertility with respect to all nominal variables. Correlations were carried out using the Pearson correlation. P values of <0.05 were considered statistically significant.
Results
A total of 200 women consented to join the study, 100 as the case group and 100 as the control group. Mean age was 34.66 ± 7.72 years for all women. There was no statistically significant difference in the mean age between the 2 groups (case group: 32.50 ± 6.39 years versus 36.82 ± 8.32 years for the control group, p=0.9953).
Abdominal pain was the most frequent symptom seen in both groups: 26% in cases and 25% in control group (p=0.871). Previous abortion was noted in one case of infertile woman, and none in the control group (p=0.50). Pelvic inflammatory disease (PID) was noted in one infertile case, none in the control group (p=0.50) (Table 1). Three cases were positive for M. genitalium (3%), and none were detected in the control group (p=0.123). One case tested positive as a mix or co-infection for M. genitalium and M. hominis (1%). These 3 positive cases constituted only 5.6% of all symptomatic cases. Furthermore, out of 21 infertile cases who presented with abnormal discharges, one (4.8%) turned out positive for M. genitalium. Out of 6 infertile cases with signs of cervicitis, one case (16.7%) turned out positive for M. genitalium. Hormonal and ovulatory factors constituted more than 50% of the primary infertility factors. There was a significant difference between hormonal factor and male factor (p=0.007). The detection rate of M. genitalium infection with the both hormonal and ovulatory factors was 10% (2 in 21 patients). Mycoplasma genitalium infection rate among tubal factor infertile women was one in 15 cases (7%). The proportion of women testing positive for M. genitalium in the primary infertility group was 3 in 57 cases (5%), and none among secondary infertility. There were no significant difference regarding the types of infertility and positive cases (p=0.181). One positive case of M. genitalium was isolated from tubal primary infertile patient (1/15; 7%, p=0.40), and 2 cases were detected from unexplained primary infertile patients (2/21; 10%). Mycoplasma genitalium was detected from 3 primary infertile cases and no positive results from secondary infertile patients (p=0.181). Among the infertility group, 2 positive cases of M. genitalium from age group 19-25 years (2/16, 13%) and one positive case from age group 26-32 years was detected (1/34,3%, p=0.331).
Table 1.
Signs and symptoms among all females in the study (case versus control).
Melting curve and melting peak analysis of M. genitalium isolates showed Cp values between cycles 19 and 36. Mycoplasma genitalium positive samples had cycles per seconds (CPs) between cycles 36.1 and 37.8. The sample with the highest template count showed an amplification curve at 36.7 with 1.14x101 DNA copies/reaction (22,800 copies/ml). The sample with the lowest template count showed an amplification curve with CP at 37.8 with 4.04 DNA copies/reaction (808 copies/ml). Absolute quantitative real time PCR run of M. genitalium positive results, their CP, and the standard dilution series of standard DNA are shown in Figure 1. Electrophoretic analysis of amplicons are shown in Figure 2.
Figure 1.
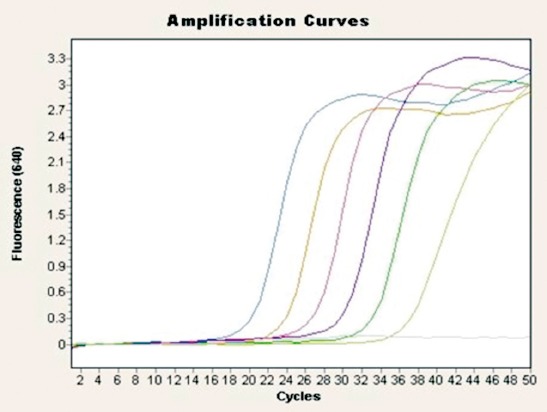
Amplification curves of Mycoplasma genitalium standard dilution series, a negative control was also included.
Figure 2.
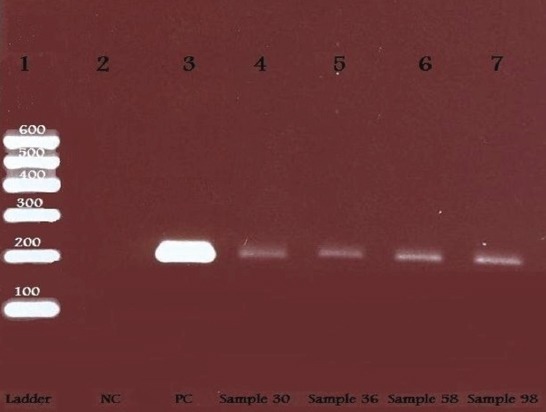
Electrophoresis analysis of Mycoplasma genitalium (M. genitalium) amplicons (224 bp bands) obtained by real time PCR with primers and probes specific for Mycoplasma genitalium. Lane 1:600 bp DNA ladder. Lane 2: negative control. Lane 3: positive control. Lanes 4-7: infertility group positive samples (sample Number 30, 36, 58, 98) of M. genitalium.
Discussion
The prevalence of M. genitalium as a cause of infection among infertile women is known to be less than that of Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum. Most of these infections present as mixed-infections of 2 or more of these agents.9 The infective profile of M. genitalium in infertility has not been thoroughly highlighted probably because its prevalence was overshadowed by the higher prevalence of the other agents. Because of this, screening for M. genitalium is not routinely carried out particularly in cases of genital infections and infertility.
This study is the first to use real time PCR with hybridization probe format by light cycler PCR protocol which showed higher detection rates and quantification of M. genitalium. This study shows that, a multitude of other infectious agents particularly those not prevalently seen and reported can be identified using this method of detection and quantification, thus an advantage to clinicians and laboratory personnel as well. Furthermore, this study highlighted the role of M. genitalium in genitourinary tract infections, that may have the potential to cause ectopic pregnancy or damage of a developing pregnancy. Age may be a significant confounding factor in the prevalence of infection in infertility since, more women nowadays wants to delay pregnancy, and so with increasing prevalence of women presenting with infertility.10 The higher percentage of infertile women in the age between 26 and 39 years old may be attributed to the fact that there is an increased awareness of the effects of aging on fertility.10 In this study, cervicitis was shown to be present only among the infertile group (6%). However, this rate was lower than that reported from a previous study where clinical cervicitis was found in 22% among the studied Saudi population.11 Our findings highlighted the hormonal factor, which is an established cause of infertility. Ovulation factor rate in our study was lower than UK rates of ovulatory disorders (25%), and lower than a study in Iran where ovarian infertility rate was 43.2%.12 The unexplained infertility rates in our study (10-20%) was slightly higher than those reported. Unexplained infertility rates in the UK was reported to be approximately 25%.12 Male factor was observed in 12% of our infertility cases, is lower compared to other studies where the rate has been reported to be between 20% and 30%.13 Our results showed that M. genitalium was found in the cervical canal in 4 infertile women, 3 as a single infection, and one was detected associated with M. hominis as a co-infection case. All 4 positive samples were detected from infertile group and none from the control group. This indicates an association between this infection and infertility as reported by previous studies.7 When compared to other studies from other countries, our rate of infection is lower than previous studies conducted in the USA and France.14 Moreover, our findings is higher than reports from other Middle East countries.15 The variation in the diagnostic tests employed in these different studies may contribute to the differences in the reported infection rates. Though M. genitalium infections are often asymptomatic, all the positive cases in our study were isolated from symptomatic women, with one positive case exhibiting signs of cervicitis. This confirms the significant role of M. genitalium as a cause of cervicitis. One interesting finding in this study is that all 3 M. genitalium infection cases were isolated from primary infertile women. This provides a strong relationship between M. genitalium infection and primary infertility.
The use of a qualitative real-time Light Cycler PCR in the detection of DNA from dead organisms has been proven advantageous compared to other standard method. The assay was found to be specific, sensitive, fast and had minimal risk of contamination. The clinical performance of the PCR was previously been evaluated and was compared to a conventional PCR. The Light Cycler PCR reproducibility was able to detect 4 positive samples. Melting peaks almost matching Tm values (Mean Tm=67.9) which determine how well the sequence of probes matches the sequence of template DNA. Real-time quantitative PCR is a powerful tool to accurately determine the M. genitalium load in clinical samples, because culture is too difficult to be an alternative. Many studies have now found significant associations with lower and upper reproductive tract disease in women. Mycoplasma genitalium should be considered an etiologic agent of cervical inflammation and upper tract disease syndromes, including infertility.
In conclusion, an association between M. genitalium infection and infertility may exist. Screening of women for M. genitalium infection is recommended as part of investigations for infertility.
References
- 1.Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA, Low N. National, Regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 Health Surveys. PLoS Med. 2012;9:e1001356. doi: 10.1371/journal.pmed.1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swift BE, Liu KE. The effect of age, ethnicity, and level of education on fertility awareness and duration of infertility. J Obstet Gynaecol Can. 2014;36:990–996. doi: 10.1016/S1701-2163(15)30412-6. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Zhao WH, Zhu Q, Cao SJ, Ping H, Xi X, et al. Risk factors for ectopic pregnancy: a multicenter case-control study. BMC Pregnancy Childbirth. 2015;15:187. doi: 10.1186/s12884-015-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Singh G, Samanta P, Sharma M. Mycoplasma genitalium : An emerging sexually transmitted pathogen. Indian J Med Res. 2012;136:942–955. [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein SA, Stiles BG. A review of the epidemiology, diagnosis and evidence-based management of Mycoplasma genitalium. Sexual Health. 2011;8:143–158. doi: 10.1071/SH10065. [DOI] [PubMed] [Google Scholar]
- 6.Averbacha SH, Hackera MR, Yiua T, Modest AM, Dimitrakoffa J, Ricciottia HA. Mycoplasma genitalium and preterm delivery at an urban community health center. Int J Gynaecol Obstet. 2013;123:54–57. doi: 10.1016/j.ijgo.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium Infection and female reproductive tract disease: A meta-analysis. Clin Infec Dis. 2015:1–25. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 8.Twin J, Taylor N, Garland SM, Hocking JS, Walker J, Bradshaw CS, Fairley CK, Tabrizi SN. Comparison of two Mycoplasma genitalium real-time PCR detection methodologies. J Clin Microbiol. 2011;49:1140–1142. doi: 10.1128/JCM.02328-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mousavi A, Farhadifar F, Mirnejad R, Ramazanzadeh R. Detection of genital mycoplasmal infections among infertile females by multiplex PCR. Iran J Microbiol. 2014;6:398–403. [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke VE, Hammarberg K. Reasons for delaying childbearing: a survey of women aged over 35 years seeking assisted reproductive technology. Aust Fam Physi. 2005;34:187–188. [PubMed] [Google Scholar]
- 11.McGowin CL, Anderson-Smits C. Mycoplasma genitalium : An emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7:e1001324. doi: 10.1371/journal.ppat.1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashidi BH, Tabriz LC, Haghollahi F, Jeddi-Tehrani M, Naghizadeh MM, Shariat M, et al. Effects of Chlamydia trachomatis infection on fertility;A case-control study. J Reprod Infert. 2013;14:67–72. [PMC free article] [PubMed] [Google Scholar]
- 13.Culley CC. Male factor infertility. Problem Based Urology. 2013:135–139. [Google Scholar]
- 14.Mobley VL, Hobbs MM, Lau K, Weinbaum BS, Getman DK, Se AC. Mycoplasma genitalium infection in women attending a sexually transmitted infection clinic: diagnostic specimen type, coinfections, and predictors. Sex Transm Dis. 2012;39:706. doi: 10.1097/OLQ.0b013e318255de03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haghighi Hasanabad M, Mohammadzadeh M, Bahador A, Fazel N, Rakhshani H, Majnooni A. Prevalence of Chlamydia trachomatis and Mycoplasma genitalium in pregnant women of Sabzevar- Iran. Iranian J Microbiol. 2011;3:123–128. [PMC free article] [PubMed] [Google Scholar]


