Abstract
Background:
In many studies, chemicals and natural materials were tested to reduce the harmful effects of radiation. It is known that Famotidine and vitamin C reduce DNA damage.
Objective:
The aim of this study was to evaluate the radioprotective effect of vitamin C, Cimetidine and Famotidine on gamma-radiation-induced damage on mouse bone marrow.
Methods:
Six-to-seven week male NMRI mice (28 g ±3) were randomly divided into fourteen groups: control, 2Gy irradiation, six group drugs without irradition (Famotidine, Cimetidine, vitaminC, Fam-Cim, Fam-Vit, Cim-Vit), six groups received drugs and 2Gy radiation with a 60Co |γ|-ray source at room temperature 22 ± 2 °C. The mice were killed 48 hours after irradiation by cervical dislocation. Slides were prepared from bone marrow cells and stained in May-Granwald and Giemsa. Finally, the cells were counted with microscope, frequencies of polychromatic erythrocyte (PCE), normochoromatic erythrocyte (NCE) and their micronuclated cell were recorded. PCE / PCE + NCE were calculated.
Results:
There were significant differences of MNPCE/1000PCE, MNNCE/1000NCE and PCE/PCE+NCE among different groups with similar radiation doses (p≤0.01). Moreover, there were significant differences of MNPCE/1000PCE and PCE/PCE+NCE among different doses of radiation (p≤0.01). While considering MNNCE/1000NCE, there were no significant differences among silimar groups with radiation dose (p˃0.05).
Conclusion:
Oral administration of Famotidine, vitamin C and Cimetidine demonstrate reliable and similar radioprotective effects. Additionally, the protective effect of single use of these drugs was similar to the combination form. Thus, the oral use of combination, 48 hours after irradiation cannot induce more radioprotective effect.
Keywords: Micronuclei , Radiation , Radioprotection , Cimetidine , Vitamin C , Famotidine
Introduction
People may be exposed to unwanted radiation during medical procedures or radiation accidents in medicine, industry or radioterrorism. Radiation causes cells clastogenic and cytotoxic damages [1], by energy transfer from radiation to macromolecules such as DNA, proteins and lipid membranes [2]. Free radical Proliferation leads to cell death by breaking or damaging chemical bonds in DNA [3]. The destructive effects of ionizing radiation in biological systems may be a direct interaction between radi¬ation and target macromolecules, or indirect due to the products released during the interaction between radiation and water. Hydroxyl radical is the most destructive type of water radiolysis product [4]. Oxygen free radicals are more destructive and responsible for about 50% of the overall damages caused by free radicals, especially DNA failures [5,6]. Radiation shielding reduces ionizing radiation damages [1]. Micronuclei test is one of the cytogenetic methods for the evaluation of radioprotective compounds [7]. It is an important indicator of protective effects of radioprotective drugs and ionizing radiation damages [8]. This test is a reliable method for assessing the clastogenic effects of materials in both in vitro and in vivo [9,10].
In order to prevent damage to human, besides rules set by international organizations, chemicals and natural materials were tested to reduce the harmful effects of radiation. Radioprotective agents are synthetic compounds or natural products that are immediately administrated before irradiation to reduce injuries caused by ionizing radiation [3]. An ideal radioprotector should provide significant protection against the effects of radiation, an acceptable route of administration, preferably oral, rapidly absorbed and distributed throughout the body, readily accesable and cheap, chemically stable to permit easy handling, storage at surrounding temperature and compatible with the wide range of other drugs which patients or personnel use. Additionally, it must have anacceptable toxicity and protective time-window effect [11,12]. There is a continued deagerness in the identification and the development of nontoxic and effective radioprotectants that can reduce the effect of ionizing radiation [13].
Vitamin C or ascorbic acid is one of the best defenses against the effects of cell damaging free radicals, demonstrated to have significantly radioprotective effects [14-17]. Also, the radioprotective effect of vitamin C on the internal and external radiation and a wide range of DRF have been reported [15,18-20]. Administration of vitamin C prior to irradiation stops micronuclei [14,21] and apoptosis [22]. Radiation induced wounds improved with administration of vitamin C [18]. It can lead to the repair of DNA failure and improved cell survival [23]. and the reduction of DNA damage in normal cells [24]. Cimetidine and Famotidine are antagonists of histamine typeII receptors used clinically for treating peptic ulcers, have been reported to be radioprotective due to its radical scavenging ability [25,26] and stimulation of the immunosystem [27,28]. Famotidine prevents DNA damage [29], apoptosis [22,30] and the formation of micronuclei [7]. Besides, the inhibition of gastric acid secretion induced by histamine is a powerful hydroxyl radical sweeper [25]. These drugs with a dose-dependent effect prevent the production of O2- and H2O2 in neutrophils [31].
According to previous studies and given the importance of following a diet of radioprotective drugs, this study investigated and compared radioprotective effects of combination and single oral administration of vitamin C, Cimetidine and Famotidine at micronuclei test.
Material and Methods
Experimental Animals
Seven or six week male NMRI mice (Pasture Institute, Amol, Iran) were housed five per cage (for one week) in climate-controlled, circadian rhythm-adjusted rooms, and they were allowed free access to food and water. The animals were, on average, seven to eight weeks old at the time of radiation an weighed between 28±3g. All experimental protocols and procedures were in agreement with the guidelines for animal experiments and the law of Shahid Beheshti University of Medical Sciences, Tehran, Islamic Republic of Iran.
Drug Treatments
Cimetidine and Famotidine powders were provided as gifts from ChemiDaru Co. (Tehran, Islamic Republic of Iran). Vitamin C powder was also as a gift from Osveh Co. (Tehran, Islamic Republic of Iran). All drugs were dissolved in distilled water and were freshly prepared for each use. All drugs were divided in three doses. Cimetidine, Famotidine and vitamin C (respectively, 15, 1.5 and 100mg/kg) were dissolved in distilled water, and were tested with gavage method along with 2 Gy of gamma rays; Gavaged three days before irradiation each 12h and also 2h before irradiation. The route of administration was per oral for in vivo studies in animals. All drugs were administred orally by gavages.
Irradiation
Irradiation was carried out with a 60Co |γ|-ray source (Theratron II, 780 C, Canada) at a dose of 2 Gy. The source-to-subject distance (SSD) was 80 cm and the dose rate was 0.98Gy/min. Mice were placed in individual ventilated Plexiglas chambers and simultaneously given a single whole-body exposure to γ -rays. Each mouse was placed in a separate and ventilated Plexigla chamber, in which they could not move. Each chamber size is 12×3×3 cm. Sham irradiation included comparable immobilization in the same irradiation chamber. The irradiation was performed without anesthetization of the mice.
Sampling, Mn Preparation and Staining
The mice were killed by cervical dislocation 48 hours after irradiation. Control groups were also killed 48 hours after the latest demo gavages. Bone marrow cells were flushed from both femurs with Fetal Bovine Serum, and a cell suspension was duly prepared. The suspension was centrifuged for 7 minutes at 1000 rpm. After centrifuging, the supernatant was removed and cells were resuspended in the remaining serum and a smear was prepared, fixed and stained by May Grunwald-Giemsa method (Schmid 1975). In this method of staining, polychromaticerythrocytes (PCEs) are stained blue-violet; while, normochromatic erythrocytes (NCEs) are stained yellow-range.
Microscopic and Statistical Analysis
OLYMPUS microscope with ×100 objective lens was used for counting the cells. For each mouce, 1000 PCEs were scored. At the same time, NCEs/1000 PCEs as well as PCEs and NCEs containing micronuclei were counted and recorded. In order to study the cytotoxic effects of gamma rays on the proliferation of the bone marrow cells, the ratio of PCEs/PCEs+NCEs was calculated. Data were expressed as mean±SEM for each experimental group. Statistical analysis was performed using SPSS 16.0 software. The significance of any intergroup differences in the number of micronucleated PCEs and NCEs as well as the ratio of PCEs/PCEs+NCEs were tested. Multiple groups were tested with analysis of variance (one-way ANOVA) followed by Tukey’s, andTwo group comparisons were performed using t-test. P-value of less than p ≤0.05 was considered significant.
Results
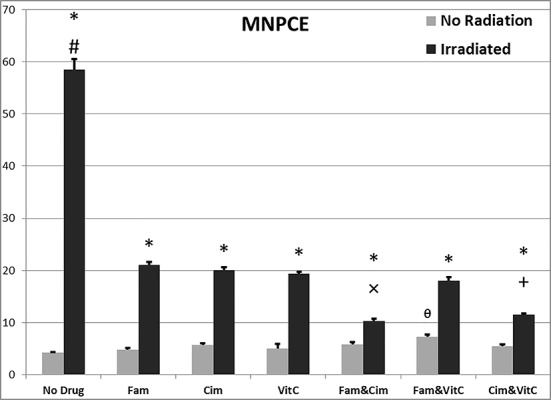
MNPCE/1000PCE after 48 hours: comparison of different groups
Data related to MNPCE/1000PCE after 48 hours are illustrated in Figure 1. There were significant differences of MNPCE/1000PCE among different groups without irradiation (0 Gy) [F (6, 29) = 4.25, p ≤0.005], and with irradiation (2 Gy) [F (6, 24) = 302.74, p ≤0.001].
Figure1.
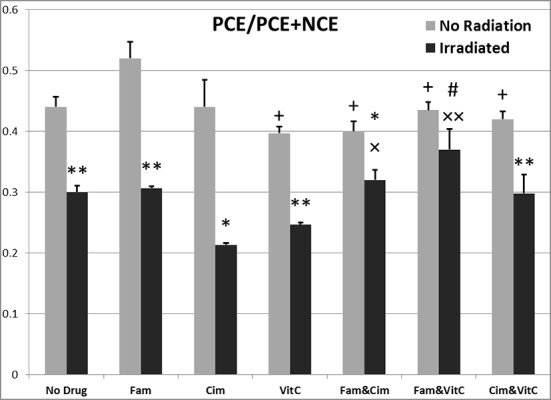
Data related to effects of vitamins C, Famotidine and Cimetidine on PCE/PCE+NCE after irradiation at the doses of 0 and 2Gy. Values represent the mean ± SEM.
*p ≤ 0.05, **p ≤ 0.001 significantly different among similar groups (0 Gy with 2 Gy).
Between different doses: # p ≤0.01 with Vit C 2Gy, ×p ≤0.05, ×× p ≤0.001 with Cim 2 Gy expect Fam&Cim, + p ≤0.001 with Fam0Gy.
MNPCE/1000PCE after 48 hours: comparison of similar groups, 0 and 2 Gy
Data related to MNPCE/1000PCE after 48 hours are illustrated in Figure 1. There were significant differences of MNPCE/1000PCE among different doses of irradiation; between control groups (p≤0.001), between vitamin groups (p≤0.001), between Famotidine groups (p≤0.001), between Cimetidine groups (p≤0.001), Famotidine +Cimetidine groups (p≤0.001), Famotidine +vitamin C groups (p≤0.001) and Cimetidine +vitamin C groups (p≤0.001).
MNNCE/1000NCE after 48 hours: comparison of different groups
Data related to MNNCE/1000NCE after 48 hours are illustrated in Figure 2. There were significant differences of MNNCE/1000NCE among different groups without irradiation [F (6, 29) = 7.77, p ≤0.001], and with irradiation [F (6, 25) = 21.76, p ≤0.001].
Figure2.
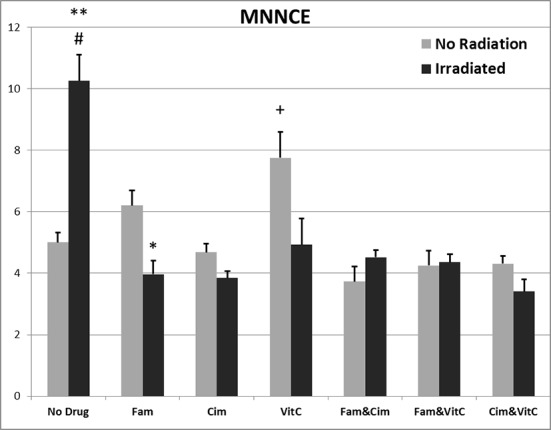
Data related to effects of vitamins C, Famotidine and Cimetidine on MNCE/1000NCE after irradiation at the doses of 0 and 2Gy. Values represent the mean ± SEM.
*p ≤ 0.05, **p ≤ 0.001 significantly different among similar groups (0 Gy with 2 Gy).
Between different doses: # p ≤0.001 with other groups 2Gy, + p ≤0.005 with other groups 2 Gy except Fam.
MNNCE/1000NCE after 48 hours: comparison of similar groups, 0 and 2 Gy
Data related to MNNCE/1000NCE after 48 hours are illustrated in Figure 2. There were significant differences of MNNCE/1000NCE among different doses of irradiation; between control groups (p≤0.001), between vitamin C groups (p>0.05), between Famotidine groups (p≤0.05), between Cimetidine groups (p>0.05), Famotidine +Cimetidine groups (p>0.05), Famotidine +vitamin C groups (p>0.05) and Cimetidine +vitamin C groups (p>0.05).
PCE/PCE+NCE after 48 hours: comparison of different groups
Data related to PCE/PCE+NCE after 48 hours are illustrated in Figure 3. There were significant differences of PCE/PCE+NCE among different groups without irradiation [F (6, 29) = 4.14, p ≤0.01], and with irradiation [F (6, 25) = 5.71, p ≤0.005].
Figure3.
Data related to effects of vitamins C, Famotidine and Cimetidine on MNPCE/1000MNPCE after irradiation at the doses of 0 and 2Gy. Values represent the mean ± SEM.
*p ≤ 0.001 significantly different among similar groups (0 Gy with 2 Gy).
Between different doses:# p ≤0.001 with other groups 2Gy,+ p ≤0.005 with other groups 2 Gy expect Fam&Cim, × p ≤0.001 with other groups 2 Gy except Cim&Vit C, ᶿp ≤0.05 with control, Fam, and Cim groups 0Gy.
PCE/PCE+NCE after 48 hours: comparison of similar groups, 0 and 2 Gy
Data related to PCE/PCE+NCE after 48 hours are illustrated in Figure 3. There were significant differences of PCE/PCE+NCE among different doses of irradiation; between control groups (p≤0.001), between vitamin C groups (p≤0.001), between Famotidine groups (p≤0.001), between Cimetidine groups (p≤0.05), Famotidine+Cimetidine groups (p≤0.05), Famotidine +vitamin C groups (p>0.05), Cimetidine+vitamin C groups (p ≤0.001).
Discussion
Ionizing radiation induces clastogenic and cytotoxic damages especially several damages in DNA, while radiation shielding reduces them [1]. Micronuclei test is an effective method for the evaluation of clastogenic effects of physical and chemical agents [14,21]. It is a reliable method to measure the protective effect of radioprotective drugs [8]. So, this study investigated the comparison of radioprotective effects of combination and single oral administration of vitamin C, Cimetidine and Famotidine by micronuclei test. These results are consistent with previous studies; they showed Gamma radiation increased the number of micronuclei in PCE and NCE (MnPCE, MnNCE) in the mouse bone marrow cells, and the PCE/PCE+NCE significantly reduced, which indicates cytotoxic gamma rays effects on the proliferation of mouse bone marrow cells, while oral use of vitamin C, Cimetidine and Famotidine did not create additional micronuclei and did not improve radiation lethality [15,16,20,27,32]. According to the present study, oral use of these drugs caused a remarkable decrease in the number of MnPCEs and MnNCEs compared to control groups at the dose of 2Gy gamma radiation. These findings are consistent with a previous study that showed decrease in the cell proliferation [33]. Other previous findings have shown that gamma ray induced micronuclei in bone marrow erythrocytes [34,35]. Histamine plays an increasing role on the bone marrow cell proliferation rate [36]. Famotidine, as a histamine H2 receptor antagonist, is a powerful hydroxyl radical sweepers [25]. Ranitidine and Famotidine have a high scavenging power for OH•, HOCl and NH2Cl [37]. Famotidine does not have immunomedulatory role in immune system, but is capable of exerting scavenging oxygen radicals [38]. Famotidine prevents DNA damage [5], inducing chromosomal aberrations [38], and micronuclei formation [7]. These results are consistent with Famotidine radioprotective effects which is against the induction of micronuclei in both in vitro and in vivo conditions [38,39], and also Cimetidine, as another histamine H2 receptor antagonist [28]. The most important protection mechanism of vitamin C is free radical and reactive oxygen scavenging [14,21]. Vitamin C modulates apoptosis in bone marrow cells increasing antiapoptosis gene expression and works against pro-apoptosis gene expression [40]. It has a direct role in the differentiation and proliferation of bone marrow cells [41,42]. Vitamin C treatment before irradiation causes the reduction of micronuclei in bone marrow, leukocyte and peripheral blood lymphocyte [14,43], and causes to prevent apoptosis in peripheral blood leukocytes [22] Vitamin C can lead to repair in a double-stranded DNA failure and improves cell surviva [23] indirectly through the restoration of glutathione help in radioprotection [44]. The results of this study showed that combination use of these drugs cannot create additional micronuclei. As was shown in comparison with the control group, these drugs combination reduced MnPCE and increased PCE/PCE+NCE at similar a manner to using in no-combination route (single use). Thus, it seems that the sweeping effect of these drugs caused the reduction of radiation harms.
Due to increased use of ionizing radiation in human life such as radiotherapy of cancer, industry, power generation and also high cost, side effects and toxicity of current available radioprotectors, we need to develop an effective and non-toxic radioprotector. Search for alternative sources such as plants, has been ongoing for several decades [45]. There are approaches to locate a potent radioprotector including mimics of antioxidant enzymes, nitroxides, gene therapy, growth factors, hyperthermia apart from natural products [46]. Although natural radioprotector sources especially medicinal plants or herbs are regarded as non-toxic even at higher concentrations, there is a growing interest among ethno-medicines and people [47]. Radioprotective activity of pure compounds isolated from the plants was previously evaluated, for example efficient superoxide radical scavenging ability of ellagitannins from Phyllanthusamarus [48], potent antioxidant activity of Ethanol extract of Ficusracemosa [49], radioprotective potential of quercetin and ethanolic extract of propolis in mice exposed to a single radiation [50]. But further investigation is needed to clarify the underlying protection mechanisms of natural radioprotectors. According to above studies and our results, these drugs in combination with natural radioprotectors could help scientists find a more effective radioprotective drug.
In conclusion, based on the results of this study, oral administration of Famotidine, vitamin C and Cimetidine showed reliable and similar radioprotective effects. Moreover, protective effect of single use of these drugs was similar to combination form. So, the oral combinational use 48 hours after irradiation cannot induce more radioprotective effects. The reduction of the incidence of micronuclei observed by the action of vitamin C, Cimetidine and Famotidine might be due to their antioxidant and radical scavenging properties. Further investigation with different organs or plans is necessary to evaluate the protective effect of these drugs, individual or in cobination with natural radioprotectors, against radiation-induced genotoxicity and cytotoxicity in radiation accident or radiation therapy
Acknowledgement
Authors take this opportunity to express their gratitude to North Research Center, Pasteur Institute of Iran members for their help and support. Prof. H. Mozdarani is supported by Iran National Science Foundation (INSF) under grant number 43800, Tehran, Iran.
Conflict of Interest:None Declared Grant Sponsor: Shahid Beheshti University of Medical Sciences, Tehran, Iran
References
- 1.Krishna G, Fiedler R, Theiss JC. Simultaneous evaluation of clastogenicity, aneugenicity and toxicity in the mouse micronucleus assay using immunofluorescence. Mutat Res. 1992;282:159–67. doi: 10.1016/0165-7992(92)90090-5. [DOI] [PubMed] [Google Scholar]
- 2.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol. 1994;65:27–33. doi: 10.1080/09553009414550041. [DOI] [PubMed] [Google Scholar]
- 3.Hosseinimehr SJ. Trends in the development of radioprotective agents. Drug Discov Today. 2007;12:794–805. doi: 10.1016/j.drudis.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 4.LaVerne JA. OH radicals and oxidizing products in the gamma radiolysis of water. Radiat Res. 2000;153:196–200. doi: 10.1667/0033-7587(2000)153[0196:ORAOPI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Mozdarani H, Nasirian B, Haeri SA. In vivo gamma-rays induced initial DNA damage and the effect of famotidine in mouse leukocytes as assayed by the alkaline comet assay. J Radiat Res. 2007;48:129–34. doi: 10.1269/jrr.06055. [DOI] [PubMed] [Google Scholar]
- 6.Imlay JA, Linn S. DNA damage and oxygen radical toxicity. Science. 1988;240:1302–9. doi: 10.1126/science.3287616. [DOI] [PubMed] [Google Scholar]
- 7.Shahidi M, Mozdarani H. Potent radioprotective effect of therapeutic doses of ranitidine and famotidine against gamma-rays induced micronuclei in vivo. Iran J Radiat Res. 2003;1:29–35. [Google Scholar]
- 8.Almassy Z, Krepinsky AB, Bianco A, Koteles GJ. The present state and perspectives of micronucleus assay in radiation protection. A review. Int J Rad Appl Instrum A 1987;38:241–9. doi: 10.1016/0883-2889(87)90033-5. [DOI] [PubMed] [Google Scholar]
- 9.Jenssen D, Ramel C. The micronucleus test as part of a short-term mutagenicity test program for the prediction of carcinogenicity evaluated by 143 agents tested. Mutat Res. 1980;75:191–202. doi: 10.1016/0165-1110(80)90014-7. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Tice RR, MacGregor JT, Anderson D, Blakey DH, Kirsh-Volders M, et al. In vivo rodent erythrocyte micronucleus assay. Mutat Res. 1994;312:293–304. doi: 10.1016/0165-1161(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Brown DS, Kao TC. Tocopherol succinate: a promising radiation countermeasure. Int Immunopharmacol. 2009;9:1423–30. doi: 10.1016/j.intimp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Joshi Y, Jadhav T, Kadam V. Radioprotective-A pharmacological intervention for protection against ionizing radiations: A review. The Internet Journal of Internal Medicine. 2010;8 [Google Scholar]
- 13.Weiss JF. Pharmacologic approaches to protection against radiation-induced lethality and other damage. Environ Health Perspect. 1997;105:1473–8. doi: 10.1289/ehp.97105s61473. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konopacka M, Widel M, Rzeszowska-Wolny J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat Res. 1998;417:85–94. doi: 10.1016/S1383-5718(98)00095-3. [DOI] [PubMed] [Google Scholar]
- 15.El-Nahas SM, Mattar FE, Mohamed AA. Radioprotective effect of vitamins C and E. Mutat Res. 1993;301:143–7. doi: 10.1016/0165-7992(93)90037-V. [DOI] [PubMed] [Google Scholar]
- 16.Mozdarani H, Nazari E. Cytogenetic damage in preimplantation mouse embryos generated after paternal and parental gamma-irradiation and the influence of vitamin C. Reproduction. 2009;137:35–43. doi: 10.1530/REP-08-0073. [DOI] [PubMed] [Google Scholar]
- 17.Kanter M, Akpolat M. Vitamin C protects against ionizing radiation damage to goblet cells of the ileum in rats. Acta Histochem. 2008;110:481–90. doi: 10.1016/j.acthis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Jagetia GC, Rajanikant GK, Baliga MS, Rao KV, Kumar P. Augmentation of wound healing by ascorbic acid treatment in mice exposed to gamma-radiation. Int J Radiat Biol. 2004;80:347–54. doi: 10.1080/09553000410001692744. [DOI] [PubMed] [Google Scholar]
- 19.Narra VR, Howell RW, Sastry KS, Rao DV. Vitamin C as a radioprotector against iodine-131 in vivo. J Nucl Med. 1993;34:637–40. [PubMed] [Google Scholar]
- 20.Mahdavi M, Mozdarani H. Protective effects of famotidine and vitamin C against radiation induced cellular damage in mouse spermatogenesis process. Iran J Radiat Res. 2011;8:223–30. [Google Scholar]
- 21.Konopacka M, Palyvoda O, Rzeszowska-Wolny J. Inhibitory effect of ascorbic acid post-treatment on radiation-induced chromosomal damage in human lymphocytes in vitro. Teratog Carcinog Mutagen. 2002;22:443–50. doi: 10.1002/tcm.10040. [DOI] [PubMed] [Google Scholar]
- 22.Mozdarani H, Ghoraeian P. Modulation of gamma-ray-induced apoptosis in human peripheral blood leukocytes by famotidine and vitamin C. Mutat Res. 2008;649:71–8. doi: 10.1016/j.mrgentox.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Fujii Y, Kato TA, Ueno A, Kubota N, Fujimori A, Okayasu R. Ascorbic acid gives different protective effects in human cells exposed to X-rays and heavy ions. Mutat Res. 2010;699:58–61. doi: 10.1016/j.mrgentox.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem 1990;265:2584–7. [PubMed] [Google Scholar]
- 25.Ching TL, Haenen GR, Bast A. Cimetidine and other H2 receptor antagonists as powerful hydroxyl radical scavengers. Chem Biol Interact. 1993;86:119–27. doi: 10.1016/0009-2797(93)90116-G. [DOI] [PubMed] [Google Scholar]
- 26.Ueda J, Saito N, Shimazu Y, Ozawa T. A comparison of scavenging abilities of antioxidants against hydroxyl radicals. Arch Biochem Biophys. 1996;333:377–84. doi: 10.1006/abbi.1996.0404. [DOI] [PubMed] [Google Scholar]
- 27.Mozdarani H, Salimi M, Froughizadeh M. Effect of cimetidine and famotidine on survival of lethally gamma irradiated mice. Iran J Radiat Res. 2008;5:187–94. [Google Scholar]
- 28.Mozdarani H, Gharbali A. Radioprotective effects of cimetidine in mouse bone marrow cells exposed to gamma-rays as assayed by the micronucleus test. Int J Radiat Biol. 1993;64:189–94. doi: 10.1080/09553009314551291. [DOI] [PubMed] [Google Scholar]
- 29.Le Caër S. Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water. 2011;3:235–53. doi: 10.3390/w3010235. [DOI] [Google Scholar]
- 30.Ghoraeian P, Mozdarani H, Akhlaghpoor S. Effect of famotidine on radiation induced apoptosis in human peripheral blood leukocytes. Iran J Radiat Res. 2005;3:31–6. [Google Scholar]
- 31.Mikawa K, Akamatsu H, Nishina K, Shiga M, Maekawa N, Obara H, et al. The effects of cimetidine, ranitidine, and famotidine on human neutrophil functions. Anesth Analg. 1999;89:218–24. doi: 10.1097/00000539-199907000-00040. [DOI] [PubMed] [Google Scholar]
- 32.Mozdarani H, Nazari E. Frequency of micronuclei in 4-8 cell mouse embryos generated after maternal gamma-irradiation in the presence and in the absence of vitamin C. Radiat Environ Biophys. 2007;46:417–22. doi: 10.1007/s00411-007-0118-z. [DOI] [PubMed] [Google Scholar]
- 33.Zangeneh M, Mozdarani H, Mahmoudzadeh A, Aghamiri MR. Effects of famotidine and vitamin C on low dose radiation-induced micronuclei in mice bone marrow cells. Journal of Paramedical Sciences. 2014;5:102–107. [Google Scholar]
- 34.Jagetia GC, Aruna R. The herbal preparation abana protects against radiation-induced micronuclei in mouse bone marrow. Mutat Res. 1997;393:157–63. doi: 10.1016/S1383-5718(97)00101-0. [DOI] [PubMed] [Google Scholar]
- 35.Hosseinimehr SJ, Tavakoli H, Pourheidari G, Sobhani A, Shafiee A. Radioprotective effects of citrus extract against gamma-irradiation in mouse bone marrow cells. J Radiat Res. 2003;44:237–41. doi: 10.1269/jrr.44.237. [DOI] [PubMed] [Google Scholar]
- 36.Medina VA, Croci M, Carabajal E, Bergoc RM, Rivera ES. Histamine protects bone marrow against cellular damage induced by ionising radiation. Int J Radiat Biol. 2010;86:283–90. doi: 10.3109/09553000903564067. [DOI] [PubMed] [Google Scholar]
- 37.Lapenna D, De Gioia, S Mezzetti, A Grossi, L Festi, D Marzio, L et. H2-receptor antagonists are scavengers of oxygen radicals. Eur J Clin Invest. 1994;24:476–81. doi: 10.1111/j.1365-2362.1994.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 38.Mozdarani H. Radioprotective properties of histamine H2 receptor antagonists: present and future prospects. J Radiat Res. 2003;44:145–9. doi: 10.1269/jrr.44.145. [DOI] [PubMed] [Google Scholar]
- 39.Ghorbani M, Mozdarani H. In vitro radioprotective effects of histamine H2 receptor antagonists against gamma-rays induced chromosomal aberrations in human lymphocytes. Iran J Radiat Res. 2003;1:99–104. [Google Scholar]
- 40.Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, Ko YH, et al. Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res. 2008;169:384–96. doi: 10.1667/RR1204.1. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi KM, Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, et al. Effect of ascorbic acid on bone marrow-derived mesenchymal stem cell proliferation and differentiation. J Biosci Bioeng. 2008;105:586–94. doi: 10.1263/jbb.105.586. [DOI] [PubMed] [Google Scholar]
- 42.Xian L, Lou M, Wu X, Yu B, Frassica F, Wan M, et al. Pretreatment with antioxidants prevent bone injury by improving bone marrow microenvironment for stem cells. 2012 [Google Scholar]
- 43.Konopacka M, Rzeszowska-Wolny J. Antioxidant vitamins C, E and beta-carotene reduce DNA damage before as well as after gamma-ray irradiation of human lymphocytes in vitro. Mutat Res. 2001;491:1–7. doi: 10.1016/S1383-5718(00)00133-9. [DOI] [PubMed] [Google Scholar]
- 44.Okunieff P, Swarts S, Keng P, Sun W, Wang W, Kim J, et al. Antioxidants reduce consequences of radiation exposure. Oxygen Transport to Tissue XXIX: Springer; 2008. pp. 165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paul P, Unnikrishnan M, Nagappa A. Phytochemicals as radioprotective agents A review. Indian Journal of Natural Products and Resources. 2011;2:137–50. [Google Scholar]
- 46.Maurya DK, Devasagayam TP, Nair CK. Some novel approaches for radioprotection and the beneficial effect of natural products. Indian J Exp Biol. 2006;44:93–114. [PubMed] [Google Scholar]
- 47.Chatterjee A, Pakrashi S. The Treatise onIndian Medicinal Plants. 1997 [Google Scholar]
- 48.Londhe JS, Devasagayam TP, Foo LY, Ghaskadbi SS. Radioprotective properties of polyphenols from Phyllanthus amarus Linn. J Radiat Res. 2009;50:303–9. doi: 10.1269/jrr.08096. [DOI] [PubMed] [Google Scholar]
- 49.Veerapur VP, Prabhakar KR, Parihar VK, Kandadi MR, Ramakrishana S, Mishra B, et al. Ficus racemosa Stem Bark Extract: A Potent Antioxidant and a Probable Natural Radioprotector. Evid Based Complement Alternat Med. 2009;6:317–24. doi: 10.1093/ecam/nem119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benkovic V, Knezevic AH, Dikic D, Lisicic D, Orsolic N, Basic I, et al. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh Hig Rada Toksikol. 2009;60:129–38. doi: 10.2478/10004-1254-60-2009-1908. [DOI] [PubMed] [Google Scholar]


