Abstract
The dentate gyrus (DG) engages in sustained Arc transcription for at least 8 hours following behavioral induction, and this time course may be functionally coupled to the unique role of the DG in hippocampus-dependent learning and memory. The factors that regulate long-term DG Arc expression, however, remain poorly understood. Animals lacking Egr3 show less Arc expression following convulsive stimulation, but the effect of Egr3 ablation on behaviorally induced Arc remains unknown. To address this, Egr3−/− and wild-type (WT) mice explored novel spatial environments and were sacrificed either immediately or after 5, 60, 240, or 480 minutes, and Arc expression was quantified by fluorescence in situ hybridization. Although short-term (i.e., within 60 min) Arc expression was equivalent across genotypes, DG Arc expression was selectively reduced at 240 and 480 minutes in mice lacking Egr3. These data demonstrate the involvement of Egr3 in regulating the late protein-dependent phase of Arc expression in the DG.
1. Introduction
The hippocampus is well established as a brain structure critical to many forms of memory. As a result, a number of studies have investigated how hippocampal neurons modify synaptic connections to permit information storage and retrieval. These synaptic changes are the result of signaling cascades that include immediate early gene (IEG) expression [1]. One of these IEGs, activity-regulated cytoskeleton-associated protein (Arc, Arg3.1), is required for both long-term potentiation (LTP) and long-term depression in the hippocampus, as well as for lasting memory formation [2–5].
Within the dentate gyrus (DG) of the hippocampus, Arc differs from most other IEGs in that it can be expressed for up to 8 hours after initial induction [6, 7]. This sustained Arc expression is required for lasting LTP in DG granule cells and thus likely critical for the synaptic changes involved in forming long-term memories that depend on the DG [5, 8]. Importantly, DG Arc expression in response to spatial learning occurs in a sequential cascade. Immediately following behavioral induction, Arc is expressed predominantly in the dorsal suprapyramidal blade (DGSP) of the DG [9]. Elevated Arc also becomes apparent in the ventral infrapyramidal blade (DGIP) after approximately 4 hours [7], and Arc transcription continues in both blades for at least 8 hours following behavioral induction. Furthermore, DG Arc expression at these long delays is correlated with spatial memory performance [6]. These observations are consistent with the idea that sustained expression of behaviorally induced Arc in the DG is functionally coupled to the formation of stable spatial memories. However, the molecular mechanism that sustains Arc expression for hours following an environmental stimulus remains unknown.
As an IEG, Arc is rapidly activated in response to neuronal activity [10]. Unlike the initial stimulation of Arc, however, the late phase of DG Arc transcription (i.e., hours after induction) requires protein synthesis. The IEG transcription factor early growth response 3 (Egr3) is required for late-phase Arc transcription in DG in response to convulsive stimulation [11]. Importantly, Egr3−/− mice do not lack the ability to transcribe Arc, as expression appears normal in these mice shortly after a pharmacologically induced seizure. However, sustained Arc expression is absent 4 hours after seizure onset. After exposure to a novel environment, Egr3 is also activated in the DG in the same cells that express Arc [11]. These findings suggest that Egr3 may also mediate enduring Arc transcription during behavior and thus play a pivotal role in both learning and plasticity as evinced by the deficits seen in animals lacking Egr3 [11, 12]. However, sustained Arc expression in mice lacking Egr3 has only been examined following supraphysiological levels of stimulation, and expression patterns of gene products in the DG produced by this type of robust stimulation can be different from those produced by behavior (e.g., [6, 13]).
To address this issue, we examined sustained Arc expression using fluorescence in situ hybridization (FISH) in the DG of Egr3−/− mice and their WT littermates immediately after a 5-minute exposure to a novel environment, as well as after 60-, 240-, and 480-minute delays. These data will establish how the time course of behaviorally induced Arc expression is altered in animals lacking Egr3.
2. Materials and Methods
2.1. Subjects
Previously generated male and female Egr3−/− mice [14] were back-crossed to C57BL/6 mice for greater than 25 generations. Animals were housed on a 14/10 h light/dark schedule with ad libitum access to food and water. Studies were conducted on littermate Egr3+/+ and Egr3−/− progeny of heterozygous breeding.
2.2. Behavioral Procedures
Animals explored a novel environment as previously described [6, 15]. Briefly, mice were divided into 5 behavioral groups (n = 6–9 mice/group/genotype) consisting of littermate-matched pairs of Egr3−/− and WT mice. The control group remained undisturbed in their cages until sacrifice. The four experimental groups were exposed to a novel open-field environment (a 78 × 37 cm clear plastic enclosure). The open field was divided into 9 equal grids. Mice were permitted to explore the environment for 5 minutes. The exploration sessions of all mice were recorded using a webcam and tracked post hoc at Wilfrid Laurier University using Any-maze tracking software (Stoelting, Kiel, WI). At the end of 5 minutes, one group (0) was immediately sacrificed. Three groups were sacrificed after a delay period of 60, 240, or 480 minutes. Mice were returned to their cages in the animal colony for the duration of the postexposure interval. Sacrifice was performed by overdose with isoflurane followed by decapitation. Brains were rapidly removed and flash frozen in isopentane submerged in slurry of ethanol and dry ice. The open-field environments were thoroughly cleaned between subjects. The caged control group was sacrificed directly from the home cage as a negative control to establish baseline levels of Arc expression. The brains were then shipped to Wilfrid Laurier University on dry ice for in situ hybridization as previously described [6, 16].
2.3. In Situ Hybridization
The frozen brains were embedded in optimal cutting temperature (OCT) medium (Fischer Scientific, Whitby, ON) in blocks that included tissue from every behavioral group. Coronal sections (20 μm thick) were obtained from each block and collected on slides treated with 3-aminopropyltriethoxysilane (Sigma-Aldrich, Oakville, ON) and stored at −80°C. Once thawed, sections were hybridized for 18 hours with full-length Arc riboprobes generated using MAXIscript kits (Ambion, Austin, TX) and digoxigenin-UTP labelling mix (Roche, Indianapolis, IN). Slides were subsequently incubated with anti-digoxigenin-peroxidase (1 : 400; Roche) for 2 h at RT, followed by Cy3 for 30 min at RT (1 : 50; PerkinElmer, Waltham, MA) and DAPI for 30 min at RT (Sigma-Aldrich) to stain nuclei. An Olympus FV1000 laser scanning confocal microscope obtained z-stacks (20 μm thickness, ~1.0 μm optical planes, and 0.7 μm interval between planes) in one random location in the DGIP and two locations in the DGSP, as well as from one random location in each of the proximal and distal regions of CA1, CA3a, and CA3c (see Figure 1). Neurons within the median of 20% of each z-stack were classified as Arc+ or Arc−. The total number of cells counted in each region is provided in Table 1.
Figure 1.
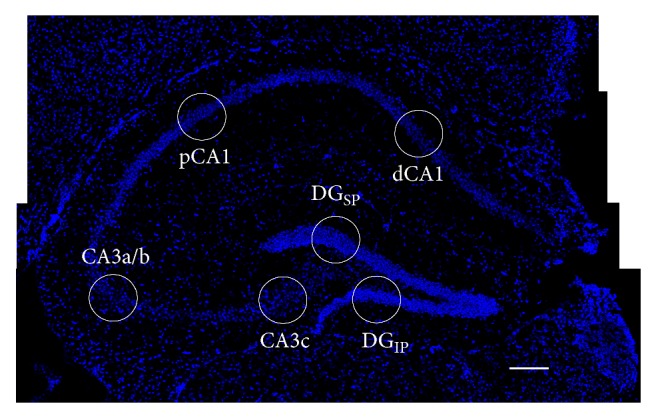
Hippocampal imaging. A sample montage of dorsal hippocampus labelled with DAPI (blue, scale bar = 100 μ) depicts representative imaging locations (circles) for the regions described in this study in both pyramidal cells (distal (dCA1) and proximal (pCA1) CA1, CA3a/b, and CA3c) and granule cells (suprapyramidal (DGSP) and infrapyramidal (DGIP) dentate gyrus).
Table 1.
Mean number of cells per animal counted across the hippocampal formation.
| Genotype | Group | Region | |||||
|---|---|---|---|---|---|---|---|
| Proximal CA11 | Distal CA11 | CA3a2 | CA3c2 | DGSP | DGIP | ||
| WT | Caged | 297.4 ± 18.3 | 353.8 ± 11.2 | 168.5 ± 15.6 | 224.6 ± 20.8 | 1782.5 ± 382.7 | 1424.3 ± 269.3 |
| 0 | 330.8 ± 14.3 | 337. 6 ± 12.1 | 202.5 ± 9.2 | 157.0 ± 9.6 | 1620.6 ± 330.5 | 1213.9 ± 386.7 | |
| 60 | 305.7 ± 20.2 | 285.4 ± 15.2 | 189.1 ± 8.7 | 212.0 ± 18.4 | 1662.5 ± 263.3 | 1487.5 ± 283.2 | |
| 240 | 263.1 ± 13.3 | 335.1 ± 9.6 | 154.5 ± 11.1 | 294.0 ± 12.1 | 1726.7 ± 280.9 | 1322.2 ± 279.1 | |
| 480 | 305.4 ± 11.4 | 319.0 ± 19.1 | 209.8 ± 9.0 | 252.8 ± 15.0 | 1905.2 ± 324.9 | 1610.8 ± 253.2 | |
| Egr3−/− | Caged | 360.8 ± 14.9 | 309.6 ± 19.0 | 135.5 ± 5.4 | 189.9 ± 13.6 | 1980.2 ± 324.9 | 1380.1 ± 254.8 |
| 0 | 348.2 ± 23.1 | 297.3 ± 18.1 | 180.8 ± 15.8 | 150.7 ± 9.3 | 1816.9 ± 317.4 | 1554.7 ± 262.6 | |
| 60 | 315.3 ± 16.3 | 347.0 ± 18.6 | 101.5 ± 18.0 | 240.8 ± 16.8 | 1722.1 ± 296.8 | 1442.5 ± 256.5 | |
| 240 | 415.1 ± 21.8 | 326.3 ± 17.8 | 216.8 ± 19.8 | 217.2 ± 9.0 | 1835.6 ± 309.1 | 1322.2 ± 234.6 | |
| 480 | 227.9 ± 27.7 | 354.9 ± 14.0 | 175.4 ± 7.4 | 194.0 ± 9.4 | 2015.6 ± 314.3 | 1493.3 ± 261.9 | |
2.4. Statistical Analyses
Statistical analyses consisted of either one- or two-way analysis of variance (ANOVA) followed by post hoc analyses using Tukey's HSD.
3. Results
3.1. Egr3 Does Not Significantly Alter Exploration Behavior in a Novel Space
Because Egr3−/− mice demonstrate both motor deficits [14] and hyperactivity [12], the locomotor behavior of all mice was analyzed. An ANOVA on the mean path length travelled by Egr3−/− (10.94 ± 2.31 m) and WT (13.73 ± 1.80 m) mice showed no significant difference (F1,78 = 1.72; p = 0.19).
3.2. Egr3 Does Not Significantly Alter Arc Expression in Hippocampal Pyramidal Cells
Figure 2(a) shows representative images of the results of Arc FISH in CA1 from Egr3−/− and WT mice at baseline and at two time points (during the early, protein synthesis-independent phase of Arc transcription, as well as during the late, protein synthesis-dependent phase of Arc transcription) following behavioral exposure to a novel environment. The transcription of Arc is observed in very few cells in mice that remain undisturbed in their home cages (baseline cage control). Five minutes of spatial exploration induces a robust increase in Arc expression in both WT and Egr3−/− mice that can be observed immediately, and that is absent at 480 minutes.
Figure 2.
Normal exploration-induced Arc expression in pyramidal cells in Egr3−/− mice. Sample confocal images are provided (a) of proximal CA1 from WT (top row) and Egr3−/− (bottom row) mice under baseline control (caged) conditions, versus 0 minutes (early) and 480 minutes (late) following a five-minute exposure to a novel environment (red = Arc, blue = DAPI, scale bar = 100 μm). Consistent with these images, graphs of (b) proximal CA1, (c) distal CA1, (d) CA3a/b, and (e) CA3c all show that novel exploration induces a robust increase in Arc expression immediately (0′) that is greatly reduced by 60′. At 240′ and 480′, Arc expression is no different from caged controls in any pyramidal cell region. No differences are observed between Egr3−/− and WT mice (∗p < 0.05 relative to caged control of the same genotype; graphs display mean ± SEM).
A two (genotype) by four (region: proximal CA1, distal CA1, CA3a/b, and CA3c) by five (behavioral group) ANOVA revealed a main effect of both behavioral group (F4,156 = 58.33; p < 0.001) and region (F4,156 = 47.84; p < 0.001), as well as a behavioral group by region interaction (F4,156 = 14.78; p < 0.001). These effects indicate that, although Arc expression was robustly increased in all pyramidal cell regions by brief spatial experience, more cells expressed Arc in the CA1 regions relative to CA3, consistent with previous literature [16–18]. No main effect of genotype (F1,156 = 2.07; p = 0.16) or genotype by behavioral group interaction (F4,156 = 1.56; p = 0.25) was observed. Thus, Arc expression remains normal in the pyramidal cells of Egr3−/− mice.
3.3. Egr3 Selectively Regulates Late Phase of Arc Transcription in DG Granule Cells
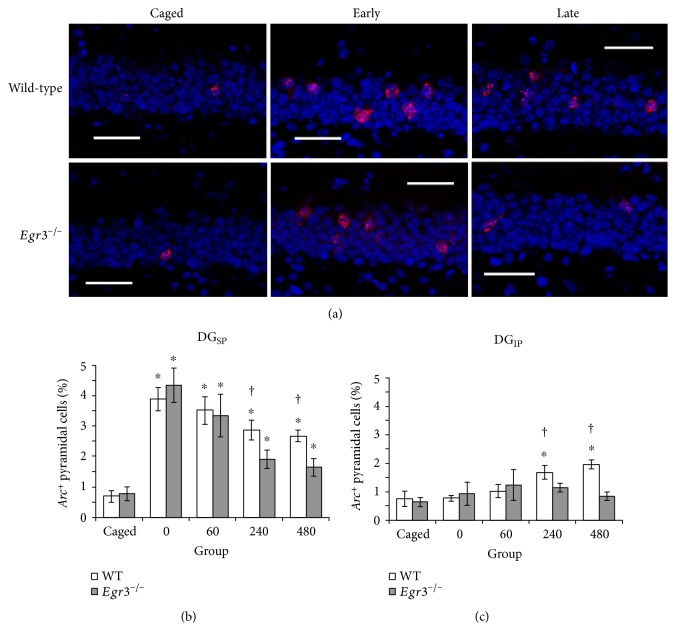
Figure 3(a) shows representative images of the results of Arc FISH in the DG suprapyramidal blade from Egr3−/− and WT mice at baseline and at two time points (one during the early, protein synthesis-independent phase of Arc transcription and one during the late, protein synthesis-dependent phase of Arc transcription) following behavioral exposure to a novel environment. Transcription of Arc is observed in very few cells in mice that remain undisturbed in their home cages (baseline cage control). Five minutes of exploration of a novel environment induces a robust increase in Arc expression in both WT and Egr3−/− mice within 60 minutes. By 480 minutes following the novel exploration, however, Arc mRNA levels decreased substantially in Egr3−/− mice compared to their WT littermates.
Figure 3.
Accelerated loss of exploration-induced Arc expression in the DG of Egr3−/− mice. Sample confocal images are provided (a) of the DG suprapyramidal blade (DGSP) from WT (top row) and Egr3−/− (bottom row) mice under baseline control (caged) conditions, versus 60 minutes (early) and 480 minutes (late) following a five-minute exposure to a novel environment (red = Arc, blue = DAPI, scale bar = 100 μm). Consistent with these images, graphs of DGSP (b) show that novel exploration induces Arc expression immediately (0′) in all mice and that in WT mice Arc transcription persists throughout all time points observed. In Egr3−/− mice, however, exploration-induced Arc expression is selectively reduced in the late, protein synthesis-dependent phase of transcription (i.e., 240′ and 480′) relative to their WT littermates. In contrast, significant induction of Arc expression in the DG infrapyramidal blade (b) is not apparent in WT mice until 240 min postexploration and is completely absent in Egr3−/− mice (∗p < 0.05 relative to caged control of the same genotype; †p < 0.05 relative to Egr3−/−; graphs display mean ± SEM).
Figure 3(b) illustrates that, in the DGSP, Arc expression is induced to the same level in Egr3−/− mice at 5 min and 60 min. As previously reported [6, 7, 9, 19], this brief exposure results in an increase in the number of granule cells expressing Arc in WT mice that perdures for at least 8 hours. However, by 240 min after exposure, the levels of Arc are reduced significantly more in the Egr3−/− mice than their WT littermates, and this effect persists at 480 min. In the DGIP, the percentage of Arc-expressing granule cells was increased in WT mice at 240 and 480 min following exploration, while no significant increase was seen in Egr3−/− mice (Figure 3(c)).
A two (genotype) by two (region: DGSP versus DGIP) by five (behavioral group) ANOVA revealed a main effect of both behavioral group (F4,138 = 16.75; p < 0.001) and region (F4,138 = 64.23; p < 0.001), as well as a behavioral group by region interaction (F4,138 = 9.27; p < 0.001). These effects indicate that the blades of the DG show unique responses to brief spatial experience, consistent with previous literature [6, 7, 9, 19]. Although no main effect of genotype was observed (F1,138 = 0.13; p = 0.72), a significant genotype by behavioral group interaction (F4,138 = 6.29; p < 0.0001) was observed. This interaction demonstrates that while Arc expression remains normal in Egr3−/− mice both during rest (i.e., in caged controls) and early after behavioral induction (i.e., in the 5′ and 60′ groups), the late phase of Arc expression (i.e., 240′ and 480′) is significantly reduced in mice lacking Egr3.
4. Discussion
The results reported here demonstrate that Egr3 selectively regulates the DG-specific perdurance of Arc transcription. In the pyramidal cell fields of the hippocampus, Arc expression was not changed in mice lacking Egr3. In contrast, the DG of Egr3−/− mice demonstrated a robust and selective knockdown in the late, protein synthesis-dependent phase of Arc transcription several hours after behavioral induction. Moreover, the blunting of the Arc transcriptional response to spatial processing was particularly pronounced in the DGIP. In WT animals, this blade only expressed more Arc than caged controls at 4 and 8 hours after behavior, and this response was absent in Egr3−/− mice. These findings reaffirm the importance of validating observations made following convulsive stimulation by investigating conditions of behavioral induction, since the time course of Arc expression in the DGSP and DGIP we observed in response to physiological conditions has not been reported in response to seizure. In the DGSP, the knockdown of behaviorally induced Arc observed is considerably more subtle (an approximately 50% knockdown, rather than a compete ablation) than that which has been described following supraphysiological stimulation [11]. This apparent discrepancy, however, may in fact be the result of methodology. Unlike previous work, which utilized semiquantitative analysis of precipitate-labelled PCR products, the current study employed single-cell analysis of fluorescence-conjugated, full-length riboprobes made by reverse transcription. Collectively, these changes result in an order of magnitude greater sensitivity in the current data, and thus greater probability of detecting low abundance Arc expression in the DG of Egr3−/− mice. This more moderate knockdown of Arc expression, however, still carries the potential to profoundly impact memory.
Our understanding of the functional role of sustained Arc transcription in the DG in supporting memory remains in its infancy. Both experimental evidence [20] and computational models [21] suggest that DG granule cells play a role in “tagging” the relative timing of events. It has been proposed that sustained Arc expression may provide a molecular mechanism to keep representations labile within the DG to sculpt and ultimately disambiguate representations for events that happen on the scale of hours [19]. Testing these ideas will be greatly facilitated by the identification of mechanisms that uniquely drive late-phase Arc transcription so that it may be selectively manipulated. The current data indicate that Egr3 is a viable target for such a manipulation to decipher the specific contribution of late-phase Arc transcription to hippocampus-dependent learning and memory in intact animals.
Acknowledgments
Support was provided by the Natural Sciences and Engineering Research Council of Canada and the Ontario Mental Health Foundation (DFM), the US National Institute of Mental Health Award MH097803 (ALG), and the Science Foundation of Arizona Bisgrove Scholarship (AMM).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Amelia L. Gallitano and Diano F. Marrone contributed equally to this work.
References
- 1.Clayton D. F. The genomic action potential. Neurobiology of Learning and Memory. 2000;74(3):185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 2.Link W., Konietzko U., Kauselmann G., et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lyford G. L., Yamagata K., Kaufmann W. E., et al. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14(2):433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 4.Steward O., Worley P. F. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30(1):227–240. doi: 10.1016/S0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 5.Plath N., Ohana O., Dammermann B., et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Marrone D. F., Satvat E., Shaner M. J., Worley P. F., Barnes C. A. Attenuated long-term Arc expression in the aged fascia dentata. Neurobiology of Aging. 2012;33(5):979–990. doi: 10.1016/j.neurobiolaging.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez-Amaya V., Angulo-Perkins A., Chawla M. K., Barnes C. A., Rosi S. Sustained transcription of the immediate early gene Arc in the dentate gyrus after spatial exploration. The Journal of Neuroscience. 2013;33(4):1631–1639. doi: 10.1523/JNEUROSCI.2916-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messaoudi E., Kanhema T., Soulé J., et al. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. The Journal of Neuroscience. 2007;27(39):10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chawla M. K., Guzowski J. F., Ramirez-Amaya V., et al. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15(5):579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- 10.Guzowski J. F., McNaughton B. L., Barnes C. A., Worley P. F. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nature Neuroscience. 1999;2(12):1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 11.Li L., Carter J., Gao X., Whitehad J., Tourtellotte W. G. The neuroplasticity-associated Arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Molecular and Cellular Biology. 2005;25(23):10286–10300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallitano-Mendel A., Izumi Y., Tokuda K., et al. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148(3):633–643. doi: 10.1016/j.neuroscience.2007.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worley P. F., Bhat R. V., Baraban J. M., Erickson C. A., McNaughton B. L., Barnes C. A. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. The Journal of Neuroscience. 1993;13(11):4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tourtellotte W. G., Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nature Genetics. 1998;20(1):87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- 15.Gheidi A., Azzopardi E., Adams A. A., Marrone D. F. Experience-dependent persistent expression of zif268 during rest is preserved in the aged dentate gyrus. BMC Neuroscience. 2013;14(1):p. 100. doi: 10.1186/1471-2202-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skinner D. M., Martin G. M., Wright S. L., et al. Hippocampal spatial mapping and the acquisition of competing responses. Hippocampus. 2014;24(4):396–402. doi: 10.1002/hipo.22233. [DOI] [PubMed] [Google Scholar]
- 17.Vazdarjanova A., Ramirez-Amaya V., Insel N., et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. The Journal of Comparative Neurology. 2006;498(3):317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- 18.Gheidi A., Satvat E., Marrone D. F. Experience-dependent recruitment of Arc expression in multiple systems during rest. Journal of Neuroscience Research. 2012;90(9):1820–1829. doi: 10.1002/jnr.23057. [DOI] [PubMed] [Google Scholar]
- 19.Meconi A., Lui E., Marrone D. F. Sustained Arc expression in adult-generated granule cells. Neuroscience Letters. 2015;603:66–70. doi: 10.1016/j.neulet.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Rangel L. M., Alexander A. S., Aimone J. B., et al. Temporally selective contextual encoding in the dentate gyrus of the hippocampus. Nature Communications. 2014;5:p. 3181. doi: 10.1038/ncomms4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aimone J. B., Wiles J., Gage F. H. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61(2):187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]