Abstract
OBJECTIVE
Older adults with diabetes and dementia are at increased risk for hypoglycemia and other adverse events associated with tight glycemic control and are unlikely to experience long-term benefits. We examined risk factors for tight glycemic control in this population and use of medications associated with a high risk of hypoglycemia in the subset with tight control.
RESEARCH DESIGN AND METHODS
This retrospective cohort study of national Veterans Affairs (VA) administrative/clinical data and Medicare claims for fiscal years (FYs) 2008–2009 included 15,880 veterans aged ≥65 years with type 2 diabetes and dementia and prescribed antidiabetic medication. Multivariable regression analyses were used to identify sociodemographic and clinical predictors of hemoglobin A1c (HbA1c) control (tight, moderate, poor, or not monitored) and, in patients with tight control, subsequent use of medication associated with a high risk of hypoglycemia (sulfonylureas, insulin).
RESULTS
Fifty-two percent of patients had tight glycemic control (HbA1c <7% [53 mmol/mol]). Specific comorbidities, older age, and recent weight loss were associated with greater odds of tight versus moderate control, whereas Hispanic ethnicity and obesity were associated with lower odds of tight control. Among tightly controlled patients, 75% used sulfonylureas and/or insulin, with higher odds in patients who were male, black, or aged ≥75 years; had a hospital or nursing home stay in FY2008; or had congestive heart failure, renal failure, or peripheral vascular disease.
CONCLUSIONS
Many older veterans with diabetes and dementia are at high risk for hypoglycemia associated with intense diabetes treatment and may be candidates for deintensification or alteration of diabetes medications.
Introduction
The prevalence of diabetes among adults aged ≥65 years is projected to increase dramatically by 2050 (1). Dementia affects up to 16% of diabetic patients aged ≥65 years and 24% aged ≥75 years (2,3), and evidence shows that the two conditions share a pathophysiological link (4–6). As described in the federally mandated National Plan to Address Alzheimer’s Disease (7), U.S. health care is poorly situated to address the needs of older adults with dementia and common comorbidities such as diabetes. This is evident in the two- to threefold increased odds of severe hypoglycemia and preventable hospitalizations in diabetic patients with comorbid dementia (2,8). Improving ambulatory care, particularly reducing known risk factors for hypoglycemia, in older patients with coexisting diabetes and dementia is critical, but to date, few such efforts have been made.
There is growing consensus that because of their increased risk for hypoglycemia and reduced potential to benefit from tight glycemic control, older diabetic patients with dementia should avoid tight glycemic control (HbA1c <7% [53 mmol/mol]) and instead pursue moderate HbA1c levels of 7% to <9% (53 to <75 mmol/mol). Older individuals with dementia often have other risk factors for hypoglycemia, including weight loss, changes in appetite and eating habits, and difficulty following prescribed regimens (9–11), and a reduced ability to recognize and respond appropriately to symptoms (9,12,13). Furthermore, older patients with dementia have an average life expectancy of 2–8 years (14–16) and are unlikely to experience benefits of tight glycemic control, which take years to accrue and are less likely in patients with long-standing diabetes (11). As such, since 2003, guidelines from the U.S. Departments of Veterans Affairs and Defense (VA/DoD) have presented a risk-stratified approach to glycemic control, recommending tight control (HbA1c <7% [53 mmol/mol]) only for patients with a life expectancy of 10–15 years or more and absent/mild microvascular complications (17,18). The American Diabetes Association and American Geriatrics Society (AGS) subsequently adopted similar recommendations (19,20), with the AGS in 2013 including the avoidance of tight glycemic control in older adults with comorbidities in its Choosing Wisely campaign (21). Although others have documented the elevated risk of hypoglycemia in older diabetic patients with dementia (2), no studies to our knowledge have focused on understanding risk factors for tight glycemic control in patients with comorbid dementia.
Choice of antidiabetic medication also may exacerbate risks to type 2 diabetic patients with dementia, especially when HbA1c is tightly controlled (22). Sulfonylureas and insulin are associated with a high risk of hypoglycemia (19), and the 2013 AGS guidelines explicitly recommend against the use of glyburide and chlorpropamide in all patients aged ≥65 years (20). However, sulfonylureas and insulin are recommended as first- and second-line diabetes therapies in the general patient population due to strong evidence of efficacy in lowering HbA1c, low cost, market longevity, and low risk of adverse events apart from hypoglycemia (17,19). As patients develop dementia, the risk of hypoglycemia increases and the risk-to-benefit balance of using these agents changes, especially if HbA1c is tightly controlled. To our knowledge, no prior studies have examined the prevalence of or risk factors for the use of medications with a high risk of hypoglycemia in patients with dementia.
In this study, we addressed these gaps in the literature by identifying risk factors for tight glycemic control in older veterans with dementia receiving antidiabetic medication therapy. We also identified the prevalence and characteristics of patients at highest potential risk for hypoglycemia through their use of sulfonylurea and/or insulin after exhibiting tight glycemic control. Such data can help to inform future interventions to improve adherence to the VA diabetes treatment guidelines and AGS Choosing Wisely guidelines recommending against tight glycemic control in this population and enhance safer prescribing for veterans with dementia previously shown to be at especially high risk for severe hypoglycemic events (2).
Research Design and Methods
Design and Data Sources
We conducted a longitudinal, retrospective cohort study using administrative data from the VA health care system and Centers for Medicare & Medicaid Services. Data sources included were VA Medical SAS Datasets, records of dispensed outpatient prescriptions, and laboratory values linked to Medicare Part A, Part B, and enrollment data obtained for a larger study (23,24). All baseline variables and HbA1c levels were based on fiscal year (FY) 2008 data; the first 120 days of FY2009 served as the follow-up period for determining antidiabetic medication use. The VA Pittsburgh Healthcare System Institutional Review Board approved this study.
Sample
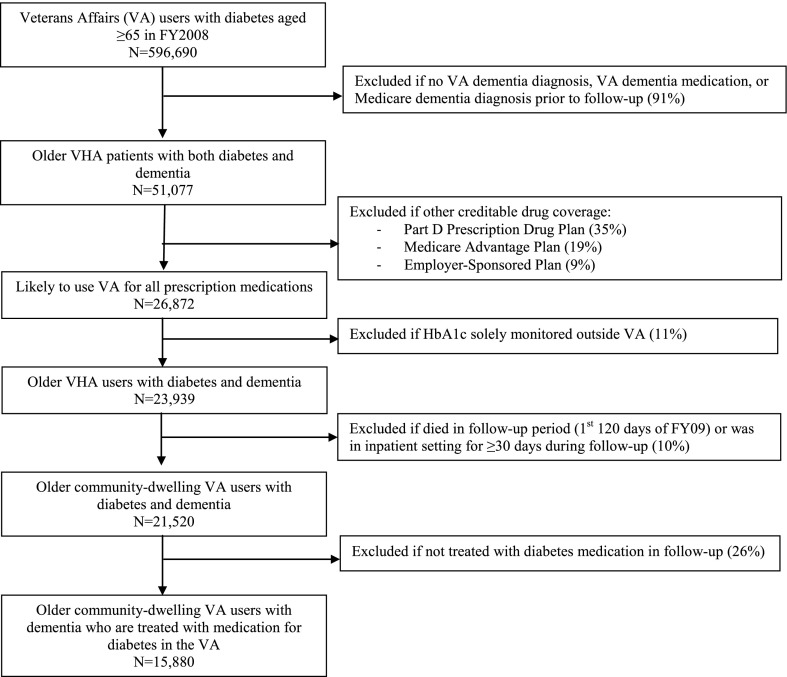
To construct the sample, we first used VA data to identify all veterans who 1) were age ≥65 years as of the start of FY2008 (1 October 2007) and 2) were given two or more inpatient or outpatient diagnoses for type 2 diabetes (ICD-9 250.x, 250.x2) in FY2008–2009 (with first diagnosis in FY2008) or received an oral diabetes medication through the VA in FY2008 (25). We then linked to Medicare claims and enrollment data to further refine the sample (Fig. 1). We applied the Medicare Chronic Conditions Warehouse ICD-9 diagnosis code list for Alzheimer’s Disease and Related Disorders (ADRD) to both VA records and Medicare claims to identify patients with dementia (26,27). The ICD-9 codes in this algorithm include Alzheimer disease, vascular dementia, and a range of other specific related disorders (see Supplementary Table 1 for full list of diagnoses). This definition contains only minor differences from an algorithm shown to have good sensitivity and specificity compared with a gold standard clinical dementia assessment (27). Patients without an ADRD ICD-9 code who filled a VA prescription for an antidementia medication (galantamine, rivastigmine, donepezil, memantine, or tacrine) in FY2008 were also included. Next, because veterans aged ≥65 years are eligible to enroll in Medicare and use non-VA health care in addition to VA care (yet we did not have access to non-VA prescription drug records), we took two steps to restrict the sample to patients with diabetes managed primarily within the VA: 1) eliminating all patients whose Medicare records indicated enrollment in a non-VA source of drug coverage during follow-up (i.e., Medicare Part D stand-alone plan, Medicare Advantage plan, employer-sponsored plan eligible for a Centers for Medicare & Medicaid Services retiree drug subsidy) and 2) excluding all remaining patients with HbA1c levels solely monitored outside the VA, indicated by no HbA1c values in VA records and at least one procedure code for an HbA1c test in Medicare claims. Next, to ensure accurate capture of outpatient medications used in the 120-day follow-up period, we excluded patients who died or resided in a VA or Medicare-covered hospital or nursing home for ≥30 days during the follow-up period and, as a result, may not have needed to refill an outpatient VA prescription during the follow-up period. Finally, we excluded all remaining patients who did not fill at least one VA prescription for antidiabetic medication during follow-up.
Figure 1.
Sample construction for older veterans with diabetes and comorbid dementia. VHA, Veterans Health Administration.
Measures
Outcomes
We extracted the last available HbA1c value in FY2008 from VA laboratory data to quantify patients’ glycemic control as tightly controlled (<7% [53 mmol/mol]), moderately controlled (7% to <9% [53 to <75 mmol/mol]), poorly controlled (≥9% [≥75 mmol/mol]), and not monitored (no HbA1c tests recorded in either VA or Medicare data). We also captured whether the HbA1c value was obtained in an outpatient versus inpatient setting for use in sensitivity analyses.
We used outpatient VA drug-dispensing records for the first 120 days of FY2009 to identify patient use of antidiabetic medication associated with a high risk of hypoglycemia, defined as having at least one fill for a sulfonylurea and/or insulin. We used a 120-day window to determine the period prevalence of use of these drugs in close relation to the last HbA1c measurement. A 120-day window was chosen because even individuals obtaining 90-day antidiabetic prescriptions would be expected to fill at least one prescription during this period, allowing for some potential nonadherence. For descriptive purposes, we also captured other specific antidiabetic medication classes (biguanides [metformin], thiazolidinediones [TZDs], α-glucosidase inhibitors, meglitinides, dipeptidyl peptidase-4 [DPP-4] inhibitors, amylin analogs, and GLP-1 receptor agonists) that patients used in this time period and created a summary variable for their overall antidiabetic regimen (noninsulin monotherapy, noninsulin multitherapy, insulin alone, or insulin plus other noninsulin agent).
Covariate Risk Factors
We assessed sociodemographic, clinical, and health care utilization factors in relation to tight glycemic control and use of high-risk medications. Patient sex, age (65–74, 75–84, or ≥85 years), and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or other) were determined from VA utilization files. Missing VA race/ethnicity values were supplemented with the Research Triangle Institute race code in the Medicare enrollment file (28). Whether patients had a copay for VA medications in the follow-up period was also extracted. We used the Elixhauser comorbidity measure (29,30) applied to diagnoses in VA and Medicare files to identify comorbidities present in ≥5% of the present sample as well as recent weight loss (3% of patients) because of an a priori hypothesis that weight loss is an important contributor to tight glycemic control. Detailed information on the specific ICD-9-CM diagnosis codes used to define each comorbid condition can be found at www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. We used VA and Medicare records to determine whether patients had at least one inpatient hospital or nursing home stay in FY2008. Finally, we determined whether dementia was documented within the VA, as indicated by either a VA ADRD diagnosis code or a VA prescription fill for an antidementia medication versus documented only in Medicare claims.
Analytic Approach
We examined descriptive statistics for all study variables in the full sample and each glycemic control group. To determine the association of sociodemographic, clinical, and health care utilization factors with HbA1c control, we used multinomial logistic regression with robust SEs. Moderate control, which reflects guideline-concordant care, was the reference category. Finally, for patients whose last HbA1c value in FY2008 indicated tight control (n = 8,276), we estimated a logistic regression model for use of medication with high hypoglycemic risk. We also conducted a sensitivity analysis using multinomial logistic regression to model use of sulfonylurea but no insulin, use of insulin but no sulfonylurea, and use of both sulfonylurea and insulin as separate outcomes, relative to use of neither agent, and report the results in Supplementary Table 2.
Results
Sample Characteristics
Table 1 shows characteristics of 15,880 community-dwelling veterans aged ≥65 years with type 2 diabetes and dementia. Almost all patients were male (99%), and 80% were non-Hispanic white. Comorbidities were common, including hypertension (81%), deficiency anemia (21%), chronic lung disease (19%), and peripheral vascular disease (17%).
Table 1.
Characteristics of community-dwelling older veterans with diabetes and comorbid dementia by level of glycemic control
| Patient characteristics | All patients (n = 15,880) | Tightly controlled (n = 8,276) | Moderately controlled (n = 5,669) | Poorly controlled (n = 1,131) | Not monitored (n = 804) |
|---|---|---|---|---|---|
| Male sex | 15,643 (99) | 8,157 (99) | 5,581 (98) | 1,115 (99) | 790 (98) |
| Race/ethnicity | |||||
| Hispanic | 1,242 (8) | 566 (7) | 475 (8) | 137 (12) | 64 (8) |
| White, non-Hispanic | 12,629 (80) | 6,742 (81) | 4,489 (79) | 776 (69) | 622 (77) |
| Black, non-Hispanic | 1,618 (10) | 781 (9) | 573 (10) | 177 (16) | 87 (11) |
| Other | 391 (2) | 187 (2) | 132 (2) | 41 (4) | 31 (4) |
| Age | |||||
| 65–74 years | 3,857 (24) | 1,882 (23) | 1,442 (25) | 367 (32) | 166 (21) |
| 75–84 years | 8,745 (55) | 4,657 (56) | 3,058 (54) | 586 (52) | 444 (55) |
| ≥85 years | 3,278 (21) | 1,737 (21) | 1,169 (21) | 178 (16) | 194 (24) |
| Has medication copay | 9,515 (60) | 4,990 (60) | 3,431 (61) | 638 (56) | 456 (57) |
| Comorbidities | |||||
| Congestive heart failure | 2,860 (18) | 1,416 (17) | 1,080 (19) | 226 (20) | 138 (17) |
| Heart valve disease | 1,168 (7) | 648 (8) | 394 (7) | 69 (6) | 57 (7) |
| Peripheral vascular disease | 2,624 (17) | 1,412 (17) | 943 (17) | 166 (15) | 103 (13) |
| Hypertension | 12,815 (81) | 6,727 (81) | 4,646 (82) | 945 (84) | 497 (62) |
| Chronic lung disease | 2,979 (19) | 1,600 (19) | 1,032 (18) | 212 (19) | 135 (17) |
| Hypothyroidism | 1,628 (10) | 884 (11) | 588 (10) | 109 (10) | 47 (6) |
| Renal failure | 2,511 (16) | 1,266 (15) | 941 (17) | 211 (19) | 93 (12) |
| Solid tumor without metastasis | 2,073 (13) | 1,110 (13) | 755 (13) | 137 (12) | 71 (9) |
| Obesity | 1,080 (7) | 517 (6) | 432 (8) | 102 (9) | 29 (4) |
| Weight loss | 436 (3) | 260 (3) | 129 (2) | 20 (2) | 27 (3) |
| Fluid and electrolyte disorder | 2,016 (13) | 1,043 (13) | 722 (13) | 166 (15) | 85 (11) |
| Deficiency anemia | 3,308 (21) | 1,821 (22) | 1,168 (21) | 203 (18) | 116 (14) |
| Psychoses | 2,065 (13) | 1,105 (13) | 709 (13) | 166 (15) | 85 (11) |
| Depression | 2,463 (16) | 1,317 (16) | 858 (15) | 189 (17) | 99 (12) |
| Inpatient stay in FY2008 | 2,416 (15) | 1,246 (15) | 837 (15) | 243 (21) | 90 (11) |
| VA documentation of dementia | 11,213 (71) | 5,869 (71) | 3,958 (70) | 818 (72) | 578 (72) |
| Last HbA1c value in baseline year (FY2008)§ | |||||
| HbA1c (%) | 6.8 (6.3–7.6) | 6.3 (6.0–6.6) | 7.5 (7.2–8.0) | 9.8 (9.3–10.7) | N/A |
| HbA1c (mmol/mol) | 51 (45–60) | 45 (42–49) | 58 (55–64) | 84 (78–93) | N/A |
| Medication use in follow-up period (first 120 days of FY2009) | |||||
| Medication regimen | |||||
| Noninsulin monotherapy | 7,298 (46) | 4,942 (60) | 1,756 (31) | 156 (14) | 444 (55) |
| Noninsulin multitherapy | 3,081 (19) | 1,438 (17) | 1,337 (24) | 180 (16) | 126 (16) |
| Insulin alone | 3,308 (21) | 1,237 (15) | 1,502 (27) | 421 (37) | 148 (18) |
| Insulin plus other agent | 2,193 (14) | 659 (8) | 1,074 (19) | 374 (33) | 86 (11) |
| Medication class# | |||||
| Insulin | 5,501 (35) | 1,896 (23) | 2,576 (45) | 795 (70) | 234 (29) |
| Sulfonylurea | 8,927 (56) | 4,690 (57) | 3,204 (57) | 548 (49) | 485 (60) |
| Metformin | 6,487 (41) | 3,593 (43) | 2,238 (39) | 382 (34) | 274 (34) |
| TZDs | 826 (5.2) | 339 (4) | 375 (7) | 74 (7) | 38 (5) |
| α-Glucosidase inhibitors | 233 (1) | 81 (1) | 116 (2) | 28 (3) | 8 (1) |
| Use of medications with high hypoglycemic risk | |||||
| No insulin/no sulfonylurea | 2,842 (18) | 2,063 (25) | 598 (11) | 42 (4) | 139 (17) |
| No insulin/yes sulfonylurea | 7,537 (47) | 4,317 (52) | 2,495 (44) | 294 (26) | 431 (54) |
| Yes insulin/no sulfonylurea | 4,111 (26) | 1,523 (18) | 1,867 (33) | 541 (48) | 180 (22) |
| Yes insulin/yes sulfonylurea | 1,390 (9) | 373 (5) | 709 (13) | 254 (22) | 54 (7) |
Data are n (%) or median (interquartile range). Tightly controlled, HbA1c <7% (53 mmol/mol); moderately controlled, HbA1c 7 to <9% (53 to <75 mmol/mol); poorly controlled, HbA1c ≥9% (≥75 mmol/mol); not monitored, no evidence of having received any FY2008 HbA1c tests in VA or Medicare records. N/A, not applicable.
§Presented for the 15,076 patients with HbA1c values in FY2008.
#Data not shown for use of meglitinides, DPP-4 inhibitors, amylin analogs, and GLP-1 agonists; <1% of the total sample used these agents.
The majority (52%; n = 8,276) of patients had tight glycemic control, 36% had moderate control, 7% had poor control, and 5% did not have HbA1c monitored in FY2008. Within the tight control group, the mean and median HbA1c value was 6.3% (45 mmol/mol; minimum 3.8% [18 mmol/mol], maximum 6.9% [52 mmol/mol], interquartile range 6.0–6.6% [42–49 mmol/mol]). More than 97% of HbA1c values were obtained in an outpatient setting, and exclusion of inpatient HbA1c values did not substantively affect the distribution of HbA1c values seen in the overall sample or within glycemic control categories.
Table 1 also provides information on the medication regimens and specific medication classes used by the sample. Overall, noninsulin monotherapy was most common (46%) followed by insulin alone (21%), noninsulin multitherapy (19%), and insulin plus another noninsulin agent (14%). Sulfonylureas, metformin, and insulin were the most common classes used, followed by TZDs and α-glucosidase inhibitors. Meglitinides, DPP-4 inhibitors, amylin analogs, and GLP-1 receptor agonists were each used very rarely (<1% of the sample).
Overall, 82% (n = 13,038) of the sample used a regimen associated with an increased risk of hypoglycemia, including 47% (n = 7,537) using sulfonylurea without insulin, 26% (n = 4,111) using insulin without sulfonylurea, and 9% (n = 1,390) using both agents (Table 1). Among tightly controlled patients, 75% (n = 6,213) used such a regimen, including 52% (n = 4,317) using sulfonylurea, 18% (n = 1,523) using insulin, and 5% (n = 373) using both agents. Of the 6,213 tightly controlled patients using either sulfonylureas or insulin, 29% (n = 1,811) also took at least one other agent not associated with a high hypoglycemic risk (data not shown).
Predictors of Glycemic Control Levels
The multinomial regression model revealed patient age, specific comorbidities, and race/ethnicity to be independently associated with having tight versus moderate glycemic control (Table 2). Compared with patients aged 65–74 years, those who were 75–84 or ≥85 years old had higher odds of HbA1c <7% (53 mmol/mol). Heart valve disease, chronic lung disease, weight loss, and deficiency anemia were associated with increased odds of HbA1c <7% (53 mmol/mol), whereas congestive heart failure, renal failure, and obesity were associated with lower odds of HbA1c <7% (53 mmol/mol). Compared with non-Hispanic white patients, Hispanic patients also had lower odds of HbA1c <7% (53 mmol/mol). Racial/ethnic minority status and having an FY2008 inpatient stay were associated with higher odds of poor glycemic control, whereas older age and deficiency anemia were associated with lower odds.
Table 2.
Factors independently associated with level of HbA1c control in community-dwelling older veterans with diabetes and comorbid dementia
| Tightly controlled
(HbA1c <7% [53 mmol/mol]) |
Poorly controlled
(HbA1c ≥9% [75 mmol/mol]) |
HbA1c not monitored |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Male sex | 1.10 | 0.83–1.46 | 0.49 | 1.02 | 0.59–1.75 | 0.94 | 0.81 | 0.45–1.46 | 0.48 |
| Race/ethnicity | |||||||||
| White non-Hispanic (reference) | — | — | — | — | — | — | — | — | — |
| Black non-Hispanic | 0.91 | 0.81–1.02 | 0.11 | 1.68 | 1.39–2.04 | <0.001 | 1.16 | 0.91–1.49 | 0.24 |
| Hispanic | 0.77 | 0.67–0.87 | <0.001 | 1.58 | 1.28–1.96 | <0.001 | 0.96 | 0.72–1.28 | 0.79 |
| Other | 0.94 | 0.75–1.19 | 0.62 | 1.73 | 1.21–2.49 | 0.003 | 1.62 | 1.07–2.44 | 0.022 |
| Age | |||||||||
| 65–74 years (reference) | — | — | — | — | — | — | — | — | — |
| 75–84 years | 1.16 | 1.07–1.26 | 0.001 | 0.81 | 0.70–0.94 | 0.005 | 1.29 | 1.06–1.56 | 0.011 |
| ≥85 years | 1.13 | 1.02–1.25 | 0.021 | 0.66 | 0.54–0.81 | <0.001 | 1.43 | 1.13–1.80 | 0.002 |
| Has medication copay | 0.97 | 0.90–1.04 | 0.34 | 0.99 | 0.86–1.13 | 0.83 | 0.84 | 0.72–0.99 | 0.036 |
| Congestive heart failure | 0.84 | 0.76–0.92 | <0.001 | 1.07 | 0.90–1.28 | 0.43 | 1.09 | 0.88–1.35 | 0.42 |
| Heart valve disease | 1.16 | 1.01–1.32 | 0.033 | 0.91 | 0.70–1.20 | 0.51 | 1.22 | 0.91–1.64 | 0.19 |
| Peripheral vascular disease | 1.03 | 0.94–1.13 | 0.56 | 0.86 | 0.72–1.03 | 0.11 | 0.84 | 0.67–1.05 | 0.13 |
| Hypertension | 0.96 | 0.88–1.05 | 0.38 | 1.08 | 0.91–1.29 | 0.37 | 0.39 | 0.33–0.45 | <0.001 |
| Chronic lung disease | 1.10 | 1.01–1.21 | 0.038 | 1.00 | 0.84–1.18 | 0.99 | 1.08 | 0.88–1.32 | 0.48 |
| Hypothyroidism | 1.03 | 0.92–1.15 | 0.62 | 0.96 | 0.77–1.19 | 0.68 | 0.60 | 0.44–0.82 | 0.001 |
| Renal failure | 0.90 | 0.82–1.00 | 0.045 | 1.13 | 0.94–1.35 | 0.21 | 0.78 | 0.61–0.99 | 0.045 |
| Solid tumor without metastasis | 0.99 | 0.90–1.09 | 0.85 | 0.90 | 0.74–1.10 | 0.31 | 0.67 | 0.52–0.87 | 0.003 |
| Obesity | 0.83 | 0.72–0.95 | 0.007 | 1.07 | 0.85–1.35 | 0.56 | 0.57 | 0.38–0.84 | 0.004 |
| Weight loss | 1.36 | 1.09–1.69 | 0.006 | 0.93 | 0.45–1.19 | 0.21 | 1.77 | 1.14–2.74 | 0.010 |
| Fluid and electrolyte disorder | 0.97 | 0.87–1.08 | 0.57 | 1.11 | 0.91–1.36 | 0.29 | 1.09 | 0.83–1.41 | 0.55 |
| Deficiency anemia | 1.12 | 1.02–1.22 | 0.016 | 0.76 | 0.64–0.92 | 0.003 | 0.78 | 0.63–0.98 | 0.030 |
| Psychoses | 1.08 | 0.97–1.20 | 0.14 | 1.04 | 0.86–1.26 | 0.68 | 0.87 | 0.68–1.11 | 0.26 |
| Depression | 1.05 | 0.95–1.15 | 0.35 | 1.05 | 0.88–1.25 | 0.60 | 0.91 | 0.72–1.15 | 0.45 |
| Inpatient stay in FY2008 | 1.04 | 0.93–1.15 | 0.50 | 1.45 | 1.21–1.73 | <0.001 | 0.90 | 0.70–1.17 | 0.44 |
| VA documentation of dementia diagnosis | 1.05 | 0.98–1.14 | 0.18 | 0.99 | 0.86–1.16 | 0.94 | 0.97 | 0.82–1.15 | 0.71 |
Multinomial logistic regression was used, with moderate control (HbA1c ≥7% [53 mmol/mol] and <9% [75 mmol/mol]) as the reference category. OR, odds ratio.
Predictors of Use of Medications With High Risk of Hypoglycemia
Among the 8,276 patients with tight control, several significant risk factors emerged for use of medications with high hypoglycemic risk (Table 3). Male sex; black race; age 75–84 or ≥85 vs. 65–74 years; presence of congestive heart failure, peripheral vascular disease, or renal failure; and having had a hospitalization or nursing home stay in FY2008 were all associated with higher odds of use of sulfonylureas and/or insulin versus neither agent. Fluid/electrolyte disorder or depression was also associated with lower odds of use of a high-risk regimen.
Table 3.
Independent predictors of use of antidiabetic medications with high risk of hypoglycemia in community-dwelling older veterans with diabetes and comorbid dementia with tight glycemic control
| OR | 95% CI | P value | |
|---|---|---|---|
| Male sex | 1.77 | 1.19–2.63 | 0.004 |
| Race/ethnicity | |||
| White non-Hispanic (reference) | — | — | — |
| Black non-Hispanic | 1.27 | 1.05–1.53 | 0.015 |
| Hispanic | 0.92 | 0.75–1.13 | 0.42 |
| Other | 0.84 | 0.60–1.16 | 0.28 |
| Age | |||
| 65–74 years (reference) | — | — | — |
| 75–84 years | 1.28 | 1.13–1.45 | <0.001 |
| ≥85 years | 1.60 | 1.37–1.88 | <0.001 |
| Has medication copay | 0.93 | 0.83–1.04 | 0.20 |
| Congestive heart failure | 1.51 | 1.28–1.78 | <0.001 |
| Valvular disease | 0.88 | 0.72–1.08 | 0.22 |
| Peripheral vascular disease | 1.2 | 1.05–1.41 | 0.010 |
| Hypertension | 1.10 | 0.97–1.26 | 0.14 |
| Chronic lung disease | 0.94 | 0.82–1.08 | 0.38 |
| Hypothyroidism | 0.91 | 0.77–1.08 | 0.28 |
| Renal failure | 4.60 | 3.67–5.78 | <0.001 |
| Solid tumor without metastasis | 0.97 | 0.83–1.13 | 0.67 |
| Obesity | 1.01 | 0.82–1.26 | 0.91 |
| Weight loss | 0.88 | 0.65–1.20 | 0.43 |
| Fluid and electrolyte disorder | 0.83 | 0.70–1.00 | 0.047 |
| Deficiency anemia | 0.96 | 0.84–1.10 | 0.59 |
| Psychoses | 0.94 | 0.81–1.10 | 0.47 |
| Depression | 0.85 | 0.74–0.98 | 0.026 |
| Inpatient stay in FY2008 | 1.26 | 1.06–1.49 | 0.008 |
| VA documentation of dementia | 1.09 | 0.97–1.22 | 0.16 |
Tight glycemic control, HbA1c <7% (53 mmol/mol). OR, odds ratio.
Conclusions
In a national cohort of >15,000 outpatients with medication-treated type 2 diabetes and dementia, we found that more than one-half had HbA1c <7% (53 mmol/mol), despite clear guidelines recommending higher glycemic targets. In addition, 75% of tightly controlled patients with dementia used medications that further exacerbated their potential risk for hypoglycemia. Even in the context of heightened hypoglycemia risk resulting from the combination of comorbid dementia and tight glycemic control, the results suggest that the large majority of providers do not substitute sulfonylureas and insulin with lower-risk antidiabetic agents. These findings highlight the need for interventions to encourage appropriate deintensification and alteration of medications when diabetic patients develop dementia, which has not been reflected in previous diabetes quality initiatives.
The study has several important strengths. Despite growing numbers of older diabetic patients with dementia and their heightened vulnerability to tight control, few studies have examined glycemic control levels partly because large enough data sources containing HbA1c values for this population are rare outside the VA. A recent study documented high prevalence (45–50%) of tight glycemic control generally among veterans with a range of risk factors for hypoglycemia, including dementia (31). The present study extends this work by focusing specifically on dementia status and examining risk factors for tight control or sulfonylurea/insulin use and by using Medicare data to help to identify patients with dementia. The incorporation of Medicare claims in identifying dementia patients is critical given past research demonstrating its importance in accurately capturing comorbidities for older veterans (32). In addition, we believe that the present study is the first to document the very high rate of use of sulfonylureas and insulin in tightly controlled patients with dementia, an important and modifiable contributor to these patients’ risk for hypoglycemia.
We identified key risk factors for tight glycemic control in patients with dementia. Age ≥75, weight loss, chronic lung disease, and deficiency anemias increase the odds of tight glycemic control, whereas obesity is protective. Providers and patients may not recognize that changes in appetite and weight associated with dementia itself, advancing age, or other comorbidities may make it easier to control blood glucose with less intense antidiabetic regimens or that medication may no longer be required. The results suggest that interventions should encourage the review and deintensification of medication regimens in patients who lose weight or reach advanced ages.
We also found that patients with concomitant congestive heart failure or renal failure exhibited lower odds of tight control compared with dementia patients without these comorbidities; these diagnoses are noted in VA/DoD guidelines as indications to avoid tight glycemic control. The explicit mention of the role of dementia in setting glycemic control targets, as in the newly revised 2013 AGS guidelines (20) and a recently proposed quality indicator for diabetes overtreatment (22), may be an essential first step in encouraging appropriate diabetes treatment deintensification in this population. Although the present findings suggest some contributing factors to the lack of appropriate deintensification of diabetes treatment in patients with dementia, future research should directly engage providers, patients, and caregivers to uncover their perceptions of how dementia should alter glycemic control targets and barriers to pursuing less intensive targets as well as what role guidelines play in these decisions.
The present findings also highlight the need for initiatives to support safer antidiabetic prescribing choices for patients with dementia. The widespread use of sulfonylureas and insulin likely reflects VA/DoD guidelines recommending both options as first-line antidiabetic therapies along with metformin and as add-on therapies to metformin or each other if glycemic goals are not achieved, with no discussion of how dementia should affect medication choice. The greatly increased odds of sulfonylureas and/or insulin use in patients with congestive heart failure and renal failure and those aged ≥75 years also likely reflect VA/DoD (and other) guidelines, which list these conditions and age ≥80 years as contraindications to the use of metformin, leaving sulfonylurea and insulin as the preferred agents over other classes of agents with lower hypoglycemic risk. Although these other antidiabetic medication classes (i.e., TZDs, DPP-4 inhibitors, GLP-1 agonists, α-glucosidase inhibitors) have lower associated risk of hypoglycemia, they have lower efficacy in reducing HbA1c and lack robust evidence regarding their safety profile and associated treatment burden in older patients with dementia. Although these alternative agents were not on the VA national formulary at the time of the study (except for acarbose), they were available to all prescribers when necessary through a nonformulary request process. Because clinical trials comparing various antidiabetic medications in older patients with dementia are unlikely to occur, well-designed observational studies are needed to guide prescribing choices for this rapidly growing population. In the meantime, the present results suggest a need for provider outreach efforts to consider medication options with a lower hypoglycemic risk and encourage the use of the minimally intensive regimen required to achieve moderate glycemic control levels.
The results of this study should be interpreted in light of several limitations. First, our focus was on understanding patient characteristics associated with two established risk factors for hypoglycemia (i.e., HbA1c <7% [53 mmol/mol], use of sulfonylureas and/or insulin), and we did not capture data on actual hypoglycemic events. Whether deintensification of therapy in response to tight glycemic control or substitution of insulin and sulfonylurea with other antidiabetic agents results in reduced hypoglycemic events in diabetic patients with dementia is an important direction for future research. In addition, the relationship between glycemic control levels and the progression of dementia is unknown. Whether tight glycemic control hinders or hastens dementia progression should be explored in future studies. Second, although guidelines recommend higher HbA1c targets for older patients with dementia, some patients with mild dementia in otherwise excellent health and good functional status may opt for tighter glycemic targets. Although we did capture extensive information on the presence of comorbid conditions, we were not able to account for patients’ functional status or preferences in the analyses. Likewise, insulin therapy may be appropriate for older dementia patients with long-standing diabetes who are unable to attain HbA1c targets with noninsulin agents alone. We did not examine dose of medications; thus, it is possible that some tightly controlled patients were prescribed very low doses. However, by limiting the analysis of hypoglycemic medication use to patients with HbA1c <7% (53 mmol/mol), such patients face a particularly high risk of hypoglycemia and may benefit from deintensification of insulin dose or a switch to a non–insulin-containing regimen.
Although this study is unique in capturing such a large number of patients with both diabetes and dementia as well as important clinical variables like HbA1c, we acknowledge that the observational study design, relying solely on administrative data, has inherent limitations. In particular, we were not able to capture the full range of covariates that may be associated with glycemic control and medication choice, such as assistance with at-home diabetes self-management from informal family caregivers or nurse-provided home care. We also did not examine interactions between covariates because of a lack of theory to guide such analyses and relatively small numbers of patients with some comorbid conditions (e.g., weight loss). Future research may consider possible multiplicative effects of specific comorbid conditions or other risk factors identified in this study. Patients may also have had HbA1c tests and medications filled outside the VA that we were unable to capture, although we reduced this possibility by limiting the sample to patients who filled antidiabetic medications in the VA and did not have another major source of drug coverage. In addition, we used a single HbA1c value to classify patients’ glycemic control at one point in time and therefore cannot determine the extent to which their glycemic control levels were transient versus stable. We also acknowledge that the data are several years old, and care of dementia patients may have improved since, especially in light of increased attention to the possible risks of tight control and hypoglycemia in older patients with comorbidities. Finally, the results reflect a primarily male veteran population and may not generalize to women or nonveterans.
In conclusion, the results show a high rate of intense treatment of diabetes in older patients with comorbid dementia, potentially placing them at elevated risk for hypoglycemia and serious adverse events. Equally disconcerting is the high frequency of use of medications known to cause hypoglycemia. The findings present a compelling need for the development of quality initiatives to encourage review of glycemic targets and antidiabetic medications in this rapidly growing group of patients, especially those aged ≥75 years and those with weight loss.
Article Information
Funding. C.T.T., S.Z., M.M., and M.J.F. are supported by VA Health Services Research & Development (CIN 13-405). W.F.G. is supported by a VA Health Services Research & Development Career Development Award (CDA 09-207).
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. C.T.T. contributed to the study concept and design, study supervision, data acquisition, data analysis and interpretation, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. W.F.G. and X.Z. contributed to the study concept and design, data acquisition, data analysis and interpretation, and critical revision of the manuscript for important intellectual content. C.B.G. contributed to the study concept and design, data analysis and interpretation, and critical revision of the manuscript for important intellectual content. S.Z. contributed to the data acquisition, data analysis and interpretation, and critical revision of the manuscript for important intellectual content. M.M. contributed to the study supervision, data acquisition, data analysis and interpretation, and critical revision of the manuscript for important intellectual content. M.J.F. contributed to the study supervision; administrative, technical, and material support; data analysis and interpretation; and critical revision of the manuscript for important intellectual content. C.T.T. is the guarantor of this work, and as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in poster form at the 2013 Annual Scientific Meeting of the Gerontological Society of America, New Orleans, LA, 20–24 November 2013.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc14-0599/-/DC1.
References
- 1.Boyle JP, Honeycutt AA, Narayan KMV, et al. . Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001;24:1936–1940 [DOI] [PubMed] [Google Scholar]
- 2.Feil DG, Rajan M, Soroka O, Tseng CL, Miller DR, Pogach LM. Risk of hypoglycemia in older veterans with dementia and cognitive impairment: implications for practice and policy. J Am Geriatr Soc 2011;59:2263–2272 [DOI] [PubMed] [Google Scholar]
- 3.Thorpe CT, Thorpe JM, Kind AJ, Bartels CM, Everett CM, Smith MA. Receipt of monitoring of diabetes mellitus in older adults with comorbid dementia. J Am Geriatr Soc 2012;60:644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaffe K, Falvey CM, Hamilton N, et al.; Health ABC Study . Association between hypoglycemia and dementia in a biracial cohort of older adults with diabetes mellitus. JAMA Intern Med 2013;173:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Falvey C, Hamilton N, et al. . Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch Neurol 2012;69:1170–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol 2006;5:64–74 [DOI] [PubMed] [Google Scholar]
- 7.National Plan to Address Alzheimer’s Disease. Washington, DC, U.S. Department of Health and Human Services , 2013 [Google Scholar]
- 8.Bynum JP, Rabins PV, Weller W, Niefeld M, Anderson GF, Wu AW. The relationship between a dementia diagnosis, chronic illness, Medicare expenditures, and hospital use. J Am Geriatr Soc 2004;52:187–194 [DOI] [PubMed] [Google Scholar]
- 9.Onder G, Landi F, Fusco D, et al. . Recommendations to prescribe in complex older adults: results of the CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project. Drugs Aging 2014;31:33–45 [DOI] [PubMed] [Google Scholar]
- 10.Feil DG, Zhu CW, Sultzer DL. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with type 2 diabetes. J Behav Med 2012;35:190–199 [DOI] [PubMed] [Google Scholar]
- 11.Kirsh SR, Aron DC. Choosing targets for glycaemia, blood pressure and low-density lipoprotein cholesterol in elderly individuals with diabetes mellitus. Drugs Aging 2011;28:945–960 [DOI] [PubMed] [Google Scholar]
- 12.Hewitt J, Smeeth L, Chaturvedi N, Bulpitt CJ, Fletcher AE. Self management and patient understanding of diabetes in the older person. Diabet Med 2011;28:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briscoe VJ, Davis SN. Hypoglycemia in type 1 and type 2 diabetes: physiology, pathophysiology, and management. Clin Diabetes 2006;24:115–121 [Google Scholar]
- 14.Xie J, Brayne C, Matthews FE; Medical Research Council Cognitive Function and Ageing Study Collaborators . Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 2008;336:258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams MM, Xiong C, Morris JC, Galvin JE. Survival and mortality differences between dementia with Lewy bodies vs Alzheimer disease. Neurology 2006;67:1935–1941 [DOI] [PubMed] [Google Scholar]
- 16.Rait G, Walters K, Bottomley C, Petersen I, Iliffe S, Nazareth I. Survival of people with clinical diagnosis of dementia in primary care: cohort study. BMJ 2010;341:c3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Department of Veterans Affairs; Department of Defense. VA/DoD Clinical Practice Guideline: Management of Diabetes Mellitus (DM). Version 4.0. Washington, DC, Veterans Health Administration, 2010 [Google Scholar]
- 18.Department of Veterans Affairs; Department of Defense. VA/DoD Clinical Practice Guideline: Management of Diabetes Mellitus in Primary Care. Version 3.0. Washington, DC, Veterans Health Administration, 2003
- 19.American Diabetes Association Standards of medical care in diabetes—2014. Diabetes Care 2014;37(Suppl. 1):S14–S80 [DOI] [PubMed] [Google Scholar]
- 20.Moreno G, Mangione CM, Kimbro L, Vaisberg E; American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus . Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. J Am Geriatr Soc 2013;61:2020–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Geriatrics Society. Choosing Wisely: Five Things Physicians and Patients Should Question. New York, American Geriatrics Society, 2013
- 22.Pogach L, Aron D. The other side of quality improvement in diabetes for seniors: a proposal for an overtreatment glycemic measure. Arch Intern Med 2012;172:1510–1512 [DOI] [PubMed] [Google Scholar]
- 23.Gellad W, Mor M, Zhao X, Donohue J, Good C. Variation in use of high-cost diabetes mellitus medications in the VA healthcare system. Arch Intern Med 2012;172:1608–1609 [DOI] [PubMed] [Google Scholar]
- 24.Gellad WF, Donohue JM, Zhao X, et al. . Brand-name prescription drug use among Veterans Affairs and Medicare Part D patients with diabetes: a national cohort comparison. Ann Intern Med 2013;159:105–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 2004;27(Suppl. 2):B10–B21 [DOI] [PubMed] [Google Scholar]
- 26.Buccaneer Computer Systems and Service, Inc. Chronic Conditions Data Warehouse user manual [Internet]. Version 3.0, 2014. Available from https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=0CCwQFjAC&url=https%3A%2F%2Fwww.ccwdata.org%2Fcs%2Fgroups%2Fpublic%2Fdocuments%2Fdocument%2Fccw_userguide.pdf&ei=QmihVMSOCoXQggTN1YH4Dg&usg=AFQjCNHhSApHILlPDoY1vvlrCP-Ckbxhzw&sig2=Lbk_uTd_AfN-P1yWz-EqDA&bvm=bv.82001339,d.eXY&cad=rja. Accessed 29 December 2014
- 27.Taylor DH Jr., Østbye T, Langa KM, Weir D, Plassman BL. The accuracy of Medicare claims as an epidemiological tool: the case of dementia revisited. J Alzheimers Dis 2009;17:807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eicheldinger C, Bonito A. More accurate racial and ethnic codes for Medicare administrative data. Health Care Financ Rev 2008;29:27–42 [PMC free article] [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27 [DOI] [PubMed] [Google Scholar]
- 30.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258–1267 [DOI] [PubMed] [Google Scholar]
- 31.Tseng CL, Soroka O, Maney M, Aron DC, Pogach LM. Assessing potential glycemic overtreatment in persons at hypoglycemic risk. JAMA Intern Med 2014;174:259–268 [DOI] [PubMed] [Google Scholar]
- 32.Byrne MM, Kuebeler M, Pietz K, Petersen LA. Effect of using information from only one system for dually eligible health care users. Med Care 2006;44:768–773 [DOI] [PubMed] [Google Scholar]