Abstract
Rationale: Prospective cohort studies have shown that chronic exposure to particulate matter and traffic-related air pollution is associated with reduced survival. However, the effects on nonmalignant respiratory mortality are less studied, and the data reported are less consistent.
Objectives: We have investigated the relationship of long-term exposure to air pollution and nonmalignant respiratory mortality in 16 cohorts with individual level data within the multicenter European Study of Cohorts for Air Pollution Effects (ESCAPE).
Methods: Data from 16 ongoing cohort studies from Europe were used. The total number of subjects was 307,553. There were 1,559 respiratory deaths during follow-up.
Measurements and Main Results: Air pollution exposure was estimated by land use regression models at the baseline residential addresses of study participants and traffic-proximity variables were derived from geographical databases following a standardized procedure within the ESCAPE study. Cohort-specific hazard ratios obtained by Cox proportional hazard models from standardized individual cohort analyses were combined using metaanalyses. We found no significant associations between air pollution exposure and nonmalignant respiratory mortality. Most hazard ratios were slightly below unity, with the exception of the traffic-proximity indicators.
Conclusions: In this study of 16 cohorts, there was no association between air pollution exposure and nonmalignant respiratory mortality.
Keywords: environmental exposure, public health, metaanalysis
At a Glance Commentary
Scientific Knowledge on the Subject
Prospective cohort studies, especially from the United States and a few single-country studies in Europe, have provided sufficient evidence that chronic exposure to traffic-related air pollution, as reflected in particulate matter and nitrogen oxides concentrations, is associated with all-cause and cardiovascular mortality. However, the evidence for long-term exposure effects on respiratory mortality is less studied, and the results reported are less consistent.
What This Study Adds to the Field
This is the largest European study bringing together data from 16 European cohorts comprising 307,553 persons with a range of follow-up from 6.3 to 18.6 years. Exposure assessment and the analysis were conducted by standardized and state-of-the-art methods. In the middle of inadequate and inconsistent evidence, this powerful and well-conducted study points to a nonsignificant association between air pollution exposure and nonmalignant respiratory mortality.
Prospective cohort studies, especially from the United States and a few single country studies in Europe, have provided sufficient evidence that chronic exposure to particulate matter (PM) and traffic-related air pollution is associated with all-cause and cardiovascular mortality (1–8). However, the evidence for long-term exposure effects on respiratory mortality is limited and inconsistent, possibly related to differences in evaluated pollutants, exposure assessment methods, and statistical analysis methods. There is more evidence that there are short-term effects of PM with an aerodynamic diameter < 2.5 μm (PM2.5) and < 10 μm (PM10) on respiratory mortality and hospital admissions, especially after relatively longer exposure; this effect is more pronounced for PM from traffic, and larger effect estimates are observed during warm periods (9–11). Short-term effects of nitrogen dioxide (NO2) on respiratory mortality have also been reported (12).
A recent review on exposure to air pollution and long-term cardiorespiratory mortality effects (13) identified 14 cohort studies, of which four were from three European countries, investigating the long-term effects of PM metrics (e.g., PM10, PM2.5, and black smoke) and/or nitrogen oxides (NOx)/NO2 on respiratory mortality. This review reports that respiratory mortality effects were investigated less in the United States compared with European cohorts, the PM effects tended to be weaker than those of NOx, and generally the evidence was heterogeneous, often with nonstatistically significant effects. A need for further investigation of respiratory effects was underlined.
Few cohort studies in Europe were initiated with the objective to study the effects of air pollution exposure. Therefore, the corresponding results were based on variable methodologies for exposure assessment, resulting in limited comparability.
Within the framework of the European Study of Cohorts for Air Pollution Effects (ESCAPE) project, a common air pollution exposure assessment and epidemiological analysis protocol was used for 16 cohorts (from 11 European countries) including nonmalignant respiratory mortality cases, providing scope to investigate the association of long-term exposure to air pollution and respiratory mortality.
Some of the results of this study have been previously reported in the form of an abstract (14).
Methods
The epidemiological analysis protocol of the ESCAPE project included a priori a two-stage analysis. First, each cohort was analyzed separately using several models under a common analysis protocol with well-defined exposures, outcomes, and confounders and with an analytical approach. A workshop for all local analysts was organized to explain the statistical methods (15). It was not possible to form a common database for pooled analysis due to confidentiality issues. Subsequently, cohort-specific effect estimates were combined by random effects metaanalysis.
Study Populations, Mortality Follow-up, and Outcome Definition
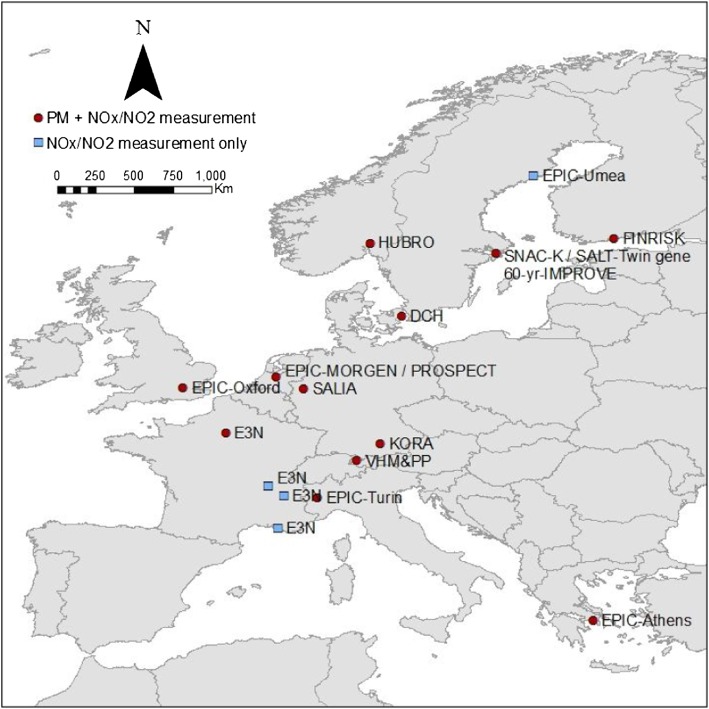
Cohorts were included in the study if the number of respiratory mortality cases exceeded seven during the follow-up period and if data for the most important potential confounders were available. Individual-level confounders were available from questionnaire data. Specifically, the cohorts were in Sweden (EPIC-Umeå, SNACK-K, SALT, and Sixty), Finland (FINRISK), Norway (HUBRO), Denmark (DCH), The Netherlands (EPIC-MORGEN and EPIC-PROSPECT), Germany (SALIA and KORA), the United Kingdom (EPIC-Oxford), Austria (VHMandPP), France (E3N), Italy (EPIC-Turin), and Greece (EPIC-Athens) (Figure 1). Six of these cohorts were part of the EPIC study. The mean follow-up period ranged from 6.3 to 18.6 years (Table 1).
Figure 1.
Location of all cohorts. Circles mark the cohort areas in which both particulate matter (PM) and nitrogen oxides were measured. Squares indicate cohort areas where only NO2 and nitrogen oxides (NOx) were measured.
Table 1:
Description of the Cohort Studies
| Cohort Name* | Subjects (n) | Respiratory Mortality Cases (n) | Baseline Recruitment Period | Total Time at Risk in Person-Years (Average Follow-up Time [yr]) | Study Area Description |
|---|---|---|---|---|---|
| EPIC-Umeå, Sweden | 22,136 | 33 | 1992–1996 | 281,711 (12.7) | City of Umeå and surrounding rural areas |
| FINRISK, Finland | 10,330 | 27 | 1992, 1997, 2002, 2007 | 109,396 (10.6) | Greater Helsinki Area and Turku city and its rural surroundings |
| HUBRO, Oslo, Norway | 18,236 | 90 | 2000–2001 | 175,062 (9.6) | City of Oslo |
| SNAC-K, Stockholm, Sweden | 2,768 | 43 | 2001–2004 | 17,311 (6.3) | City of Stockholm |
| SALT, Stockholm, Sweden | 5,511 | 43 | 1998–2002 | 47,887 (8.7) | Stockholm Count |
| Sixty, Stockholm, Sweden | 3,758 | 18 | 1997–1999 | 42,168 (11.2) | Stockholm County |
| DCH, Copenhagen, Denmark | 35,458 | 265 | 1993–1997 | 469,571 (13.2) | City of Copenhagen and surrounding areas |
| EPIC-MORGEN, The Netherlands | 16,446 | 36 | 1993–1997 | 217,722 (13.2) | Cities of Amsterdam, Maastricht and Doetinchem and surrounding rural areas |
| EPIC-PROSPECT, The Netherlands | 15,670 | 79 | 1993–1997 | 202,809 (12.9) | City of Utrecht and surrounding rural areas |
| SALIA, Germany | 4,352 | 32 | 1985–1987, 1990–1994 | 81,093 (18.6) | Areas in the cities of Dortmund, Duisburg, Essen, Gelsenkirchen and Herne situated in the Ruhr Area and adjacent towns Borken and Dulmen |
| EPIC-Oxford, UK | 38,941 | 151 | 1993–2001 | 491,542 (12.6) | Urban and rural areas in a buffer of 400 km around London-Oxford area |
| KORA, Germany | 8,402 | 52 | 1995–1995, 1999–2001 | 97,168 (11.6) | City of Augsburg and two adjacent rural counties |
| VHM&PP, Voralberg, Austria | 103,097 | 619 | 1985–2005 | 1,769,491 (17.2) | State of Vorarlberg, excluding high mountain areas (>600 m) and areas within 300 m of state border |
| E3N, France† | 10,993 (8,356) | 29 (22) | 1993–1996 | 151,423 (13.8) (115,269 [13.8]) | Cities of Paris, Grenoble, Lyon, and Marseille and surrounding rural areas |
| EPIC-Turin, Italy | 7,263 | 8 | 1993–1998 | 93,515 (12.9) | City of Turin |
| EPIC-Athens, Greece | 4,192 | 12 | 1994–1999 | 46,852 (11.2) | 16 municipalities of the greater Athens area |
The order of cohorts is based on a north-to-south gradient in Europe.
Numbers for analyses for which particulate matter data were available.
Nonmalignant respiratory mortality was defined on the basis of the underlying cause of death recorded on death certificates. Nonmalignant respiratory mortality included ICD-9 codes 460 to 519 or ICD-10 codes J00 to J99. In most cohorts, follow-up was based on linkage to national mortality registries; for EPIC-Athens, active follow-up was implemented (16).
Air Pollution Exposure Assessment
Air pollution concentrations were assigned to baseline residential addresses of study participants after extensive and standardized pollutant measurement campaigns and the use of standardized Land Use Regression (LUR) modeling procedures in all study areas, which are described in detail elsewhere (17, 18). In brief, air pollution monitoring campaigns were performed between October 2008 and May 2011 in all study areas to obtain annual average concentrations of nitrogen oxides (NO2 and NOx), PM2.5 absorbance (determined as the reflectance of PM2.5 filters), and PM2.5 and PM10 (19, 20). The measurement campaigns involved 20 sites for PM and 40 sites for NOx using the same measurement technique and covered all seasons. All instruments were prepared and analyzed by one central laboratory at the Institute for Risk Assessment Sciences, Utrecht, using the ESCAPE protocols. A detailed description of sampling and analysis in the ESCAPE project can be found in Eeftens and colleagues (18) and Cyrys and colleagues (19). PMcoarse was calculated as PM10 minus PM2.5. PM measurements were restricted to 15 of the 16 study areas. Only NOx and NO2 were measured in Umea (EPIC-UMEA), Sweden for budgetary reasons.
Study-area specific LUR models were developed to explain the within-area spatial variation of measured annual average air pollution concentrations using traffic and land use predictor variables in a Geographic Information System (17, 20). A standardized approach described in the ESCAPE exposure manual (21) was used to develop LUR models in all areas. Briefly, for each site an annual average concentration was calculated. The coordinates of the monitoring sites were used to derive the values for the predictor variables through Europe-wide and local Geographic Information System databases. LUR models were developed using linear regression, with the annual average concentrations as the dependent variable and an extensive list of geographical attributes as possible predictors related to traffic intensity, road length, population density and proximity to natural or urban green areas, proximity to a port or industrial areas, and altitude; these parameters maximize the adjusted percentage explained variance (R2) in a supervised, forward, stepwise procedure. The set of predictors chosen depended on the study area and the specific pollutant.
The results of the LUR models were used to estimate ambient air pollutant concentrations at the participants’ baseline addresses. An evaluation of the fit of LUR models developed within the ESCAPE project has been described elsewhere (22). In addition to air pollution concentrations, traffic intensity on the nearest road (vehicles/d) and total traffic load on all major roads within a 100-m buffer around each subject’s residence (vehicles × m/d) were used as indicators of traffic at the residence. Air pollution measurements were performed from 2008 to 2011, but baseline addresses were in earlier time periods. Therefore, the predicted concentrations were extrapolated back at the recruitment time (1985–2007 with most studies in the mid-90s) using the absolute difference and the ratio method between the two periods (21). In the present analysis, only data for NO2 and NOx were available from routine background monitoring network site(s) to perform back-extrapolation of the predicted concentrations. Moreover, the procedure was applicable to only some cohorts: 10 cohorts with back-extrapolated concentrations for NO2 and eight cohorts for NOx.
Statistical Analyses
Cohort-specific analyses.
Cox proportional hazards models were used for the cohort-specific analyses. Age was used as the underlying time scale because of evidence of better adjustment for potential confounding (23). Censoring occurred at the time of death, emigration, loss of follow-up, or end of follow-up, whichever came first. Air pollution exposure was analyzed as a linear variable in three a priori specified confounder models (see below), which had an increasing level of adjustment for individual and area-level confounders.
Model 1 included gender, calendar time (year(s) of enrollment), and age (time axis). Model 2 included, in addition to the variables in Model 1, smoking status (never/former/current), smoking intensity (as life-time average tobacco smoking intensity, in g/d), smoking duration (the net number of smoking years), environmental tobacco smoke exposure (various definitions in different cohorts), occupational exposure (a white/blue collar classification), employment status (employed, self-employed, unemployed, stay at home, retired), fruit intake (continuous in g/d), vegetables intake (continuous in g/y), marital status (as single, married/living with a partner, divorced/separated, widowed), educational level (defined as “low” [primary education], “medium” [secondary education], and “high” [tertiary education]) and body mass index (BMI) (BMI [kg/m2] and BMI2 were used to take into account nonlinear effects). Model 3 included, in addition to model 2, area-level socioeconomic status variables (e.g., mean income or educational level on a neighborhood or municipality scale). Model 3 was chosen as the main confounder model.
As sensitivity analyses, we tested whether back-extrapolation of the modeled concentrations to the baseline year had any impact on the results. Sensitivity analysis, restricted to subjects who did not move during follow-up (information available for eight cohorts), was also conducted. Effect modification by age during follow-up (<60, 60–75, ≥75 yr), gender, smoking status, fruit intake (<150, 150–300, ≥300 g/d), and BMI (<25, 25–30, ≥30 kg/m2) was investigated by including interaction terms in the main model 3. For some cohorts, specific models investigating effect modification were not applied because there were not enough respiratory mortality cases in each category of the corresponding effect modifier.
A common STATA script was available for all cohorts (STATA versions 10–12; StataCorp, College Station, TX).
Metaanalysis
Results from cohort-specific analyses were sent to the Department of Hygiene, Epidemiology and Medical Statistics, Medical School, University of Athens, Greece for evaluation. Metaanalyses of cohort-specific effect estimates were conducted applying the Der Simonian-Laird method with random effects (24). Fixed exposure increments were used to calculate metaanalysis hazard ratios (HRs) and 95% confidence intervals (CIs): 10 μg/m3 for NO2, 20 μg/m3 for NOx, 5 μg/m3 for PM2.5, 10−5 m−1 for PM2.5 absorbance, 10 μg/m3 for PM10, 5 μg/m3 for PMcoarse, 5,000 motor vehicles/d for the traffic intensity on the nearest road, and 4,000,000 motor vehicles × m/d for the total traffic load on all major roads within a 100-m buffer. A metaanalysis was repeated as sensitivity analysis after excluding a cohort in situations where the specific cohort contributed over 50% of the weight in the metaanalysis. Effect modification was tested by metaanalyzing the pooled estimates from the different strata with the χ2 test of heterogeneity. The I2 statistic and P value for the χ2 test from Cochran’s Q were calculated to investigate the heterogeneity among cohort-specific effect estimates (25). All metaanalyses were conducted in STATA.
Results
Table 1 shows the number of subjects, the number of respiratory mortality cases, the recruitment period, and the follow-up time in person-years and the study area per cohort. The total number of subjects, after excluding subjects with missing values for any of the confounder variables, was 307,553, with 33.5% contributed by the VHM&PP cohort. There were 1,559 respiratory deaths in all cohorts, ranging from 8 (EPIC-Turin) to 619 (VHM&PP). Most of the study areas were large cities with adjacent suburban or smaller rural communities. Five cohorts (DCH, EPIC-Oxford, VHM&PP, E3N, and EPIC-Athens) (Table 1) were part of larger cohorts that could not be included as a whole because exposures could not be assigned to all the areas of residence covered by the full cohort. The subjects included in the analyses (i.e., those without missing values in the confounders) had practically identical mean age and gender proportion. More details on the cohorts and restrictions of exposure assignment are given in the online supplement.
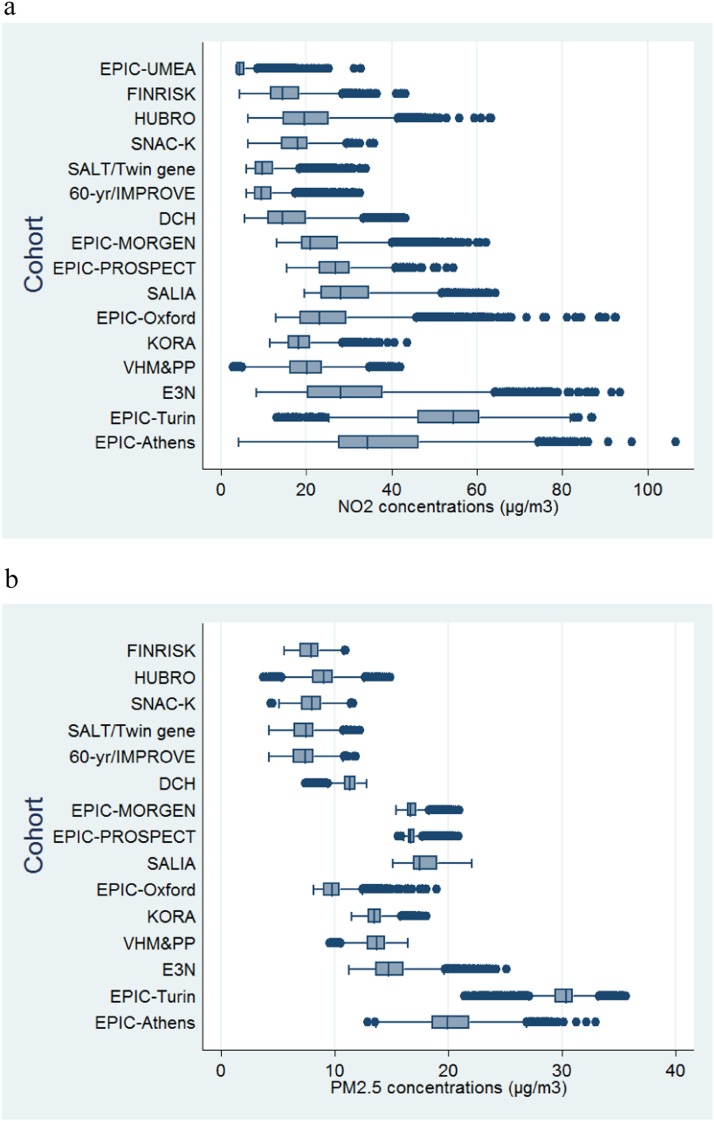
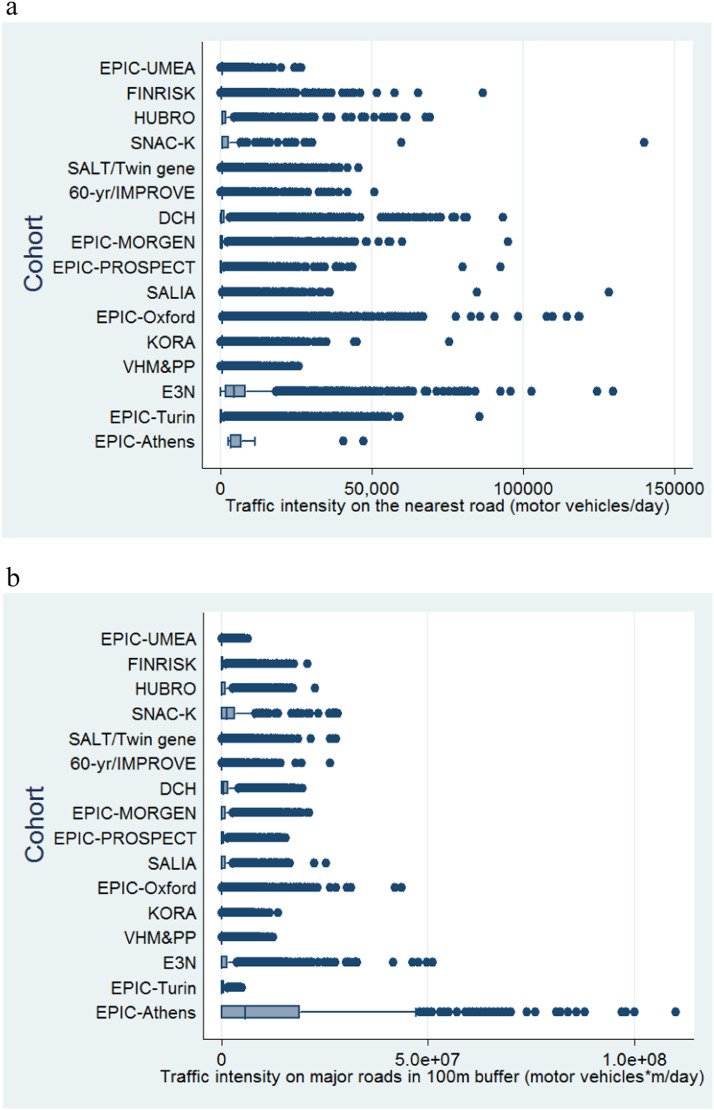
The levels of air pollution and traffic indicators characterizing the subjects’ residences are shown in Figures 2 and 3. A general geographical trend with higher concentrations of pollutants in southern countries compared with northern is observed (see Table E2.1 and Figures E2.1–E2.8 in the online supplement). The same is not true for traffic load around the residences, which varies according to the degree of urbanization of the areas studied in each cohort. Table 2 shows the sociodemographic characteristics of study subjects by cohort at baseline. The mean age at enrolment varied from 42 to 73 years. Three cohorts (EPIC-PROSPECT, SALIA, and E3N) consisted of women only. Age, gender, and smoking status were available for all participating cohorts. Information on confounder availability is given in the online supplement.
Figure 2.
Description of modeled outdoor concentrations of NO2 (a) and particulate matter with an aerodynamic diameter < 2.5 μm (PM2.5) (μg/m3) (b) at participant addresses in each cohort. Particulate matter data not available for EPIC-Umeå.
Figure 3.
Description of traffic intensity on the nearest road (motor vehicles/d) (a) and traffic load on major roads in a 100-m buffer (motor vehicles × m/d) (b) at participant addresses in each cohort.
Table 2:
Population Study Characteristics at Baseline
| Cohort* | Baseline Age (yr) | Cigarettes/d | Years of Smoking | BMI (kg/m2) | Fruit Intake† (g/d) | Vegetable Intake (g/d)‡ | Women (%) | Never-smokers (%) | Married (%) | Low Educational Level (%) | Employed (%) | ETS§ | White Collar (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EPIC-Umeå, Sweden | 46.0 (10.2)|| | 2.4 (5.6) | 8.8 (13.0) | 25.0 (4.0) | 163.0 (132.6) | 93.5 (90.1) | 52.2 | 62.0 | 82.3 | 28.0 | 85.4 | — | — |
| FINRISK, Finland | 48.0 (13.2) | 3.8 (7.8) | 14.0 (17.2) | 26.4 (4.6) | 67.8% | 68.2% | 53.7 | 45.1 | 70.1 | 31.0 | 69.1 | 18.5 | 53.1 |
| HUBRO, Oslo, Norway | 48.3 (15.2) | 6.7 (8.5) | 11.5 (14.4) | 25.7 (4.1) | 39.9% | 14.5% | 56.1 | 46.1 | 49.9 | 17.7 | 73.2 | — | — |
| SNAC-K, Stockholm, Sweden | 73.0 (10.3) | 6.6 (9.2) | 15.9 (19.4) | 25.7 (4.1) | — | — | 62.0 | 46.9 | 49.7 | 24.9 | 24.8 | 51.4 (65.0) | 81.4 |
| SALT, Stockholm, Sweden | 58.4 (10.4) | 2.9 (6.5) | 16.3 (17.2) | 28.5 (4.1) | — | — | 56.4 | 39.2 | 67.5 | 21.8 | — | — | — |
| Sixty, Stockholm, Sweden | 60.3 (0.1) | 8.0 (9.1) | 15.3 (16.4) | 23.3 (2.3) | 64.4% | 13.2% | 52.5 | 40.7 | 71.5 | 27.7 | 50.2 | 47.5 | 77.3 |
| DCH, Copenhagen, Denmark | 56.7 (4.4) | 6.3 (10.4) | 18.7 (17.1) | 26.0 (4.1) | 183.2 (151.2) | 175.9 (99.2) | 54.1 | 36.3 | 69.2 | 29.6 | 80.1 | 64.3 | — |
| EPIC-MORGEN, Netherlands | 43.9 (10.9) | 10.4 (11.1) | 14.3 (13.7) | 25.2 (4.0) | 171.9 (129.2) | 126.6 (51.8) | 54.4 | 35.0 | 67.6 | 11.9 | — | — | — |
| EPIC-PROSPECT, Netherlands | 57.7 (6.0) | 5.7 (7.4) | 15.2 (16.5) | 25.5 (4.1) | 231.6 (139.2) | 136.3 (52.5) | 100.0 | 45.0 | 76.9 | 22.2 | — | — | — |
| SALIA, Germany | 54.5 (0.6) | 2.6 (6.6) | 4.4 (10.5) | — | — | — | 100.0 | 74.5 | — | 28.8 | — | 50.8 | — |
| EPIC-Oxford, UK | 45.8 (13.7) | 5.0 (8.3) | 6.7 (11.2) | 24.0 (3.9) | 259.9 (204.5) | 281.0 (156.4) | 77.5 | 63.3 | 70.8 | 36.5 | 72.5 | — | — |
| KORA, Germany | 49.5 (13.8) | 9.2 (13.3) | 12.0 (14.2) | 27.2 (4.6) | 59.5% | 47.1% | 50.8 | 43.7 | 75.7 | 12.6 | 58.3 | 23.9 (24.7) | — |
| VHM&PP, Voralberg, Austria | 41.9 (14.9) | — | — | 24.8 (4.3) | — | — | 56.1 | 69.9 | 68.4 | — | 69.3 | — | 56.3 |
| E3N, France | |
||||||||||||
| Paris | 52.9 (6.7) | 3.7 (6.9) | 11.8 (16.0) | 22.8 (3.2) | 238.4 (163.2) | 239.1 (127.0) | 100.0 | 63.0 | — | 10.6 | — | — | — |
| Grenoble, Lyon, and Marseille | 52.9 (6.7) | 3.5 (6.7) | 11.4 (15.8) | 22.7 (3.2) | 244.8 (166.3) | 242.7 (127.4) | 100.0 | 64.2 | — | 11.3 | — | — | — |
| EPIC-Turin, Italy | 50.4 (7.5) | 7.2 (8.2) | 17.6 (16.3) | 25.3 (3.8) | 318.3 (182.3) | 181.8 (100.2) | 47.7 | 42.6 | 85.6 | 43.6 | — | — | — |
| EPIC-Athens, Greece | 49.4 (11.7) | 12.7 (15.0) | 10.8 (13.1) | 27.5 (4.5) | 402.6 (258.2) | 609.5 (288.6) | 55.0 | 39.5 | 78.0 | 23.6 | 66.9 | — | 47.5 |
Definition of abbreviations: BMI = body mass index; ETS = environmental tobacco smoke.
The order of cohorts is based on a north-to-south gradient in Europe
Mean (SD) (g/d) or percentage with daily fruit consumption.
Mean (SD) (g/d) or percentage with daily vegetable consumption.
Percentage of any exposure at home and/or work (values in parentheses indicate the percentage of ETS at work).
Mean (SD) unless otherwise noted.
Table 3 summarizes the association of the modeled air pollution concentrations at the subjects’ residences and traffic-proximity variables with respiratory mortality. HRs using model 1 (only adjusted for calendar year of recruitment, age, and gender) were the highest; they decreased after adjusting for individual level confounders (model 2). Inclusion of area-level socioeconomic status variables led to a further, smaller, decrease in the HRs (model 3). From model 3, only one of the estimated pooled HRs was statistically significant, and most were slightly below 1. Only traffic-proximity variables resulted in estimates above 1 for respiratory mortality, not reaching the nominal level of statistical significance (HR,1.03 and 95% CI, 0.93–1.13 for an increase in total traffic load on all major roads within a 100-m buffer per 4,000,000 motor vehicles × m/d; and HR,1.01 and 95% CI, 0.95–1.06 for larger traffic intensity on the nearest road by 5,000 motor vehicles/d). Figure 4 shows the cohort-specific HRs and the pooled HR from the metaanalyses for NO2 and PM2.5. There is no statistically significant HR in any cohort. Figure 5 shows the cohort-specific HRs and the pooled HR from the metaanalyses for traffic intensity on the nearest road and total traffic load on all major roads within a 100-m buffer around the subjects’ residence. Forest plots of cohort-specific and pooled HRs for the association between respiratory mortality and all exposures, using main model 3, are given in Figures E4.1 to E4.8. No significant heterogeneity between the cohorts was noted in any of the metaanalyses (I2 < 15%; P > 0.05).
Table 3:
Pooled Hazard Ratios and 95% Confidence Intervals from Cox Proportional Hazards Models Including Various Confounders for the Association between Modeled Outdoor Concentrations of Various Air Pollutants and Traffic Indicators Included Alternatively in the Models (Expressed per Fixed Increment as Specified) and Nonmalignant Respiratory Mortality (ICD-9 Codes 460–519 or ICD-10 Codes J00–J99)
| Exposure (Fixed Increment) | No. of Cohorts | Cox Proportional Hazards Models* |
Measures of Heterogeneity (Model
3) |
|||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | I2 (%) | P Value | ||
| NO2 (10 μg/m3)† | 16 | 1.02 (0.94–1.11)‡ | 0.98 (0.90–1.06) | 0.97 (0.89–1.05) | 0.0 | 0.849 |
| NOx (20 μg/m3)† | 16 | 1.05 (0.95–1.15) | 1.00 (0.92–1.08) | 0.99 (0.90–1.09) | 14.6 | 0.286 |
| PM10 (10 μg/m3)† | 15§ | 0.87 (0.69–1.04) | 0.90 (0.72–1.08) | 0.86 (0.67–1.04) | 0.0 | 0.980 |
| PMcoarse (5 μg/m3)† | 15§ | 1.01 (0.82–1.20) | 0.95 (0.77–1.14) | 0.95 (0.76–1.14) | 0.0 | 0.931 |
| PM2.5 (5 μg/m3)† | 15§ | 0.87 (0.65–1.10) | 0.91 (0.68–1.14) | 0.89 (0.66–1.12) | 0.0 | 0.999 |
| PM2.5 (5 μg/m3)† | 14|| | 0.92 (0.53–1.30) | 0.92 (0.53–1.31) | 0.89(0.50–1.29) | 0.0 | 0.997 |
| PM2.5ABS (10−5/m)† | 15§ | 0.82 (0.62–1.03) | 0.83 (0.62–1.03) | 0.70 (0.47–0.93) | 12.8 | 0.310 |
| Traffic intensity on the nearest road (5,000 motor vehicles/d) | 16 | 1.01 (0.97–1.06) | 1.01 (0.96–1.06) | 1.01 (0.95–1.06) | 11.6 | 0.321 |
| Total traffic load on all major roads within a 100-m buffer (4,000,000 motor vehicles × m/d) | 16 | 1.06 (0.96–1.16) | 1.02 (0.92–1.12) | 1.03 (0.93–1.12) | 0.0 | 0.999 |
Definition of abbreviations: NOx = nitrogen oxides; PM2.5 = particulate matter with an aerodynamic diameter < 2.5 μm; PM10 = particulate matter with an aerodynamic diameter < 10 μm; PMcoarse = PM10 − PM2.5.
Model 1: gender, calendar time. Model 2: model 1 + smoking status, smoking intensity, smoking duration, environmental tobacco smoke, occupation, employment status, intake of fruit, intake of vegetables, marital status, educational level, and body mass index. Model 3: model 2 + socioeconomic status at an area level (i.e. of the municipality or neighborhood).
Estimated concentrations using Land Use Regression models.
Values are hazard ratios with 95% confidence intervals in parentheses.
Without the EPIC-UMEA cohort, where a particulate matter measurement campaign was not implemented.
Without the VHM&PP cohort (contributed >50% of the weight in metaanalysis, conducted as sensitivity analysis).
Figure 4.
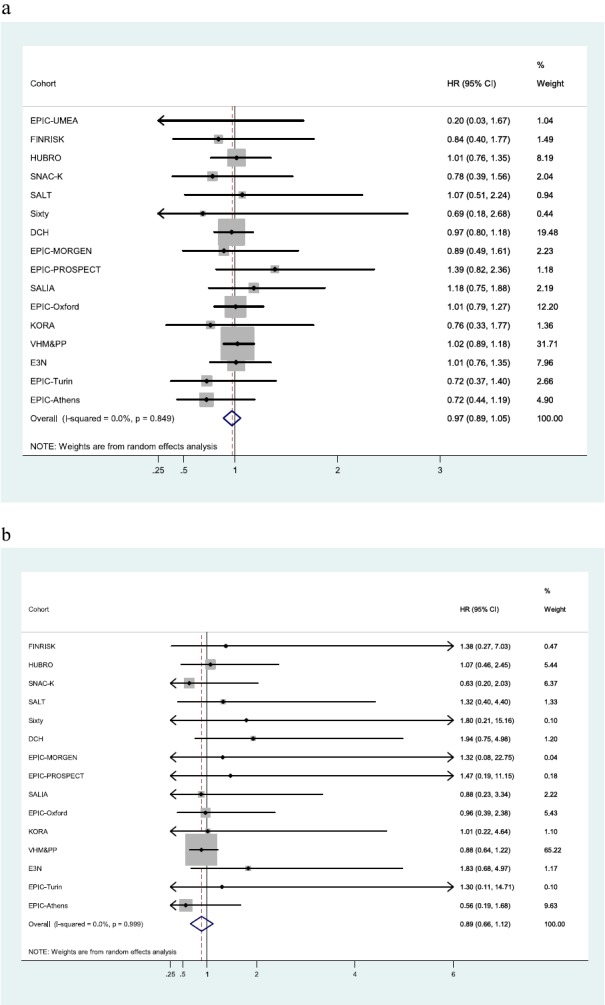
Cohort-specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) based on confounder model 3 for nonmalignant respiratory mortality in association with NO2 (per 10 μg/m3) (a) and particulate matter with an aerodynamic diameter < 2.5 μm (PM2.5) (per 5 μg/m3) (b) modeled outdoor concentrations. Model 3: gender, calendar time + smoking status, smoking intensity, smoking duration, environmental tobacco smoke, occupation, employment status, intake of fruit, intake of vegetables, marital status, educational level, body mass index + socioeconomic status at an area level (i.e., of the municipality or neighborhood).
Figure 5.
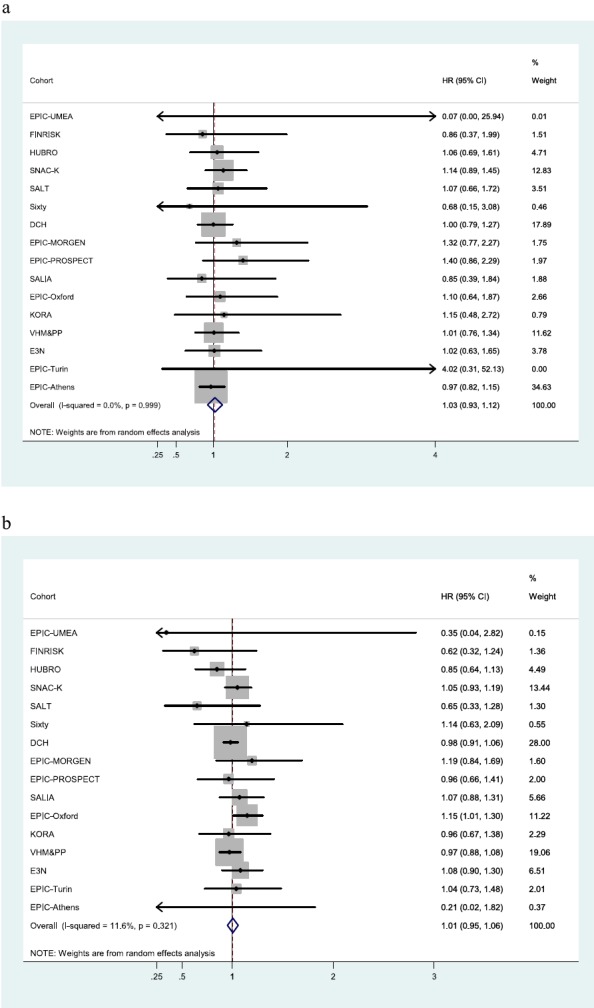
Cohort-specific and pooled hazard ratios (HRs) and 95% confidence intervals (CIs) based on confounder model 3 for nonmalignant respiratory mortality in association with total traffic load on all major roads within a 100-m buffer (4,000,000 motor vehicles × m/d) around the subjects’ residences (a) and with traffic intensity on the nearest road (per 5,000 motor vehicles/d) (b). Model 3: gender, calendar time + smoking status, smoking intensity, smoking duration, environmental tobacco smoke, occupation, employment status, intake of fruit, intake of vegetables, marital status, educational level, body mass index + socioeconomic status at an area level (i.e., of the municipality or neighborhood).
When metaanalysis was conducted for the back-extrapolated NO2 and NOx modeled concentrations, the pooled HRs were the same compared with the results of the main model 3 (Table E5.1). Analysis restricted to subjects who did not move during follow-up resulted in similar HRs as for the main model 3 results (Table E6.1). No statistically significant effect modification for any of the variables evaluated was observed (Table 4). Only a subset of the cohorts was included in this analysis. There was a tendency for effects of NO2 and traffic load on all major roads within a 100-m buffer to be larger in the older age group.
Table 4:
Adjusted Association between Respiratory Mortality and Modeled Outdoor Concentrations of NO2, PM2.5, and Traffic Intensity on the Nearest Road Stratified by Age, Sex, Smoking Status, Fruit Consumption, and Body Mass Index (Results from Random-Effects Metaanalyses)
| NO2 (10 μg/m3) | PM2.5 (5 μg/m3) | Traffic Intensity on the Nearest Road (5,000 Motor Vehicles/d) | Total Traffic Load on All Major Roads within a 100-m Buffer (4,000,000 Motor Vehicles × m/d) | |
|---|---|---|---|---|
| Age,* yr | ||||
| <60 | 0.72 (0.42–1.01)† | 0.45 (0.41–1.31) | 1.09 (0.91–1.26) | 0.94 (0.25–1.63) |
| 60–75 | 0.99 (0.85–1.12) | 1.18 (0.58–1.78) | 0.96 (0.89–1.04) | 0.94 (0.73–1.14) |
| ≥75 | 1.02 (0.88–1.16) | 0.74 (0.44–1.03) | 1.05 (0.97–1.13) | 1.14 (0.89–1.40) |
| P value‡ | 0.191 | 0.158 | 0.186 | 0.451 |
| Sex§ | ||||
| Female | 0.91 (0.78–1.05) | 0.64 (0.34–0.94) | 0.94 (0.78–1.10) | 1.02 (0.86–1.18) |
| Male | 0.90 (0.76–1.03) | 0.87 (0.52–1.23) | 1.03 (0.95–1.12) | 1.02 (0.88–1.17) |
| P value‡ | 0.854 | 0.325 | 0.289 | 0.976 |
| Smoking status|| | ||||
| Current | 1.09 (0.88–1.30) | 1.25 (0.56–1.94) | 1.01 (0.90–1.11) | 1.03 (0.87–1.19) |
| Former | 0.96 (0.75–1.17) | 0.65 (0.01–1.29) | 0.85 (0.62–1.09) | 0.97 (0.65–1.29) |
| Never | 0.88 (0.71–1.04) | 0.47 (0.05–0.88) | 1.00 (0.87–1.13) | 0.97 (0.82–1.12) |
| P value‡ | 0.273 | 0.163 | 0.486 | 0.850 |
| Fruit intake,¶ g/d | ||||
| <150 | 0.97 (0.79–1.16) | 0.88 (0.40–2.16) | 1.02 (0.83–1.20) | 1.08 (0.79–1.36) |
| 150–300 | 0.95 (0.75–1.15) | 0.94 (0.20–2.08) | 1.03 (0.92–1.14) | 0.92 (0.57–1.27) |
| ≥300 | 0.92 (0.68–1.16) | 1.65 (0.90–4.20) | 1.08 (0.82–1.34) | 1.18 (0.80–1.57) |
| P value‡ | 0.933 | 0.710 | 0.920 | 0.599 |
| BMI,** kg/m2 | ||||
| <25 | 0.98 (0.84–1.11) | 0.98 (0.57–1.40) | 1.00 (0.92–1.08) | 1.05 (0.83–1.28) |
| 25–30 | 1.08 (0.90–1.25) | 0.94 (0.45–1.43) | 1.05 (0.97–1.13) | 0.99 (0.71–1.27) |
| ≥30 | 0.82 (0.59–1.06) | 0.48 (0.01–1.00) | 0.62 (0.07–1.17) | 0.95 (0.49–1.41) |
| P value‡ | 0.245 | 0.252 | 0.225 | 0.905 |
Definition of abbreviations: BMI = body mass index; PM2.5 = particulate matter with an aerodynamic diameter < 2.5 μm.
Included the following cohorts: EPIC-UMEA, FINRISK, SALT, DCH, EPIC-PROSPECT, SALIA, EPIC-Oxford, VHM&PP, and E3N.
Values are hazard ratios with 95% confidence intervals in parentheses.
Meta regression P value that denotes whether there is statistical difference between strata.
Included the following cohorts: EPIC-UMEA, FINRISK, SALT, Sixty, DCH, EPIC-MORGEN, EPIC-Oxford, KORA, VHM&PP, EPIC-Turin, and EPIC-Athens, Greece.
Included the following cohorts: EPIC-UMEA, SNAC-K, SALT, Sixty, DCH, EPIC-MORGEN, EPIC-PROSPECT, SALIA, EPIC-Oxford, KORA, and VHM&PP.
Included the following cohorts: FINRISK, SALT, DCH, EPIC-PROSPECT, EPIC-Oxford, VHM&PP, and EPIC-Turin.
Included the following cohorts: FINRISK, DCH, EPIC-PROSPECT, EPIC-Oxford, E3N, and EPIC-Turin.
Discussion
We report results from the largest European study on long-term effects of air pollution and nonmalignant respiratory mortality. Limited and inconsistent evidence has been reported to date on this issue (13). We studied the effects of long-term exposure to NO2, NOx, various indices of particulate pollution (PM2.5, PM absorbance, PM10, and PMcoarse), proximity to traffic intensity, and traffic load on major roads around the residence of 307,553 subjects in 16 cohorts across Europe on nonmalignant respiratory mortality. We did not find statistically significant adverse associations between the studied exposures and health outcomes. Other outcomes (natural mortality, cardiovascular mortality, and lung cancer incidence) were included in previous publications from the ESCAPE project (15, 26, 27).
In United States cohort studies, generally no significant associations between PM10 and PM2.5 and nonmalignant respiratory mortality have been found (28–32). NO2 effects were also generally not statistically significant, with some HRs below 1 (28, 31) and some indicating an adverse effect (29, 30). One Canadian study (33) reported a significantly increased risk of COPD mortality (HR, 1.07; 95% CI, 1.00–1.13) associated with increased black carbon concentrations.
In a Dutch Cohort study (1), a statistically significant effect of NO2 and of traffic intensity around the subjects’ residence on nonmalignant respiratory mortality was reported (HR, 1.37 and 95% CI, 1.00–1.87; HR, 1.21; and 95% CI, 1.02–1.44, respectively), whereas PM2.5 exposure had an adverse but nonsignificant effect. A study in Norway (34) reported associations of exposure to NOx and respiratory deaths (HR, 1.23; 95% CI, 1.13–1.35) for the more recent period of follow-up. In a study in Scotland (35), effects of BS on respiratory mortality were inconsistent between the two cohorts analyzed: in one study the results indicated a strong adverse effect, whereas in the other study the effect was in the inverse direction. In the Rome cohort (36), a statistically significant HR of 1.03 (95% CI, 1.00–1.06) for respiratory mortality was observed per 10 μg/m3 increase in NO2 and a similar size, but not statistically significant, effect of PM2.5. In this cohort and in a recent German study (37), there was also an indication of increased HR associated with traffic intensity near the residences of the subjects. Furthermore, the study in Dublin, evaluating the beneficial effects of a considerable reduction in PM concentrations (measured as black smoke), found a subsequent significant decrease in respiratory mortality more pronounced than the corresponding decrease in total or cardiovascular mortality (38).
In a Chinese cohort (39), a statistically nonsignificant adverse effect of NOx was found on respiratory mortality. In contrast, in the Shenyang cohort study (40), statistically significant relative risks of 1.67 (95% CI, 1.60–1.74) for respiratory mortality were reported per 10 μg/m3 increase in PM10; a relative risk of 2.97 (95% CI, 2.69–3.27) was reported for a similar increase in NO2. The study in Japan (6) found a statistically significant adverse effect of exposure to NO2 and pulmonary disease mortality (HR, 1.19; 95% CI, 1.02–1.38) per 10 μg/m3 increase in the pollutant.
The accumulated evidence (13) and the present results do not support a clear long-term effect of traffic-related pollutants on nonmalignant respiratory mortality. This evidence may be considered to be inconsistent with the statistically significant short-term effects that have been repeatedly reported (9–11) and that have indicated that effects at longer lags (e.g., up to 40 d) (41) on respiratory mortality were higher. In principle, cohort studies are able to detect cumulative short-term and long-term effects. However, in contrast to time series studies, which have very high statistical power, cohort studies are not able to detect very small effects unless the sample size is considerably larger than even our current effort. Hoek and colleagues (13) found a pooled estimate per 10 μg/m3 for PM2.5 of 1.029 (95% CI, 0.941–1.126) and moderate heterogeneity (I2 = 59%) among individual study results. The hypothesis that NO2 and soot concentrations, as traffic-related pollutants, could be associated more with respiratory mortality than PM2.5 was not supported by the ESCAPE results. However, another explanation of this apparently discordant results may be that in time series studies a very large proportion of the daily events analyzed are contributed by the very elderly proportion of the population, which may be more sensitive to the short-term effects of air pollution. In several of our cohorts, very elderly subjects are not well represented, but the relative risk for a long-term effect is not expected to be higher; rather, it is expected to be lower because of the high baseline risk.
One possible explanation of our findings is that an effect exists but our study was unable to detect it. The main reason for such an explanation would be limited statistical power. However, our study has assembled the largest database of cohorts across Europe, and the number of cases analyzed here is not small. Moreover, the lack of heterogeneity in the results must be underlined. The lung cancer incidence analysis, within ESCAPE, analyzing a somewhat larger number of cases, has been able to detect statistically significant effect estimates (15). However, coding practices on death certificates differ among countries, especially for respiratory causes, possibly leading to misclassification in the outcome. Residual confounding is an unlikely explanation of the null findings because the combined estimate and the cohort-specific estimates were uniformly nonsignificant, and residual confounding in different cohort studies will probably not to lead to the same bias.
In our project, exposure assessment was based on an extensive and harmonized measurement campaign and statistical modeling. However, the possibility of some exposure misclassification exists, resulting in biasing our effect estimates toward the null. Proximity variables may not reflect differential dispersion of traffic-related air pollutants in specific situations, but exposure estimates based on LUR models address this in a better way. There was no baseline air pollution exposure measurement in the included cohorts. Exposure assessment was done from 2008 to 2011, and exposure was retrospectively estimated for all subjects to periods more relevant for the induction of long-term respiratory problems. This methodology has been shown to be valid (36, 42–44). The same methodology using 2008–2011 air pollution data to develop exposure models and applying them to the participants’ baseline addresses was used in other ESCAPE publications (15, 26, 27).
Exposure assessment was only based on the subjects’ residence because their working address and commuting habits were not available. This probably led to more exposure misclassification, possibly biasing the effect estimates toward the null. Furthermore, in our study we focused on traffic-related pollutants, which may not be those affecting the respiratory system, and did not study the effects of ozone, a secondary pollutant, often spatially negatively correlated with primary pollutants that has been shown to be associated specifically with respiratory mortality effects (45).
Another possible explanation is that no association exists between air pollution effects and nonmalignant respiratory mortality, at least at the age range studied. In the majority of our cohorts mean age is less than 70 years of age, whereas nonmalignant respiratory mortality increases at ages above 80 years old (46). In our study we have observed increased nonstatistically significant effects in the elderly population. However, not all cohorts covered the “≥75 years” age group, and some of those belonged to the most polluted areas.
The effects of air pollution on mortality have been studied within single or combined categories such as cardiorespiratory mortality. The role of competing risks, which may be even more important for the relatively younger age groups represented in our study, as well as multimorbidity may be considered in future research to shed light on the implications of local effects in the lung combined with underlying systemic effects potentially affecting multiple chronic diseases.
In conclusion, we did not find significant associations between a comprehensive set of major air pollutants and long-term risk for nonmalignant respiratory mortality in 16 European cohort studies. In light of the contrasting evidence and the reported short-term effects, more investigations on long-term respiratory effects, possibly involving more prolonged follow-up periods, are needed. New studies need to be large to have sufficient power to detect small risks and should include elderly subjects.
Acknowledgments
Acknowledgment
The authors thank Marjan Tewis, Marieke Oldenwening, Marta Cirach, Audrey de Nazelle, Bernhard Anwander, Paolo Crosignani, Jon Wickmann, Daniela Raffaele, Marco Gilardetti, Ulrich Quass, Mohammad Vossoughi, Simone Bucci, L.-J. Sally Liu, Tarja Yli-Tuomi, Pekka Taimisto, and Arto Pennanen (from the Department of Environmental Health at National Institute for Health and Welfare) for help with exposure assessment and data management within ESCAPE and Jon Wickmann for his efforts to coordinate our team and to assure the quality of exposure assessment in the HUBRO cohort. Mortality, area-level socioeconomic status, and building data were provided by Statistics Finland. For HUBRO, the data collection was conducted as part of the Oslo Health Study 2000–2001.
Footnotes
This work was supported by the following institutions. The Finnish part of the study was funded by the Academy of Finland (project number 129317). For HUBRO, the data collection was conducted as part of the Oslo Health Study 2000-2001 and financed by the Norwegian Institute of Public Health, the University of Oslo and the Municipality of Oslo. Financial support for the combined work with the Stockholm studies was received from the Swedish Environmental Protection Agency, the Swedish Heart-Lung Foundation and the Swedish Council for Working Life and Social Research. The Swedish Ministry for Higher Education financially supports the Swedish Twin Register. SALT was supported by the Swedish Council for Working Life and Social Research and by National Institutes of Health grant AG-08724. TwinGene was supported by the Swedish Research Council (M-2005-1112), GenomEUtwin (EU/QLRT-2001-01254; QLG2-CT-2002-01254), National Institutes of Health grant DK U01-066134, The Swedish Foundation for Strategic Research, and Heart and Lung Foundation grant 20070481. Financial support and mortality data for EPIC-MORGEN and EPIC-PROSPECT were received by the Dutch Ministry of Public Health, Welfare and Sports (V.W.S.), Netherlands Cancer Registry (N.K.R.), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), and Statistics Netherlands (The Netherlands). The baseline study and the mortality follow-up of SALIA were funded by the Ministry of the Environment of North-Rhine-Westfalia (Germany). The KORA research platform and the MONICA Augsburg studies were initiated and financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education and Research and by the State of Bavaria. The VHM&PP is supported by the State of Vorarlberg, Austria.
Author Contributions: K.D. contributed to the design, exposure assessment, statistical script, data analyses, and drafted the manuscript. E.S. and C.S. contributed to the statistical script. R.B. contributed to the design, exposure assessment, statistical script, and data analyses. M. Stafoggia and G.W. contributed to the statistical script and data analyses. Z.J.A. contributed to the design, statistical script, and data analyses. B.H. contributed to the statistical script and provided local cohort data. O.R.-N. and B.B. contributed to the design. P.J., N.A., A. Tjønneland, T.A.J.K., M.V., B.J., M. Stempfelet, and A.V. contributed to data analyses. P.F., M.N., L.M., M. Korek, K.T.E., M.E., K.M., M.W., K.d.H., A.I., and M.-Y.T. contributed to exposure assessment. P.V. contributed to the design and provided local cohort data. W.X. contributed to the design and data analyses. A.O., A. Turunen, B.O., J.P., F.R., M. Katsoulis contributed to the data analyses. B.F., P.N., U.d.F., N.P., L.F., P.H.P., B.B.-d.-M., U.K., J.H., T.K., A.P., H.C., and A. Trichopoulou provided local cohort data. T.L. contributed to exposure assessment and provided local cohort data. P.E.S. and T.B. provided local cohort data. R.H. and X.P. contributed to exposure assessment and data analyses. A.M. contributed to exposure assessment. G.N. contributed to data analyses and provided local cohort data. G.H. contributed to the design and statistical script. K.K. contributed to the design and drafted the manuscript. All authors contributed to critical reading of and comments to the manuscript and interpretation of data, and approved the final draft.
This article has an online data supplement, which is accessible from the issue's table of content online at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201310-1777OC on February 12, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Jerrett M, Hughes E, Armstrong B, Brunekreef B. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study) Environ Health Perspect. 2008;116:196–202. doi: 10.1289/ehp.10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eftim SE, Samet JM, Janes H, McDermott A, Dominici F. Fine particulate matter and mortality: a comparison of the six cities and American Cancer Society cohorts with a medicare cohort. Epidemiology. 2008;19:209–216. doi: 10.1097/EDE.0b013e3181632c09. [DOI] [PubMed] [Google Scholar]
- 3.Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000-2005) Environ Health Perspect. 2008;116:1614–1619. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 5.Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, Henderson KD, Bernstein L. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118:363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yorifuji T, Kashima S, Tsuda T, Takao S, Suzuki E, Doi H, Sugiyama M, Ishikawa-Takata K, Ohta T. Long-term exposure to traffic-related air pollution and mortality in Shizuoka, Japan. Occup Environ Med. 2010;67:111–117. doi: 10.1136/oem.2008.045542. [DOI] [PubMed] [Google Scholar]
- 7.Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120:965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raaschou-Nielsen O, Andersen ZJ, Jensen SS, Ketzel M, Sørensen M, Hansen J, Loft S, Tjønneland A, Overvad K. Traffic air pollution and mortality from cardiovascular disease and all causes: a Danish cohort study. Environ Health. 2012;11:60. doi: 10.1186/1476-069X-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katsouyanni K, Samet JM, Anderson HR, Atkinson R, Le Tertre A, Medina S, Samoli E, Touloumi G, Burnett RT, Krewski D, et al. HEI Health Review Committee. Air pollution and health: a European and North American approach (APHENA) Res Rep Health Eff Inst. 2009;142:5–90. [PubMed] [Google Scholar]
- 10.Samoli E, Stafoggia M, Rodopoulou S, Ostro B, Declercq C, Alessandrini E, Díaz J, Karanasiou A, Kelessis AG, Le Tertre A, et al. MED-PARTICLES Study Group. Associations between fine and coarse particles and mortality in Mediterranean cities: results from the MED-PARTICLES project. Environ Health Perspect. 2013;121:932–938. doi: 10.1289/ehp.1206124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stafoggia M, Samoli E, Alessandrini E, Cadum E, Ostro B, Berti G, Faustini A, Jacquemin B, Linares C, Pascal M, et al. MED-PARTICLES Study Group. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: results from the MED-PARTICLES project. Environ Health Perspect. 2013;121:1026–1033. doi: 10.1289/ehp.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samoli E, Aga E, Touloumi G, Nisiotis K, Forsberg B, Lefranc A, Pekkanen J, Wojtyniak B, Schindler C, Niciu E, et al. Short-term effects of nitrogen dioxide on mortality: an analysis within the APHEA project. Eur Respir J. 2006;27:1129–1138. doi: 10.1183/09031936.06.00143905. [DOI] [PubMed] [Google Scholar]
- 13.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio-respiratory mortality: a review. Environ Health. 2013;12:43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimakopoulou K, Samoli E, Beelen R, Raaschou-Nielsen O, Andersen ZJ, Vineis P, Xun W, Hoek G, Katsouyanni K. Effects of long-term exposure to air pollution on respiratory mortality; results of the ESCAPE Project. Environment and Health, August 19–23, 2013, Basel, Switzerland. Abstract 4495 [Google Scholar]
- 15.Raaschou-Nielsen O, Andersen ZJ, Beelen R, Samoli E, Stafoggia M, Weinmayr G, Hoffmann B, Fischer P, Nieuwenhuijsen MJ, Brunekreef B, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE) Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 16.Benetou V, Trichopoulou A, Orfanos P, Naska A, Lagiou P, Boffetta P, Trichopoulos D Greek EPIC cohort. Conformity to traditional Mediterranean diet and cancer incidence: the Greek EPIC cohort. Br J Cancer. 2008;99:191–195. doi: 10.1038/sj.bjc.6604418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, Tsai M-Y, Künzli N, Schikowski T, Marcon A, et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe: the ESCAPE project. Atmos Environ. 2013;72:10–23. [Google Scholar]
- 18.Eeftens M, Beelen R, de Hoogh K, Bellander T, Cesaroni G, Cirach M, Declercq C, Dėdelė A, Dons E, de Nazelle A, et al. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas: results of the ESCAPE project. Environ Sci Technol. 2012;46:11195–11205. doi: 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- 19.Cyrys J, Eeftens M, Heinrich J, Ampe C, Armengaud A, Beelen R, Bellander T, Beregszaszi T, Birk M, Cesaroni G. Variation of NO2 and NOx concentrations between and within 36 European study areas: results from the ESCAPE study. Atmos Environ. 2012;62:374–390. [Google Scholar]
- 20.Eeftens M, Tsai M-Y, Ampe C, Anwander B, Beelen R, Bellander T, Cesaroni G, Cirach M, Cyrys J, de Hoogh K. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2-Results of the ESCAPE project. Atmos Environ. 2012;62:303–317. [Google Scholar]
- 21.ESCAPE–European Study of Cohorts for Air Pollution Effects. ESCAPE manuals [accessed 2013 June 10]. Available from: http://www.escapeproject.eu/manuals/
- 22.Wang M, Beelen R, Basagana X, Becker T, Cesaroni G, de Hoogh K, Dedele A, Declercq C, Dimakopoulou K, Eeftens M, et al. Evaluation of land use regression models for NO2 and particulate matter in 20 European study areas: the ESCAPE project. Environ Sci Technol. 2013;47:4357–4364. doi: 10.1021/es305129t. [DOI] [PubMed] [Google Scholar]
- 23.Thiébaut AC, Bénichou J.Choice of time-scale in Cox's model analysis of epidemiologic cohort data: a simulation study Stat Med 2004. 30;23:3803–3820. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, et al. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project Lancet [online ahead of print] 6 Dec 2013; DOI: 10.1016/S0140-6736(13)62158-3 [DOI] [PubMed] [Google Scholar]
- 27.Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, Dimakopoulou K, Brunekreef B, Weinmayr G, Hoffmann B, et al. on behalf of the ESCAPE group. Long-term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts within the ESCAPE project. Epidemiology. 2013;56:1696–1704. doi: 10.1097/EDE.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 28.Abbey DE, Nishino N, McDonnell WF, Burchette RJ, Knutsen SF, Lawrence Beeson W, Yang JX. Long-term inhalable particles and other air pollutants related to mortality in nonsmokers. Am J Respir Crit Care Med. 1999;159:373–382. doi: 10.1164/ajrccm.159.2.9806020. [DOI] [PubMed] [Google Scholar]
- 29.Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183:73–78. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: extended follow-up of the Harvard Six Cities study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, Smith DF, Garcia C, Chang ET, Bernstein L. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 33.Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- 34.Nafstad P, Håheim LL, Wisløff T, Gram F, Oftedal B, Holme I, Hjermann I, Leren P. Urban air pollution and mortality in a cohort of Norwegian men. Environ Health Perspect. 2004;112:610–615. doi: 10.1289/ehp.6684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beverland IJ, Cohen GR, Heal MR, Carder M, Yap C, Robertson C, Hart CL, Agius RM. A comparison of short-term and long-term air pollution exposure associations with mortality in two cohorts in Scotland. Environ Health Perspect. 2012;120:1280–1285. doi: 10.1289/ehp.1104509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cesaroni G, Porta D, Badaloni C, Stafoggia M, Eeftens M, Meliefste K, Forastiere F. Nitrogen dioxide levels estimated from land use regression models several years apart and association with mortality in a large cohort study. Environ Health. 2012;11:48. doi: 10.1186/1476-069X-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinrich J, Thiering E, Rzehak P, Krämer U, Hochadel M, Rauchfuss KM, Gehring U, Wichmann HE. Long-term exposure to NO2 and PM10 and all-cause and cause-specific mortality in a prospective cohort of women. Occup Environ Med. 2013;70:179–186. doi: 10.1136/oemed-2012-100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dockery DW, Rich DQ, Goodman PG, Clancy L, Ohman-Strickland P, Prethibha G, Kotlov T. Boston, MA: Health Effects Institute; 2013. Effect of air pollution control on mortality and hospital admissions in Ireland. Research report 176. [PubMed] [Google Scholar]
- 39.Cao J, Yang C, Li J, Chen R, Chen B, Gu D, Kan H.Association between long-term exposure to outdoor air pollution and mortality in China: a cohort study J Hazard Mater 2011. 281861594–1600. [DOI] [PubMed] [Google Scholar]
- 40.Dong GH, Zhang P, Sun B, Zhang L, Chen X, Ma N, Yu F, Guo H, Huang H, Lee YL, et al. Long-term exposure to ambient air pollution and respiratory disease mortality in Shenyang, China: a 12-year population-based retrospective cohort study. Respiration. 2012;84:360–368. doi: 10.1159/000332930. [DOI] [PubMed] [Google Scholar]
- 41.Zanobetti A, Schwartz J, Samoli E, Gryparis A, Touloumi G, Peacock J, Anderson RH, Le Tertre A, Bobros J, Celko M, et al. The temporal pattern of respiratory and heart disease mortality in response to air pollution. Environ Health Perspect. 2003;111:1188–1193. doi: 10.1289/ehp.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eeftens M, Beelen R, Fischer P, Brunekreef B, Meliefste K, Hoek G. Stability of measured and modelled spatial contrasts in NO(2) over time. Occup Environ Med. 2011;68:765–770. doi: 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- 43.Gulliver J, de Hoogh K, Hansell A, Vienneau D.Development and back-extrapolation of NO2 land use regression models for historic exposure assessment in Great Britain Environ Sci Technol 2013. 16;477804–7811. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Henderson SB, Sbihi H, Allen RW, Brauer M. Temporal stability of land use regression models for traffic-related air pollution. Atmos Environ. 2013;64:312–319. [Google Scholar]
- 45.Jerrett M, Burnett RT, Pope CA, III, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health OrganizationGlobal surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. Geneva, Switzerland: WHO Press; 2007. NLM classification: WF 140 [Google Scholar]