Abstract
Rationale: Rifapentine has potent activity in mouse models of tuberculosis chemotherapy but its optimal dose and exposure in humans are unknown.
Objectives: We conducted a randomized, partially blinded dose-ranging study to determine tolerability, safety, and antimicrobial activity of daily rifapentine for pulmonary tuberculosis treatment.
Methods: Adults with sputum smear-positive pulmonary tuberculosis were assigned rifapentine 10, 15, or 20 mg/kg or rifampin 10 mg/kg daily for 8 weeks (intensive phase), with isoniazid, pyrazinamide, and ethambutol. The primary tolerability end point was treatment discontinuation. The primary efficacy end point was negative sputum cultures at completion of intensive phase.
Measurements and Main Results: A total of 334 participants were enrolled. At completion of intensive phase, cultures on solid media were negative in 81.3% of participants in the rifampin group versus 92.5% (P = 0.097), 89.4% (P = 0.29), and 94.7% (P = 0.049) in the rifapentine 10, 15, and 20 mg/kg groups. Liquid cultures were negative in 56.3% (rifampin group) versus 74.6% (P = 0.042), 69.7% (P = 0.16), and 82.5% (P = 0.004), respectively. Compared with the rifampin group, the proportion negative at the end of intensive phase was higher among rifapentine recipients who had high rifapentine areas under the concentration–time curve. Percentages of participants discontinuing assigned treatment for reasons other than microbiologic ineligibility were similar across groups (rifampin, 8.2%; rifapentine 10, 15, or 20 mg/kg, 3.4, 2.5, and 7.4%, respectively).
Conclusions: Daily rifapentine was well-tolerated and safe. High rifapentine exposures were associated with high levels of sputum sterilization at completion of intensive phase. Further studies are warranted to determine if regimens that deliver high rifapentine exposures can shorten treatment duration to less than 6 months.
Clinical trial registered with www.clinicaltrials.gov (NCT 00694629).
Keywords: mycobacterium, rifamycins, rifapentine, therapeutics, tuberculosis
At a Glance Commentary
Scientific Knowledge on the Subject
Rifamycins are key sterilizing agents for tuberculosis treatment, but recommended doses are at the low end of the dose–response curve. Optimizing rifamycin dose and exposure is a strategy for optimizing regimen potency and shortening duration of therapy required for cure. Rifapentine, a ring-substituted rifamycin, has potent antituberculosis activity when administered daily in animal models, but its optimal dose and exposure in humans is unknown.
What This Study Adds to the Field
In this phase 2 study, the substitution of high-dose daily rifapentine for rifampin improved the antimicrobial activity of combination chemotherapy during the intensive phase of pulmonary tuberculosis treatment, and this activity was driven by rifapentine exposure. The observed safety and tolerability, high levels of antimicrobial activity observed in the groups with the higher rifapentine exposures, magnitude of the activity differences versus rifampin, and consistency across end points and media types provide support for the evaluation of high-dose daily rifapentine-containing regimens of less than 6 months duration in phase 3 clinical trials.
An obstacle to tuberculosis (TB) control is the long treatment duration (at least 6 mo) required for cure of drug-susceptible pulmonary TB. Potent regimens of shorter duration would facilitate treatment completion and therefore improve individual and public health (1). Strategies for increasing regimen potency include development of new drugs and optimization of the use of existing drugs. Of the drugs in current use, rifamycins hold promise for shortening treatment through pharmacodynamic optimization. Rifamycins have durable sterilizing activity against Mycobacterium tuberculosis, and the currently recommended 10 mg/kg dosage of rifampin, the most commonly used rifamycin, is at the low end of the dose–response curve (2–9). Historically, selection of this rifampin dose seems to have been influenced by cost in the setting of incomplete dose-finding studies (10).
Rifapentine is a cyclopentyl ring-substituted rifamycin. Compared with rifampin, rifapentine has a longer half-life and lower minimum inhibitory concentration against M. tuberculosis (11–15). Preclinical studies using mouse models have shown that regimens containing daily rifapentine can cure TB after only 3 months of treatment (16–18). A phase 1 clinical trial showed that rifapentine doses up to 20 mg/kg administered daily were well-tolerated and safe in healthy volunteers (19). A previous phase 2 clinical trial found 10 mg/kg of daily rifapentine to be as safe as, but not more efficacious than, 10 mg/kg of daily rifampin during the first 8 weeks of combination TB chemotherapy (20). We conducted a dose-ranging clinical trial to determine the optimal dose of daily rifapentine during the first 8 weeks (intensive phase) of combination treatment for pulmonary TB. Some of the results of this trial have been reported previously in the form of an abstract (21).
Methods
This was a randomized, multicenter, partially blinded clinical trial. The primary end point was discontinuation of assigned treatment during the first 8 weeks (tolerability). The frequency and severity of adverse events were also determined (safety). Efficacy end points were sputum culture status at completion of intensive phase as assessed separately on solid and liquid culture medium, and time to stable culture conversion.
Setting, Population, and Design
Participants were enrolled at 18 sites (nine in North America, four in Africa, two in South America, two in Asia, one in Europe). Adults with suspected pulmonary TB and acid-fast bacilli detected by microscopy in a stained sputum specimen were eligible. Individuals were excluded if they had received more than 5 days of anti-TB treatment in the preceding 6 months, or had current or planned therapy in the subsequent 8 weeks with antiretroviral medications. Detailed eligibility criteria are in Table E1 of the online supplement. All participants underwent HIV testing. This study was approved by the Centers for Disease Control and Prevention and site institutional ethics review boards. Participants gave written informed consent.
Participants were randomly assigned to receive rifampin (∼10 mg/kg) or rifapentine (∼10, 15, or 20 mg/kg) administered once daily for 7 day per week, in addition to daily isoniazid, pyrazinamide, ethambutol, and pyridoxine for the intensive phase of TB treatment (8 wk). Randomization was performed centrally, in 1:1:1:1 allocation between arms, stratified by the presence of cavitation on baseline chest radiograph and by enrollment site, and restricted to limit imbalance between arms to no more than two subjects (22).
Dosages of isoniazid, rifampin, pyrazinamide, ethambutol, and pyridoxine were in accordance with published guidelines; additional details are in Table E2 (23). With respect to clinical care providers and participants, rifapentine dose was double-blinded but assignment of rifapentine versus rifampin was open-label because of different food requirements; mycobacteriology laboratory staffs were fully blinded with respect to treatment assignment. On at least 5 of 7 days per week, study medicines were administered by directly observed therapy. To increase rifapentine bioavailability, rifapentine regimens were administered within 1 hour after a high-fat meal (target, ≥28 g fat); rifampin regimens were administered mostly without food because food delays rifampin absorption (24, 25). After completing intensive phase treatment, participants continued treatment with a conventional continuation phase regimen, typically isoniazid plus rifampin for 4 additional months (23).
Information on symptoms, blood for alanine aminotransferase, bilirubin, creatinine, and complete blood count, and a sputum specimen were collected at baseline and at completion of 2, 4, 6, and 8 weeks of treatment. An additional sputum specimen was collected at Week 8. Sputa were collected monthly during continuation phase treatment unless two or more consecutive prior cultures were already negative for M. tuberculosis. At local site laboratories sputa were processed using conventional N-acetyl-l-cysteine-NaOH methods and cultured using both Lowenstein-Jensen solid media and BACTEC Mycobacterial Growth Indicator Tube (MGIT; Becton Dickinson and Co., Franklin Lakes, NJ) liquid media with the MGIT 960 system. Each laboratory used its own programmatic source for Lowenstein-Jensen media. M. tuberculosis isolates underwent drug susceptibility testing at site laboratories; confirmatory testing for all isolates was performed at a single laboratory using the indirect agar proportion method for isoniazid, rifampin, and ethambutol, and MGIT medium for pyrazinamide.
Pharmacokinetic Analysis
Pharmacokinetic sampling was performed 3–8 weeks after treatment initiation. Intensive pharmacokinetic sampling (seven samples over 24 h) was performed at designated sites with capacity to do so; for other participants, one to three samples were obtained. Plasma concentrations of rifapentine were determined by a validated high-pressure liquid chromatography method in a single laboratory (26). Population pharmacokinetic models were developed using nonlinear mixed effects modeling (NONMEM, version 7; ICON plc, Dublin, Ireland) (27, 28). Post hoc Bayesian estimates of individual areas under the concentration–time curve (AUC) were derived from the models.
Data Analysis
A “well-tolerated” regimen was prespecified as one for which the upper bound of the 90% one-sided confidence interval of the percentage of participants discontinuing treatment during intensive phase was less than 30% (twice the rate observed for the rifampin regimen in prior Tuberculosis Trials Consortium studies) (29, 30). Using the Clopper-Pearson method we calculated that a sample size of 70 microbiologically eligible participants per arm was required to assess tolerability (31). To obtain 70 microbiologically eligible participants per arm, target enrollment was 80 per arm (320 total) to account for baseline cultures that grew drug-resistant M. tuberculosis or were negative for M. tuberculosis growth; confidence intervals were constructed from observed data using the Wilson score method (32).
Culture status was considered to be negative at completion of intensive phase if neither of the two sputa collected at that time grew M. tuberculosis. Stable culture conversion was defined as having occurred at the time of collection of the first of two consecutive specimens, collected at least 2 weeks apart, that were culture-negative for M. tuberculosis, with no subsequent specimen culture-positive for M. tuberculosis. The intention-to-treat analysis group was comprised of all randomized participants and was used for tolerability and safety analyses. For efficacy analyses, a modified intention-to-treat (MITT) group included participants with growth in a baseline culture of M. tuberculosis that was susceptible to isoniazid, rifampin, and pyrazinamide. For MITT efficacy analyses of culture status at completion of intensive phase, participants with cultures that were missing or contaminated were considered as failures. For efficacy analyses a per-protocol subset of the MITT group was defined as participants who completed assigned intensive phase treatment (56 doses within 56–70 calendar days) and had an end of intensive phase culture that was evaluable (i.e., not missing or contaminated). The primary efficacy analysis was by assigned treatment group; secondary analyses were performed by dosage (in milligrams) of rifapentine administered and by rifapentine AUC tertile.
Differences in the percentage of participants found to be culture-negative at the end of intensive phase were calculated comparing each rifapentine group with the rifampin group; confidence intervals for differences between arms were constructed using the Wald method. We assessed differences in time to stable culture conversion visually by graphing the Kaplan-Meier product-limit estimates at Days 15, 29, 43, 57, 85, and 113 after start of therapy, and we compared them formally with the log-rank test extended to interval-censored data (33, 34). Calculations were performed in SAS (v 9.3; SAS Institute, Cary, NC) and R (v 2.12; R Development Core Team, Vienna, Austria).
Results
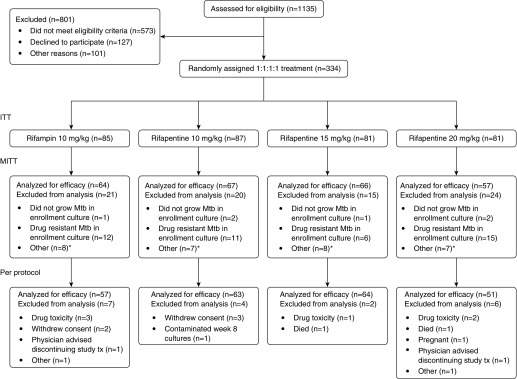
Between November 2011 and October 2012, 334 participants were enrolled (Figure 1). Table 1 shows participant characteristics at enrollment. A total of 190 of 334 (56.9%) were enrolled at African sites, 26 of 334 (7.8%) had HIV, and 257 of 334 (77.0%) had cavitation on baseline chest radiograph. By chance, the rifapentine 20 mg/kg group contained a larger percentage of HIV-infected persons (13.6%) than the other groups.
Figure 1.
Enrollment and disposition of study participants. ITT = intention-to-treat; MITT = modified intention-to-treat; Mtb = Mycobacterium tuberculosis. *See Results: Efficacy section.
Table 1.
Baseline Characteristics of Participants in the Intention-to-Treat Analysis Population
| Characteristic | Overall (n = 334) | Rifampin (n = 85) | Rifapentine 10 mg/kg (n = 87) | Rifapentine 15 mg/kg (n = 81) | Rifapentine 20 mg/kg (n = 81) |
|---|---|---|---|---|---|
| Enrolled at African site, n (%) | 190 (56.9) | 45 (52.9) | 49 (56.3) | 48 (59.3) | 48 (59.3) |
| Cavitation on chest radiograph at enrollment, n (%) | 257 (77.0) | 69 (81.2) | 67 (77.0) | 61 (75.3) | 60 (74.1) |
| Median (range) age, yr | 31 (18–78) | 33 (19–78) | 29 (19–66) | 31 (18–69) | 31 (19–70) |
| Male, n (%) | 230 (68.9) | 55 (64.7) | 63 (72.4) | 58 (71.6) | 54 (66.7) |
| History of smoking cigarettes, n (%) | 142 (42.5) | 45 (52.9) | 32 (36.8) | 30 (37.0) | 35 (43.2) |
| HIV-positive, n (%) | 26 (7.8) | 5 (5.9) | 6 (6.9) | 4 (4.9) | 11 (13.6) |
| Median (IQR) CD4 count for HIV-positive participants, cells/μl | 321 (196–429) | 277 (257–400) | 428 (415–434) | 353 (134–474) | 283 (156–414) |
| Median (IQR) # days of prestudy TB treatment | 2 (0–3) | 2 (0–4) | 2 (0–4) | 2 (0–3) | 1 (0–3) |
| Median (IQR) body mass index, kg/m2 | 19.4 (17.8–21.4) | 19.2 (17.5–21.2) | 19.1 (17.6–21.1) | 19.5 (17.9–21.5) | 19.7 (18.1–22.0) |
| Serum or plasma ALT > ULN, n (%) | 35 (10.5) | 9 (10.6) | 7 (8.1) | 11 (13.6) | 8 (9.9) |
| High sputum smear grade, n (%) | 186 (56.0) | 50 (59.5) | 47 (54.0) | 39 (48.2) | 50 (62.5) |
| Median (IQR) days to detection in MGIT culture | 6.6 (5.0–9.0) | 6.9 (5.5–8.5) | 7.0 (5.1–10.5) | 7.0 (4.8–9.3) | 6.4 (4.7–8.6) |
| Rifapentine dose in mg, n (%) | |||||
| 450 mg | — | — | 49 (56.3) | 0 (0) | 0 (0) |
| 600 mg | — | — | 37 (42.5) | 38 (46.9) | 0 (0) |
| 900 mg | — | — | 1 (1.2) | 39 (48.2) | 44 (54.3) |
| 1,200 mg | — | — | 0 (0) | 4 (4.9) | 33 (40.7) |
| 1,500 mg | — | — | 0 (0) | 0 (0) | 4 (4.9) |
Definition of abbreviations: ALT = alanine aminotransferase; IQR = interquartile range; MGIT = Mycobacterial Growth Indicator Tube; TB = tuberculosis; ULN = upper limit of normal for the testing laboratory.
Tolerability and Safety
Percentages of participants discontinuing assigned treatment during the first 8 weeks, by arm, were as follows: rifampin, 12.9% (11 of 85; upper bound of the 90% one-sided confidence interval, 19.0%); rifapentine 10 mg/kg, 5.7% (5 of 87; 10.5%); rifapentine 15 mg/kg, 6.2% (5 of 81; 11.3%); and rifapentine 20 mg/kg, 11.1% (9 of 81; 17.1%) (Table 2). None of the upper 90% confidence intervals exceeded the prespecified 30% rate for unacceptable tolerability, and 40% of all discontinuations were caused by newly demonstrated microbiologic ineligibility (i.e., absence of M. tuberculosis growth in baseline cultures or the presence of initial drug resistance). There were two deaths, one in the rifapentine 15 mg/kg group attributed to TB and one in the rifapentine 20 mg/kg group from sudden death; neither was attributed to study treatment. Discontinuation of assigned treatment because of toxicity other than death occurred in three participants in the rifampin group (two with hepatitis and one with drug allergy), one participant in the rifapentine 15 mg/kg group (grade 2 nausea), and two participants in the rifapentine 20 mg/kg group (one with hepatitis, one with drug allergy).
Table 2.
Discontinuations during the Intensive Phase of Tuberculosis Treatment, and Adverse Events within the First 70 Days after the Initial Dose of Study Drugs
| Rifampin (n = 85) | Rifapentine 10 mg/kg (n = 87) | Rifapentine 15 mg/kg (n = 81) | Rifapentine 20 mg/kg (n = 81) | |
|---|---|---|---|---|
| Regimen permanently discontinued, n (%; upper bound of 90% one-sided CI) | 11 (12.9; 19.0) | 5 (5.7; 10.5) | 5 (6.2; 11.3) | 9 (11.1; 17.1) |
| Based on microbiologic findings*, n (% of group n) | 4 (4.7) | 2 (2.2) | 3 (3.7) | 3 (3.7) |
| Based on reasons other than microbiologic findings, n (% of group n) | 7 (8.2) | 3 (3.4) | 2 (2.5) | 6 (7.4) |
| Death, n | 0 | 0 | 1† | 1‡ |
| Toxicity other than death, n | 3§ | 0 | 1|| | 2¶ |
| Withdrawal of consent, n | 2 | 3 | 0 | 0 |
| Other, n | 2 | 0 | 0 | 3 |
| Any SAE, n (%) | 3 (3.5) | 3 (3.4) | 3 (3.7) | 8 (9.9) |
| SAE attributed to study treatment, n | 2** | 1†† | 0 | 1‡‡ |
| SAE not attributed to study treatment, n | 1§§ | 2|||| | 3¶¶ | 7*** |
| Any adverse event, n (%) | 20 (23.5) | 29 (33.3) | 25 (30.9) | 26 (32.1) |
| Hepatitis, n | 2 | 1 | 3 | 2 |
| Absolute neutrophil count < 1,000 cells/mm3, n | 2 | 2 | 1 | 3 |
| Pruritis and/or rash, n | 3 | 1 | 1 | 1 |
Definition of abbreviations: CI = confidence interval by Wilson score method; SAE = serious adverse event.
Hepatitis was defined as transaminases greater than or equal to five times upper limit of normal or greater than or equal to three times upper limit of normal with symptoms, or bilirubin greater than or equal to three times upper limit of normal, or determined by site investigator to have a new diagnosis of hepatitis.
Discontinued from study regimen in response to mycobacteriology laboratory results showing, at baseline, no growth of M. tuberculosis in cultures, or growth of a drug-resistant strain of M. tuberculosis.
Death caused by hematemesis after nine doses of study medicines.
Sudden death after seven doses of study medicines.
Two participants with hepatitis, one participant with drug allergy.
Participant with grade 2 nausea.
One participant with hepatitis, one participant with drug allergy.
Hepatitis, drug allergy.
Leukocytosis.
Hepatitis.
Pleural effusion.
Gastroparesis in participant with preexisting diabetes mellitus, hemoptysis.
Pneumonia in a patient with diabetes mellitus, hematemesis with death as per footnote a, hemoptysis.
CD4 lymphopenia (<50 cells/mm3) in HIV-positive participant, pneumonia in HIV-positive participant, lung cancer, hyperglycemia in participant with preexisting diabetes mellitus, fevers, failure to thrive, sudden death after seven doses of study medicines as per footnote ‡. For the participant with lung cancer, the diagnosis was made during the intensive phase of study treatment and the participant died of lung cancer at 161 d after enrollment.
Adverse events are shown in Table 2, with additional detail in Table E3. There were eight serious adverse events in the rifapentine 20 mg/kg group and three in each of the other groups. Only one (hepatitis) of the serious adverse events in the rifapentine 20 mg/kg group was attributed to study treatment. There were no clinically significant dose-related trends in adverse events among participants who received rifapentine.
Efficacy
Among 334 enrolled participants, 50 were excluded from efficacy analyses because baseline cultures failed to grow M. tuberculosis (n = 6) or grew drug-resistant M. tuberculosis (n = 44) (Figure 1). In addition, all 30 participants from one site were excluded from efficacy analyses (but not from safety and tolerability analyses); that site had a substantial proportion of culture results that were not evaluable, mainly because of contamination, precluding interpretation of bacteriologic outcomes.
Efficacy results by assigned treatment group
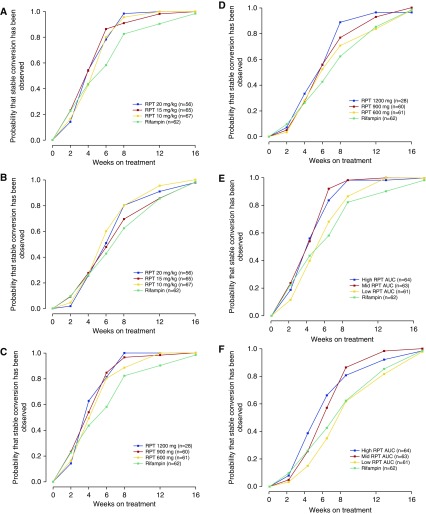
For the MITT analysis group, 81.3% (52 of 64) of participants in the rifampin group had negative cultures on solid medium at completion of intensive phase versus 92.5% (62 of 67; P = 0.097 vs. rifampin), 89.4% (59 of 66; P = 0.29 vs. rifampin), and 94.7% (54 of 57; P = 0.049 vs. rifampin) in the rifapentine 10, 15, and 20 mg/kg groups (Table 3), respectively. On liquid media, 56.3% (36 of 64) of participants in the rifampin group had negative cultures versus 74.6% (50 of 67; P = 0.042 vs. rifampin), 69.7% (46 of 66; P = 0.16 vs. rifampin), and 82.5% (47 of 57; P = 0.004 vs. rifampin) in the rifapentine 10, 15, and 20 mg/kg group, respectively. Time to stable culture conversion was significantly shorter for each of the rifapentine arms versus the rifampin arm using solid media, but there were no differences using liquid media (see Table E7 and Figures 2A and 2B). Similar trends were observed in the per-protocol analysis (see Tables E4 and E8, and Figures E1A and E1B).
Table 3.
Percentages of Participants with Negative Cultures at Completion of Intensive Phase Treatment, by Treatment Assignment, for the Modified Intention-to-Treat Analysis Group
| Rifampin | Rifapentine 10 mg/kg | Rifapentine 15 mg/kg | Rifapentine 20 mg/kg | |
|---|---|---|---|---|
| Solid culture medium | ||||
| % (n/n) with negative cultures | 81.3 (52/64) | 92.5 (62/67) | 89.4 (59/66) | 94.7 (54/57) |
| % difference vs. rifampin (95% CI) | 11.3 (−1.7 to 24.3) | 8.1 (−5.5 to 21.8) | 13.5 (0.6 to 26.3) | |
| P value | 0.097 | 0.29 | 0.049 | |
| Liquid culture medium | ||||
| % (n/n) with negative cultures | 56.3 (36/64) | 74.6 (50/67) | 69.7 (46/66) | 82.5 (47/57) |
| % difference vs. rifampin (95% CI) | 18.4 (0.8 to 35.9) | 13.4 (−4.5 to 31.4) | 26.2 (8.9 to 43.5) | |
| P value | 0.042 | 0.16 | 0.004 |
Definition of abbreviation: CI = confidence interval.
Figure 2.
Time to stable culture conversion for the modified intention-to-treat analysis group: by assigned treatment group, and as assessed using solid culture medium (P = 0.010) (A) and liquid culture medium (P = 0.32) (B); by administered rifapentine dose, as assessed using solid culture medium (P = 0.011) (C) and liquid culture medium (P = 0.38) (D); by rifapentine area under the concentration–time curve (AUC) tertile, as assessed using solid culture medium (P < 0.001) (E) and liquid culture medium (P < 0.001) (F). The x-axes are the time in study, calculated at baseline and at Days 15, 29, 43, 57, 85, and 113 (corresponding to Weeks 2, 4, 6, 8, 12, and 16, respectively). Stable culture conversion was considered to have occurred at the time a sputum specimen was obtained that was found to be negative for Mycobacterium tuberculosis in culture, with at least one additional subsequent sputum that was culture-negative and no subsequent sputa that were culture-positive for M. tuberculosis. RPT = rifapentine.
Efficacy results by administered rifapentine dose (in milligrams)
For the MITT analysis group, negative cultures on solid medium at completion of intensive phase occurred in 87.1% (54 of 62; P = 0.51 vs. rifampin), 96.7% (58 of 60; P = 0.015 vs. rifampin), and 89.7% (26 of 29; P = 0.47 vs. rifampin) of participants receiving rifapentine 600, 900, and 1,200 mg, respectively (Table 4). Negative cultures on liquid media occurred in 75.8% (47 of 62; P = 0.033 vs. rifampin), 75.0% (45 of 60; P = 0.045 vs. rifampin), and 82.8% (24 of 29; P = 0.025 vs. rifampin) of participants receiving rifapentine 600, 900, and 1,200 mg, respectively. Time to stable culture conversion on solid media was significantly shorter for the rifapentine 900-mg arm versus the rifampin arm (see Table E7 and Figures 2C and 2D). Similar trends were observed in the per-protocol analysis (see Tables E5 and E8, and Figures E1C and E1D).
Table 4.
Percentages of Participants with Negative Cultures at Completion of Intensive Phase Treatment, by Administered Rifapentine Dose, for the Modified Intention-to-Treat Analysis Group
| Rifampin | Rifapentine 600 mg | Rifapentine 900 mg | Rifapentine 1,200 mg | |
|---|---|---|---|---|
| Solid culture medium | ||||
| % (n/n) with negative cultures | 81.3 (52/64) | 87.1 (54/62) | 96.7 (58/60) | 89.7 (26/29) |
| % difference vs. rifampin (95% CI) | 5.8 (−8.4 to 20.1) | 15.4 (3.2 to 27.6) | 8.4 (−8.7 to 25.5) | |
| P value | 0.51 | 0.015 | 0.47 | |
| Liquid culture medium | ||||
| % (n/n) with negative cultures | 56.3 (36/64) | 75.8 (47/62) | 75.0 (45/60) | 82.8 (24/29) |
| % difference vs. rifampin (95% CI) | 19.6 (1.8 to 37.3) | 18.8 (0.8 to 36.7) | 26.5 (5.7 to 47.4) | |
| P value | 0.033 | 0.045 | 0.025 |
Definition of abbreviations: CI = confidence interval.
Efficacy results by rifapentine AUC tertile
For the MITT analysis group, negative cultures on solid medium at completion of intensive phase occurred in 83.9% (52 of 62; P = 0.88 vs. rifampin), 100.0% (63 of 63; P < 0.001 vs. rifampin), and 92.3% (60 of 65; P = 0.11 vs. rifampin) in the lowest, mid, and highest rifapentine AUC tertiles, respectively (Table 5). Negative cultures on liquid media occurred in 54.8% (34 of 62; P = 1.00 vs. rifampin), 90.5% (57 of 63; P < 0.001 vs. rifampin), and 80.0% (52 of 65; P = 0.007 vs. rifampin) in the lowest, mid, and highest rifapentine tertiles, respectively. Time to stable culture conversion was significantly shorter for each of the mid and high rifapentine exposure groups versus the rifampin group (see Table E7 and Figures 2E and 2F). Results for the rifapentine lowest tertile exposure group and the rifampin group were strikingly similar regardless of the end point or media type; this trend was also observed in the per-protocol analysis (see Tables E6 and E8, and Figures E1E and E1F).
Table 5.
Percentages of Participants with Negative Cultures at Completion of Intensive Phase Treatment, by Rifapentine Area under the Concentration–Time Curve Tertile, for the Modified Intention-to-Treat Analysis Group
| Rifampin | Rifapentine AUC ≤ 323 μg ⋅ h/ml | Rifapentine AUC 324–513 μg ⋅ h/ml | Rifapentine AUC > 513 μg ⋅ h/ml | |
|---|---|---|---|---|
| Solid culture medium | ||||
| % (n/n) with negative cultures | 81.3 (52/64) | 83.9 (52/62) | 100.0 (63/63) | 92.3 (60/65) |
| % difference vs. rifampin (95% CI) | 2.6 (−12.2 to 17.4) | 18.8 (7.6 to 29.9) | 11.1 (−2.0 to 24.2) | |
| P value | 0.88 | <0.001 | 0.11 | |
| Liquid culture medium | ||||
| % (n/n) with negative cultures | 56.3 (36/64) | 54.8 (34/62) | 90.5 (57/63) | 80.0 (52/65) |
| % difference vs. rifampin (95% CI) | −1.4 (−20.4 to 17.5) | 34.2 (18.5 to 50.0) | 23.8 (6.6 to 40.9) | |
| P value | 1.00 | <0.001 | 0.007 |
Definition of abbreviations: AUC = areas under the concentration–time curve; CI = confidence interval.
Discussion
Results of this dose-ranging study showed that rifapentine doses of up to 20 mg/kg once daily, administered with food to optimize rifapentine absorption and exposure, were well tolerated and safe during the first 8 weeks of combination chemotherapy for pulmonary TB. Although the study was not powered for efficacy, all rifapentine arms achieved high rates of sputum culture conversion at completion of intensive phase. Most strikingly, antimicrobial activity was strongly associated with rifapentine exposure. Among participants with higher rifapentine exposures (AUC ≥324 μg*h/ml), 80–90% had negative cultures on liquid media at the completion of intensive phase, compared with 56% in the control group in this study and 54–65% in control groups in other recent phase 2 trials conducted by our consortium in similar populations (20, 30).
Is the antimycobacterial activity observed with the higher rifapentine exposures sufficient to achieve durable cure in less than 6 months and thereby shorten the duration of treatment for drug-susceptible pulmonary TB? Previously conducted trials provide some guidance with respect to the use of the surrogate end point of proportion of participants with negative cultures on solid media at completion of intensive phase. In earlier randomized trials conducted by the British Medical Research Council, the addition of pyrazinamide to regimens including isoniazid plus rifampin increased the proportion of participants culture-negative for M. tuberculosis on solid media by an average of 12.7% (range, 7–17%). This increase correlated clinically with the ability to decrease the duration of therapy from 9 months to the current 6 months, while maintaining acceptably low relapse rates (35–41). In our study, using this surrogate end point, substituting rifapentine for rifampin (as randomized) increased the proportion of participants culture negative for M. tuberculosis by 8–13%, and the difference was 11–19% when rifapentine AUC was greater than or equal to 324 μg ⋅ h/ml. Therefore, for the higher-exposure rifapentine regimens in our study, the potency is in range for shortening treatment based on this surrogate bacteriologic end point.
With respect to selecting the optimal dose of rifapentine to use in a future phase 3 trial of treatment shortening, we first analyzed antimicrobial activity by treatment assignment (as randomized). In solid and in liquid media the percentage with negative cultures was highest in the rifapentine 20 mg/kg group, but there was no clear trend across the rifapentine arms, whether assessed using the end point of culture status at end of intensive phase or time to stable culture conversion. However, pharmacokinetic-pharmacodynamic evaluations provided important insights. Regardless of the efficacy end point, the antimicrobial activity was indistinguishable between participants with rifapentine AUC in the lowest tertile compared with participants receiving rifampin. Efficacy was substantially greater in participants with rifapentine AUC values in the second and third exposure tertiles. This suggests that the tested doses of rifapentine resulted in exposures that were on the steep part of the exposure–response curve, a situation similar to that for rifampin at the doses used clinically today (4, 8, 9, 42).
Interindividual variability in rifapentine pharmacokinetics is substantial, particularly with mg/kg, because weight does not significantly impact rifapentine clearance (27). In our trial, overlap of exposures may have obscured the relationship between antimicrobial activity and either treatment assignment or administered dose. Because all rifapentine doses in our study seemed to be safe and the limits of tolerability were not reached, the decision as to rifapentine dose to move forward into a phase 3 trial will be made based on efficacy in the context of a full pharmacokinetic-pharmacodynamic model. Fixed dosing in milligrams, not mg/kg, will be used to reduce variability in exposures and a dose will be selected that ensures that most participants reach target AUC, especially given that therapeutic drug monitoring is not feasible in most high TB burden settings.
It is worth noting that two recent phase 3 clinical trials failed to demonstrate noninferiority of daily 4-month TB regimens that substituted a fluoroquinolone for ethambutol (43, 44). Leading up to that phase 3 trial, three phase 2 studies substituting a fluoroquinolone for ethambutol had shown inconsistent results with respect to the differences (between investigational and control arms) in percentages of participants with negative cultures on solid media at completion of intensive phase, differences of 0–18% (29, 45, 46). One of these studies also incorporated liquid media MGIT cultures and reported statistically nonsignificant differences of 4–8% between investigational and control arms in percentages of participants with negative cultures on liquid media at completion of intensive phase (45). In our study the groups with the higher rifapentine exposures had very high percentages of participants with negative cultures in liquid media at completion of intensive phase (80.0–90.5%) corresponding to differences versus rifampin of 23.8–34.2%. Although liquid culture has not been well-validated for use as a surrogate marker for durable cure, the robustness of our results is encouraging. Additionally, in our study, it is reassuring that results were consistent for both culture media used, both bacteriologic end points assessed (i.e., culture status at end of intensive phase and time to stable culture conversion), and in both the MITT and per-protocol analysis groups.
A limitation of our study is that we did not investigate rifapentine doses above 20 mg/kg; the highest dose administered in our study was 1,500 mg daily. However, several lines of evidence suggest that the optimal rifapentine dose is unlikely to be substantially greater than approximately 1,200 mg daily. A study of the early bactericidal activity of rifapentine showed an apparent maximal bactericidal effect between doses of 900 and 1,200 mg (8). Preliminary pharmacodynamic modeling of our results also showed that the maximal improvement in efficacy with rifapentine substitution for rifampin was achieved at rifapentine AUC values between the medians for 900 and 1,200 mg daily doses (28). With respect to tolerability, in a phase 1 study of healthy volunteers administered daily rifapentine, five of seven (71%) participants who received 1,800 mg discontinued drug early because of toxicity (47). In our study, we administered rifapentine-containing regimens with a high-fat meal to increase bioavailability (25). We used staple foodstuffs readily available in the communities in which the trial was conducted. Whether provision of food with drug doses would be feasible in routine practice is uncertain.
To be programmatically relevant, the dose selection for rifapentine in phase 3 trials should take into account the fact that patients may or may not take their doses with food under routine conditions. HIV-positive individuals who were on antiretroviral therapy or in whom antiretroviral therapy was indicated were underrepresented, thereby limiting generalizability of our findings in this group. Ongoing work to characterize drug-drug interactions between rifapentine and antiretroviral agents may help to identify strategies for using rifapentine with certain antiretrovirals. There was no blinding with respect to rifampin versus rifapentine, and this could have had an impact on tolerability.
We conclude that the substitution of high-dose daily rifapentine for rifampin improves the antimicrobial activity of combination chemotherapy during the intensive phase of pulmonary TB treatment, and that this activity is driven by rifapentine exposure. The observed safety and tolerability, high levels of antimicrobial activity observed in the groups with the higher rifapentine exposures, magnitude of the activity differences versus rifampin, and consistency across end points and media types provide support for the evaluation of high-dose daily rifapentine-containing regimens of less than 6 month duration in phase 3 clinical trials of durable cure.
Acknowledgments
Acknowledgment
The authors thank all of the study participants who contributed their time to this study. The authors are grateful to Drs. Kenneth Castro and Phil LoBue for their continued support of the Tuberculosis Trials Consortium within the Centers for Disease Control and Prevention, to Dr. Margarita Elsa Villarino for her role in the development of this study, and to local TB program staff who assisted in the clinical management of some study participants.
Participating clinical sites (principal investigators, study coordinators, and microbiologists, with numbers of participants randomized in parentheses) were as follows:
Uganda–Case Western Reserve University Research Collaboration, Kampala (109): John L. Johnson, M.D., Roy D. Mugerwa, M.B. Ch.B., M.Med., Harriet Mayanja-Kizza, M.B. Ch.B., M.Med., Grace Muzanyi, M.B. Ch.B., Phineas Gitta, M.B. Ch.B., Alphonse Okwera, M.B. Ch.B., M.Sc., Dorcas Lamunu, B.S.N., Pheona Nsubuga, B.Pharm., Moses Joloba, M.B. Ch.B., Ph.D., Karen Morgan, M.S., M.P.H., Sam Ogwang, M.S., Yusuf Mulumba, M.S., Paul Mubiri, B.Stat., Joseph G. Nakibali, James Kimera, and Elias Ssaku.
Vietnam National TB Program–University of California, San Francisco Research Collaboration, Hanoi (48): Nguyen Viet Nhung, M.D., Ph.D., Payam Nahid, M.D., M.P.H., Dinh Ngoc Sy, M.D., Ph.D., Luu Thi Lien, M.D., Pham Huu Thuong, M.D., Nguyen Van Hung, M.D., Ph.D., Vu Cao Cuong, M.D., M.Sc., Nguyen Phuong Hoang, M.D., Ha Phan, M.D., Dr.PH., Nguyen Thi Thuy Hanh, Nguyen Luu Van Trang, and Cindy Merrifield, R.N.
Stellenbosch University, Cape Town (30): Anneke Hesseling, M.D., Ph.D., Mark Cotton, M.Med., Ph.D., Andreas Diacon, M.D., Ph.D., Norma Groenewald, Charlotte Lawn, Vera Simmonds, Zoja Noveljic, MB. Ch.B., M.Med., and Amour Venter.
KEMRI-CDC, Kisumu, Kenya (28): Kevin Cain, M.D., Kayla Laserson, Sc.D., Lena Matata, M.D., Elisha Okeyo, M.D., Victor Mudhune, B.Pharm, Albert Okumu, Jeremiah Khayumbi, and Janet Agaya.
Perinatal HIV Research Unit, Soweto (23): Richard E. Chaisson, M.D., Neil Martinson, M.B. B.Ch., M.P.H., Bernardine Friese, B.Sc. Hons., Jennifer Hoffmann, M.S.N., M.P.H., Anne Efron, M.S.N., M.P.H., and Jessica Trussler, M.B. Ch.B., M.Med.
TB and Chest Service of Hong Kong, China (19): Chi-Chiu Leung, M.B. B.S., Kwok-Chiu Chang, M.B. B.S., M.Sc., Sik-Wai Tam, Cheuk-Ming Tam, M.B. B.S., M.Sc., Sau-Yin Tam, Ka-Yun Mak, Ka-Lin Fong, Nai-Chung Lee, Chi-Wai Yip, Ph.D., Judy Yee-Man Lam, M.B. B.S., Chi-Wai Ng, M.B. B.S., Oi-Wah Fong, Edman Tin-Keung Lam, M.B. Ch.B., Chung-Ying Wong, and Ka-Ling Leung.
South Texas-Audie Murphy VA Hospital Research Collaboration, Harlingen (16): Marc H. Weiner, M.D., Richard Wing, M.D., Diane Wing, R.N., Juan Uribe, R.N., Josefina Gonzalez, L.V.N., Melissa Engle, C.R.T., C.C.R.C., Lee C. Sadkowski, M.T. (A.S.C.P.), Suzanne Salinas, M.T. (A.S.C.P.), S.M., and Mildred Manzano, A.M.T.
Brazil-Johns Hopkins University Research Collaboration, Rio de Janeiro (11): Susan Dorman, M.D., Marcus B. Conde, M.D., Anne Efron, M.S.N., M.P.H., Carla Loredo, Fernanda Mello, M.D., Rafael Duarte, Ph.D., M.D., Gisele Betzler de Oliveira Vieira, Milene Barty, and Derek Armstrong.
University of North Texas Health Science Center, Fort Worth (10): Michel Fernandez, M.D., Stephen Weis, D.O., Barbara King, R.N., Le Turk, R.N., Gloria Stevenson, Joseph Helal, R.Ph., Norma Shafer, L.V.N., Denise Dunbar, M. (A.S.C.P.), and Ken Jost, M.T. (A.S.C.P.).
Audie L. Murphy VA Hospital, San Antonio (9): Marc H. Weiner, M.D., Melissa Engle, C.R.T., C.C.R.C., Hipolito Pavon, M.P.H., Jose Jimenez, B.S., Lee C. Sadkowski, M.T. (A.S.C.P.), Suzanne Salinas, M.T. (A.S.C.P.), S.M., and Mildred Manzano, A.M.T.
Universidad Peruana Cayetano Heredia, Lima (8): Eduardo Jose Gotuzzo, M.D., F.A.C.P., Carlos Zamudio, M.D., Carlos Seas, M.D., Cinthia Hurtado, M.D., Leslie Levano, M.D., and Celer Pantoja.
Baylor College of Medicine, Houston (8): Elizabeth Guy, M.D., Richard Hamill, M.D., Ruby Nickson, R.N., and Kathleen Goodrich, M.T., M.S., S.M. (A.S.C.P.) M.
Spain TB Investigation Unit of Barcelona-University of North Texas Research Collaboration, Barcelona (7): Joan A. Cayla, M.D., Ph.D., Jose M. Miró, M.D., Ph.D., Antonio Moreno, M.D., Joan P. Millet, M.D., Ph.D., Lucía del Baño, R.N., Laia Fina, M.Sc., Llanos Roldán, R.N., Àngels Orcau, M.D., José A. Martinez M.D., Ph.D., M. Antònia Sambeat M.D., Ph.D., Virginia Pomar, M.D., Hernando Knobel, M.D., Ph.D., M. Luiza de Souza, M.D., M. Angeles Jiménez, M.D., Celia Milà, M.D., Xavier Martínez Lacasa, M.D., Adrià Curran, M.D., Ph.D., Israel Molina, M.D., Miguel Santín, M.D., Ph.D., Laura Muñoz, M.D., Fernando Alcaide, M.D., Ph.D., Raquel Moure, Ph.D., Julian Gonzalez, M.D., Ph.D., Griselda Tudo Ph.D., Mª Teresa Tórtola, M.D., Lucía González, R.N., Jessica Muñoz, R.N., Àngels Fontanet, R.N., Núria Saborit, R.N., Elisa Lara, R.N., Carmen Ligero, R.N., Neus Jové, R.N., Roser Font, R.N., Marisa Manzanares, and Adela Cantos, R.N.
Columbia University College of Physicians and Surgeons and the New York City Department of Health and Mental Hygiene Tuberculosis Control Program, New York (4): Neil W. Schluger, M.D., Joseph Burzynski, M.D., M.P.H., Vilma Lozano, R.N., Magda Wolk, R.N., and Adeleh Ebrahimzadeh, Ph.D.
University of Medicine and Dentistry of New Jersey, Newark (1): Bonita T. Mangura, M.D., Lee B. Reichman, M.D., M.P.H., Alfred A. Lardizabal, M.D., Amee Patrawalla, M.D., Maria Corazon Leus, R.N., Veronica Anokute, R.N., Michelle Burday, Ph.D., and Debra Sickles, B.S.
Denver Health and Hospitals, Denver (1): Randall Reves, M.D., William Burman, M.D., Laurie Luna, R.N, Robert Belknap, M.D., Jacquie Moore, and Ginger Hildred, M.T. (A.S.C.P.) S.M.
University of California, San Francisco (1): Payam Nahid, M.D., M.P.H., Philip Hopewell, M.D., Cindy Merrifield, R.N., Irina Rudoy, M.D., Jill Israel, R.N., and Anna Babst.
Vanderbilt University Medical Center, Nashville (1): Timothy R. Sterling, M.D., Amy Kerrigan, M.S.N., R.N., C.C.R.P., Teresa Smith, and Alicia Wright, M.S.
FHI360/Duke University/VA Durham (0): Carol Dukes Hamilton, M.D., M.H.S., Jason Stout, M.D., M.H.S., David Holland, M.D., M.H.S., Ann Mosher, R.N., M.P.H., F.N.P.-B.C., and Emily Hecker, R.N., M.S.N.
Washington, DC VA Medical Center, Prince George’s County and Montgomery County, MD TB Control Programs, District of Columbia TB Control Program, and the George Washington University Medical Center (0): Debra Benator, M.D., Donna Sepulveda Conwell, R.N., and Fred M. Gordin, M.D.
Application development was done by Jay Kim, Neelima Kandukuri, Silver Wang, M.S., Kumar Batra, P.M.P., Howard Davis, D.B.A., Jody Haney, Brandon Campbell, Max Mirabito, Pam Fedrick, and Sharon Burks, M.A.
Data management and analyses were performed by Pei-Jean Feng, M.P.H., Ruth Moro, M.D., M.P.H., Lorna Bozeman M.S., Erin Bliven-Sizemore, M.P.H., Patricia Bessler, M.P.H., and Yan Yuan, M.S.
Study drug supplies were coordinated by Patricia Bessler, M.P.H. and managed at the CDC Drug Service by Lori Hall, Pharm.D., Chris Allen, R.Ph., Cindy Doherty, Pharm.D., and Julian Jolly, Pharm.D.
Site monitoring was performed by Westat, which was represented by Betiel Haile on the protocol team.
Trial administration and data management were performed at Centers for Disease Control and Prevention by Patricia Bessler, M.P.H., Yan Yuan, M.S., Kimberley Chapman, M.P.H., Crystal Carter, Melissa Fagley, Marie Hannett, Constance Henderson, Margaret Jackson, and Beverly DeVoe Payton.
Microbiology data collection was supervised by Lorna Bozeman, M.S., with assistance from William (Kit) Whitworth, Dorothy Kaminski, Yan Yuan, M.S., Sean Toney, and Denise Hartline.
CDC laboratory testing was performed or supervised by Beverly Metchock, Dr.P.H., David Sikes, and Denise Hartline.
Independent data and safety monitoring were performed by James Neaton, Ph.D., John Kaplan, M.D., and David Ashkin, M.D.
Footnotes
Supported by the U.S. Government Division of Tuberculosis Elimination, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention. Sanofi donated rifapentine and rifampin, and since 2007 donated over $1.7 million to the Centers for Disease Control and Prevention Foundation to supplement available U.S. federal funding for rifapentine research; these funds included salary support for R.N.M.
Author Contributions: All authors contributed to the intellectual content of the manuscript and approved the manuscript version submitted for publication. S.E.D., S.G., J.E.S., N.S., G.M., J.L.J., P.N., E.J.H., C.M.H., L.B., W.M., E.L.N., A.V., M.W., and members of the Tuberculosis Trials Consortium made substantial contributions to the study concept and study design. S.G., G.M., E.J.H., L.B., P.-J.I.F., R.N.M., and the Tuberculosis Trials Consortium made substantial contributions to study implementation. S.E.D., R.M.S., S.G., J.E.S., N.S., J.L.J., P.N., C.M.H., L.B., P.-J.I.F., R.N.M., W.M., K.E.D., E.L.N., A.V., and M.W. made substantial contributions to data analysis and synthesis.
Originally Published in Press as DOI: 10.1164/rccm.201410-1843OC on December 9, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Jr, Dye C, Halloran ME. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA. 2009;106:13980–13985. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosset J. Bacteriologic basis of short-course chemotherapy for tuberculosis. Clin Chest Med. 1980;1:231–241. [PubMed] [Google Scholar]
- 3.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4:796–806. [PubMed] [Google Scholar]
- 4.Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, et al. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob Agents Chemother. 2003;47:2118–2124. doi: 10.1128/AAC.47.7.2118-2124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji B, Truffot-Pernot C, Lacroix C, Raviglione MC, O’Brien RJ, Olliaro P, Roscigno G, Grosset J. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–1546. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 6.Long MW, Snider DE, Jr, Farer LS. U.S. Public Health Service Cooperative trial of three rifampin-isoniazid regimens in treatment of pulmonary tuberculosis. Am Rev Respir Dis. 1979;119:879–894. doi: 10.1164/arrd.1979.119.6.879. [DOI] [PubMed] [Google Scholar]
- 7.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 8.Sirgel FA, Fourie PB, Donald PR, Padayatchi N, Rustomjee R, Levin J, Roscigno G, Norman J, McIlleron H, Mitchison DA. The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis. Am J Respir Crit Care Med. 2005;172:128–135. doi: 10.1164/rccm.200411-1557OC. [DOI] [PubMed] [Google Scholar]
- 9.Diacon AH, Patientia RF, Venter A, van Helden PD, Smith PJ, McIlleron H, Maritz JS, Donald PR. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob Agents Chemother. 2007;51:2994–2996. doi: 10.1128/AAC.01474-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Ingen J, Aarnoutse RE, Donald PR, Diacon AH, Dawson R, Plemper van Balen G, Gillespie SH, Boeree MJ. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin Infect Dis. 2011;52:e194–e199. doi: 10.1093/cid/cir184. [DOI] [PubMed] [Google Scholar]
- 11.Heifets LB, Lindholm-Levy PJ, Flory MA. Bactericidal activity in vitro of various rifamycins against Mycobacterium avium and Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141:626–630. doi: 10.1164/ajrccm/141.3.626. [DOI] [PubMed] [Google Scholar]
- 12.Keung A, Eller MG, McKenzie KA, Weir SJ. Single and multiple dose pharmacokinetics of rifapentine in man: part II. Int J Tuberc Lung Dis. 1999;3:437–444. [PubMed] [Google Scholar]
- 13.Langdon G, Wilkins JJ, Smith PJ, McIlleron H. Consecutive-dose pharmacokinetics of rifapentine in patients diagnosed with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2004;8:862–867. [PubMed] [Google Scholar]
- 14.Bemer-Melchior P, Bryskier A, Drugeon HB. Comparison of the in vitro activities of rifapentine and rifampicin against Mycobacterium tuberculosis complex. J Antimicrob Chemother. 2000;46:571–576. doi: 10.1093/jac/46.4.571. [DOI] [PubMed] [Google Scholar]
- 15.Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin Pharmacokinet. 2001;40:327–341. doi: 10.2165/00003088-200140050-00002. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal IM, Zhang M, Williams KN, Peloquin CA, Tyagi S, Vernon AA, Bishai WR, Chaisson RE, Grosset JH, Nuermberger EL. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal IM, Zhang M, Almeida D, Grosset JH, Nuermberger EL. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am J Respir Crit Care Med. 2008;178:989–993. doi: 10.1164/rccm.200807-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob Agents Chemother. 2012;56:4331–4340. doi: 10.1128/AAC.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, Fuchs EJ, Melia MT, Burman WJ, Dorman SE. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin Pharmacol Ther. 2012;91:881–888. doi: 10.1038/clpt.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dorman SE, Goldberg S, Stout JE, Muzanyi G, Johnson JL, Weiner M, Bozeman L, Heilig CM, Feng PJ, Moro R, et al. Tuberculosis Trials Consortium. Substitution of rifapentine for rifampin during intensive phase treatment of pulmonary tuberculosis: study 29 of the tuberculosis trials consortium. J Infect Dis. 2012;206:1030–1040. doi: 10.1093/infdis/jis461. [DOI] [PubMed] [Google Scholar]
- 21.Dorman SCDC Tuberculosis Trials ConsortiumA dose-ranging study of daily rifapentine-containing regimens for intensive phase treatment of pulmonary TB: Tuberculosis Trials Consortium Study 29X. Presented at the 44th Union World Conference on Lung Health. November 2, 2013. Paris, France. Abstract OP-230-02 [Google Scholar]
- 22.Soares JF, Wu CFJ. Some restricted randomization rules in sequential designs. Commun Stat Theory Methods. 1983;12:2017–2034. [Google Scholar]
- 23.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, et al. American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 24.Peloquin CA, Namdar R, Singleton MD, Nix DE. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest. 1999;115:12–18. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 25.Zvada SP, Van Der Walt JS, Smith PJ, Fourie PB, Roscigno G, Mitchison D, Simonsson US, McIlleron HM. Effects of four different meal types on the population pharmacokinetics of single-dose rifapentine in healthy male volunteers. Antimicrob Agents Chemother. 2010;54:3390–3394. doi: 10.1128/AAC.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiner M, Bock N, Peloquin CA, Burman WJ, Khan A, Vernon A, Zhao Z, Weis S, Sterling TR, Hayden K, et al. Tuberculosis Trials Consortium. Pharmacokinetics of rifapentine at 600, 900, and 1,200 mg during once-weekly tuberculosis therapy. Am J Respir Crit Care Med. 2004;169:1191–1197. doi: 10.1164/rccm.200311-1612OC. [DOI] [PubMed] [Google Scholar]
- 27.Savic RM, Lu Y, Bliven-Sizemore E, Weiner M, Nuermberger E, Burman W, Dorman SE, Dooley KE. Population pharmacokinetics of rifapentine and desacetyl rifapentine in healthy volunteers: nonlinearities in clearance and bioavailability. Antimicrob Agents Chemother. 2014;58:3035–3042. doi: 10.1128/AAC.01918-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savic RM, Weiner M, Mac Kenzie W.PKPD analysis of rifapentine in patients during intensive phase treatment for tuberculosis from Tuberculosis Trial Consortium Studies 29 and 29x. Presented at the 6th International Workshop on Clinical Pharmacology of Tuberculosis Drugs. September 9, 2013. Denver, CO. Abstract 11
- 29.Burman WJ, Goldberg S, Johnson JL, Muzanye G, Engle M, Mosher AW, Choudhri S, Daley CL, Munsiff SS, Zhao Z, et al. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am J Respir Crit Care Med. 2006;174:331–338. doi: 10.1164/rccm.200603-360OC. [DOI] [PubMed] [Google Scholar]
- 30.Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, Heilig CM, Bernardo J, Choudhri S, Grosset JH, et al. Tuberculosis Trials Consortium. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180:273–280. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- 31.Clopper C, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 32.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 33.Sun J. A non-parametric test for interval-censored failure time data with application to AIDS studies. Stat Med. 1996;15:1387–1395. doi: 10.1002/(SICI)1097-0258(19960715)15:13<1387::AID-SIM268>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 34.Fay MP, Shaw PA. Exact and asymptotic weighted logrank tests for interval censored data: the interval R package. J Stat Softw. 2010;36:1–34. doi: 10.18637/jss.v036.i02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.East African/British Medical Research Council. Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1972;1:1079–1085. [PubMed] [Google Scholar]
- 36.East African/British Medical Research Council. Controlled clinical trial of four short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1974;2:1100–1106. [PubMed] [Google Scholar]
- 37.East African/British Medical Research Council. Controlled clinical trial of four short-course regimens of chemotherapy for two durations in the treatment of pulmonary tuberculosis: first report: Third East African/British Medical Research Councils study. Am Rev Respir Dis. 1978;118:39–48. doi: 10.1164/arrd.1978.118.1.39. [DOI] [PubMed] [Google Scholar]
- 38.Hong Kong Chest Service/British Medical Research Council. Controlled trial of 6-month and 8-month regimens in the treatment of pulmonary tuberculosis: the results up to 24 months. Tubercle. 1979;60:201–210. doi: 10.1016/0041-3879(79)90001-1. [DOI] [PubMed] [Google Scholar]
- 39.Hong Kong Chest Service/British Medical Research Council. Controlled trial of four thrice-weekly regimens and a daily regimen all given for 6 months for pulmonary tuberculosis. Lancet. 1981;1:171–174. [PubMed] [Google Scholar]
- 40.British Thoracic Association. A controlled trial of six months chemotherapy in pulmonary tuberculosis. Second report: results during the 24 months after the end of chemotherapy. Am Rev Respir Dis. 1982;126:460–462. doi: 10.1164/arrd.1982.126.3.460. [DOI] [PubMed] [Google Scholar]
- 41.Combs DL, O’Brien RJ, Geiter LJ. USPHS Tuberculosis Short-Course Chemotherapy Trial 21: effectiveness, toxicity, and acceptability. The report of final results. Ann Intern Med. 1990;112:397–406. doi: 10.7326/0003-4819-76-3-112-6-397. [DOI] [PubMed] [Google Scholar]
- 42.Boeree M, Diacon A, Dawson R.PanACEA Consortium. What is the ‘right’ dose of rifampin? Presented at the 20th Conference on Retroviruses and Opportunistic Infections. March 3–6, 2013. Atlanta, GA. Abstract 148LB
- 43.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, Pappas F, Phillips PP, Nunn AJ REMoxTB Consortium. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med. 2014;371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, Odhiambo J, Amukoye E, Bah B, Kassa F, et al. OFLOTUB/Gatifloxacin for Tuberculosis Project. A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med. 2014;371:1588–1598. doi: 10.1056/NEJMoa1315817. [DOI] [PubMed] [Google Scholar]
- 45.Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, Reddy C, Sturm AW, Sirgel FA, Allen J, et al. Gatifloxacin for TB (OFLOTUB) study team. A phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:128–138. [PubMed] [Google Scholar]
- 46.Conde MB, Efron A, Loredo C, De Souza GR, Graça NP, Cezar MC, Ram M, Chaudhary MA, Bishai WR, Kritski AL, et al. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet. 2009;373:1183–1189. doi: 10.1016/S0140-6736(09)60333-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dooley KE, Savic R, Park J-G, Cramer Y, Hafner R, Janik J, Marzinke MA, Weiner M, Dorman S, Haas DW, et al. Safety and pharmacokinetics of novel dosing strategies to increase rifapentine exposures for TB: preliminary results from ACTG Study A5311. Presented at the 21st Conference on Retroviruses and Opportunistic Infections. March 3–6, 2014. Boston, MA. Abstract 816 [Google Scholar]