Abstract
Rationale: Although substantial scientific evidence suggests that chronic exposure to ambient air pollution contributes to premature mortality, uncertainties exist in the size and consistency of this association. Uncertainty may arise from inaccurate exposure assessment.
Objectives: To assess the associations of three types of air pollutants (fine particulate matter, ozone [O3], and nitrogen dioxide [NO2]) with the risk of mortality in a large cohort of California adults using individualized exposure assessments.
Methods: For fine particulate matter and NO2, we used land use regression models to derive predicted individualized exposure at the home address. For O3, we estimated exposure with an inverse distance weighting interpolation. Standard and multilevel Cox survival models were used to assess the association between air pollution and mortality.
Measurements and Main Results: Data for 73,711 subjects who resided in California were abstracted from the American Cancer Society Cancer Prevention II Study cohort, with baseline ascertainment of individual characteristics in 1982 and follow-up of vital status through to 2000. Exposure data were derived from government monitors. Exposure to fine particulate matter, O3, and NO2 was positively associated with ischemic heart disease mortality. NO2 (a marker for traffic pollution) and fine particulate matter were also associated with mortality from all causes combined. Only NO2 had significant positive association with lung cancer mortality.
Conclusions: Using the first individualized exposure assignments in this important cohort, we found positive associations of fine particulate matter, O3, and NO2 with mortality. The positive associations of NO2 suggest that traffic pollution relates to premature death.
Keywords: air pollution, mortality, survival analyses, GIS, spatial analyses
At a Glance Commentary
Scientific Knowledge on the Subject
Several cohort studies have examined whether long-term exposure to air pollution is associated with premature death. The results of these studies have been mixed, possibly due to errors introduced in the exposure assessment process.
What This Study Adds to the Field
To address this potential problem, this study assigned members of the American Cancer Society Cancer Prevention Study II Cohort residing in California more precise exposure assignments at their home address using advanced exposure models. The study provides the first evidence that ozone is significantly associated with cardiovascular mortality, particularly from ischemic heart disease; shows a strong association between nitrogen dioxide (NO2) and lung cancer; and demonstrates that that fine particulate matter with aerodynamic diameter of 2.5 μm or less (PM2.5) and NO2 associate independently with premature death from all causes and cardiovascular disease. The findings from this study confirm earlier evidence on PM2.5 associations with mortality and expand the evidence base markedly on associations between ozone or NO2 and premature death.
A substantial body of evidence suggests that long-term exposure to combustion-related air pollution contributes to the development of chronic disease and can lead to premature death (1–6). Exposure to air pollution affects huge populations globally. As a result, the public health impact can be large (7, 8).
Using data from the American Cancer Society’s (ACS) Cancer Prevention Study II (CPS-II), a nationwide cohort study of nearly 1.2 million adults who have been followed for mortality since 1982, several studies have been published examining associations of metropolitan-level air pollution and mortality (3, 9–11). In those studies, exposure data were derived at the metropolitan scale, relying on between-city exposure contrasts using central monitor data.
In addition, two studies using CPS-II data evaluated within-city (i.e., Los Angeles and New York) exposure contrasts in fine particulate matter with aerodynamic diameter of 2.5 μm or less (PM2.5) (2, 3). Both studies assigned exposure to the ZIP code postal area of residence, but in the study from Los Angeles (2), the PM2.5–mortality dose–response relationship was stronger than that for the full nationwide cohort, and in the study from New York City, the relationship was weaker (3). Although the ZIP code areas were more specific than the metropolitan area, they may have introduced error in the exposure assignment that led to the inconsistent results. Another recent study based on individualized exposures found little association between PM2.5 exposure and mortality in a cohort of male health professionals (12); however, in that study if home address records were missing, then workplace addresses were used for exposure assignment, possibly leading to measurement error. Conversely, an earlier study based on a large cohort of nurses reported strong and significant associations of PM2.5 with mortality, using essentially the same exposure model but with complete home address information for exposure assignment (13). Viewed together, these findings suggest that uncertainties in the characterization of the dose–response relationship may be due partly to the errors in exposure estimates arising from the lack of specificity of the coordinates used to link addresses to the exposure estimates. A need therefore exists to investigate how individualized estimates of exposure at the home address influence the observed dose–response function.
In the present analysis, individualized exposure estimates were developed and assigned to the home address for more than 73,000 California residents enrolled in CPS-II. These estimates were used to assess the association of three types of air pollutants (PM2.5, ozone [O3], and nitrogen dioxide [NO2]) with risk of mortality. We also sought to understand the joint effects of the pollutants in co- and multipollutant models. Although CPS-II is a nationwide cohort, we limited this analysis to California because the state has a wide range of pollution exposures and a good monitoring network.
Methods
The ACS CPS-II cohort was enrolled in 1982 (details are presented in References 3 and 14). For the purposes of this paper, vital status was ascertained through to 2000. Subjects with valid postal addresses had their residential locations geocoded. After limiting to residence in the State of California and making exclusions for missing data on key covariates, there were 73,711 subjects available for analysis.
We assigned exposure for PM2.5, NO2, and O3. Monthly average monitoring data for PM2.5 were available at 112 sites between 1998 and 2002. NO2 and O3 data were available over the period 1988 to 2002 at 138 and 262 sites, respectively. PM2.5 and NO2 exposures were assessed using land use regression (LUR) models that were selected from more than 70 possible land use covariates (15). The PM2.5 model included an advanced remote sensing model coupled with atmospheric modeling (16). LUR models were selected with the deletion/substitution/addition algorithm (17). The deletion/substitution/addition algorithm, which aggressively tests nearly all polynomial covariate combinations, uses v-fold cross-validation to evaluate potential models. In this instance of v-fold cross-validation, data are first partitioned into 10 roughly equal parts (i.e., folds). The model is then trained on nine folds and cross-validated on the left out fold. This is repeated 10 times so every fold is used as a cross-validation data set. The model selection method avoids the potential problems of over-fitting on all the data or on a large training set and then using a cross-validation subset (details presented References 15 and 18). For O3, we extracted monthly averaged values from 1988 to 2002 and calculated the inverse distance weighting (IDW) models with the decay parameter set to the inverse of the square of the distance from all sites within a 50-km radius of operational monitors during any particular month. Estimates for all pollutants were then assigned to geocoded baseline residential addresses of the CPS-II subjects, and the monthly values were averaged for the entire time period available.
We used a comprehensive set of individual risk factor variables operationalized through 42 covariates similar to those used in previous studies of the CPS-II cohort (3, 18). Individual-level variables controlled for lifestyle, dietary, demographic, occupational, and educational factors, and ecological variables extracted from the 1990 US Census in the ZIP code of residence were used to control for potential “contextual” neighborhood confounding (including unemployment, poverty, income inequality, and racial composition).
We assessed the association between air pollution and mortality using standard and multilevel Cox proportional hazards regression models. Control for place of residence was also applied in the five largest conurbations—defined by the four consolidated metropolitan statistical areas of California and the metropolitan statistical area of San Diego—that potentially have lower mortality rates than nonmetropolitan areas. This pattern is consistent with what has been termed the “nonmetropolitan mortality penalty,” where nonmetropolitan areas tend to have higher death rates compared with metropolitan areas (19). Because metropolitan areas generally have higher pollution, failure to control for residence in large urban areas has the potential to confound associations between mortality and air pollution.
We evaluated the association between air pollution and several causes of death, including cardiovascular disease (CVD), ischemic heart disease (IHD), stroke, respiratory disease, and lung cancer. We also evaluated “all other” causes of death, excluding the preceding causes, to serve as a negative control. Finally, we evaluated mortality from all causes combined.
Results
Table 1 compares characteristics of the nationwide CPS-II cohort used in previous analyses to the subset selected for this analysis (a detailed description of exclusions and sample selection is provided in Reference 18). Minor differences in alcohol consumption and education are apparent, but overall the California cohort appears to have characteristics similar to the nationwide cohort. Subjects included in this analysis were widely distributed across California, giving comprehensive coverage for much of the State’s population (54/58 California counties were represented).
TABLE 1.
PARTICIPANT CHARACTERISTICS IN THE NATIONWIDE STUDY COMPARED WITH THE CALIFORNIA COHORT
| Variable | Nationwide | California |
|---|---|---|
| Participants, n | 485,426 | 73,711 |
| Participants died from, % | ||
| All causes | 26.4 | 26.8 |
| CPD | 13.1 | 13.6 |
| CVD | 10.9 | 10.9 |
| IHD | 6.1 | 6.2 |
| Respiratory | 2.2 | 2.7 |
| Lung cancer | 2.0 | 2.0 |
| All other causes | 11.3 | 11.2 |
| Demographics | ||
| Mean (SD) age, yr | 56.6 (10.5) | 57.4 (10.6) |
| Female, % | 56.6 | 56.2 |
| White, % | 94.2 | 91.6 |
| Education, % | ||
| <High school | 12.1 | 8.7 |
| High school | 31.3 | 22.9 |
| >High school | 56.6 | 68.4 |
| Alcohol consumption, % | ||
| Beer | 22.9 | 24.1 |
| No beer | 9.5 | 10.9 |
| Missing beer | 67.6 | 65.0 |
| Liquor | 27.6 | 35.1 |
| No liquor | 8.7 | 8.9 |
| Missing liquor | 63.7 | 56.0 |
| Wine | 23.1 | 37.3 |
| No Wine | 8.9 | 7.7 |
| Missing wine | 68.0 | 55.0 |
| Smoking status | ||
| Current smoker, % | 21.6 | 19.4 |
| Cigarettes per day | 22.1 (12.4) | 21.5 (12.6) |
| Years of smoking | 33.5 (11.0) | 34.1 (11.4) |
| Former smoker, % | 25.9 | 28.9 |
| Cigarettes per day | 21.4 (14.7) | 20.8 (14.7) |
| Years of smoking | 22.2 (12.6) | 22.1 (12.7) |
| Age when started smoking, % | ||
| <18 yr (current smoker) | 8.9 | 7.7 |
| <18 yr (former smoker) | 10.0 | 10.3 |
| Hours per day exposed to smoking | 3.2 (4.4) | 2.7 (4.1) |
Definition of abbreviations: CPD = cardiopulmonary disease; CVD = cardiovascular disease; IHD = ischemic heart disease.
Table 2 shows the mean, variance, and percentiles of each pollutant as estimated by the different models used in this study. All models display considerable variation in the exposures assigned to the home address. Most pollutants show moderate to high positive correlations (Table 3). The exception is between interpolated ozone and NO2 estimates, which displays a weak negative correlation.
TABLE 2.
DISTRIBUTION OF AIR POLLUTANTS AT THE INDIVIDUAL LEVEL*
| Air Pollution | Subjects (n) | Mean | Variance | Percentiles |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 25 | 50 | 75 | 90 | 95 | 100 | ||||
| PM2.5 LUR, μg/m3 | 73,711 | 14.09 | 12.42 | 4.25 | 8.29 | 9.45 | 11.60 | 14.03 | 16.90 | 18.42 | 19.36 | 25.09 |
| NO2 LUR, ppb | 73,711 | 12.27 | 8.54 | 3.04 | 7.93 | 8.81 | 10.21 | 12.12 | 14.33 | 16.22 | 17.09 | 21.94 |
| Ozone IDW, ppb | 73,711 | 50.35 | 212.18 | 17.11 | 28.81 | 31.13 | 36.83 | 50.80 | 61.00 | 68.56 | 74.18 | 89.33 |
Definition of abbreviations: IDW = inverse distance weighting model; LUR = land use regression.
Years represented by air pollution exposure estimates: PM2.5 LUR, 1998–2002; NO2 LUR and ozone IDW, 1988–2002.
TABLE 3.
PEARSON CORRELATIONS (×100) BETWEEN AIR POLLUTANTS (CALIFORNIA OVERALL)
| PM2.5 LUR | NO2 LUR | |
|---|---|---|
| PM2.5 LUR | — | — |
| NO2 LUR | 55.10 | — |
| Ozone IDW | 55.81 | −0.71 |
Definition of abbreviations: IDW = inverse distance weighting model; LUR = land use regression; PM2.5 = particulate matter with aerodynamic diameter of 2.5 μm or less.
Estimates of adjusted relative risk (RR) and 95% confidence intervals (CIs) are reported in Table 4. All RR estimates are given over the interquartile range of each pollutant. We assessed residual spatial autocorrelation in the health effect estimates with a multilevel Cox model (3). Because the multilevel clustering and autocorrelation analysis had minimal impact on the risk estimates, only results for the standard Cox models are reported.
TABLE 4.
AMERICAN CANCER SOCIETY COHORT WITH FOLLOW-UP FROM 1982 TO 2000, ADJUSTING FOR 42 INDIVIDUAL-LEVEL COVARIATES, FIVE CONSOLIDATED METROPOLITAN STATISTICAL AREA CITY INDICATORS, SEVEN 1990 ECOLOGIC COVARIATES STRATIFYING THE BASELINE HAZARD FUNCTION BY AGE (1-YR GROUPINGS), GENDER, AND RACE USING THE STANDARD COX SURVIVAL MODEL
| Air Pollutant | Cause of Death |
||||||
|---|---|---|---|---|---|---|---|
| All Causes (n = 19,733) | Cardiovascular (n = 8,046) | Ischemic Heart (n = 4,540) | Stroke (n = 3,068) | Respiratory (n = 1,973) | Lung Cancer (n = 1,481) | All Others (n = 8,233) | |
| PM2.5 LUR | 1.032 (1.002–1.062)* | 1.064 (1.016–1.114) | 1.111 (1.045–1.181) | 1.065 (0.988–1.148) | 1.046 (0.953–1.148) | 1.062 (0.954–1.183) | 0.994 (0.950–1.040) |
| NO2 LUR | 1.031 (1.008–1.056) | 1.048 (1.010–1.087) | 1.066 (1.015–1.119) | 1.078 (1.016–1.145) | 0.999 (0.927–1.077) | 1.111 (1.020–1.210) | 1.009 (0.973–1.046) |
| Ozone IDW | 0.998 (0.960–1.036) | 1.045 (0.986–1.109) | 1.104 (1.021–1.194) | 1.011 (0.919–1.112) | 1.017 (0.902–1.147) | 0.861 (0.747–0.992) | 0.967 (0.911–1.027) |
Definition of abbreviations: IDW = inverse distance weighting model; LUR = land use regression; PM2.5 = particulate matter with aerodynamic diameter of 2.5 μm or less.
Relative risks are shown for the interquartile range of exposure in each pollutant (i.e., 5.3037 μg/m3 for PM2.5, 4.1167 ppb NO2, and 24.1782 ppb for O3). Values in parentheses are 95% confidence intervals.
For PM2.5 we observed significantly elevated RR for mortality from all causes (RR, 1.032; 95% CI, 1.002–1.068), CVD (RR, 1.064; 95% CI, 1.016–1.114), and IHD (RR, 1.111; 95% CI, 1.045–1.181). Deaths from stroke, respiratory causes, and lung cancer had positive RRs with less precision and CIs that included unity. No association is present with other causes.
NO2 is significantly and positively associated with all-cause (RR, 1.031; 95% CI, 1.008–1.056), CVD (RR, 1.048; 95% CI, 1.010–1.087), IHD (RR, 1.066; 95% CI, 1.015–1.119), stroke (RR, 1.078; 95% CI, 1.016–1.145), and lung cancer (RR, 1.111; 95% CI, 1.020–1.210) mortality. Respiratory deaths and those from all other causes were not associated with NO2.
Although there was no association between O3 and all-cause mortality, there was a positive association with CVD mortality (RR, 1.045; 95% CI, 0.986–1.108) and a significantly elevated risk for IHD death (RR, 1.104; 95% CI, 1.021–1.194). O3 had a positive association with stroke and respiratory deaths that lacked precision and a marginally significant negative association with deaths from lung cancer. There was no association with other causes.
We compared the risk estimates obtained from single-pollutant models with risk estimates from two-pollutant and multipollutant models (Table 5). In models that included PM2.5 and NO2, the PM2.5 associations with mortality from all causes were reduced to about half the size of those in the single pollutant models, and the estimates became insignificant. When O3 and PM2.5 were included in the same all-cause mortality model, the effects from PM2.5 remained significantly elevated and became slightly larger. A similar pattern was observed with CVD and IHD, where the effects of PM2.5 were attenuated with NO2 but remained unchanged in the presence of the O3 estimates (Figure 1).
TABLE 5.
TWO-POLLUTANT AND MULTIPOLLUTANT MODEL RESULTS FROM THE AMERICAN CANCER SOCIETY COHORT WITH FOLLOW-UP FROM 1982 TO 2000, ADJUSTING FOR 42 INDIVIDUAL-LEVEL COVARIATES, FIVE CONSOLIDATED METROPOLITAN STATISTICAL AREA CITY INDICATORS, SEVEN 1990 ECOLOGIC COVARIATES STRATIFYING THE BASELINE HAZARD FUNCTION BY AGE (1-YR GROUPINGS), GENDER, AND RACE USING THE STANDARD COX SURVIVAL MODEL
| Air Pollutant | Cause of Death |
||||||
|---|---|---|---|---|---|---|---|
| All Causes (n = 19,733) | Cardiovascular (n = 8,046) | Ischemic Heart (n = 4,540) | Stroke (n = 3,068) | Respiratory (n = 1,973) | Lung Cancer (n = 1,481) | All Others (n = 8,233) | |
| PM2.5 LUR | 1.015 (0.980–1.050)† | 1.043 (0.989–1.101) | 1.090 (1.015–1.170) | 1.019 (0.934–1.112) | 1.064 (0.954–1.185) | 0.985 (0.867–1.119) | 0.984 (0.933–1.038) |
| NO2 LUR | 1.025 (0.997–1.054) | 1.030 (0.987–1.075) | 1.029 (0.972–1.090) | 1.070 (0.998–1.147) | 0.973 (0.891–1.063) | 1.118 (1.010–1.236) | 1.016 (0.973–1.060) |
| PM2.5 LUR | 1.035 (1.004–1.067) | 1.057 (1.008–1.109) | 1.093 (1.027–1.165) | 1.067 (0.987–1.153) | 1.045 (0.949–1.151) | 1.103 (0.985–1.234) | 1.002 (0.955–1.050) |
| Ozone IDW | 0.985 (0.947–1.025) | 1.025 (0.964–1.089) | 1.070 (0.987–1.161) | 0.988 (0.894–1.091) | 1.001 (0.883–1.134) | 0.832 (0.719–0.964) | 0.966 (0.908–1.029) |
| NO2 LUR | 1.032 (1.008–1.057) | 1.055 (1.016–1.095) | 1.082 (1.029–1.137) | 1.082 (1.019–1.150) | 1.001 (0.928–1.080) | 1.097 (1.006–1.196) | 1.006 (0.970–1.043) |
| Ozone IDW | 1.006 (0.968–1.046) | 1.062 (1.000–1.127) | 1.132 (1.045–1.227) | 1.034 (0.938–1.140) | 1.017 (0.901–1.149) | 0.882 (0.764–1.019) | 0.968 (0.912–1.029) |
| PM2.5 LUR | 1.015 (0.977–1.055) | 1.024 (0.965–1.086) | 1.048 (0.969–1.133) | 1.008 (0.915–1.110) | 1.070 (0.949–1.207) | 1.040 (0.902–1.198) | 0.995 (0.938–1.056) |
| NO2 LUR | 1.025 (0.995–1.056) | 1.044 (0.996–1.093) | 1.059 (0.995–1.126) | 1.079 (1.000–1.163) | 0.969 (0.881–1.066) | 1.078 (0.967–1.201) | 1.008 (0.963–1.056) |
| Ozone IDW | 0.999 (0.957–1.042) | 1.050 (0.982–1.122) | 1.106 (1.012–1.209) | 1.031 (0.925–1.149) | 0.984 (0.860–1.126) | 0.866 (0.739–1.015) | 0.971 (0.908–1.038) |
Definition of abbreviations: IDW = inverse distance weighting model; LUR = land use regression model; PM2.5 = particulate matter with aerodynamic diameter of 2.5 μm or less.
Relative risks are shown for the interquartile range of exposure in each pollutant (i.e., 5.3037 μg/m3 for PM2.5; 4.1167 ppb NO2; and 24.1782 ppb for O3). Values in parentheses are 95% confidence intervals.
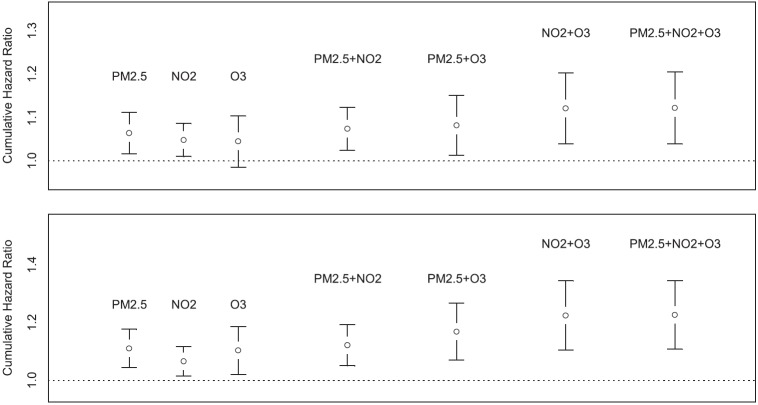
Figure 1.
Summary plot of individual and multipollutant cumulative hazard ratios. Top: Cardiovascular mortality. Bottom: Ischemic heart disease mortality.
The NO2 associations with CVD and IHD were attenuated when PM2.5 was included in the model, but they became slightly larger when O3 was included. O3 continued to show elevated risks for CVD and IHD in the two-pollutant models with either NO2 or PM2.5 included. For respiratory deaths, PM2.5 continued to have elevated but insignificant risk estimates, whereas neither of the other pollutants was associated with respiratory mortality. For lung cancer, NO2 consistently displayed significantly elevated risks in two-pollutant models. When combined with O3, PM2.5 associations with lung cancer increased but remained insignificant.
In multipollutant models containing all three pollutants, NO2 had the strongest associations with all-cause mortality and CVD and with lung cancer, whereas PM2.5 tended to have stronger effects on deaths from IHD. Intercorrelations among the various pollutants, however, likely contribute to bias in individual pollutant risk estimates in such simultaneous pollutant models, so these results must be interpreted with caution. In multipollutant models, PM2.5 continued to produce elevated risks for all-cause, CVD, IHD, and respiratory mortality, but none of these estimates were statistically significant. O3 had elevated risks on CVD and remained a significant predictor of IHD deaths even with the other pollutants in the model.
There was little evidence of associations with the other causes of death in the two-pollutant or multipollutant models.
Figure 1 presents results from cumulative risk index (CRI) models for CVD and IHD mortality that show the extent to which one pollutant confounds the others (details of the CRI methods are provided in the online supplement). Comparisons of CRI based on combinations of pollutants estimated jointly and independently can also provide a means of understanding the joint impacts of the atmospheric mixture on survival. For example, with CVD mortality, the combined hazard ratio (HR) of NO2 and O3 assuming independence is 1.048 × 1.045 = 1.095. However, the combined HR based on the two-pollutant survival model is 1.121, suggesting a synergy of effect among the pollutants. A similar pattern of synergy is also observed for IHD mortality.
Such a comparative assessment is illustrated in Figure 1 for three pollutants (NO2, O3, and PM2.5) and two causes of death (CVD and IHD). The HRs evaluated at their respective interquartile ranges for the three pollutants are presented singly, based on the three possible two-pollutant models, and based on the single three-pollutant model. There is some modest increase in the CRI for models containing PM2.5 and either NO2 or O3 compared with each of the single-pollutant models. The model with NO2 and O3, however, is larger than either of the other two-pollutant models and has a similar CRI to the three-pollutant model, suggesting that a combination of NO2 and O3 is sufficient to characterize the toxicity of the pollutant mixture in this study, at least with respect to the three pollutants considered.
The CRI implies that there is little marginal contribution to CVD and IHD mortality from the addition of PM2.5 in the presence of the mixture represented by NO2 and O3. We also caution that in this interpretation the CIs clearly overlap each of the CRIs we have calculated. This limits our ability to infer the set of minimally sufficient pollutants required to fully capture the toxicity of the atmosphere in California.
Discussion
We sought to estimate the effects of three criteria air pollutants on premature death in California. This study was motivated by earlier research from Los Angeles that showed PM2.5 exerted a large significant effect on all-cause mortality and mortality from CVD. Other studies, including those based on data from the ACS CPS-II, showed heterogeneous health effect estimates that potentially resulted from a lack of precision in the exposure assessment. To address this problem, we developed detailed exposure assessment models that included auxiliary information and assigned resulting estimates of exposure to the baseline residential address of more than 73,000 subjects with valid data from the ACS CPS-II cohort.
Several important results deserve mention. First, findings of associations of PM2.5 with all-cause and cardiovascular mortality are consistent with those reported from our previous analyses of the full, nationwide CPS-II cohort (3). Table 6 shows that results for all-cause, CVD, and IHD mortality from the current study are similar, although they are slightly weaker than from the study of the nationwide cohort. The difference in exposure metrics had little impact on the risk estimates for PM2.5. We also fit models specifically for Los Angeles to compare with earlier results (2). Although the sample size is different here due to limitations in the geocoding, the results show that the effects in Los Angeles continue to be higher than those in the national study or in the rest of the state. We also examined the dose–response function for nonlinearity because levels in Los Angeles are generally higher than in many other parts of the state, but we found no evidence of nonlinearity in the dose–response function based on visual inspection of spline plots and formal measures of model fit (Akaike information criteria and Bayesian information criteria results not shown). This suggests that the population of Los Angeles is more susceptible to air pollution, that the air pollution there is more toxic, or both.
TABLE 6.
COMPARISON OF RELATIVE RISK ESTIMATES FROM THE CALIFORNIA AND NATIONAL AMERICAN CANCER SOCIETY COHORTS FOR PM2.5 USING A 10 μg/m3 EXPOSURE INCREMENT*
| California† | National Level‡ | Los Angeles Only† | |
|---|---|---|---|
| All-cause | 1.060 (1.003–1.120)§ | 1.065 (1.035–1.096) | 1.104 (0.968–1.260) |
| CVD | 1.122 (1.030–1.223) | 1.141 (1.086–1.198) | 1.124 (0.918–1.375) |
| IHD | 1.217 (1.085–1.365) | 1.248 (1.160–1.342) | 1.385 (1.058–1.814) |
Definition of abbreviations: CVD = cardiovascular disease; IHD = ischemic heart disease; PM2.5 = particulate matter with aerodynamic diameter of 2.5 μm or less.
Models for both risk estimates control for an identical set of individual risk factors (e.g., smoking) and contextual risk factors (e.g., unemployment in area of residence) and are stratified by age, race, and sex. Results for the California cohort are additionally adjusted for place of residence in five major urban conurbations. The follow-up period for all studies was from 1982 to 2000.
California and Los Angeles use residential address with a land use regression estimate of exposure results using standard Cox model.
The national-level study uses metropolitan area of residence with the average of all fine particulate matter (PM2.5) monitors within the metropolitan area as the exposure estimate; results were determined using two-level random effects assuming no spatial autocorrelation.
Values are relative risk with 95% confidence interval in parentheses.
The strongest associations with mortality appear to be for exposures that are markers for traffic-related air pollution. The largest predictors of NO2 in the LUR model were measures of roadway length near the monitors, although we cannot rule out other contributions to the modeled concentrations, such as heating and industrial sources, particularly given the generally higher concentrations of NO2 during the winter when home heating contributes to emissions of NO2 precursors (20). This exposure measure demonstrated significant associations with all-cause, CVD, IHD, and lung cancer mortality. In multipollutant models, these associations remained elevated but became insignificant in some models, possibly due to multicollinearity among the pollutants. We also examined direct measures of proximity to roadways in earlier studies (18) and found these markers of traffic had positive coefficients, but the findings were null, suggesting that the improved exposure estimates with the LUR model may have reduced exposure measurement error.
Our results are broadly consistent with several studies from Europe in which NO2 exposure was positively associated with mortality (21, 22). In an American study of male truck drivers, NO2 was found to be independently associated with all-cause and cause-specific mortality even after controlling for occupational exposures (23). In a comprehensive review by the Health Effects Institute, effects of traffic-related pollution on mortality were identified as suggestive but insufficient to establish a causal association (24). When viewed in the context of the emerging literature, our results strengthen the evidence base on the effects of traffic-related air pollution on mortality.
Although acute exposure to O3 has been related to mortality (25), here we observed a significant positive association between long-term O3 exposure and CVD mortality, notably for IHD. The strength of association for O3 was similar to that of PM2.5 and NO2. The association of O3 with IHD was mildly confounded by PM2.5; however, the two exposures had moderately high correlation, and, given the extensive auxiliary information in the PM2.5 model, the PM2.5 estimates may have dominated by virtue of lower exposure measurement error (26). Nevertheless, O3 continued to exhibit a significant association with IHD, even with PM2.5 in the model.
Positive RR estimates for O3 became larger when NO2 was included in the model (see Figure 1). We hypothesize that this results from the negative correlation between the two pollutants due to the atmospheric chemistry, such that in areas where O3 is high, NO2 tends to be low, and vice versa (27, 28). If both pollutants represent harmful constituents of the complex mixture of ambient air pollutions, each would contaminate the comparison for calculating “clean” atmospheres when assessing the risk of the other pollutant. In such instances, the comparison groups with lower pollution levels may also have higher mortality, resulting in part from higher levels of the other pollutant that occupies the opposite spatial pattern. We found a negative, significant association between O3 and lung cancer, which became insignificant when NO2 was included in the model. These findings together suggest the importance of having both O3 and NO2 in models that attempt to predict health effects from either pollutant. We did observe a weak negative correlation between the two pollutants; however, subsequent analyses showed that in four of the five major urban regions of California, NO2 had moderately high negative correlations with O3 (details are provided in the online supplement), which supports the possibility of the positive confounding we have observed here and of the hypothesis that both pollutants need to be in the model for correct inference on either.
Unlike previous analyses (14), we did not see a significant association between respiratory disease and O3. In the present analysis, however, the number of respiratory deaths was much smaller than in the earlier national study. The point estimate here was elevated and of similar size to that reported in an earlier analysis of the nationwide cohort (3); consequently, the lack of significant association may have resulted from the lower event numbers. In contrast to earlier results, PM2.5 did have a positive association with respiratory mortality, which tended to get stronger with the inclusion of copollutants, particularly O3. In the correlational analyses done by major urban regions (see Appendix), we observed significant negative correlations between O3 and PM2.5 suggesting again the potential for positive confounding.
Several strengths and limitations merit mention. For NO2 and PM2.5, we used advanced exposure assessment models informed by auxiliary information that had good predictive capacity. These models, however, were based on government monitoring data, and the placement of the government monitoring sites might be less representative of all exposure domains because they are chosen to represent background conditions. For the most part, near-road environments are not well represented in this network, limiting the ability to predict small-area variations near roadways. Our estimates of O3 exposure likely do not capture the small area variation that can occur in open space areas and other areas away from roadways (27). Nonetheless, by assigning exposures that vary among individuals within cities, this study extends the applicability of the risk estimates to support studies that have an interest in assessing the health impacts of air pollutants within cities, which is being increasingly done to justify the health benefits of urban planning and climate mitigation interventions (29, 30).
Regarding limitations, there were no follow-up surveys conducted in the full CPS-II, and key lifestyle characteristics may have changed during the follow-up (e.g., smoking rates declined precipitously across California between 1982 and 2000) (31). If the declines in smoking rates were spatially associated with the air pollution levels, these would have the potential to confound our air pollution risk estimates. We also lacked information on mobility during the follow-up and on key microenvironments such as in-transit exposures, which contribute substantially to interindividual variability in air pollution exposures (32).
In conclusion, our results suggest that several components of the combustion-related air pollution mixture are significantly associated with increased all-cause and cause-specific mortality. Associations with CVD deaths in general and with IHD in particular stand out as most consistent in our analyses. The strong associations of NO2 with all-cause, CVD, and lung cancer mortality are suggestive of traffic-related pollution as a cause of premature death. The potential for positive confounding between O3 and NO2 requires increased attention in future research. Given the indications that O3 may relate significantly to CVD mortality, future research may lead to refined O3 exposure assessment with lower measurement error. In sum, the associations observed here reduce key uncertainties regarding the relationship between air pollution and mortality and confirm that air pollution is a significant risk factor for mortality.
Footnotes
This work was supported in part by a contract with the California Air Resources Board. Additional funding came from the Environmental Public Health Tracking Program of the Centers for Disease Control. G.T. was also supported in part by the NYU-NIEHS Center of Excellence Grant ES00260.
Author Contributions: M.J. conceived the study, led all analyses, contributed to the development of the exposure models, drafted much of the text, and responded to comments from co-author reviewers. B.S.B. ran many of the statistical models that led to the exposure assessments, conducted geographic analyses, contributed text, and assisted with interpreting the results. R.T.B. supplied expert statistical advice on the analyses, drafted sections of the paper, and assisted with the interpretation of the results. E.H. developed the statistical programs used to interpret the random effects models, helped to interpret the results, and supplied key statistical advice on the interpretation. D.K. contributed to the original grant proposal, assisted with interpretation of the results, and wrote sections of the paper. C.A.P. contributed to the statistical analyses, wrote sections of the text, and assisted with interpreting the results. S.M.G. is the Principal Investigator of the ACS CPS-II cohort and commented on the final draft of the paper. She also oversaw the geocoding process for exposure assignment. M.J.T. assisted with interpretation of the statistical models and supplied expert medical epidemiological advice on the results. G.T. assisted with the conception of the study, supplied key information on interpreting the pollution models, and commented on several drafts of the paper, which changed the interpretation of the results. M.C.T. contributed text and tables, helped to assemble supporting data, assisted with the statistical modeling, interpreted the results, and served as a liaison with the American Cancer Society for code review and data access. R.V.M. and A.v.D. contributed the remote sensing models used to derive estimates of PM2.5, supplied text, edited versions of the paper, and gave advice on atmospheric chemistry issues. Y.S. ran the statistical models, managed the data, prepared code for review by the American Cancer Society, prepared all of the tables and associated text, and assisted with the interpretation of the results.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201303-0609OC on June 27, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brook RD, Rajagopalan S, Pope CA, III, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Jerrett M, Burnett RT, Ma R, Pope CA, III, Krewski D, Newbold KB, Thurston G, Shi Y, Finkelstein N, Calle EE, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. doi: 10.1097/01.ede.0000181630.15826.7d. [DOI] [PubMed] [Google Scholar]
- 3.Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, Turner MC, Pope CA, III, Thurston G, Calle EE, et al. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality Res Rep Health Eff Inst 20091405–114.discussion 115–136 [PubMed] [Google Scholar]
- 4.Pope CA, III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 5.Pope CA, III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect. 2011;119:1616–1621. doi: 10.1289/ehp.1103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Goldberg MS, Villeneuve PJ. A systematic review of the relation between long-term exposure to ambient air pollution and chronic diseases. Rev Environ Health. 2008;23:243–297. doi: 10.1515/reveh.2008.23.4.243. [DOI] [PubMed] [Google Scholar]
- 7.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 8.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krewski D, Burnett RT, Goldberg MS, Hoover K, Siemiatycki J, Abrahamowicz M, White WH.Part I: Replication and validation. In: Reanalysis of the Harvard Six Cities Study and the American Cancer Society Study of particulate air pollution and mortality: a special report of the Institute's Particle Epidemiology Reanalysis ProjectCambridge, MA: Health Effects Institute20001–295. [Google Scholar]
- 10.Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope CA, III, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 12.Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119:384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, Speizer FE, Laden F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses’ Health Study. Environ Health Perspect. 2009;117:1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jerrett M, Burnett RT, Pope CA, III, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. N Engl J Med. 2009;360:1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckerman BS, Jerrett M, Martin RV, van Donkelaar A, Ross Z, Burnett RT. Application of the deletion/substitution/addition algorithm to selecting land use regression models for interpolating air pollution measurements. Atmos Environ. 2013;77:172–177. [Google Scholar]
- 16.van Donkelaar A, Martin RV, Brauer M, Kahn R, Levy R, Verduzco C, Villeneuve PJ. Global estimates of ambient fine particulate matter concentrations from satellite-based aerosol optical depth: development and application. Environ Health Perspect. 2010;118:847–855. doi: 10.1289/ehp.0901623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinisi SE, van der Laan MJ.Deletion/substitution/addition algorithm in learning with applications in genomics Stat Appl Genet Mol Biol 20043Article18 [DOI] [PubMed] [Google Scholar]
- 18.Jerrett M, Burnett RT, Pope A, III, Krewski D, Thurston G, Christakos G, Hughes E, Ross Z, Shi Y, Thun M, et al. Sacramento, CA: California Air Resources Board; 2011. Spatiotemporal analysis of air pollution and mortality in California based on the American Cancer Society Cohort: final report. [Google Scholar]
- 19.Cosby AG, Neaves TT, Cossman RE, Cossman JS, James WL, Feierabend N, Mirvis DM, Jones CA, Farrigan T. Preliminary evidence for an emerging nonmetropolitan mortality penalty in the United States. Am J Public Health. 2008;98:1470–1472. doi: 10.2105/AJPH.2007.123778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spengler J, Schwab M, Ryan PB, Colome S, Wilson AL, Billick I, Becker E. Personal exposure to nitrogen dioxide in the Los Angeles Basin. Air Waste. 1994;44:39–47. doi: 10.1080/1073161x.1994.10467236. [DOI] [PubMed] [Google Scholar]
- 21.Brunekreef B. Health effects of air pollution observed in cohort studies in Europe. J Expo Sci Environ Epidemiol. 2007;17:S61–S65. doi: 10.1038/sj.jes.7500628. [DOI] [PubMed] [Google Scholar]
- 22.Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, Forastiere F. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121:324–331. doi: 10.1289/ehp.1205862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long-term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183:73–78. doi: 10.1164/rccm.200912-1903OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Health Effects Institute Panel on the Health Effects of Traffic-Related Air Pollution. Traffic-related air pollution: a critical review of the literature on emissions, exposure and health effects. Special report 17. Boston, MA: HEI; 2009
- 25.Henrotin JB, Zeller M, Lorgis L, Cottin Y, Giroud M, Béjot Y. Evidence of the role of short-term exposure to ozone on ischaemic cerebral and cardiac events: the Dijon Vascular Project (DIVA) Heart. 2010;96:1990–1996. doi: 10.1136/hrt.2010.200337. [DOI] [PubMed] [Google Scholar]
- 26.Zidek JV, Wong H, Le ND, Burnett R. Causality, measurement error and multicollinearity in epidemiology. Environmetrics. 1996;7:441–451. [Google Scholar]
- 27.Beckerman B, Jerrett M, Brook JR, Verma DK, Arain MA, Finkelstein MM. Correlation of nitrogen dioxide with other traffic pollutants near a major expressway. Atmos Environ. 2008;42:275–290. [Google Scholar]
- 28.McConnell R, Berhane K, Yao L, Lurmann FW, Avol E, Peters JM. Predicting residential ozone deficits from nearby traffic. Sci Total Environ. 2006;363:166–174. doi: 10.1016/j.scitotenv.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 29.Woodcock J, Edwards P, Tonne C, Armstrong BG, Ashiru O, Banister D, Beevers S, Chalabi Z, Chowdhury Z, Cohen A, et al. Public health benefits of strategies to reduce greenhouse-gas emissions: urban land transport. Lancet. 2009;374:1930–1943. doi: 10.1016/S0140-6736(09)61714-1. [DOI] [PubMed] [Google Scholar]
- 30.Rojas-Rueda D, de Nazelle A, Tainio M, Nieuwenhuijsen MJ. The health risks and benefits of cycling in urban environments compared with car use: health impact assessment study. BMJ. 2011;343:d4521. doi: 10.1136/bmj.d4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.California Department of Public Health California Tobacco Control ProgramSmoking prevalence among California adults, 1984-2010 [prepared 2011 Apr; accessed 2012 Sep 11]. Available from: http://www.cdph.ca.gov/Pages/NR11-031SmokingChart.aspx
- 32.de Nazelle A, Nieuwenhuijsen MJ, Antó JM, Brauer M, Briggs D, Braun-Fahrlander C, Cavill N, Cooper AR, Desqueyroux H, Fruin S, et al. Improving health through policies that promote active travel: a review of evidence to support integrated health impact assessment. Environ Int. 2011;37:766–777. doi: 10.1016/j.envint.2011.02.003. [DOI] [PubMed] [Google Scholar]