Abstract
The diffuse cystic lung diseases have a broad differential diagnosis. A wide variety of pathophysiological processes spanning the spectrum from airway obstruction to lung remodeling can lead to multifocal cyst development in the lung. Although lymphangioleiomyomatosis and pulmonary Langerhans cell histiocytosis are perhaps more frequently seen in the clinic, disorders such as Birt-Hogg-Dubé syndrome, lymphocytic interstitial pneumonia, follicular bronchiolitis, and light-chain deposition disease are increasingly being recognized. Obtaining an accurate diagnosis can be challenging, and management approaches are highly disease dependent. Unique imaging features, genetic tests, serum studies, and clinical features provide invaluable clues that help clinicians distinguish among the various etiologies, but biopsy is often required for definitive diagnosis. In part II of this review, we present an overview of the diffuse cystic lung diseases caused by lymphoproliferative disorders, genetic mutations, or aberrant lung development and provide an approach to aid in their diagnosis and management.
Keywords: Birt-Hogg-Dubé syndrome, Sjögren syndrome, high-resolution computed tomography, lymphoid interstitial pneumonia, follicular bronchiolitis
The diffuse cystic lung diseases (DCLDs) encompass a broad set of disorders characterized by the formation of multiple thin-walled parenchymal lucencies. In the first part of this review, we proposed a classification for DCLDs and discussed neoplastic, infectious, smoking-related, and interstitial lung disease–related etiologies, with a focus on lymphangioleiomyomatosis (LAM) and pulmonary Langerhans cell histiocytosis (PLCH). In part two, we describe DCLDs with genetic, developmental, lymphoproliferative, and other etiologies. We conclude by discussing the mechanisms of pulmonary cyst formation and the radiological and pathological evaluation of cystic lung disease and present an approach to the diagnosis and management of the DCLDs.
Cystic Lung Diseases Associated with Genetic Mutations
Birt-Hogg-Dubé Syndrome
Birt-Hogg-Dubé syndrome (BHD) is a rare, autosomal-dominant disorder characterized by the development of hair follicle tumors, renal neoplasms, and pulmonary cysts. Cystic lung lesions from BHD are typically seen in the fourth to fifth decade of life (1, 2) but have been described in teenagers and octogenarians (3). By 50 years of age, there is greater than 80% penetrance of pulmonary cysts in affected subjects (4). There is limited information regarding the development or profusion of cysts in younger age groups. Pneumothorax in BHD has no sex predilection and has been reported in the absence of radiographic evidence of cysts on high-resolution computed tomography (HRCT) of the chest (5).
The presence of pulmonary cysts predisposes patients with BHD to the development of pneumothoraces. Although present in the majority of the adult patients with BHD, only 24% of patients with lung cysts ever develop a pneumothorax, albeit with a very high (75%) recurrence rate (4). The incidence of pneumothorax in patients with BHD is 32-fold higher than the general population, extending up to 50-fold higher after adjusting for age (6).
Pathogenesis
BHD is caused by mutations in the folliculin (FLCN) gene encoding the tumor suppressor protein, folliculin. FLCN mutations are believed to cause dysregulation in mechanistic target of rapamycin (mTOR) signaling, although it remains unclear whether the dominant effect of the mutations is mTOR suppression or activation (7, 8). Other pathways have also been implicated in the tumor-suppressive actions of FLCN, including transforming growth factor-β signaling (9) and the “differentially expressed in normal cells and neoplasia” (DENN) proteins that control intracellular trafficking (9, 10). The role of FLCN in the maintenance of pulmonary structural integrity is not well established, but possible mechanisms of cyst formation include activation of the mTOR pathway (11), leading to cell dropout or adhesion protein defects or deficiencies that increase the vulnerability of the alveolar-septal junction to tearing by mechanical forces during the respiratory cycle (12).
Pathology
The primary differentiating radiological feature of cysts in BHD is the predominantly basilar location, which contrasts with the largely apical location of dilated airspaces and blebs in emphysema and primary spontaneous pneumothorax (13). Characteristic HRCT findings include round to lentiform-shaped, thin-walled pulmonary cysts of various sizes, distributed in the basilar and subpleural regions of the lung (Figure 1A) (14, 15). In the few available pathological descriptions of BHD cysts, the histological features have generally been found to be indistinguishable from those of emphysema. Recently, however, in a study of 229 cysts from 50 patients with BHD, the majority of the cysts (88%) abutted the interlobular septa, and 14% contained intracystic structures composed of interlobular septa (12). The cysts were surrounded by normal lung parenchyma in all patients and lacked evidence of neoplastic cell proliferations or significant inflammation (Figure 1B) (12). In many cases the cysts did not appear to communicate with the airway or were subtended by a very small airway (12). With deeper understanding of the molecular pathways involved, it is possible that distinctive morphological and immunohistochemical characteristics of BHD will emerge and enable the expert pathologist to distinguish BHD pathology from emphysema.
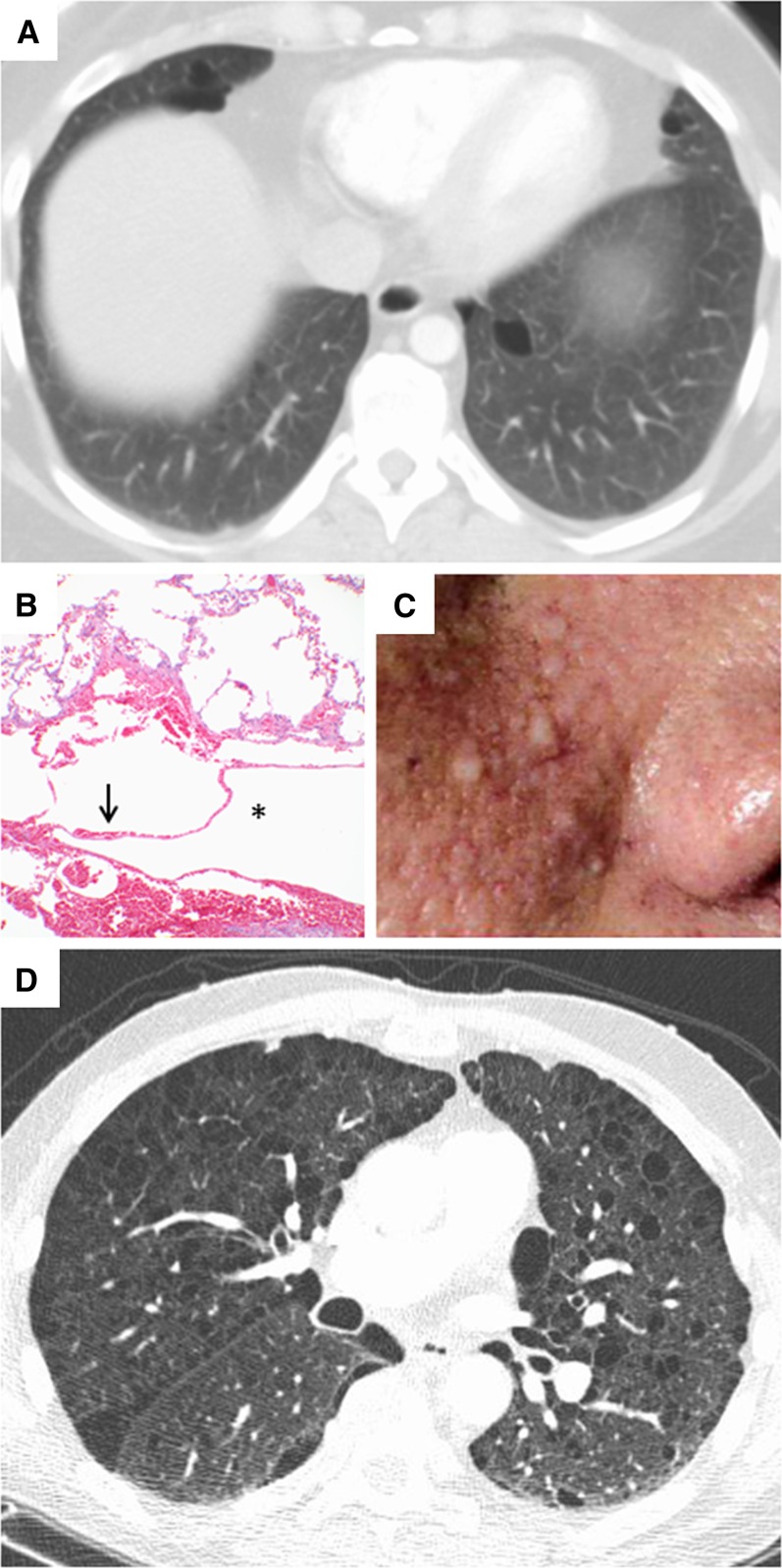
Figure 1.
Computed tomography (CT) and histopathologic images of diffuse cystic lung disease associated with genetic mutations. (A) Chest CT showing lentiform cysts in a basilar distribution in a patient with Birt-Hogg-Dubé (BHD) syndrome. (B) Histologic sections of a lung biopsy from a patient with BHD syndrome showing intraparenchymal cysts (*) surrounded by normal parenchyma with an intracystic septum (arrow) and lacking abnormal cellular proliferations or a significant fibroinflammatory component. Original magnification, ×10. (C) Facial photograph of a patient with BHD syndrome showing multiple dome-shaped, whitish papules consistent with fibrofolliculomas in the nasolabial fold area. (D) Chest CT showing multiple thin-walled cysts and paraseptal emphysema in a patient with neurofibromatosis (courtesy of Dr. Cristopher Meyer, University of Wisconsin, Madison, WI).
Diagnostic approach
The presence of spontaneous pneumothorax in a young patient, especially with a personal or family history of pneumothorax, skin lesions, or renal tumors, should prompt an evaluation for BHD. The prevalence of BHD among young patients presenting to the emergency ward with pneumothorax may be as high as 5 to 10%, based on studies from China and Holland (16, 17). A detailed skin examination should be performed, as many patients with BHD will have characteristic skin fibrofolliculomas (Figure 1C) or trichodiscomas (4). When performing skin biopsies, care should be taken to avoid shallow sampling. Punch biopsies, rather than shave biopsies, should be performed (18), and tissues from multiple locations may be required to establish the correct diagnosis (19).
Chest HRCT is the most useful modality for the initial evaluation of a patient with suspected pulmonary BHD but is not usually sufficient to establish the diagnosis with certainty. Pulmonary cysts occur with 80 to 100% penetrance in BHD (4, 6, 11) and are commonly confused with blebs or bullae and attributed to emphysema. The predominantly basilar and paraseptal location of BHD-associated cysts, however, differs from the apical-predominant distribution of dilated airspaces that typically occur in emphysema.
Renal cancer is the most threatening manifestation of BHD, seen in more than 25% of patients, with a mean age at presentation of 50.4 years (range, 31–74 yr) (20). Chromophobe adenomas and oncocytomas are the most common renal neoplasms in BHD and are bilateral and multifocal in more than half of patients (20). Other histologies, including clear cell, papillary, and mixed pattern carcinomas, can also be seen (20–22). BHD should be strongly suspected in patients presenting with early-onset (<50 yr) multifocal or bilateral renal cancer or renal cancer of mixed chromophobe and oncocytic histology (19).
It is important to note that not all patients with pulmonary manifestations of BHD have characteristic skin and/or renal findings or a positive family history. Multiple studies have revealed FLCN gene mutations in patients presenting with pulmonary cysts and/or pneumothoraces without any skin or renal involvement (2, 23, 24). Conversely, pneumothorax has been reported in BHD in the absence of visible cysts on CT (5).
Genetic testing for FLCN mutations should be offered to patients suspected to have BHD to confirm the diagnosis and to facilitate screening of family members. We recommend referral to a genetic counselor before BHD screening, for discussion of issues of insurability, employability, and anxiety associated with genetic disease testing. Given the high penetrance of pulmonary cysts and renal tumors in BHD (4, 11), we recommend that even asymptomatic patients with genetically confirmed BHD undergo imaging to evaluate for potential lung and kidney involvement. Our group and others have previously proposed algorithms for establishing a diagnosis of BHD (18, 19).
Management
The rate of progression of lung disease in BHD is incompletely understood, but it seems clear that BHD cystic lung disease does not typically result in respiratory failure. In a small cross-sectional study of patients with BHD, pulmonary function tests revealed preserved spirometric values along with a mild reduction in diffusion capacity for carbon monoxide (DlCO) (14). Further studies are needed to evaluate the natural history of lung function decline in patients with BHD.
Patients with BHD with a sentinel pneumothorax have a high (75%) recurrence rate (4), and pleurodesis should be considered with the first event to avoid morbidity associated with repeated episodes (18). It seems prudent to discourage patients from diving due to the risk of pneumothorax secondary to expansion of cysts associated with hydraulic pressure fluxes (18). Air travel is generally considered safe in patients with diffuse cystic lung diseases (25), but delayed pneumothorax after flying has recently been reported in patients with BHD (26). Patients with extensive cystic change, history of prior pneumothoraces, or new/worsening symptoms of chest pain or dyspnea may require evaluation by a pulmonologist before air travel (18). Use of tobacco should be discouraged (18), although no clear relationship between smoking history and development of pneumothoraces or lung function decline has been reported in patients with BHD (4).
Once the diagnosis of BHD is established, patients should be screened for the presence of renal tumors. The age of initiation of screening and the best imaging modality for this purpose is not clear. Because the earliest renal tumors detected in BHD occur in young adulthood, it is recommended to begin screening at about 20 years of age (19, 27). Ultrasonography suffers from a lack of sensitivity for detecting small lesions (28). The cumulative radiation exposure from repeated CT scans through life can become prohibitive; thus, magnetic resonance imaging may be the best modality for serial screening of renal tumors (27). In the absence of any abnormality on the initial scan, a typical repeat imaging interval is 3 years (27). Fortunately, renal cancer associated with BHD is indolent, and development of metastases is rare with proper monitoring (19). However, the risk of metastasis increases with tumor size, and nephron-sparing resection is recommended for tumors larger than 3 cm (19, 27).
Other Genetic Etiologies of DCLD
Genetic connective tissue disorders such as neurofibromatosis, Ehlers-Danlos syndrome, and Proteus syndrome can produce DCLD patterns on HRCT. Numerous, upper lobe–predominant cysts have been described in neurofibromatosis (Figure 1D) (29), most commonly in smokers (30). Multiple parenchymal cysts and cavitary lesions have rarely been reported in cases of Ehlers-Danlos syndrome (31, 32). The hallmark of Proteus syndrome is vascular malformations and postnatal asymmetric overgrowth of connective tissues, but emphysematous and cystic pulmonary changes have also been described (33–35). Recent literature has shown an association between activating mutations in AKT1 and the Proteus syndrome (36).
Cystic Lung Diseases Associated with Aberrant Lung Development and Growth
Congenital pulmonary airway malformation (CPAM), formerly known as congenital cystic adenomatoid malformation, can present as a DCLD in childhood (37) or, more rarely, in adulthood (38). CPAM has been further categorized into subtypes based on the size and location of the cysts as well as other associated congenital abnormalities (39). Reports of various malignancies developing within CPAMs lead many experts to recommend resection (40).
Bronchopulmonary dysplasia, the most frequent cause of cystic lung disease in children (41), was initially described in 1967 as a manifestation of oxygen toxicity after recovery from severe respiratory distress syndrome (42). It is now recognized as a chronic lung disease caused by disruption in distal lung growth in association with barotrauma induced by mechanical ventilation in premature infants. The clinical presentation of bronchopulmonary dysplasia can be subtle in exposed and at-risk older children and young adults who do not manifest obvious underlying lung disease and/or the need for supplemental oxygen (43). Cystic changes in bronchopulmonary dysplasia can be seen within a few days of birth and are usually bilateral (41).
Other rare congenital causes of cystic lung disease include congenital lobar emphysema, congenital bulla, congenital bronchiectasis, and bronchial atresia (44).
Cystic Lung Diseases Associated with Lymphoproliferative Disorders
Lymphocytic Interstitial Pneumonia/Follicular Bronchiolitis and Sjögren Syndrome
Lymphocytic interstitial pneumonia (LIP) is a clinicopathologic term that describes diffuse involvement of lung parenchyma by reactive pulmonary lymphoid tissue (45). Follicular bronchiolitis (FB) refers to a pattern of lymphoid follicular hyperplasia centered on airways, vessels, and interlobular septa consistent with a lymphatic distribution (46). FB and LIP can be idiopathic or associated with a variety of underlying conditions, most commonly autoimmune disorders like Sjögren syndrome (SS), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), or immunodeficiency states such as HIV and common variable immune deficiency (47). SS is a chronic inflammatory autoimmune exocrinopathy that can occur in isolation (primary SS) or in combination with other rheumatologic conditions, such as rheumatoid arthritis, SLE, and systemic sclerosis (secondary SS) (48). Among the connective tissue disorders, SS is most commonly associated with LIP/FB.
Pathogenesis
FB and LIP represent a pathophysiological continuum of lymphocytic infiltration from hyperplasia of bronchus-associated lymphoid tissue to cellular expansion of the interstitium with fibrosis. Cysts in FB/LIP may result from ischemia due to vascular obstruction, postobstructive bronchiolar ectasia, or bronchiolar compression by lymphoid tissues resulting in subsegmental overinflation due to a check-valve mechanism (49).
Pathology
LIP is characterized by the presence of dense interstitial lymphocytic infiltrates composed of small lymphocytes admixed with variable numbers of plasma cells. Nodular lymphoid aggregates with reactive germinal centers are present in up to 50% of patients with the idiopathic and connective tissue–associated forms of LIP but are less common in patients with HIV. The lymphocytic infiltrate is composed of a mixture of B and T cells, with the B cells often localized to nodular lymphoid follicles and T cells most prominent in the interstitium. Occasional nonnecrotizing granulomas may be seen, but they are usually inconspicuous (46).
Histologically, FB is characterized by numerous peribronchial and peribronchiolar lymphoid follicles with reactive germinal centers (Figures 2A and 2B). An accompanying interstitial inflammatory infiltrate is present in approximately 20% of cases that show overlapping LIP and FB patterns (50).
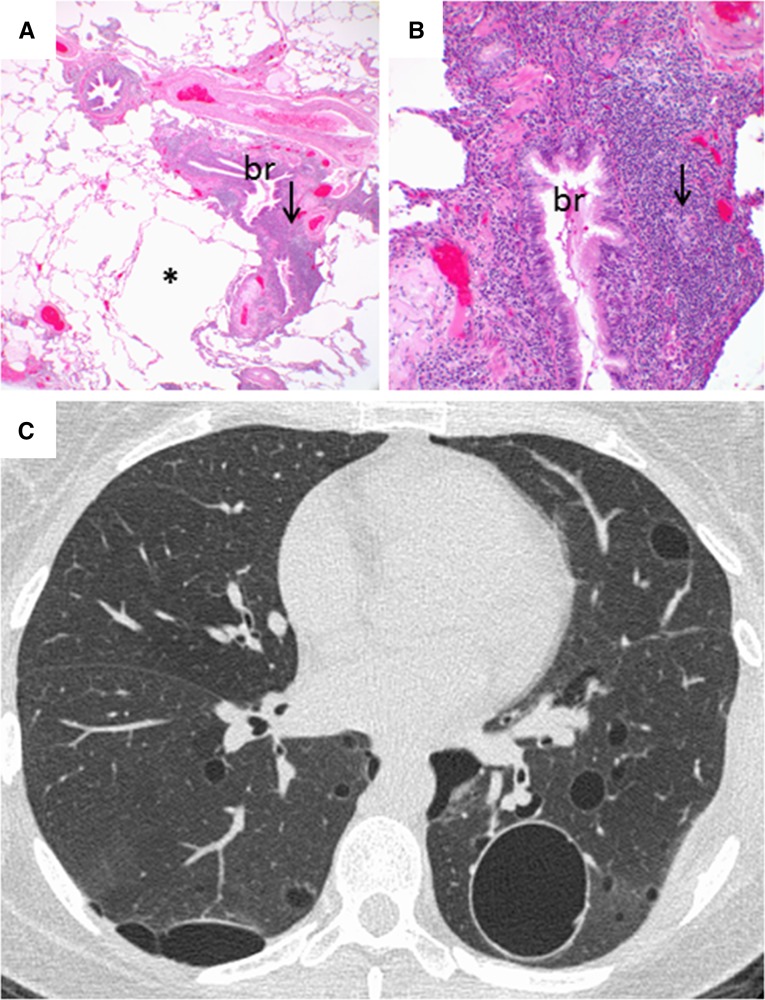
Figure 2.
Computed tomography and histopathologic images of follicular bronchiolitis. (A) Histologic sections showing parenchymal cysts (*) associated with follicular lymphoid hyperplasia (arrow) centered around bronchioles (br). Original magnification, ×2. (B) Higher-power view of histologic image in A showing follicular hyperplasia (arrow) surrounding the bronchiole (br). Original magnification, ×20. (C) Chest computed tomography showing multiple cysts of varying sizes associated with minimal ground-glass opacification.
Diagnostic approach
Interstitial lung disease is frequently observed in patients with SS, and patterns of involvement include FB, LIP, nonspecific interstitial pneumonia, amyloidosis, and lymphoproliferative disorders including lymphoma. Multiple studies have attempted to better define the radiologic abnormalities of pulmonary involvement in SS (48, 51–53), and diffuse cystic lung disease has been noted in varying proportions. In patients with SS, HRCT abnormalities have been identified in 58 to 90% of patients, with cystic changes seen in 12 to 46% (48, 51, 52). The cysts associated with SS tend to be random in distribution, contain internal structure (which is rare in LAM and PLCH), and are often bordered by an eccentric vessel (Figure 2C). The average cyst size is about 16 mm (range, 3–52 mm) (52). Cystic lung disease in the presence of SS can also be associated with amyloidosis (54–56).
LIP/FB can present with various pulmonary manifestations, including ground-glass opacification, centrilobular nodules, and cystic change. Johkoh and colleagues studied the CT findings in 22 patients with LIP (57). Although the predominant abnormalities in all patients consisted of ground-glass attenuation and poorly defined centrilobular nodules, cystic changes were observed in 68% (15/22) of patients, with an average cyst size of 6.4 mm (range, 1–30 mm). Cysts were bilateral in 10 patients and unilateral in 5 patients and had a random distribution involving less than 10% of the lung parenchyma (57). Honda and colleagues found that cysts were present in 82% (14/17) of patients with LIP and only 2% (1/44) of patients with lymphoma by HRCT scanning, suggesting that the presence of cysts may help differentiate LIP from lymphoma (58). More studies are needed, however, to confirm this finding. Pulmonary function tests reveal restrictive physiology with reduced diffusion capacity in the majority of LIP cases (47, 59), whereas FB is characterized by an obstructive pattern often associated with evidence of air trapping and reduction in DlCO (60).
Tissue confirmation is often required to establish the diagnosis of LIP/FB, although patterns of cystic change in patients with SLE, SS, or other autoimmune diseases can be sufficiently characteristic to obviate the need for biopsy (given the lack of a proven intervention for cystic destruction). The diagnostic yield of transbronchial lung biopsy in LIP/FB is low, and surgical lung biopsy is the procedure of choice to establish pathologic diagnosis (47). Careful examination of the biopsy specimen is needed to exclude the presence of low-grade malignant lymphoproliferative disorders, such as mucosal-associated lymphoid tissue (MALT) lymphoma (49). Immunohistochemical studies and flow cytometry performed on the lung tissue can help distinguish between the polyclonal populations of lymphocytes seen in LIP and a monotypic cell population seen in malignant lymphoproliferative disorders (49). A thorough evaluation for an underlying systemic condition, especially autoimmune and immunodeficiency states, should be undertaken after a histopathological diagnosis of LIP is established (49).
Management
The natural history of lung function decline in cystic lung disease due to LIP or FB is poorly understood. Improvement or disease stabilization with immunosuppressive regimens (corticosteroids, either alone or in combination with steroid-sparing agents) has been reported in LIP (47, 61). Steroid regimens for treatment of interstitial changes due to LIP have been proposed (49). Successful treatment, sometimes with near-complete clinical and radiological resolution of LIP, has been reported with the use of antiretroviral drugs in cases of LIP secondary to HIV (61). Unfortunately, the benefits of treatment for the cystic changes of LIP and FB are unclear. Johkoh and colleagues reported 14 patients with LIP followed for a median of 13 months (range, 4–82 mo) including 10 with cystic lung disease, 9 of whom developed new cysts (62) despite improvement in interstitial abnormalities.
Cystic lung disease in the presence of SS may be associated with low-grade malignancies, such as MALT lymphoma (63). In general, malignant transformation to lymphoma from LIP is a rare phenomenon (45). However, clinicians should consider lung biopsy of consolidated or ground-glass opacities that may represent a lymphoproliferative disorder, especially when there is evidence of increasing lesion size. We recommend close monitoring with repeat imaging in patients with SS and cystic lung disease, but further studies are needed to determine the optimal interval and duration of follow up. The prognosis of patients with interstitial disease due to LIP is variable, and reported median survival times range from 5 years (49) to 11.5 years (47), but there are no survival data available for patients where cystic change is the predominant abnormality.
Amyloidosis
Amyloidosis refers to a heterogenous group of disorders characterized by extracellular deposition of proteins in an abnormal fibrillary fashion. It can occur as a systemic disease or as a localized lesion affecting one particular organ. Localized pulmonary amyloidosis typically presents with multiple pulmonary nodules that may cavitate. Rarely, pulmonary amyloidosis can present as diffuse cystic lung disease (Figure 3A) (64), either alone or in the presence of coexisting SS (65). Baqir and colleagues reported eight cases of primary SS–associated amyloidosis, with six patients demonstrating multiple, asymmetric bilateral cysts of varying sizes along with nodules of varying attenuation (63). Amyloidosis was associated with MALT lymphoma in 38% (3/8) of these patients (63). Lantuejoul and colleagues described a case of pulmonary nodular amyloidosis with cystic lung disease in the presence of MALT lymphoma (66).
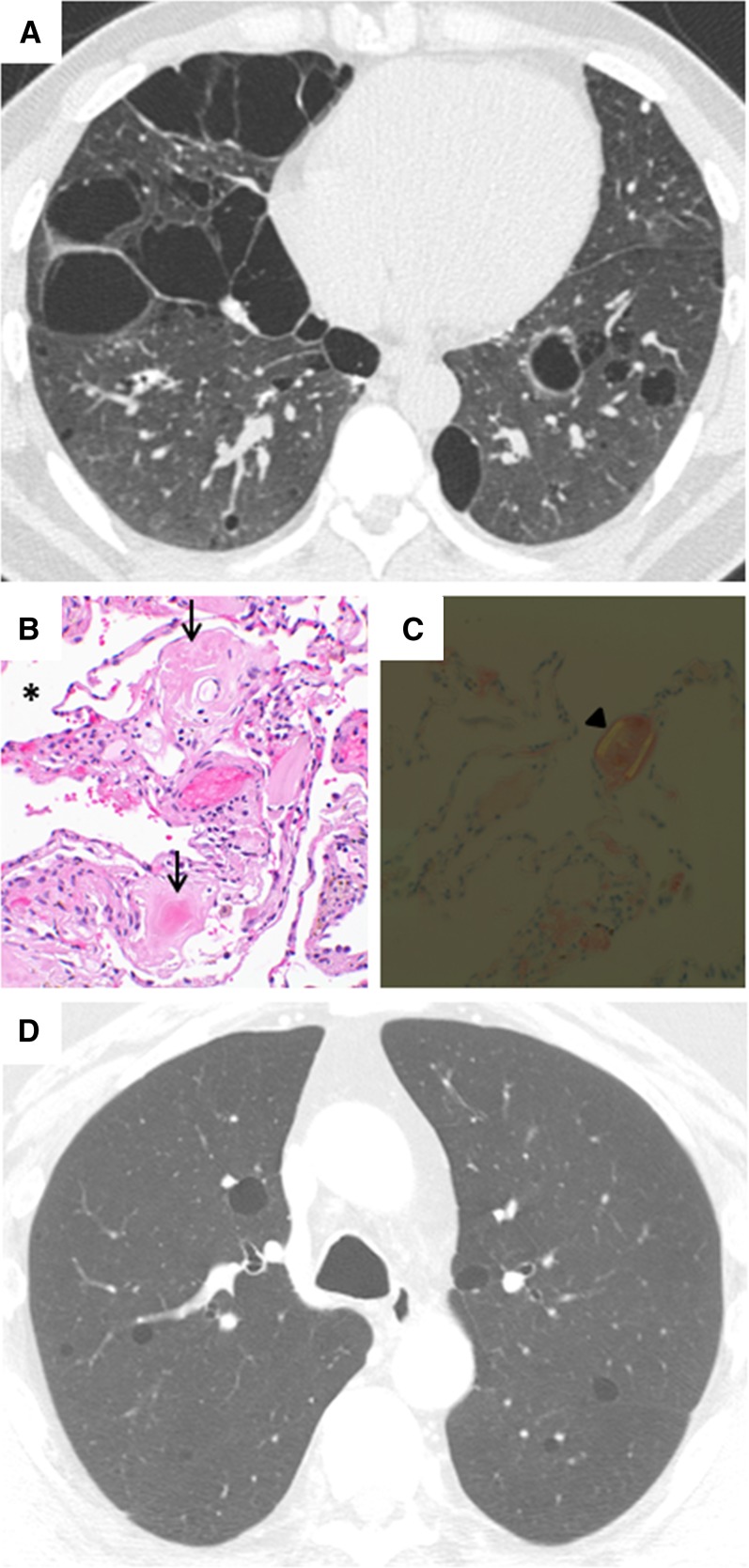
Figure 3.
Computed tomography (CT) and histopathologic images of diffuse cystic lung disease associated with lymphoproliferative disorders with protein deposition. (A) Chest CT showing diffuse, irregular cysts in a patient with amyloidosis. (B) Histologic sections of a lung biopsy from a patient with amyloidosis showing intraparenchymal cysts (*) and accumulation of amyloid (arrows) in the surrounding parenchyma and vessels. Original magnification, ×40. (C) The amyloid deposition in this biopsy shows the characteristic apple-green birefringence under polarized light (arrowhead). Original magnification, ×40. (D) Chest CT showing multiple round, thin-walled cysts in a patient with light-chain deposition disease (courtesy of Dr. Jonathan Chung, National Jewish Health, Denver, CO).
Although the exact mechanism of cyst formation is unclear in pulmonary amyloidosis, narrowing of the airways from surrounding inflammation or amyloid protein deposits may lead to development of a ball valve phenomenon (54, 56). Alternatively, an ischemic process with amyloid deposition causing capillary disruption may lead to alveolar wall destruction and cyst formation (65).
The diagnosis of cystic lung disease resulting from amyloidosis requires a lung biopsy and demonstration of fibrillar deposits exhibiting apple-green birefringence when Congo red stained sections are viewed by polarizing microscopy (Figures 3B and 3C). Electron microscopy shows a typical mesh of nonbranching, 7.5- to 12.0-nm–diameter fibrils arrayed in a disorderly fashion. Immunoglobulin light-chain protein deposition can also be seen by immunohistochemistry (63).
Light-Chain Deposition Disease
Light-chain deposition disease (LCDD) was first described by Randall and colleagues in 1976 (67). It is characterized by the presence of nonfibrillary, amorphous material that does not have a β-pleated sheet configuration and therefore does not bind Congo red or exhibit apple-green birefringence under polarized light as occurs with amyloidosis-associated protein deposits (67). The majority of systemic LCDD cases are associated with lymphoproliferative disorders and have renal involvement as a consistent feature (68).
LCDD presenting as an isolated pulmonary disease with cystic manifestations was first described by Colombat and colleagues in 2006 in three cases of diffuse cystic lung disease initially believed to represent PLCH or LAM (69). HRCT findings of LCDD can vary from multiple small (<2 cm), round cysts in a diffuse distribution (mimicking LAM) (Figure 3D) to large cystic spaces associated with reticulonodular opacities (mimicking PLCH) (69). Histologically, LCDD is characterized by monotypic kappa light chain deposition in the alveolar walls, small airways, and vessels accompanied by emphysematous changes and small airway dilation. Electron microscopy reveals coarsely granular deposits along the basement membrane (69). Early involvement of major airways followed later by lung parenchymal changes has also been described in LCDD (70). In cases where LCDD presents as diffuse thin-walled moniliform bronchiectasis, endobronchial biopsies can provide a definitive diagnosis (71). Colombat and colleagues identified a dominant B-cell clone isolated to the lungs in three patients with LCDD, suggesting that LCDD is a primary pulmonary lymphoproliferative disorder that could arguably be classified as a neoplastic cause of cystic lung disease together with PLCH and LAM (72). The mechanisms for cyst formation in LCDD are incompletely understood, but increased matrix metalloproteinase (MMP) activity leading to elastolysis and subsequent cyst formation has been proposed (73). LCDD is a progressive disease that often leads to respiratory failure. Treatment involves identification and treatment of the underlying hematological disease (if present) (71), with lung transplantation reserved for advanced cases and end-stage disease (69). The safety and preliminary outcomes of lung transplantation in a small series of patients with LCDD has recently been reported (74).
Other Cystic Lung Diseases
Accidental aspiration of petroleum derivatives during fire-eating demonstrations can lead to development of a chemical pneumonitis (75). Fire-eater's lung can occasionally present with a predominantly cystic pattern on chest CT due to multiple pneumatoceles. The clinical course is usually benign, with radiographic resolution over the course of a few weeks to months (75).
Hyper-IgE syndrome (HIS) is a rare primary immunodeficiency condition characterized by multiple cutaneous and sinopulmonary infections associated with markedly elevated serum IgE levels (76, 77). Recently, mutations in the signal transducer and activator of transcription 3 (STAT3) gene have been discovered in the sporadic and dominant forms of HIS (78, 79). Staphylococcal infections are responsible for the majority of skin and pulmonary infections in patients with HIS and are a likely etiology for formation of multiple pulmonary cysts. Radiographic features of this syndrome include multiple pneumatoceles, which can become infected and form abscesses. The clinical course is variable and ranges from spontaneous pneumatocele resolution to development of pneumothoraces and bronchopleural fistulas (76, 77).
Blunt trauma to the chest can lead to laceration of alveoli and interstitium leading to formation of cystic air spaces known as post-traumatic pseudocysts or pneumatoceles. Trauma-related cysts tend to be more common in children and young adults and follow a benign course, with complete resolution over a period of a few weeks (80, 81).
Mechanisms of Pulmonary Cyst Formation
The pathogenesis of pulmonary cyst formation is not well understood in any of the cystic lung diseases. Proposed mechanisms include: (1) check-valve obstruction with distal overinflation, (2) ischemia, and (3) remodeling induced by MMPs and other matrix-degrading enzymes (37). One-way obstruction of airflow in small airways that allows air entry but not emptying can result in balloon-like expansion of distal airspaces. This mechanism has been implicated in cyst formation in FB, metastatic neoplasms, and pneumatoceles due to infectious processes (82, 83). It has also been proposed as an etiology for cyst formation in other DCLDs, including LAM and PLCH, but cysts in these disorders are known to freely communicate with the airways and to fluctuate with respiration, suggesting some degree of bidirectional flow (84). Infiltration and obstruction of small vessels and capillaries supplying the terminal bronchioles can lead to necrosis and ischemic dilation of small airways and alveoli, progressing to cyst formation (65). Chronic destruction of the bronchiolar wall and subsequent dilation of the lumen has been proposed as a mechanism for cystic changes seen in cases of PLCH (85). Lung remodeling due to connective tissue degradation and elastolysis by matrix-degrading enzymes is believed to drive cyst formation in a number of DCLDs, and increased activity of MMPs and other proteases has been described in LAM, PLCH, and LCDD (73, 86, 87).
Radiological Evaluation of Pulmonary Cysts
HRCT of the chest is the cornerstone of diagnosis for the DCLDs. The radiographic definition of a cyst is a thin-walled (<2 mm), air-filled, spherical lucency with a well-defined lung/air interface (88). Features of cysts that can help identify and distinguish DCLDs include shape, wall thickness, distribution within the lung parenchyma and secondary lobule, presence of internal structures, association with adjacent structures, and rate of development and progression. Cysts are frequently confused with other air-filled structures in the lung parenchyma such as cavities, blebs, bullae, and pneumatoceles. Cavities generally have walls that are greater than 2 mm in thickness and are typically more irregularly shaped than cysts (89). Table 1 describes the salient features of cystic air spaces encountered on chest radiography that can be diagnostically useful. Septa and vessels can be seen within cysts due to FB, LIP, and emphysema, but generally not those due to LAM or PLCH. An eccentric vessel is often seen on the margin of cysts due to FB and BHD. Bronchiectasis can occasionally lead to confusion with DCLDs, but airway dilation and distortion can usually be distinguished from true cystic change by careful examination of contiguous sections on a volumetric CT that demonstrate the tubular rather than spherical dimensions.
Table 1.
Fleischner Society Definitions of Cystic Changes Seen on Chest Radiographs
| Lesion | Definition |
|---|---|
| Cyst | Thin-walled (<2 mm), spherical parenchymal lucency interfaced with normal lung. |
| Cavity | Gas-filled space within pulmonary consolidation, mass, or nodule, typically thick walled (>2 mm) and more irregularly shaped than cysts. |
| Bulla | Spherical focal lucency, ≥1 cm in diameter, bounded by a thin wall (usually <1 mm). It is usually accompanied by emphysematous changes in the adjacent lung. |
| Bleb | Cystic air space bounded by a thin wall adjacent to the visceral pleura, typically <1 cm in size. |
| Pneumatocele | Approximately round, thin-walled, air-filled space in the lung. Most frequently caused by infections, trauma, or aspiration of hydrocarbon fluid and is usually transient. |
Definitions from Reference 88.
Airspace dilation that mimics cystic change can also occur in pulmonary emphysema and lead to confusion with true DCLDs. Typical centrilobular emphysema secondary to smoking has an upper lobe predilection. α1-Antitrypsin deficiency, in contrast, has a panacinar distribution and classically (but not commonly) presents with basilar predominant airspace dilation (90). In contrast to true cysts, the cyst-like airspaces due to centrilobular emphysema usually have indiscernible walls, and the airspace dilation in panlobular emphysema often presents radiographically as hyperinflation alone (91).
Pathological Evaluation of Pulmonary Cysts
Systematic pathologic evaluation is often required to establish the correct diagnosis and underlying etiology in the DCLDs. Familiarity with the spectrum of diseases in the differential diagnosis is especially important when the biopsies are small (such as those obtained by bronchoscopy) and the diagnostic histopathologic features are subtle. The craniocaudal, central/peripheral, and intralobular localization, and structural characteristics of the cysts, along with associated histopathologic findings, such as inflammation, abnormal cellular proliferations, nodule formation, protein deposition, and fibrosis, are useful features for differentiating between the DCLDs. Pulmonary cysts detected by radiographic imaging can represent true cysts, cavities, or dilated airways, as defined pathologically. True cysts have an epithelial cell lining and thus need to be differentiated from cyst-like spaces with a discontinuous epithelial lining resulting from parenchymal loss, as can be seen in emphysema or post-traumatic pseudocysts. Distribution of the cysts is also extremely helpful in arriving at the correct diagnosis. For example, cystic changes in BHD are frequently intraparenchymal, basilar in distribution, and abutting interlobular septae, which differs from the apical, centrilobular-predominant pattern of emphysema (12). Abnormal cellular proliferations accompanying cystic changes are key diagnostic features in LAM, PLCH, and other neoplasms. The presence of a prominent chronic inflammatory infiltrate and/or fibrosis can lead to suspicion of chronic hypersensitivity pneumonitis or a connective tissue disease etiology. The presence of granulomatous inflammation, acute inflammation, and/or microorganisms on histopathological evaluation can belie an infectious etiology. Finally, disease-specific features such as abnormal protein deposits in amyloidosis and LCDD, HMB-45–positive cells in LAM, CD1a-positive cell aggregates in PLCH, and monoclonal lymphocytic proliferations in pulmonary lymphomas can be pivotal for establishing the diagnosis in the DCLDs. Multidisciplinary integration of the histopathologic features with the clinical, radiographic, and serologic findings facilitates accurate diagnosis and optimal patient management.
Diagnostic Approach to DCLDs
As with all diseases, the first step in establishing the correct diagnosis in the DCLDs is a detailed history and physical examination. The development of acute, rapidly progressive cystic change is suggestive of an infectious, inflammatory, or traumatic origin, whereas chronic processes are more likely to be secondary to neoplastic, congenital, vascular, or other slowly progressive disorders. A history of tobacco exposure or the presence of sicca symptoms can provide insights into smoking and connective tissue disease etiologies, respectively. A detailed family history, especially history of pneumothoraces, skin lesions, and renal tumors in children, siblings, parents, and more distant blood relatives, is useful information for establishing the diagnosis of LAM and BHD. As part of the detailed pulmonary examination, particular attention must be given to signs of a connective tissue disease or skin findings suggestive of BHD or tuberous sclerosis complex, which is often associated with LAM.
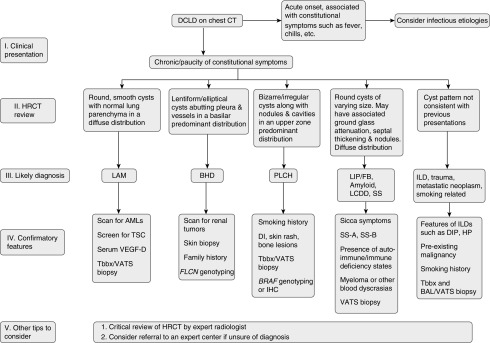
Critical review of the HRCT by an expert radiologist is essential for accurate diagnosis of DCLDs. The cyst characteristics and associated radiographic findings can be quite suggestive of the underlying disease. Radiological information can be further supplemented by serum biomarkers such as vascular endothelial growth factor-D, SS-A, SS-B, and α1-antitrypsin. Genetic studies on peripheral blood can be helpful in BHD. Lung biopsy with histopathologic evaluation in conjunction with special studies may be required to establish a definitive diagnosis, given the extensive overlap of clinical and radiographic features in some cases. Tables 2–4 summarize the demographic, clinical, radiologic, and histopathologic features of common DCLDs. A proposed diagnostic algorithm for DCLDs is shown in Figure 4.
Table 2.
Radiologic and Pathologic Characteristics of Cysts Seen in Selected Diffuse Cystic Lung Diseases
| LAM | PLCH | BHD | LIP/FB | Amyloid/LCDD | |
|---|---|---|---|---|---|
| Distribution | Diffuse, random | Upper & middle lung zones; sparing costophrenic angles | Basilar/peripheral/subpleural and near vessels | Diffuse, random, often near vessels | Diffuse, random |
| Size | 2 mm to 2 cm | Variable, 2 mm to >2 cm | 75% <1 cm | Average size 3 mm to 1 cm | 4 to 45 mm, majority larger than 1 cm |
| Shape | Round, uniform | Bizarre, irregular | Elliptical, lentiform | Round, variable | Round, variable |
| Pathological examination diagnostic | Yes | Yes | No | Yes | Yes |
| Pathologic findings | Infiltration by HMB-45–positive LAM cells with smooth muscle phenotype | S100- and CD1a-positive Langerhans cells with intracellular Birbeck granules by electron microscopy; stellate fibrotic scars in late stages | Intraparenchymal and subpleural cysts abutting interlobular septae and lacking abnormal cell proliferations or significant fibroinflammatory component | LIP: diffuse interstitial polyclonal lymphocytic infiltrate | Amyloid: amorphous protein deposits with fibrillar ultrastructure and apple-green birefringence by Congo red stain viewed under polarized light |
| FB: peribronchiolar polyclonal follicular lymphoid hyperplasia with germinal centers | LCDD: typically monotypic kappa light chain deposition with finely granular ultrastructure lacking apple-green birefringence by Congo red stain and polarized light | ||||
| Other associated findings on HRCT | Pleural effusions | Micro and macro nodules with or without cavitation, thick-walled cysts, cavities and reticulation | Cysts frequently abut pleura and proximal vessels | Ground-glass attenuation, poorly defined centrilobular nodules, interlobular septal thickening, cysts may contain internal structure | Multiple nodules of varying attenuation and random distribution; nodules abut cyst walls |
Definition of abbreviations: BHD = Birt-Hogg-Dubé syndrome; FB = follicular bronchiolitis; HMB-45 = human melanoma black-45; HRCT = high-resolution computed tomography; LAM = lymphangioleiomyomatosis; LCDD = light-chain deposition disease; LIP = lymphoid interstitial pneumonia; PLCH = pulmonary Langerhans cell histiocytosis.
Table 4.
Summary of Clinical and Diagnostic Features of Selected Diffuse Cystic Lung Diseases
| LAM | PLCH | BHD | LIP/FB | Amyloid | LCDD | |
|---|---|---|---|---|---|---|
| Personal history | Pneumothorax, angiomyolipomas, chylous effusions, and cortical tubers, seizures, skin lesions if TSC | Pneumothorax, smoking | Pneumothorax, skin lesions, renal tumors | HIV, autoimmune diseases, sicca symptoms, Raynaud's phenomenon | Sicca symptoms, autoimmune diseases | Lymphoproliferative disorders |
| Family history | TSC | Not relevant | Pneumothoraces, skin lesions, renal cancers | Not relevant | Not relevant | Not relevant |
| Extrapulmonary manifestations & other associations | Renal angiomyolipomas, chylous effusions, TSC manifestations | Diabetes insipidus, cutaneous & osteolytic bone lesions | Renal tumors, skin fibrofolliculomas | SS & other CTDs, HIV, EBV, CVID | SS & other CTDs, systemic amyloidosis | Lymphoproliferative disorders, renal failure |
| Laboratory testing | Serum VEGF-D | Serum & urine studies for diabetes insipidus | Genetic testing for FLCN mutations | Polyclonal dysproteinemia | Monoclonal dysproteinemia | Lymphoproliferative disorders, renal failure |
| Diagnostic yield of bronchoscopy (BAL, TBBx) | >50% | 30–50% | 0 | Low yield | Low yield | Low yield |
| Consider surgical lung biopsy | Yes | Yes | No | Yes | Yes | Yes |
| Genetic testing | TSC mutations, but usually not clinically indicated | BRAF mutation | FLCN gene mutation | No | No | No |
| Treatment | Sirolimus | Smoking cessation, immunosuppression, cladribine | None available | Corticosteroids & other immunosuppressive agents for LIP | None available | None available |
Definition of abbreviations: BAL = bronchioalveolar lavage; BHD = Birt-Hogg-Dubé syndrome; BRAF = v-Raf murine sarcoma viral oncogene homolog B; CTD = connective tissue disease; CVID = common variable immune deficiency; EBV = Epstein-Barr virus; FB = follicular bronchiolitis; FLCN = folliculin; LAM = lymphangioleiomyomatosis; LCDD = light-chain deposition disease; LIP = lymphoid interstitial pneumonia; PLCH = pulmonary Langerhans cell histiocytosis; SS = Sjögren syndrome; TBBx = transbronchial biopsy; TSC = tuberous sclerosis complex; VEGF-D = vascular endothelial growth factor-D.
Figure 4.
Algorithm to guide approach to the diagnosis of diffuse cystic lung diseases. AML = angiomyolipoma; BAL = bronchioalveolar lavage; BHD = Birt-Hogg-Dubé syndrome; BRAF = v-Raf murine sarcoma viral oncogene homolog B; CT = computed tomography; DCLD = diffuse cystic lung disease; DI = diabetes insipidus; DIP = desquamative interstitial pneumonia; FB = follicular bronchiolitis; FLCN = folliculin; HRCT = high-resolution computed tomography; HP = hypersensitivity pneumonitis; IHC = immunohistochemistry; ILD = interstitial lung disease; LAM = lymphangioleiomyomatosis; LCDD = light-chain deposition disease; LIP = lymphoid interstitial pneumonia; PLCH = pulmonary Langerhans cell histiocytosis; SS = Sjögren syndrome; Tbbx = transbronchial biopsy; TSC = tuberous sclerosis complex; VATS = video-assisted thoracoscopic surgery; VEGF-D = vascular endothelial growth factor-D.
Table 3.
Demographic Features of Selected Diffuse Cystic Lung Diseases
| LAM | PLCH | BHD | LIP/FB | Amyloid/LCDD | |
|---|---|---|---|---|---|
| Inheritance pattern | Autosomal dominant or sporadic | Not heritable | Autosomal dominant | Not heritable | Not heritable |
| Genetic mutation implicated | TSC | BRAF, MAP2K1 | FLCN | N/A | N/A |
| Nature of mutation | Somatic in S-LAM and germline in TSC-LAM | Somatic | Germline | N/A | N/A |
| Prevalence of pneumothorax, % | 70 | 10–20 | 24 | Unknown | Unknown |
| Average age at first pneumothorax | 35 | 27 | 38 | Unknown | Unknown |
| Rate of recurrent pneumothorax, % | 73 | 63 | 75 | Unknown; likely rare | Unknown; likely rare |
| Exacerbation by pregnancy | Yes | No | No | No | Unknown |
| Smoking related | No | Yes | No | No | No |
| Sex | Women ≫ men | Women = men | Women = men | Women > men | Women = men |
Definition of abbreviations: BHD = Birt-Hogg-Dubé syndrome; BRAF = v-Raf murine sarcoma viral oncogene homolog B; FB = follicular bronchiolitis; FLCN = folliculin; LAM = lymphangioleiomyomatosis; LCDD = light-chain deposition disease; LIP = lymphoid interstitial pneumonia; MAP2K1 = mitogen-activated protein kinase kinase 1; N/A = not applicable; PLCH = pulmonary Langerhans cell histiocytosis; S-LAM = sporadic LAM; TSC = tuberous sclerosis complex.
Conclusions
A variety of pathophysiological processes and diseases can present as diffuse cystic lung disease. Establishment of a correct diagnosis is of paramount importance, as the clinical course, treatment, and prognosis vary widely among the DCLDs. Chest HRCT remains the single most useful noninvasive diagnostic test for evaluation of DCLDs. Confirmatory tests, by either tissue or genetic analysis, are recommended in most cases of DCLDs, given the considerable overlap in radiographic findings. Better understanding of the pathophysiology of cyst formation is needed for us to develop targeted therapies for these etiologies.
Footnotes
Author Contributions: N.G. led the writing group; N.G., R.V., K.A.W.-B., and F.X.M. wrote the manuscript; and K.A.W.-B. provided the pathology cases and pathological descriptions.
CME will be available for this article at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201411-2096CI on April 23, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kunogi M, Kurihara M, Ikegami TS, Kobayashi T, Shindo N, Kumasaka T, Gunji Y, Kikkawa M, Iwakami S, Hino O, et al. Clinical and genetic spectrum of Birt-Hogg-Dube syndrome patients in whom pneumothorax and/or multiple lung cysts are the presenting feature. J Med Genet. 2010;47:281–287. doi: 10.1136/jmg.2009.070565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunji Y, Akiyoshi T, Sato T, Kurihara M, Tominaga S, Takahashi K, Seyama K. Mutations of the Birt Hogg Dube gene in patients with multiple lung cysts and recurrent pneumothorax. J Med Genet. 2007;44:588–593. doi: 10.1136/jmg.2007.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomassetti S, Carloni A, Chilosi M, Maffè A, Ungari S, Sverzellati N, Gurioli C, Casoni G, Romagnoli M, Gurioli C, et al. Pulmonary features of Birt-Hogg-Dubé syndrome: cystic lesions and pulmonary histiocytoma. Respir Med. 2011;105:768–774. doi: 10.1016/j.rmed.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, Wei MH, Schmidt LS, Davis L, Zbar B, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onuki T, Goto Y, Kuramochi M, Inagaki M, Bhunchet E, Suzuki K, Tanaka R, Furuya M. Radiologically indeterminate pulmonary cysts in Birt-Hogg-Dubé syndrome. Ann Thorac Surg. 2014;97:682–685. doi: 10.1016/j.athoracsur.2013.05.120. [DOI] [PubMed] [Google Scholar]
- 6.Zbar B, Alvord WG, Glenn G, Turner M, Pavlovich CP, Schmidt L, Walther M, Choyke P, Weirich G, Hewitt SM, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. [PubMed] [Google Scholar]
- 7.Baba M, Hong SB, Sharma N, Warren MB, Nickerson ML, Iwamatsu A, Esposito D, Gillette WK, Hopkins RF, III, Hartley JL, et al. Folliculin encoded by the BHD gene interacts with a binding protein, FNIP1, and AMPK, and is involved in AMPK and mTOR signaling. Proc Natl Acad Sci USA. 2006;103:15552–15557. doi: 10.1073/pnas.0603781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartman TR, Nicolas E, Klein-Szanto A, Al-Saleem T, Cash TP, Simon MC, Henske EP. The role of the Birt-Hogg-Dubé protein in mTOR activation and renal tumorigenesis. Oncogene. 2009;28:1594–1604. doi: 10.1038/onc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong SB, Oh H, Valera VA, Stull J, Ngo DT, Baba M, Merino MJ, Linehan WM, Schmidt LS. Tumor suppressor FLCN inhibits tumorigenesis of a FLCN-null renal cancer cell line and regulates expression of key molecules in TGF-beta signaling. Mol Cancer. 2010;9:160. doi: 10.1186/1476-4598-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nookala RK, Langemeyer L, Pacitto A, Ochoa-Montaño B, Donaldson JC, Blaszczyk BK, Chirgadze DY, Barr FA, Bazan JF, Blundell TL. Crystal structure of folliculin reveals a hidDENN function in genetically inherited renal cancer. Open Biol. 2012;2:120071. doi: 10.1098/rsob.120071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuya M, Tanaka R, Koga S, Yatabe Y, Gotoda H, Takagi S, Hsu YH, Fujii T, Okada A, Kuroda N, et al. Pulmonary cysts of Birt-Hogg-Dubé syndrome: a clinicopathologic and immunohistochemical study of 9 families. Am J Surg Pathol. 2012;36:589–600. doi: 10.1097/PAS.0b013e3182475240. [DOI] [PubMed] [Google Scholar]
- 12.Kumasaka T, Hayashi T, Mitani K, Kataoka H, Kikkawa M, Tobino K, Kobayashi E, Gunji Y, Kunogi M, Kurihara M, et al. Characterization of pulmonary cysts in Birt-Hogg-Dubé syndrome: histopathological and morphometric analysis of 229 pulmonary cysts from 50 unrelated patients. Histopathology. 2014;65:100–110. doi: 10.1111/his.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butnor KJ, Guinee DG., Jr Pleuropulmonary pathology of Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2006;30:395–399. doi: 10.1097/01.pas.0000183571.17011.06. [DOI] [PubMed] [Google Scholar]
- 14.Tobino K, Hirai T, Johkoh T, Kurihara M, Fujimoto K, Tomiyama N, Mishima M, Takahashi K, Seyama K. Differentiation between Birt-Hogg-Dubé syndrome and lymphangioleiomyomatosis: quantitative analysis of pulmonary cysts on computed tomography of the chest in 66 females. Eur J Radiol. 2012;81:1340–1346. doi: 10.1016/j.ejrad.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 15.Tobino K, Gunji Y, Kurihara M, Kunogi M, Koike K, Tomiyama N, Johkoh T, Kodama Y, Iwakami S, Kikkawa M, et al. Characteristics of pulmonary cysts in Birt-Hogg-Dubé syndrome: thin-section CT findings of the chest in 12 patients. Eur J Radiol. 2011;77:403–409. doi: 10.1016/j.ejrad.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Johannesma PC, Reinhard R, Kon Y, Sriram JD, Smit HJ, van Moorselaar RJ, Menko FH, Postmus PE Amsterdam BHD working group. Prevalence of Birt-Hogg-Dubé syndrome in patients with apparently primary spontaneous pneumothorax. Eur Respir J. 2015;45:1191–1194. doi: 10.1183/09031936.00196914. [DOI] [PubMed] [Google Scholar]
- 17.Ren HZ, Zhu CC, Yang C, Chen SL, Xie J, Hou YY, Xu ZF, Wang DJ, Mu DK, Ma DH, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74:178–183. doi: 10.1111/j.1399-0004.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Seyama K, McCormack FX. Pulmonary manifestations of Birt-Hogg-Dubé syndrome. Fam Cancer. 2013;12:387–396. doi: 10.1007/s10689-013-9660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menko FH, van Steensel MA, Giraud S, Friis-Hansen L, Richard S, Ungari S, Nordenskjöld M, Hansen TV, Solly J, Maher ER European BHD Consortium. Birt-Hogg-Dubé syndrome: diagnosis and management. Lancet Oncol. 2009;10:1199–1206. doi: 10.1016/S1470-2045(09)70188-3. [DOI] [PubMed] [Google Scholar]
- 20.Pavlovich CP, Grubb RL, III, Hurley K, Glenn GM, Toro J, Schmidt LS, Torres-Cabala C, Merino MJ, Zbar B, Choyke P, et al. Evaluation and management of renal tumors in the Birt-Hogg-Dubé syndrome. J Urol. 2005;173:1482–1486. doi: 10.1097/01.ju.0000154629.45832.30. [DOI] [PubMed] [Google Scholar]
- 21.Kluijt I, de Jong D, Teertstra HJ, Axwijk PH, Gille JJ, Bell K, van Rens A, van der Velden AW, Middelton L, Horenblas S. Early onset of renal cancer in a family with Birt-Hogg-Dubé syndrome. Clin Genet. 2009;75:537–543. doi: 10.1111/j.1399-0004.2009.01159.x. [DOI] [PubMed] [Google Scholar]
- 22.Fahmy W, Safwat AS, Bissada NK, Curry N, Guirguis N, Clarke HS, Fraig M, Finkbeiner A. Multiple/bilateral renal tumors in patients with Birt-Hogg-Dubé syndrome. Int Urol Nephrol. 2007;39:995–999. doi: 10.1007/s11255-006-9129-y. [DOI] [PubMed] [Google Scholar]
- 23.Graham RB, Nolasco M, Peterlin B, Garcia CK. Nonsense mutations in folliculin presenting as isolated familial spontaneous pneumothorax in adults. Am J Respir Crit Care Med. 2005;172:39–44. doi: 10.1164/rccm.200501-143OC. [DOI] [PubMed] [Google Scholar]
- 24.Painter JN, Tapanainen H, Somer M, Tukiainen P, Aittomäki K. A 4-bp deletion in the Birt-Hogg-Dubé gene (FLCN) causes dominantly inherited spontaneous pneumothorax. Am J Hum Genet. 2005;76:522–527. doi: 10.1086/428455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, Cowl CT, Baqir M, Ryu JH. Air travel and pneumothorax. Chest. 2014;145:688–694. doi: 10.1378/chest.13-2363. [DOI] [PubMed] [Google Scholar]
- 26.Postmus PE, Johannesma PC, Menko FH, Paul MA. In-flight pneumothorax: diagnosis may be missed because of symptom delay. Am J Respir Crit Care Med. 2014;190:704–705. doi: 10.1164/rccm.201404-0698LE. [DOI] [PubMed] [Google Scholar]
- 27.Stamatakis L, Metwalli AR, Middelton LA, Marston Linehan W. Diagnosis and management of BHD-associated kidney cancer. Fam Cancer. 2013;12:397–402. doi: 10.1007/s10689-013-9657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamis-Dow CA, Choyke PL, Jennings SB, Linehan WM, Thakore KN, Walther MM. Small (< or = 3-cm) renal masses: detection with CT versus US and pathologic correlation. Radiology. 1996;198:785–788. doi: 10.1148/radiology.198.3.8628872. [DOI] [PubMed] [Google Scholar]
- 29.Zamora AC, Collard HR, Wolters PJ, Webb WR, King TE. Neurofibromatosis-associated lung disease: a case series and literature review. Eur Respir J. 2007;29:210–214. doi: 10.1183/09031936.06.00044006. [DOI] [PubMed] [Google Scholar]
- 30.Ryu JH, Parambil JG, McGrann PS, Aughenbaugh GL. Lack of evidence for an association between neurofibromatosis and pulmonary fibrosis. Chest. 2005;128:2381–2386. doi: 10.1378/chest.128.4.2381. [DOI] [PubMed] [Google Scholar]
- 31.Dowton SB, Pincott S, Demmer L. Respiratory complications of Ehlers-Danlos syndrome type IV. Clin Genet. 1996;50:510–514. doi: 10.1111/j.1399-0004.1996.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 32.Murray RA, Poulton TB, Saltarelli MG, Dweik RA, Litwin DK, Kirby TJ, Meziane MA, O’Donovan PB. Rare pulmonary manifestation of Ehlers-Danlos syndrome. J Thorac Imaging. 1995;10:138–141. doi: 10.1097/00005382-199521000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MM., Jr Proteus syndrome review: molecular, clinical, and pathologic features. Clin Genet. 2014;85:111–119. doi: 10.1111/cge.12266. [DOI] [PubMed] [Google Scholar]
- 34.Newman B, Urbach AH, Orenstein D, Dickman PS. Proteus syndrome: emphasis on the pulmonary manifestations. Pediatr Radiol. 1994;24:189–193. doi: 10.1007/BF02012188. [DOI] [PubMed] [Google Scholar]
- 35.Turner JT, Cohen MM, Jr, Biesecker LG. Reassessment of the Proteus syndrome literature: application of diagnostic criteria to published cases. Am J Med Genet A. 2004;130A:111–122. doi: 10.1002/ajmg.a.30327. [DOI] [PubMed] [Google Scholar]
- 36.Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordier JF, Johnson SR. Multiple cystic lung diseases. Eur Respir Mon. 2011;54:46–83. [Google Scholar]
- 38.Plit ML, Blott JA, Lakis N, Murray J, Plit M. Clinical, radiographic and lung function features of diffuse congenital cystic adenomatoid malformation of the lung in an adult. Eur Respir J. 1997;10:1680–1682. doi: 10.1183/09031936.97.10071680. [DOI] [PubMed] [Google Scholar]
- 39.Cantin L, Bankier AA, Eisenberg RL. Multiple cystlike lung lesions in the adult. AJR Am J Roentgenol. 2010;194:W1–W11. doi: 10.2214/AJR.09.3540. [DOI] [PubMed] [Google Scholar]
- 40.Azizkhan RG, Crombleholme TM. Congenital cystic lung disease: contemporary antenatal and postnatal management. Pediatr Surg Int. 2008;24:643–657. doi: 10.1007/s00383-008-2139-3. [DOI] [PubMed] [Google Scholar]
- 41.Godwin JD, Webb WR, Savoca CJ, Gamsu G, Goodman PC. Multiple, thin-walled cystic lesions of the lung. AJR Am J Roentgenol. 1980;135:593–604. doi: 10.2214/ajr.135.3.593. [DOI] [PubMed] [Google Scholar]
- 42.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 43.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–1431. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 44.Odev K, Guler I, Altinok T, Pekcan S, Batur A, Ozbiner H. Cystic and cavitary lung lesions in children: radiologic findings with pathologic correlation. J Clin Imaging Sci. 2013;3:60. doi: 10.4103/2156-7514.124087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholson AG. Lymphocytic interstitial pneumonia and other lymphoproliferative disorders in the lung. Semin Respir Crit Care Med. 2001;22:409–422. doi: 10.1055/s-2001-17384. [DOI] [PubMed] [Google Scholar]
- 46.Guinee DG., Jr Update on nonneoplastic pulmonary lymphoproliferative disorders and related entities. Arch Pathol Lab Med. 2010;134:691–701. doi: 10.5858/134.5.691. [DOI] [PubMed] [Google Scholar]
- 47.Cha SI, Fessler MB, Cool CD, Schwarz MI, Brown KK. Lymphoid interstitial pneumonia: clinical features, associations and prognosis. Eur Respir J. 2006;28:364–369. doi: 10.1183/09031936.06.00076705. [DOI] [PubMed] [Google Scholar]
- 48.Matsuyama N, Ashizawa K, Okimoto T, Kadota J, Amano H, Hayashi K. Pulmonary lesions associated with Sjögren’s syndrome: radiographic and CT findings. Br J Radiol. 2003;76:880–884. doi: 10.1259/bjr/18937619. [DOI] [PubMed] [Google Scholar]
- 49.Swigris JJ, Berry GJ, Raffin TA, Kuschner WG. Lymphoid interstitial pneumonia: a narrative review. Chest. 2002;122:2150–2164. doi: 10.1378/chest.122.6.2150. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson AG, Wotherspoon AC, Diss TC, Hansell DM, Du Bois R, Sheppard MN, Isaacson PG, Corrin B. Reactive pulmonary lymphoid disorders. Histopathology. 1995;26:405–412. doi: 10.1111/j.1365-2559.1995.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 51.Lohrmann C, Uhl M, Warnatz K, Ghanem N, Kotter E, Schaefer O, Langer M. High-resolution CT imaging of the lung for patients with primary Sjogren’s syndrome. Eur J Radiol. 2004;52:137–143. doi: 10.1016/j.ejrad.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Watanabe M, Naniwa T, Hara M, Arakawa T, Maeda T. Pulmonary manifestations in Sjogren’s syndrome: correlation analysis between chest computed tomographic findings and clinical subsets with poor prognosis in 80 patients. J Rheumatol. 2010;37:365–373. doi: 10.3899/jrheum.090507. [DOI] [PubMed] [Google Scholar]
- 53.Koyama M, Johkoh T, Honda O, Mihara N, Kozuka T, Tomiyama N, Hamada S, Nakamura H. Pulmonary involvement in primary Sjögren’s syndrome: spectrum of pulmonary abnormalities and computed tomography findings in 60 patients. J Thorac Imaging. 2001;16:290–296. doi: 10.1097/00005382-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Jeong YJ, Lee KS, Chung MP, Han J, Chung MJ, Kim KI, Seo JB, Franquet T. Amyloidosis and lymphoproliferative disease in Sjögren syndrome: thin-section computed tomography findings and histopathologic comparisons. J Comput Assist Tomogr. 2004;28:776–781. doi: 10.1097/00004728-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Desai SR, Nicholson AG, Stewart S, Twentyman OM, Flower CD, Hansell DM. Benign pulmonary lymphocytic infiltration and amyloidosis: computed tomographic and pathologic features in three cases. J Thorac Imaging. 1997;12:215–220. doi: 10.1097/00005382-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi H, Matsuoka R, Kitamura S, Tsunoda N, Saito K. Sjögren’s syndrome with multiple bullae and pulmonary nodular amyloidosis. Chest. 1988;94:438–440. doi: 10.1378/chest.94.2.438. [DOI] [PubMed] [Google Scholar]
- 57.Johkoh T, Müller NL, Pickford HA, Hartman TE, Ichikado K, Akira M, Honda O, Nakamura H. Lymphocytic interstitial pneumonia: thin-section CT findings in 22 patients. Radiology. 1999;212:567–572. doi: 10.1148/radiology.212.2.r99au05567. [DOI] [PubMed] [Google Scholar]
- 58.Honda O, Johkoh T, Ichikado K, Tomiyama N, Maeda M, Mihara N, Higashi M, Hamada S, Naito H, Yamamoto S, et al. Differential diagnosis of lymphocytic interstitial pneumonia and malignant lymphoma on high-resolution CT. AJR Am J Roentgenol. 1999;173:71–74. doi: 10.2214/ajr.173.1.10397102. [DOI] [PubMed] [Google Scholar]
- 59.Shi JH, Liu HR, Xu WB, Feng RE, Zhang ZH, Tian XL, Zhu YJ. Pulmonary manifestations of Sjögren’s syndrome. Respiration. 2009;78:377–386. doi: 10.1159/000214841. [DOI] [PubMed] [Google Scholar]
- 60.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 61.Dufour V, Wislez M, Bergot E, Mayaud C, Cadranel J. Improvement of symptomatic human immunodeficiency virus-related lymphoid interstitial pneumonia in patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2003;36:e127–e130. doi: 10.1086/374665. [DOI] [PubMed] [Google Scholar]
- 62.Johkoh T, Ichikado K, Akira M, Honda O, Tomiyama N, Mihara N, Kozuka T, Koyama M, Hamada S, Nakamura H. Lymphocytic interstitial pneumonia: follow-up CT findings in 14 patients. J Thorac Imaging. 2000;15:162–167. doi: 10.1097/00005382-200007000-00002. [DOI] [PubMed] [Google Scholar]
- 63.Baqir M, Kluka EM, Aubry MC, Hartman TE, Yi ES, Bauer PR, Ryu JH. Amyloid-associated cystic lung disease in primary Sjögren’s syndrome. Respir Med. 2013;107:616–621. doi: 10.1016/j.rmed.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Chew KM, Clarke MJ, Dubey N, Seet JE. Nodular pulmonary amyloidosis with unusual, widespread lung cysts. Singapore Med J. 2013;54:e97–e99. doi: 10.11622/smedj.2013062. [DOI] [PubMed] [Google Scholar]
- 65.Ohdama S, Akagawa S, Matsubara O, Yoshizawa Y. Primary diffuse alveolar septal amyloidosis with multiple cysts and calcification. Eur Respir J. 1996;9:1569–1571. doi: 10.1183/09031936.96.09071569. [DOI] [PubMed] [Google Scholar]
- 66.Lantuejoul S, Moulai N, Quetant S, Brichon PY, Brambilla C, Brambilla E, Ferretti GR. Unusual cystic presentation of pulmonary nodular amyloidosis associated with MALT-type lymphoma. Eur Respir J. 2007;30:589–592. doi: 10.1183/09031936.00136605. [DOI] [PubMed] [Google Scholar]
- 67.Randall RE, Williamson WC, Jr, Mullinax F, Tung MY, Still WJ. Manifestations of systemic light chain deposition. Am J Med. 1976;60:293–299. doi: 10.1016/0002-9343(76)90440-x. [DOI] [PubMed] [Google Scholar]
- 68.Buxbaum J, Gallo G. Nonamyloidotic monoclonal immunoglobulin deposition disease: light-chain, heavy-chain, and light- and heavy-chain deposition diseases. Hematol Oncol Clin North Am. 1999;13:1235–1248. doi: 10.1016/s0889-8588(05)70123-4. [DOI] [PubMed] [Google Scholar]
- 69.Colombat M, Stern M, Groussard O, Droz D, Brauner M, Valeyre D, Mal H, Taillé C, Monnet I, Fournier M, et al. Pulmonary cystic disorder related to light chain deposition disease. Am J Respir Crit Care Med. 2006;173:777–780. doi: 10.1164/rccm.200510-1620CR. [DOI] [PubMed] [Google Scholar]
- 70.Colombat M, Gounant V, Mal H, Callard P, Milleron B. Light chain deposition disease involving the airways: diagnosis by fibreoptic bronchoscopy. Eur Respir J. 2007;29:1057–1060. doi: 10.1183/09031936.00134406. [DOI] [PubMed] [Google Scholar]
- 71.Girard N, Vasiljevic A, Cottin V, Falchero L, Meyronet D, Thivolet-Bejui F, Cordier JF. Respiratory failure with diffuse bronchiectases and cryoglobulinaemia. Eur Respir J. 2008;31:1374–1378. doi: 10.1183/09031936.00004408. [DOI] [PubMed] [Google Scholar]
- 72.Colombat M, Mal H, Copie-Bergman C, Diebold J, Damotte D, Callard P, Fournier M, Farcet JP, Stern M, Delfau-Larue MH. Primary cystic lung light chain deposition disease: a clinicopathologic entity derived from unmutated B cells with a stereotyped IGHV4-34/IGKV1 receptor. Blood. 2008;112:2004–2012. doi: 10.1182/blood-2007-11-123596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colombat M, Caudroy S, Lagonotte E, Mal H, Danel C, Stern M, Fournier M, Birembaut P. Pathomechanisms of cyst formation in pulmonary light chain deposition disease. Eur Respir J. 2008;32:1399–1403. doi: 10.1183/09031936.00132007. [DOI] [PubMed] [Google Scholar]
- 74.Hirschi S, Colombat M, Kessler R, Reynaud-Gaubert M, Stern M, Chenard MP, Métivier AC, Jeung MY, Mal H. Lung transplantation for advanced cystic lung disease due to nonamyloid kappa light chain deposits. Ann Am Thorac Soc. 2014;11:1025–1031. doi: 10.1513/AnnalsATS.201404-137OC. [DOI] [PubMed] [Google Scholar]
- 75.Gentina T, Tillie-Leblond I, Birolleau S, Saidi F, Saelens T, Boudoux L, Vervloet D, Delaval P, Tonnel AB. Fire-eater’s lung: seventeen cases and a review of the literature. Medicine (Baltimore) 2001;80:291–297. doi: 10.1097/00005792-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Donabedian H, Gallin JI. The hyperimmunoglobulin E recurrent-infection (Job’s) syndrome: a review of the NIH experience and the literature. Medicine (Baltimore) 1983;62:195–208. doi: 10.1097/00005792-198307000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Jhaveri KS, Sahani DV, Shetty PG, Shroff MM. Hyperimmunoglobulinaemia E syndrome: pulmonary imaging features. Australas Radiol. 2000;44:328–330. doi: 10.1046/j.1440-1673.2000.00823.x. [DOI] [PubMed] [Google Scholar]
- 78.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 79.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, Kawamura N, Ariga T, Pasic S, Stojkovic O, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 80.Moore FA, Moore EE, Haenel JB, Waring BJ, Parsons PE. Post-traumatic pulmonary pseudocyst in the adult: pathophysiology, recognition, and selective management. J Trauma. 1989;29:1380–1385. doi: 10.1097/00005373-198910000-00016. [DOI] [PubMed] [Google Scholar]
- 81.Yazkan R, Ozpolat B, Sahinalp S. Diagnosis and management of post-traumatic pulmonary pseudocyst. Respir Care. 2009;54:538–541. [PubMed] [Google Scholar]
- 82.Dines DE. Diagnostic significance of pneumatocele of the lung. JAMA. 1968;204:1169–1172. [PubMed] [Google Scholar]
- 83.Kikuchi E, Kinoshita I, Yamazaki K, Itoh T, Shimizu T, Shimizu H, Nishimura M. Epithelioid sarcoma presenting as pulmonary cysts with cancer antigen 125 expression. Respirology. 2006;11:826–829. doi: 10.1111/j.1440-1843.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- 84.Lee KN, Yoon SK, Choi SJ, Goo JM, Nam KJ. Cystic lung disease: a comparison of cystic size, as seen on expiratory and inspiratory HRCT scans. Korean J Radiol. 2000;1:84–90. doi: 10.3348/kjr.2000.1.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kambouchner M, Basset F, Marchal J, Uhl JF, Hance AJ, Soler P. Three-dimensional characterization of pathologic lesions in pulmonary langerhans cell histiocytosis. Am J Respir Crit Care Med. 2002;166:1483–1490. doi: 10.1164/rccm.2201050. [DOI] [PubMed] [Google Scholar]
- 86.Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124:267–275. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi T, Rush WL, Travis WD, Liotta LA, Stetler-Stevenson WG, Ferrans VJ. Immunohistochemical study of matrix metalloproteinases and their tissue inhibitors in pulmonary Langerhans’ cell granulomatosis. Arch Pathol Lab Med. 1997;121:930–937. [PubMed] [Google Scholar]
- 88.Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 89.Ryu JH, Tian X, Baqir M, Xu K. Diffuse cystic lung diseases. Front Med. 2013;7:316–327. doi: 10.1007/s11684-013-0269-z. [DOI] [PubMed] [Google Scholar]
- 90.Lee KH, Lee JS, Lynch DA, Song KS, Lim TH. The radiologic differential diagnosis of diffuse lung diseases characterized by multiple cysts or cavities. J Comput Assist Tomogr. 2002;26:5–12. doi: 10.1097/00004728-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 91.Matsumoto Y, Horiba K, Usuki J, Chu SC, Ferrans VJ, Moss J. Markers of cell proliferation and expression of melanosomal antigen in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 1999;21:327–336. doi: 10.1165/ajrcmb.21.3.3693. [DOI] [PubMed] [Google Scholar]
- 92.Brauner MW, Grenier P, Mouelhi MM, Mompoint D, Lenoir S. Pulmonary histiocytosis X: evaluation with high-resolution CT. Radiology. 1989;172:255–258. doi: 10.1148/radiology.172.1.2787036. [DOI] [PubMed] [Google Scholar]
- 93.Travis WD, Borok Z, Roum JH, Zhang J, Feuerstein I, Ferrans VJ, Crystal RG. Pulmonary Langerhans cell granulomatosis (histiocytosis X). A clinicopathologic study of 48 cases. Am J Surg Pathol. 1993;17:971–986. doi: 10.1097/00000478-199310000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, Maurer J, McCormack FX, Sahn SA. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 95.Brown NA, Furtado LV, Betz BL, Kiel MJ, Weigelin HC, Lim MS, Elenitoba-Johnson KS. High prevalence of somatic MAP2K1 mutations in BRAF V600E-negative Langerhans cell histiocytosis. Blood. 2014;124:1655–1658. doi: 10.1182/blood-2014-05-577361. [DOI] [PubMed] [Google Scholar]
- 96.Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest. 2004;125:1028–1032. doi: 10.1378/chest.125.3.1028. [DOI] [PubMed] [Google Scholar]
- 97.Sahm F, Capper D, Preusser M, Meyer J, Stenzinger A, Lasitschka F, Berghoff AS, Habel A, Schneider M, Kulozik A, et al. BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood. 2012;120:e28–e34. doi: 10.1182/blood-2012-06-429597. [DOI] [PubMed] [Google Scholar]
- 98.Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, Reynaud-Gaubert M, Boehler A, Brauner M, Popper H, et al. Review Panel of the ERS LAM Task Force. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. Eur Respir J. 2010;35:14–26. doi: 10.1183/09031936.00076209. [DOI] [PubMed] [Google Scholar]
- 99.McCormack FX. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133:507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 100.McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al. National Institutes of Health Rare Lung Diseases Consortium; MILES Trial Group. Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med. 2011;364:1595–1606. doi: 10.1056/NEJMoa1100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meraj R, Wikenheiser-Brokamp KA, Young LR, Byrnes S, McCormack FX. Utility of transbronchial biopsy in the diagnosis of lymphangioleiomyomatosis. Front Med. 2012;6:395–405. doi: 10.1007/s11684-012-0231-5. [DOI] [PubMed] [Google Scholar]
- 102.Young LR, Vandyke R, Gulleman PM, Inoue Y, Brown KK, Schmidt LS, Linehan WM, Hajjar F, Kinder BW, Trapnell BC, et al. Serum vascular endothelial growth factor-D prospectively distinguishes lymphangioleiomyomatosis from other diseases. Chest. 2010;138:674–681. doi: 10.1378/chest.10-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harari S, Torre O, Cassandro R, Taveira-DaSilva AM, Moss J. Bronchoscopic diagnosis of Langerhans cell histiocytosis and lymphangioleiomyomatosis. Respir Med. 2012;106:1286–1292. doi: 10.1016/j.rmed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grobost V, Khouatra C, Lazor R, Cordier JF, Cottin V. Effectiveness of cladribine therapy in patients with pulmonary Langerhans cell histiocytosis. Orphanet J Rare Dis. 2014;9:191. doi: 10.1186/s13023-014-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]