Abstract
Rationale: The use of 6-minute-walk distance (6MWD) as an indicator of exercise capacity to predict postoperative survival in lung transplantation has not previously been well studied.
Objectives: To evaluate the association between 6MWD and postoperative survival following lung transplantation.
Methods: Adult, first time, lung-only transplantations per the United Network for Organ Sharing database from May 2005 to December 2011 were analyzed. Kaplan-Meier methods and Cox proportional hazards modeling were used to determine the association between preoperative 6MWD and post-transplant survival after adjusting for potential confounders. A receiver operating characteristic curve was used to determine the 6MWD value that provided maximal separation in 1-year mortality. A subanalysis was performed to assess the association between 6MWD and post-transplant survival by disease category.
Measurements and Main Results: A total of 9,526 patients were included for analysis. The median 6MWD was 787 ft (25th–75th percentiles = 450–1,082 ft). Increasing 6MWD was associated with significantly lower overall hazard of death (P < 0.001). Continuous increase in walk distance through 1,200–1,400 ft conferred an incremental survival advantage. Although 6MWD strongly correlated with survival, the impact of a single dichotomous value to predict outcomes was limited. All disease categories demonstrated significantly longer survival with increasing 6MWD (P ≤ 0.009) except pulmonary vascular disease (P = 0.74); however, the low volume in this category (n = 312; 3.3%) may limit the ability to detect an association.
Conclusions: 6MWD is significantly associated with post-transplant survival and is best incorporated into transplant evaluations on a continuous basis given limited ability of a single, dichotomous value to predict outcomes.
Keywords: lung transplantation, exercise tolerance, patient outcome assessment
At a Glance Commentary
Scientific Knowledge on the Subject
Six-minute-walk distance (6MWD) has been associated with waitlist mortality in patients with end-stage lung disease awaiting transplantation and has therefore been incorporated into the Lung Allocation Score and become a standard component of pretransplant evaluation. Although these data are routinely recorded for lung transplant candidates (because of waitlist implications), the use of 6MWD as an indicator of exercise capacity to predict postoperative survival has not been well studied, nor have sufficient data been reported in the literature to identify optimal thresholds to distinguish high-risk surgical candidates based on exercise tolerance.
What This Study Adds to the Field
The authors analyzed 6MWD as it pertains to postoperative outcomes in a United States cohort of more than 9,500 patients. The results of this work potentially impact policy decisions regarding the use of 6MWD in the Lung Allocation Score, clinical decisions regarding an individual’s risk/benefit ratio for proceeding with transplantation, optimization of organ distribution, and also may guide preparations and recipient optimization in advance of surgery.
The numbers of lung transplantations performed in the United States continue to increase, with more than 1,800 transplants performed in 2011 (1); however, 200–400 deaths continue to occur each year for those with end-stage pulmonary disease awaiting lung transplant (2). Given the scarcity of lung allografts (3), identifying measures to risk-stratify potential transplant recipients based on expected outcome following lung transplantation is of critical importance.
Six-minute-walk distance (6MWD) is a commonly used and reproducible measure to evaluate exercise capacity in patients with severe cardiac or pulmonary disease (4). This metric has been shown to accurately predict survival in several pulmonary diseases including chronic obstructive pulmonary disease (5, 6), idiopathic pulmonary fibrosis (7), and primary pulmonary hypertension (8, 9). Accordingly, 6MWD has been associated with waitlist mortality in patients with end-stage lung disease awaiting transplantation (10–14), and has therefore been incorporated into the Lung Allocation Score (LAS) and become a standard component of pretransplant evaluation (dichotomized variable over/under 150 ft) (15).
The use of 6MWD as an indicator of exercise capacity to predict postoperative survival, however, has not been well studied. In a two-center analysis reported in 2008, Martinu and colleagues (14) demonstrated a relationship between 6MWD and both pretransplant and post-transplant survival; however, the post-transplant cohort consisted of only 172 patients. These results have not been evaluated on a larger scale, nor have sufficient data been reported in the literature to identify optimal thresholds to distinguish high-risk surgical candidates based on exercise tolerance. The objectives of the current study are therefore to evaluate the association between 6MWD and postoperative survival following lung transplantation, and determine a threshold distance to distinguish high-risk surgical candidates based on 6MWD. Some of the results of these studies have been previously reported in the form of an abstract (16).
Methods
The Institutional Review Board at Duke University Medical Center approved this study.
Data Source
The Organ Procurement and Transplantation Network’s national computerized database as maintained by the United Network of Organ Sharing (UNOS) was used for this analysis (referred to as the UNOS database) (17). This contains data regarding every organ donation and transplant event occurring in the United States since October 1, 1987 (1). A supplemental file was obtained from UNOS containing 6MWD recordings for all lung transplant candidates since May 4, 2005 as used in the calculation of the LAS for these candidates.
Study Population
All lung transplant recipients per the UNOS database were included for analysis. Pediatric recipients (age < 18 yr), multiorgan, en bloc, lobar, and repeat transplants were excluded. The study period included transplants performed from May 4, 2005 when 6MWD was first recorded as part of the LAS, through December 31, 2011 with follow-up through March 2012. Patients for whom 6MWD was not recorded were excluded.
Variable Definitions
Predictor variables
The primary predictor for our analysis was the most recent 6MWD recorded before transplantation. This is defined as the distance in feet the candidate can walk in 6 minutes while receiving supplemental oxygen required to maintain an oxygen saturation of 88% (18).
Baseline characteristics and risk factors
The UNOS database includes donor, recipient, and transplant-related characteristics. The following characteristics and risk factors were extracted from the dataset for analysis:
-
1.
Donor characteristics: Age, diabetes, hypertension, smoking history (>20 pack-years ever), cocaine use ever, terminal serum creatinine (mg/dl), body mass index (BMI) kg/m2, and Po2 on 100% inspired oxygen.
-
2.
Recipient characteristics: Age, sex, race/ethnicity, etiology of lung failure (obstructive disease, restrictive disease, cystic fibrosis/immunodeficiency, or pulmonary vascular disease), history of diabetes, history of hypertension, history of cerebrovascular disease, serum creatinine at the time of transplant (mg/dl), BMI (kg/m2), chronic steroid use pretransplant, pretransplant status (hospitalized, intensive care, or neither), requiring life support at the time of transplant (includes ventilator, extracorporeal membrane oxygenation, intravenous inotropes, intraaortic balloon pump, or inhaled nitric oxide), ventilator dependence at the time of transplant, LAS, pulmonary function and hemodynamic metrics, and days on the waitlist.
-
3.
Transplant characteristics: Type of transplant (single vs. double lung), human leukocyte antigen mismatch level, donor/recipient sex mismatch, donor/recipient race mismatch, donor/recipient cytomegalovirus mismatch (defined as donor cytomegalovirus positive and recipient cytomegalovirus negative), total ischemic time (hours), transplant year, and transplant center volume (calculated as a continuous variable based on the total number of lung transplants performed during the study period).
Outcome measures and follow-up
The primary outcome variable was overall survival. Survival information for each patient was ascertained from the date of transplantation until patient death, date of last follow-up, or the end of study period (March 31, 2012).
Study Design and Statistical Analysis
We performed a retrospective, observational cohort analysis of lung transplant recipients subject to inclusion/exclusion criteria as described previously. Baseline characteristics were described for the overall study population, with medians and 25th–75th percentiles (Q1–Q3) reported for continuous variables and proportions (frequency, percentage) for discrete variables.
The study population was stratified by 6MWD quartile and unadjusted patient survival rates were estimated using the product-limit (Kaplan-Meier) method (19) and compared among groups by the log-rank test. Multivariable Cox proportional hazards modeling (20) was used to assess the simultaneous effect of 6MWD (analyzed as a continuous variable) on risk of patient death, while adjusting for donor, recipient, and transplant-related risk factors. Covariates for risk adjustment were based on the Scientific Registry of Transplant Recipients adult 1-year lung transplant risk model (21). The Scientific Registry of Transplant Recipients 1-year survival model was chosen for consistency with the outcome measure used in the LAS calculation. Single versus double lung transplant and center volume were also included in the model. A listing of these covariates is provided with the results of the multivariable model. Hazard ratio and 95% confidence interval (CI) were calculated as measures of strength of association and precision, respectively.
Threshold values for 6MWD with respect to survival outcomes were further assessed by modeling 6MWD as a nominal variable in 200-ft increments and calculating Kaplan-Meier estimates of 30-day and 90-day mortality as well as multivariable-adjusted Cox proportional hazards ratio for cumulative risk of death.
The discriminatory accuracy of predicting 1-year mortality was evaluated by the area under the curve of the receiver operating characteristic (ROC) curve (22). Patients with a 6MWD of zero or patients with less than 1 year of follow-up time were censored.
A subanalysis was performed to assess the association between 6MWD and post- transplant survival by disease category (obstructive disease, restrictive disease, cystic fibrosis/immunodeficiency, or pulmonary vascular disease). Kaplan-Meier methods were used to estimate 30-day, 90-day, 1-year, and 3-year mortality (note that the sample size of the subgroups precluded use of the multivariable model and therefore these data are presented descriptively).
Statistical analyses were performed using JMP Version 10.0 (SAS Institute Inc., Cary, NC) and R version 2.15.1 (R Core Team 2012). For all comparisons, P values less than or equal to 0.05 were considered statistically significant. All P values are two-sided.
Results
Baseline Characteristics
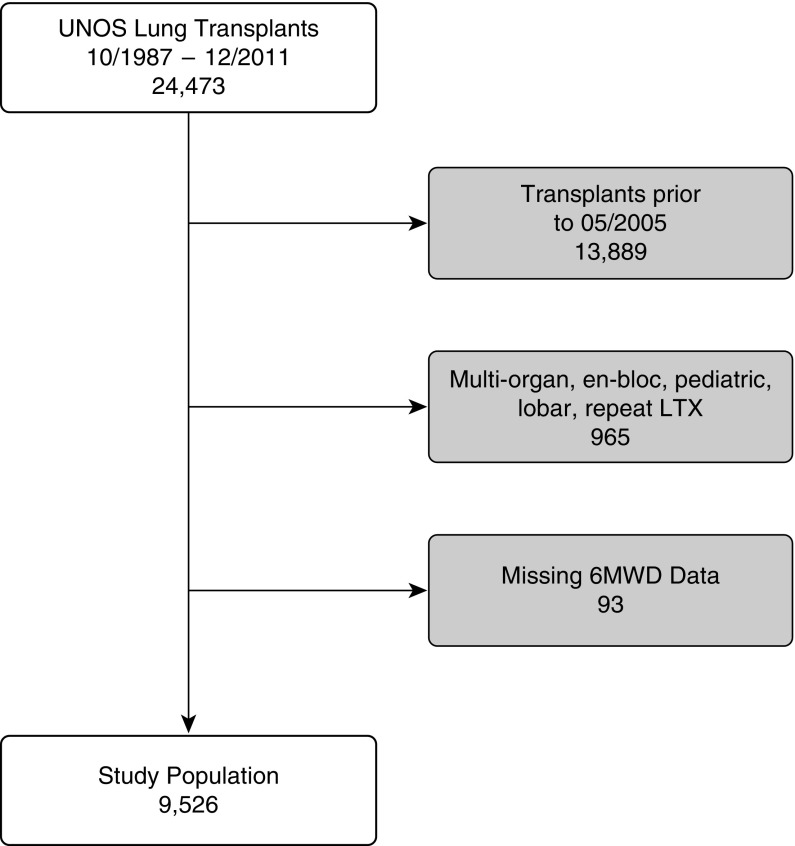
A total of 9,526 lung transplant recipients met inclusion criteria (see Figure 1 for study inclusion algorithm). Baseline donor, recipient, and transplant characteristics are demonstrated in Table 1. The median donor age was 32 years (Q1–Q3, 21–46) with a median BMI of 24.7 kg/m2 (Q1–Q3: 22.1–28.0) and 6.2% reported as having diabetes. Laboratory parameters demonstrated a terminal serum creatinine of 1.0 mg/dl (Q1–Q3, 0.8–1.3) and partial pressure of oxygen on 100% inspired oxygen of 422 mm Hg (Q1–Q3, 248–493).
Figure 1.
Study inclusion algorithm. 6MWD = 6-minute-walk distance; LTX = lung transplant; UNOS = United Network for Organ Sharing.
Table 1.
Baseline Characteristics for Entire Cohort (n = 9,526)
| Donor characteristics | |
| Donor age | 32 (21–46) |
| Donor diabetes | 592 (6.2%) |
| Donor smoking history (>20 pack‐years ever) | 1,168 (12.4%) |
| Donor cocaine use (ever) | 1,054 (11.3%) |
| Terminal serum creatinine, mg/dl | 1.0 (0.8–1.3) |
| Donor BMI, kg/m2 | 24.7 (22.1–28.0) |
| Po2 on 100% inspired oxygen, mm Hg | 422 (248–493) |
| Recipient characteristics | |
| Age, yr | 58 (49–64) |
| Age ≥ 60 yr | 4,276 (44.9%) |
| Female sex | 3,932 (41.3%) |
| Race | |
| White | 8,064 (84.7%) |
| Black | 818 (8.6%) |
| Hispanic | 463 (4.9%) |
| Asian | 117 (1.2%) |
| Other/unknown | 64 (0.7%) |
| Etiology of lung failure | |
| Obstructive disease | 3,363 (35.3%) |
| Restrictive disease | 4,650 (48.8%) |
| CF or immunodeficiency | 1,201 (12.6%) |
| Pulmonary vascular disease | 312 (3.3%) |
| Comorbidities | |
| Diabetes | 1,674 (17.7%) |
| Hypertension | 498/2,063 (24.1%) |
| Cerebrovascular disease | 19/2,048 (0.9%) |
| Creatinine at transplant, mg/dl | 0.8 (0.7–1.0) |
| BMI at transplant, kg/m2 | 25.1 (21.3–28.5) |
| Chronic steroid use pretransplant | 4,333 (47.5%) |
| Pretransplant status | |
| Hospitalized | 720 (7.6%) |
| Intensive care unit | 685 (7.3%) |
| Requiring life support at transplant* | 753 (8.%) |
| Ventilator dependent at transplant | 514 (5.4%) |
| Lung Allocation Score | 38.9 (34.3–47.7) |
| Pulmonary function and hemodynamics | |
| Oxygen requirement, L (n = 1,894) | 3 (2–4) |
| FEV1, % predicted | 34 (20–53) |
| FVC, % predicted | 46 (36–59) |
| FEV/FVC | 0.86 (0.46–1.09) |
| Mean PA pressure, mm Hg (n = 8,393) | 25 (20–31) |
| PVR, Wood units (n = 7,570) | 2.7 (1.9–3.9) |
| Cardiac index, L/min/m2 (n = 7,962) | 2.8 (2.4–3.3) |
| Days on waitlist | 76 (22–234) |
| Transplant characteristics | |
| Bilateral transplant | 6,170 (64.8%) |
| HLA mismatch level 3+ | 8,054/8,361 (96.3%) |
| Donor/recipient sex mismatch | 2,954 (31.%) |
| Donor/recipient race mismatch | 3,977 (41.8%) |
| Donor/recipient CMV mismatch | 2,254/8,867 (25.4%) |
| Ischemic time, h (n = 8,919) | 4.9 (3.9–6.0) |
Definition of abbreviations: BMI = body mass index; CF = cystic fibrosis; CMV = cytomegalovirus; PA = pulmonary artery; PVR = peripheral vascular resistance.
Median (25th–75th percentile) for nonparametric continuous variables and n (%) for categorical variables. If data are missing for >5% of the study population, the denominator is given for categorical variables and “n” given for continuous variables.
Includes ventilator, extracorporeal membrane oxygenation, intravenous inotropes, intraaortic balloon pump, or inhaled nitric oxide.
Recipients had a median age of 58 years (Q1–Q3, 49–64), 41.3% female, 8.6% black persons, 64.8% receiving bilateral transplant, with the most common etiology of lung failure as restrictive disease (48.8%) (Table 1). Diabetes was present in 17.7% of recipients. At the time of transplant, 7.3% of recipients required intensive care preoperatively with 5.4% ventilator dependent. The median LAS was 38.9 (Q1–Q3, 34.3–47.7), median FVC (% predicted) 46 (Q1–Q3, 36–59), and median days on the waitlist of 76 (Q1–Q3, 22–234).
Survival Outcomes
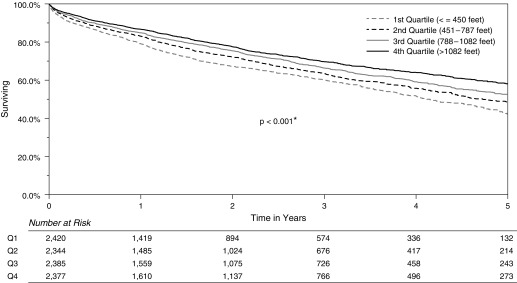
Using Kaplan-Meier methods, the median unadjusted survival was 4.1 years for patients in the first quartile for 6MWD (≤450 ft), 4.7 years for the second quartile (451–787 ft), 5.5 years for the third quartile (788–1,082 ft), and 5.8 years for the fourth quartile (>1,082 ft) (P < 0.001) (Figure 2). The results of the multivariable Cox proportional hazards model are demonstrated in Table 2. When analyzed as a continuous variable, the association between increased 6MWD and improved survival retained statistical significance (adjusted hazard ratio [AHR] per 200-ft increments, 0.96; 95% CI, 0.94–0.98; P < 0.001).
Figure 2.
Unadjusted Kaplan-Meier survival curves by 6-minute-walk distance quartiles. Survival information includes all deaths from the date of transplantation until the end of the study period. *Statistically significant.
Table 2.
Results of Multivariable Cox Proportional Hazards Model for Cumulative Risk of Post-transplant Death*
| Characteristic† | Adjusted Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Primary predictor variable | |||
| Six-minute-walk distance (per 200-ft increment) | 0.96 | 0.94–0.98 | <0.001‡ |
| Covariates | |||
| Donor characteristics | |||
| Age (per 5-yr increment) | 1.01 | 0.99–1.02 | 0.536 |
| Body surface area, m2 (per 0.1-m2 increment) | 0.97 | 0.96–0.99 | 0.001‡ |
| Diabetes (ref = no diabetes) | 1.30 | 1.12–1.51 | <0.001‡ |
| Black ethnicity (ref = white) | 1.26 | 1.15–1.38 | <0.001‡ |
| Recipient and transplant characteristics | |||
| Age (per 5-yr increment) | 1.06 | 1.04–1.08 | <0.001‡ |
| Male sex (ref = female) | 1.07 | 0.99–1.16 | 0.108 |
| Functional status no assistance (ref = full assistance) | 0.83 | 0.77–0.90 | <0.001‡ |
| Diagnosis category (ref = obstructive) | |||
| Pulmonary vascular | 1.33 | 1.07–1.65 | 0.012‡ |
| Cystic fibrosis or immunodeficiency | 1.21 | 1.02–1.44 | 0.025‡ |
| Restrictive | 1.07 | 0.98–1.17 | 0.125 |
| Sarcoidosis, mean PA pressure ≤30 mm Hg | 1.31 | 0.92–1.87 | 0.136 |
| Creatinine at transplant (per incremental mg/dl) | 1.15 | 1.09–1.21 | <0.001‡ |
| Ventilator dependent at transplant | 1.07 | 0.89–1.27 | 0.481 |
| Ischemic time squared (per 1 h increase) | 1.01 | 1.003–1.02 | 0.006‡ |
| Single lung transplant (ref = bilateral transplant) | 1.18 | 1.08–1.29 | <0.001‡ |
| Center volume (per increase of 10 LTX/yr) | 0.97 | 0.96–0.99 | <0.001‡ |
Definition of abbreviations: CI = confidence interval; LTX = lung transplant; PA = pulmonary artery.
Survival information includes all deaths from the date of transplantation until the end of the study period.
Covariates per the Scientific Registry of Transplant Recipients adult 1-year lung transplant risk model. Variables included in the model but not listed above are as follows: donor cause of death, specific diagnoses (separate variable for each: Eisenmenger syndrome, lymphangioleiomyomatosis, bronchiolitis obliterans in nonretransplants, bronchiectasis, pulmonary fibrosis, sarcoidosis with mean PA pressure >30 mm Hg, FVC % predicted), intensive care preoperatively versus hospitalized versus neither, oxygen requirement at rest, recipient body mass index, and recipient race/ethnicity.
Statistically significant.
Other covariates associated with improved survival were functional status requiring no assistance (AHR, 0.83; 95% CI, 0.77–0.90; P < 0.001) and increased center volume (AHR per increase in center volume of 10 lung transplants per year, 0.97; 95% CI, 0.96–0.99; P < 0.001).
Assessment of Threshold Values for 6MWD
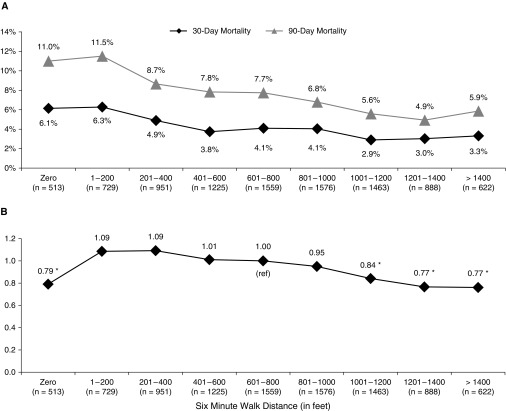
When analyzing threshold values based on 6MWD as a nominal variable in 200-ft increments, unadjusted 30-day mortality steadily decreased from 6.3% in those 1–200 ft to 2.9% and 3.0% in groups 1,001–1,200 ft and 1,201–1,400 ft, respectively (Figure 3A). Unadjusted 30-day mortality was slightly lower in the group with 6MWD of 0 ft (6.1%) compared with those 1–200 ft (6.3%). Similarly, unadjusted 90-day mortality decreased from 11.5% in those 1–200 ft to a low of 4.9% for the group 1,200–1,400 ft (Figure 3A). Unadjusted 90-day mortality was slightly lower in the group with 6MWD of 0 ft (11.0%) compared with those 1–200 ft (11.5%). The adjusted Cox proportional hazards ratio for each 200-ft range was calculated using the group 601–800 ft as the reference because this contains the median 6MWD of 787 ft. The AHR continuously declined from 1.09 for the group 1–200 ft to 0.77 in groups 1,201–1,400 and greater than 1,400 ft (Figure 3B). The AHR of those with a 6MWD of 0 ft (hazard ratio, 0.79) was similar to higher walk distance groups of 1,201–1,400 ft (0.77) and greater than 1,400 ft (0.77).
Figure 3.
(A) Perioperative mortality and (B) cumulative risk of death per 200-ft increments of 6-minute-walk distance (6MWD). Mortality rates for A were estimated using Kaplan-Meier methods. Please refer to Figure 2 for statistical comparison of Kaplan-Meier survival curves. The group 601–800 ft was chosen as a reference because it contains the median 6MWD (787 ft). Survival information for B includes all deaths from the date of transplantation until the end of the study period. Survival information for B represents adjusted risk of death based on covariates per the Scientific Registry of Transplant Recipients adult 1-year lung transplant risk model. This analysis of threshold values is based on 6MWD as a nominal variable in 200-ft increments compared to the median reference group. Ranges that are statistically significant (P < 0.05) when compared to the reference group are marked with an asterisk. ref = reference.
After censoring patients with a 6MWD of 0 (n = 513) or patients with less than 1-year of follow-up time (n = 1,944), a total of 7,069 patients were included in the dichotomization analysis. The median follow-up time was 2.1 years. The distance associated with maximal discriminative accuracy in predicting 1-year mortality on the ROC curve was 740 ft (area under the curve, 0.55977) (Figure 4).
Figure 4.
Receiver operating characteristics curve of 6-minute-walk distance prediction of 1-year mortality. Patients with 6-minute-walk distance = 0 or less than 1 year of follow-up time were censored. *The distance associated with maximal discriminative accuracy in predicting 1-year mortality based on sensitivity and specificity.
Subanalysis by Disease Category
Unadjusted 30-day, 90-day, 1-year, and 3-year Kaplan-Meier mortality estimates by disease category are presented in Figure 5. All disease categories demonstrated significantly longer survival with increasing 6MWD (P ≤ 0.009) except pulmonary vascular disease (P = 0.74).
Figure 5.
(A–D) Subanalysis by disease category of perioperative mortality per 200-ft increments of 6-minute-walk distance. Mortality rates were estimated using Kaplan-Meier methods. Statistical comparisons are based on the log-rank test using the following grouping: ≤450 ft, 451–787 ft, 788–1,082 ft, and >1,082 ft. *Statistically significant.
Discussion
Understanding and optimizing factors associated with successful lung transplantation is of particular importance with traits that may be modifiable. The principal findings of the current study indicate that increased 6MWD is strongly associated with lower perioperative mortality and longer overall survival after adjusting for potential confounders. Continuous increase in walk distance through 1,200–1,400 ft conferred an incremental survival advantage. These findings have important implications for evaluating an individual’s risk/benefit ratio for proceeding with transplantation, and also may guide preparations and recipient optimization in advance of surgery.
In the lung transplant population, the use of 6MWD has primarily focused on pretransplant mortality and evaluation of end-stage lung disease (10–14); however, cardiopulmonary exercise testing has previously been evaluated as an indicator of operative risk and predictor of postoperative mortality in other major surgical procedures (23–27). Extending these findings to lung transplant recipients provides important information for clinicians given the dramatic changes in cardiopulmonary physiology following transplantation. Additionally, a variety of measures have been used to assess fitness, including anaerobic threshold (23), maximum oxygen transport at peak exercise (24), and incremental shuttle walking (25). Our results indicate that 6MWD, despite dependence on patient motivation and “nonmaximal” nature of the test (4, 23, 25), also provides an appropriate metric for fitness given the strong association with survival in our data as well as ease of test administration. It has been proposed that exercise testing closely mimics the requirement for increased cardiac output in the postoperative situation to satisfy the increased oxygen demand as part of the neurohumoral stress response to surgery (23, 28), which may in part explain the association between 6MWD and survival observed in the current study.
Additionally, it is important to note that in our analysis, preoperative 6MWD is associated not only with perioperative outcomes but also long-term survival. The long-term association is perhaps less intuitive, and potentially relates to the well-documented association between a patient’s early postoperative course and overall long-term outcomes. This has been demonstrated with patients undergoing major abdominal surgery (29–31), cardiac surgery (32), and in the lung transplant literature (33, 34).
Threshold Values for Assessing Fitness to Undergo Major Surgery
A threshold 6MWD to stratify high-risk transplant candidates has not been well established. Cutoffs for waitlist mortality have been proposed ranging from 150 to 1,150 ft (6, 13, 15); however, these studies did not evaluate post-transplant survival thresholds. For major noncardiac surgery, 1,400 ft has been suggested for dichotomization of high perioperative risk in a single center study of 110 patients (35); however, this has not been validated in a larger sample nor confirmed in the lung transplant setting. It is important to note in our results that continuous increase in walk distance through 1,200–1,400 ft conferred an incremental survival advantage. Additionally, detecting marginal benefit to 6MWD greater than or equal to 1,400 could be limited in our dataset given the relatively low number of subjects in that range (<7%).
Furthermore, the area under the ROC curve of 0.55977 indicates relatively low accuracy of dichotomization in predicting 1-year mortality based on common measures used to assess discriminative ability (22). This indicates that although 6MWD strongly correlates with post- transplant survival, there is limited ability of a single value to predict mortality, and therefore the need to evaluate this variable continuously. Cumulatively, these findings suggest that 6MWD as a predictor of postoperative risk should be evaluated on a continuous basis in conjunction with other clinical parameters, and we would accordingly caution against dichotomization of this variable.
This is particularly important given that a survey of clinical practice of lung transplantation in North America by Levine (36) demonstrated that 58% of transplant programs had a minimum exercise capacity threshold to be listed for lung transplantation, with the most frequent cutoff value of a 6MWD of 600 ft. Although common practice, there is little evidence to support such a threshold. In our study, patients with the lowest 6MWD of 1–200 ft did not necessarily exhibit prohibitive risk compared with the median group (AHR, 1.09); however, when compared with groups above 1,200 ft (AHR, 0.77), the difference is substantial. This further emphasizes the importance of the current study to provide a basis for incorporating 6MWD into transplant decisions.
Other methods to assess fitness for undergoing major surgery may warrant further study in the lung transplant population. Composite frailty index values have been reported for major cardiovascular surgery, for example based on advanced age, low BMI, anemia, history of stroke, and low total psoas volume (37), or impairment in activities of daily living, ambulation, and documented history of dementia (38). Additionally, comprehensive measures of overall burden of disease, such as sarcopenia, have demonstrated strong correlation with mortality after liver transplant (39). These measures are not well studied in lung transplant, and may warrant further research to provide insight in appropriate candidates for listing and optimal allocations of scarce allograft resources.
It is important to note that although the 6MWD value of over/under 150 ft used in the LAS was not predictive of adjusted cumulative risk of death in our results (data not shown), the purpose of this variable in the LAS calculation is solely related to waitlist mortality and not post-transplant survival (15). Evaluating waitlist mortality is outside the scope of the current study precluding comment on the appropriateness of this cutoff in the LAS calculation. However, the disparity between the 6MWD of 150 ft used in the LAS calculation and the results of our study demonstrating incremental survival benefit through 1,400 ft further highlights the complex balance of waitlist mortality versus post-transplant survival inherent in lung allograft allocation. Our study may therefore inform the debate regarding future revisions to the lung allocation system to optimize organ distribution.
At our institution we advocate aggressive pulmonary rehabilitation before listing for lung transplantation. In general, our protocol involves targeted physical therapy (typically performed on our campus after relocation as necessary) with a goal 6MWD of 1,000 ft, although exceptions are made if clinically necessary. The results of the current study support this practice and may warrant consideration of similar protocols by other centers. The subanalysis by disease category seems to indicate a clear advantage to higher 6MWD for obstructive disease, restrictive disease, and cystic fibrosis/immunodeficiency. Although a statistically significant benefit to higher 6MWD was not apparent in pulmonary vascular disease, the relatively low volume of patients in this category (n = 312; 3.3%) may limit the ability to detect an association in this subgroup.
6MWD of Zero
It is interesting to note that on multivariable-adjusted analysis, patients in our data set with 6MWD of 0 ft demonstrated reduced risk of death relative to the median walk distance that was similar to that of patients in the highest walk-distance categories greater than 1,200 ft. Despite extensive studies regarding 6MWD in the transplant (10, 11, 13, 14) and nontransplant literature (4, 7, 9, 40, 41), to our knowledge patients with 6MWD of zero have not previously been analyzed as a distinct subgroup. Furthermore, there is no guidance on how to address this population for purposes of the LAS (15). The current analysis therefore provides novel information to the transplant community not only to highlight the association between 6MWD of zero and survival compared with other 6MWD distance categories, but also to call attention to the importance of determining why a patient has a 6MWD of zero to stratify risk for transplant priority and guide patient and clinician decisions on proceeding with transplantation.
Additional data provided by UNOS reveal that of the 513 recipients with a 6MWD of zero, 218 (42%) had a previous 6MWD recorded that was greater than zero, and 206 of the 218 (94%) were within 1 year of transplant. Of these, 30% (66 of 218) had a previous 6MWD greater than the study population median of 787 ft, and 16% (35 of 218) had a previous 6MWD greater than 1,000 ft, ranging from 25 days to 1 year before transplantation. Although definitive conclusions cannot be drawn from these data, and analysis of the trend in 6MWD preceding transplant is outside the scope of the current study, this does highlight the need to better understand the surrounding circumstances contributing to a 6MWD of zero. The integrity of these recordings would also warrant scrutiny. Details as to why a given patient had a 6MWD of zero are unavailable in our dataset. Therefore, care should be taken when interpreting the results of this group given the inability to distinguish whether zero walk distance is related to frailty, ventilator dependence, reliance on extracorporeal membrane oxygenation, or some other impediment to ambulation. The results of this study are not significantly altered when the 6MWD of 0 ft (n = 513) is excluded (data not shown).
Study Limitations
These results should be interpreted in the context of the study’s limitations. First, trends in a given patient’s 6MWD before surgery are not analyzed. Pretransplant implications of 6MWD are not evaluated. Additionally, outcomes other than survival are not included in our study, and given the retrospective nature of this review, unmeasured confounders may exist. Despite these limitations, however, our series of more than 9,500 lung transplant recipients represents the largest clinical study evaluating a consistently measured metric of exercise fitness for purposes of evaluating postoperative outcomes. These data may better enable clinicians to evaluate operative risk, guide discussions with patients and families regarding treatment options, and aid clinical decision making for lung transplant candidates and patients considering other major surgical procedures.
Conclusions
6MWD is significantly associated with postoperative survival following lung transplantation, with a continuous increase in walk distance through 1,200–1,400 ft conferring an incremental survival advantage. Although 6MWD strongly correlated with survival, the impact of a single dichotomous value to predict outcomes was limited, and therefore caution against dichotomization of this variable is advised. Strategies to improve patient functional status preoperatively, including aggressive rehabilitation efforts, could potentially impact postoperative survival. Evaluation of trends in 6MWD preceding transplant, implications regarding waitlist mortality, and other methods to evaluate fitness to undergo major surgery require further study.
Footnotes
In accordance with the United Network of Organ Sharing and U.S. Lung Allocation score metrics, distances are quantified in feet. The conversion from feet to meters is 3.28 ft = 1 m.
By use of the UNOS database, this work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content herein is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author Contributions: A.W.C., B.R.E., and M.W. contributed to conception and design, acquisition of data, analysis and interpretation of data, and drafting and revising the article. A.A.O. and B.C.G. contributed to acquisition of data, analysis and interpretation of data, and drafting and revising the article. L.D.S., S.M.P., R.D.D., and M.G.H. contributed to conception and design, interpretation of data, and writing and/or revising the article critically for important intellectual content. All authors provided final approval.
Originally Published in Press as DOI: 10.1164/rccm.201409-1698OC on June 11, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration; 2012. (SRTR) 2011 Annual Data Report; p. 13. [Google Scholar]
- 2.Yusen RD, Shearon TH, Qian Y, Kotloff R, Barr ML, Sweet S, Dyke DB, Murray S. Lung transplantation in the United States, 1999-2008. Am J Transplant. 2010;10:1047–1068. doi: 10.1111/j.1600-6143.2010.03055.x. [DOI] [PubMed] [Google Scholar]
- 3.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report—2011. J Heart Lung Transplant. 2011;30:1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 5.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 6.Cote CG, Casanova C, Marín JM, Lopez MV, Pinto-Plata V, de Oca MM, Dordelly LJ, Nekach H, Celli BR. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J. 2008;31:571–578. doi: 10.1183/09031936.00104507. [DOI] [PubMed] [Google Scholar]
- 7.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Sahn SA, Szwarcberg J, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, Nakanishi N, Miyatake K. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 9.Paciocco G, Martinez FJ, Bossone E, Pielsticker E, Gillespie B, Rubenfire M. Oxygen desaturation on the six-minute walk test and mortality in untreated primary pulmonary hypertension. Eur Respir J. 2001;17:647–652. doi: 10.1183/09031936.01.17406470. [DOI] [PubMed] [Google Scholar]
- 10.Kadikar A, Maurer J, Kesten S. The six-minute walk test: a guide to assessment for lung transplantation. J Heart Lung Transplant. 1997;16:313–319. [PubMed] [Google Scholar]
- 11.Kawut SM, O’Shea MK, Bartels MN, Wilt JS, Sonett JR, Arcasoy SM. Exercise testing determines survival in patients with diffuse parenchymal lung disease evaluated for lung transplantation. Respir Med. 2005;99:1431–1439. doi: 10.1016/j.rmed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Belkin RA, Henig NR, Singer LG, Chaparro C, Rubenstein RC, Xie SX, Yee JY, Kotloff RM, Lipson DA, Bunin GR. Risk factors for death of patients with cystic fibrosis awaiting lung transplantation. Am J Respir Crit Care Med. 2006;173:659–666. doi: 10.1164/rccm.200410-1369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lederer DJ, Arcasoy SM, Wilt JS, D’Ovidio F, Sonett JR, Kawut SM. Six-minute-walk distance predicts waiting list survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:659–664. doi: 10.1164/rccm.200604-520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinu T, Babyak MA, O’Connell CF, Carney RM, Trulock EP, Davis RD, Blumenthal JA, Palmer SM INSPIRE Investigators. Baseline 6-min walk distance predicts survival in lung transplant candidates. Am J Transplant. 2008;8:1498–1505. doi: 10.1111/j.1600-6143.2008.02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 16.Castleberry AW, Englum BR, Snyder LD, Worni M, Osho AW, Pietrobon R, Palmer SM, Davis RD, Hartwig MG. Utility of six-minute walk distance in predicting outcomes after lung transplant: a nationwide survival analysis [abstract] J Heart Lung Transpl. 2013;32(Suppl 4S):S147. [Google Scholar]
- 17.Brown RS, Belton AM, Martin JM, Simmons DD, Taylor GJ, Willard E. Evolution of quality at the Organ Center of the Organ Procurement and Transplantation Network/United Network for Organ Sharing. Prog Transplant. 2009;19:221–226. doi: 10.1177/152692480901900306. [DOI] [PubMed] [Google Scholar]
- 18.Policy 3.7.6.4 - Organ Procurement and Transplantation Network [accessed 2013 April 12]. Available from: http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_9.pdf
- 19.Kaplan EL. Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Cox DR. Regression models and life-tables (with discussion) J R Stat Soc Ser B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 21.The SRTR Risk Model Documentation. Lung, adult, one-year patient survival [accessed 2014 Apr 21]. Available from: http://www.srtr.org/csr/current/Centers/201402_1401/modtabs/Risk/LUADA2P.pdf
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 23.Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999;116:355–362. doi: 10.1378/chest.116.2.355. [DOI] [PubMed] [Google Scholar]
- 24.Win T, Jackson A, Sharples L, Groves AM, Wells FC, Ritchie AJ, Laroche CM. Cardiopulmonary exercise tests and lung cancer surgical outcome. Chest. 2005;127:1159–1165. doi: 10.1378/chest.127.4.1159. [DOI] [PubMed] [Google Scholar]
- 25.Murray P, Whiting P, Hutchinson SP, Ackroyd R, Stoddard CJ, Billings C. Preoperative shuttle walking testing and outcome after oesophagogastrectomy. Br J Anaesth. 2007;99:809–811. doi: 10.1093/bja/aem305. [DOI] [PubMed] [Google Scholar]
- 26.Carlisle J, Swart M. Mid-term survival after abdominal aortic aneurysm surgery predicted by cardiopulmonary exercise testing. Br J Surg. 2007;94:966–969. doi: 10.1002/bjs.5734. [DOI] [PubMed] [Google Scholar]
- 27.Struthers R, Erasmus P, Holmes K, Warman P, Collingwood A, Sneyd JR. Assessing fitness for surgery: a comparison of questionnaire, incremental shuttle walk, and cardiopulmonary exercise testing in general surgical patients. Br J Anaesth. 2008;101:774–780. doi: 10.1093/bja/aen310. [DOI] [PubMed] [Google Scholar]
- 28.Ridgway ZA, Howell SJ. Cardiopulmonary exercise testing: a review of methods and applications in surgical patients. Eur J Anaesthesiol. 2010;27:858–865. doi: 10.1097/EJA.0b013e32833c5b05. [DOI] [PubMed] [Google Scholar]
- 29.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–341, discussion 341–343. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chok KS, Ng KK, Poon RT, Lo CM, Fan ST. Impact of postoperative complications on long-term outcome of curative resection for hepatocellular carcinoma. Br J Surg. 2009;96:81–87. doi: 10.1002/bjs.6358. [DOI] [PubMed] [Google Scholar]
- 31.Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–2566. doi: 10.1245/s10434-007-9434-4. [DOI] [PubMed] [Google Scholar]
- 32.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16:195–200. doi: 10.1681/ASN.2003100875. [DOI] [PubMed] [Google Scholar]
- 33.Broekroelofs J, Navis GJ, Stegeman CA, van der Bij W, de Boer WJ, de Zeeuw D, de Jong PE. Long-term renal outcome after lung transplantation is predicted by the 1-month postoperative renal function loss. Transplantation. 2000;69:1624–1628. doi: 10.1097/00007890-200004270-00017. [DOI] [PubMed] [Google Scholar]
- 34.Castleberry AW, Worni M, Kuchibhatla M, Lin SS, Snyder LD, Shofer SL, Palmer SM, Pietrobon R, Davis RD, Hartwig MG. A comparative analysis of bronchial stricture after lung transplantation in recipients with and without early acute rejection. Ann Thorac Surg. 2013;96:1008–1017, discussion 1017–1018. doi: 10.1016/j.athoracsur.2013.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinclair RCF, Batterham AM, Davies S, Cawthorn L, Danjoux GR. Validity of the 6 min walk test in prediction of the anaerobic threshold before major non-cardiac surgery. Br J Anaesth. 2012;108:30–35. doi: 10.1093/bja/aer322. [DOI] [PubMed] [Google Scholar]
- 36.Levine SM Transplant/Immunology Network of the American College of Chest Physicians. A survey of clinical practice of lung transplantation in North America. Chest. 2004;125:1224–1238. doi: 10.1378/chest.125.4.1224. [DOI] [PubMed] [Google Scholar]
- 37.Ganapathi AM, Englum BR, Hanna JM, Schechter MA, Gaca JG, Hurwitz LM, Hughes GC. Frailty and risk in proximal aortic surgery. J Thorac Cardiovasc Surg. 2014;147:186–191.e1. doi: 10.1016/j.jtcvs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee DH, Buth KJ, Martin B-J, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 39.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Feo S, Tramarin R, Lorusso R, Faggiano P. Six-minute walking test after cardiac surgery: instructions for an appropriate use. Eur J Cardiovasc Prev Rehabil. 2009;16:144–149. doi: 10.1097/HJR.0b013e328321312e. [DOI] [PubMed] [Google Scholar]
- 41.Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med. 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]