To the Editor:
Blau syndrome, a rare autosomal dominant disorder, presents in early childhood with granulomatous arthritis, dermatitis, and uveitis (1). Blau has been strongly associated with mutations in the NOD2 gene (nucleotide-binding oligomerization domain–containing protein 2) (2), a member of the NOD-like receptor family involved in innate immunity. Blau shares similarities in phenotype and histological findings (presence of noncaseating granulomas) with sarcoidosis, another multisystemic granulomatous disorder. However, it differs in mode of inheritance, onset age, and pattern of organ involvement. Sarcoidosis rarely presents in childhood (3) and involves the lungs in more than 90% of cases, although any organ may be affected (e.g., bone/joints, skin) (3).
Despite these similarities, studies of patients with adult sarcoidosis have failed to find an association with NOD2 (4). In this study, we assess the role of genes within the NOD2 pathway in patients with adult sarcoidosis exhibiting similar pattern of organ involvement to Blau (eye, skin, bone/joint).
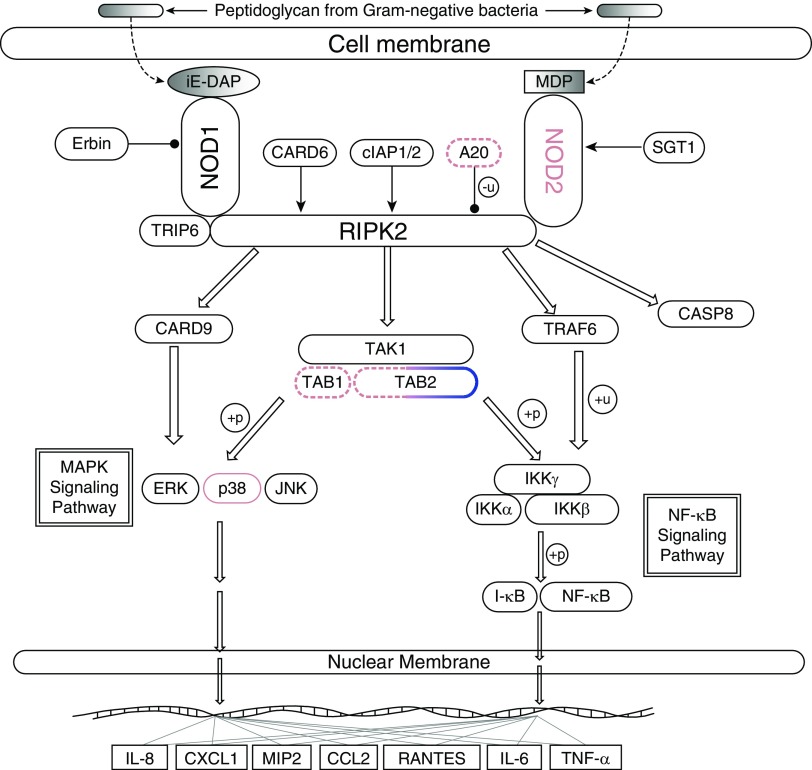
Methods
Analysis was performed independently for European American (EA) and African American (AA) sarcoidosis cases. AA cases (n = 1273) were compiled from the Sarcoidosis Genetic Analysis study population (5) and the National Heart, Lung, and Blood Institute’s ACCESS (A Case Controlled Etiologic Study of Sarcoidosis) study (6), and a cohort from the Detroit Henry Ford Health System. EA cases (n = 442) were obtained from the ACCESS study. Analysis of each cohort involved the comparison of cases positive for both skin and bone/joint involvement to those negative for both. Using EMMAX (Efficient Mixed-Model Association eXpedited) (7), single-marker sex-adjusted association tests were performed for the single-nucleotide polymorphisms (SNPs) in a number of genes selected from the NOD2 pathway (Figure 1). The NOD2 pathway has a total of 30 components, but our analysis excluded genes encoding pathway byproducts (e.g., chemokines), leaving 23 genes (covering 7,615 SNPs for AA and 2,612 for EA). The effective number of independent tests (Neff) was estimated by computing the number of linkage disequilibrium (LD) blocks (LD threshold: r2 = 0.5) in our SNP data, and Bonferroni correction was used to adjust for multiple testing. Thresholds for suggestive associations in each analysis were chosen on the basis of the expectation of a single false-positive association per analysis.
Figure 1.
NOD2 pathway. Genes found to harbor significant/suggestive single-nucleotide polymorphism (SNPs) are highlighted with pink (for African Americans) or blue (for European Americans) borders. Dashed borders are used to denote genes with only suggestive SNPs. Lines with circular tips denote inhibitory effects. A20 = TNFAIP3 (tumor necrosis factor-α–induced protein 3); MAPK = mitogen-activated protein kinase; NOD2 = nucleotide-binding oligomerization domain–containing protein 2; p38 = p38 MAPK; TAB = TAK1-binding protein.
Results and Discussion
No significant associations were seen in NOD2 in both AA and EA analyses. For EA, we observed novel significant associations in four variants within the TAB2 gene. These variants were in complete LD (r2 = 1). For AA, we observed a significant genetic association in a MAPK13, a gene encoding p38-δ, one of the four isoforms of the p38 MAP kinase.
Table 1 provides information on the SNPs, and Figure 1 provides a graphical overview of the pathway components harboring significant and/or suggestive variants in either cohort.
Table 1.
NOD2 Pathway SNPs Exhibiting Significant or Suggestive Associations with a Blau Syndrome–like Phenotype in Adult Sarcoidosis Cases
| Cohort | Chromosome | Gene | SNP | Position (hg19) | Reference/Alternate Allele | P Value |
|---|---|---|---|---|---|---|
| European American | 6 | TAB2 | rs76778446 | 149646830 | C/T | 1.30 × 10−5* |
| rs79995379 | 149650461 | A/G | ||||
| rs111447766 | 149689465 | A/G | ||||
| rs111576955 | 149697332 | C/T | ||||
| African American | 6 | TAB2 | rs9404026 | 149616799 | C/G | 1.91 × 10−4 |
| MAPK13 | rs138268427 | 36109934 | G/A | 8.45 × 10−7* | ||
| 22 | TAB1 | rs35506409 | 39812207 | C/G | 4.71 × 10−5 | |
| rs34804656 | 39827556 | G/A | 5.37 × 10−4 |
Definition of abbreviations: MAPK13 = mitogen-activated protein kinase 13; NOD2 = nucleotide-binding oligomerization domain–containing protein 2; SNP = single-nucleotide polymorphism; TAB = TAK1-binding protein.
Statistically significant P values.
The four perfectly correlated SNPs identified in the EA cohort analysis reside in a large haplotype block within the TAB2 (TAK1-binding protein 2) gene. In the AA cohort, we also discovered a suggestive association in the TAB2 gene. Although there is no overlap between the TAB2 SNPs identified in either cohort, the association of this gene in both race groups suggests its potential relevance to the subphenotype of interest and provides evidence of “gene-level replication.” Our AA analysis also revealed suggestive associations for SNPs in TAB1 (TAK1-binding protein 1), a functional counterpart of TAB2. TAB1 and TAB2 bind to TAK1 to form a complex that functions as a single unit. These results suggest a key role for the TAK1–TAB1–TAB2 complex in the intracellular signaling cascade initiated by NOD2. The complex is known to be involved in macrophage (a component of granulomas) activation, and deletion of TAB1/TAB2 results in macrophage death (8). Studies have also shown this complex plays regulatory roles in the epidermal and intestinal epithelium (9) and in joint and cartilage development (10).
We also observed a suggestive associated variant in TNFAIP3 (tumor necrosis factor α–induced protein 3), a gene encoding A20, a ubiquitin-editing enzyme that has been shown to inhibit NF-κB activation as well as TNF-mediated apoptosis.
All variants showing significant/suggestive associations were located in noncoding regions. Functional annotation using HaploReg and RegulomeDB revealed enrichment for transcription factor binding sites, enhancer/promoter histone modification, and DNase hypersensitivity in different cell types.
This study is the first to fully explore the NOD2 signaling pathway in search of downstream components that might play a role in the granulomatous inflammation common to Blau and sarcoidosis. Our findings will guide further investigations into the mechanisms leading to Blau-like manifestations of sarcoidosis. Although we further confirm that the NOD2 gene itself is not a contributor to sarcoidosis, we do provide evidence indicating the NOD2 pathway is involved.
Footnotes
Author Contributions: Study conception and design, C.M., M.C.I., and B.A.R.; data acquisition, B.A.R.; data analysis, G.A.B. and G.G.D.; drafting and editing of the manuscript, G.A.B., I.A., B.A.R., A.M.L., M.C.I., and C.M.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Rybicki BA, Maliarik MJ, Bock CH, Elston RC, Baughman RP, Kimani AP, Sheffer RG, Chen KM, Major M, Popovich J, Jr, et al. The Blau syndrome gene is not a major risk factor for sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:203–208. [PubMed] [Google Scholar]
- 2.Wouters CH, Maes A, Foley KP, Bertin J, Rose CD. Blau syndrome, the prototypic auto-inflammatory granulomatous disease. Pediatr Rheumatol Online J. 2014;12:33. doi: 10.1186/1546-0096-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 4.Adrianto I, Lin CP, Hale JJ, Levin AM, Datta I, Parker R, Adler A, Kelly JA, Kaufman KM, Lessard CJ, et al. Genome-wide association study of African and European Americans implicates multiple shared and ethnic specific loci in sarcoidosis susceptibility. PLoS One. 2012;7:e43907. doi: 10.1371/journal.pone.0043907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybicki BA, Hirst K, Iyengar SK, Barnard JG, Judson MA, Rose CS, Donohue JF, Kavuru MS, Rabin DL, Rossman MD, et al. A sarcoidosis genetic linkage consortium: the sarcoidosis genetic analysis (SAGA) study. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22:115–122. [PubMed] [Google Scholar]
- 6.ACCESS Research Group. Design of a case control etiologic study of sarcoidosis (ACCESS) J Clin Epidemiol. 1999;52:1173–1186. doi: 10.1016/s0895-4356(99)00142-0. [DOI] [PubMed] [Google Scholar]
- 7.Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, Sabatti C, Eskin E. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42:348–354. doi: 10.1038/ng.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mihaly SR, Morioka S, Ninomiya-Tsuji J, Takaesu G. Activated macrophage survival is coordinated by TAK1 binding proteins. PLoS One. 2014;9:e94982. doi: 10.1371/journal.pone.0094982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem. 2006;281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunnell LM, Jonason JH, Loiselle AE, Kohn A, Schwarz EM, Hilton MJ, O'Keefe RJ. TAK1 regulates cartilage and joint development via the MAPK and BMP signaling pathways. J Bone Miner Res. 2010;25:1784–1797. doi: 10.1002/jbmr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]