Abstract
Rationale: Lung cancer (LC) screening using low-dose chest computed tomography is now recommended in several guidelines using the National Lung Screening Trial (NLST) entry criteria (age, 55–74; ≥30 pack-years; tobacco cessation within the previous 15 yr for former smokers). Concerns exist about their lack of sensitivity.
Objectives: To evaluate the performance of NLST criteria in two different LC screening studies from Europe and the United States, and to explore the effect of using emphysema as a complementary criterion.
Methods: Participants from the Pamplona International Early Lung Action Detection Program (P-IELCAP; n = 3,061) and the Pittsburgh Lung Screening Study (PLuSS; n = 3,638) were considered. LC cumulative frequencies, incidence densities, and annual detection rates were calculated in three hypothetical cohorts, including subjects who met NLST criteria alone, those with computed tomography–detected emphysema, and those who met NLST criteria and/or had emphysema.
Measurements and Main Results: Thirty-six percent and 59% of P-IELCAP and PLuSS participants, respectively, met NLST criteria. Among these, higher LC incidence densities and detection rates were observed. However, applying NLST criteria to our original cohorts would miss as many as 39% of all LC. Annual screening of subjects meeting either NLST criteria or having emphysema detected most cancers (88% and 95% of incident LC of P-IELCAP and PLuSS, respectively) despite reducing the number of screened participants by as much as 52%.
Conclusions: LC screening based solely on NLST criteria could miss a significant number of LC cases. Combining NLST criteria and emphysema to select screening candidates results in higher LC detection rates and a lower number of cancers missed.
Keywords: National Lung Screening Trial, lung cancer screening, emphysema, low-dose computed tomography
At a Glance Commentary
Scientific Knowledge on the Subject
Lung cancer screening using low-dose chest computed tomography is now recommended by several guidelines and mainly relies on National Lung Screening Trial entry criteria. Concerns exist about their lack of sensitivity in detecting lung cancer cases.
What This Study Adds to the Field
In comparison with screening based solely on National Lung Screening Trial criteria, complementing these with radiographic emphysema improves lung cancer detection rates in annual lung cancer screening rounds with fewer cancers undetected.
Recently, the National Lung Screening Trial (NLST) showed that lung cancer screening using low-dose computed tomography (LDCT) of the chest resulted in a significant reduction in lung cancer mortality (1). Since then, the U.S. Preventive Services Task Force has recommended LDCT screening for lung cancer (2), joining the chorus of other stakeholders who had previously advocated for lung cancer screening (3–8). Most of the recommendations rely on the NLST entry criteria. However, several researchers have argued that these criteria might lack sensitivity (9), and that additional variables not considered in the NLST could improve the selection process (10). For instance, Pinsky and Berg (11) reported that only 27% of patients with lung cancer from several U.S. registries met NLST criteria. One approach to improve this is to implement individualized risk assessment in those with the highest risk for lung cancer for whom LDCT screening is more efficient (10, 12).
Airway obstruction (AO) and emphysema have been proposed as important risk factors for developing lung cancer in a lung cancer screening setting (13–15). However, both have been neglected by current guidelines identifying the target population that should undergo screening. Recently, Kovalchik and colleagues (10) used emphysema as a criterion to further refine NLST entry criteria, narrowing even more the trial’s selection criteria. Considering the NLST’s sensitivity limitations, this could be an important issue.
There are no data available on how NLST inclusion criteria perform in existing LDCT screening programs. Our study explores the impact of NLST criteria on lung cancer detection rates in two different lung cancer screening studies from Europe and the United States, and investigates the potential role of emphysema as a complementary selection criterion for lung cancer screening. Some of the results of this study have been previously reported in the form of an abstract (16).
Methods
Participants
Participants included in this study were recruited into two different lung cancer screening studies using LDCT: the Pamplona International Early Lung Cancer Detection Program (P-IELCAP), in Spain; and the Pittsburgh Lung Screening Study (PLuSS) in the United States.
Individuals entering the P-IELCAP were enrolled between September 2000 and September 2014, as part of the International Early Lung Cancer Action Program (17). Detailed information regarding individual enrollment, emphysema, AO, and lung cancer assessments has been previously described (13, 17). Briefly, men and women 40 years of age or older, who were current or former smokers with a tobacco history of more than 10 pack-years, and without lung cancer symptoms, were included. LDCT and spirometry were performed at baseline, and at annual or earlier follow-up visits according to the established protocol (18). There was no limit to the number of LDCT screening rounds per participant. Only lung cancers diagnosed within 12 months of a suspicious LDCT were included in the analysis (screen-detected cancers). Emphysema was visually assessed by two chest radiologists (κ coefficient, 0.91) (19). Any trace of emphysema was considered positive. AO was defined according to the Global Initiative for Chronic Obstructive Lung Disease recommendations (20). The ethics committee of the University of Navarra approved the study protocol and all subjects signed an informed consent prior to entry.
Details regarding subject enrollment, AO, emphysema, and lung cancer assessment in the PLuSS have also been previously described (14, 21). The study enrolled subjects between January 2002 and April 2005, who were between 50 and 79 years of age, current or former smokers with at least 12.5 pack-years of smoking, and with no personal lung cancer history. All participants underwent a baseline and one annual LDCT screening round. A high-risk subgroup was selected to continue further screening rounds (calculated 5-yr lung cancer risk >2.5% [22] based on the presence of moderate or severe emphysema [>25% and 50% emphysema on LDCT, respectively] [14], chronic obstructive pulmonary disease [COPD] Global Initiative for Chronic Obstructive Lung Disease grades 3 or 4, or more than two first-degree relatives with lung cancer). Only screen-detected lung cancers were included in the analysis. Emphysema was visually assessed by three readers with an overall kappa statistic of 0.68 (14). Any trace of emphysema was considered positive. All subjects signed an informed consent and the institutional review board for the University of Pittsburgh approved the study.
NLST Testing
NLST inclusion criteria (age between 55 and 74 yr, at least 30 pack-years of smoking history, and if former smokers had quit within the previous 15 yr) were applied to both cohorts (1).
Emphysema as an Intermediate Selection Criterion
Once the first screening round was completed, the presence or absence of emphysema was assessed in all participants. Each cohort was then reassessed regarding further annual screening, defining two subgroups as those who had emphysema independent of NLST criteria (P-IELCAP[E] and PLuSS[E]); and a combined subgroup of those who met NLST criteria and/or had emphysema on baseline CT imaging (NLST/E).
Data Analysis
Quantitative data with normal distribution are presented as mean ± SD, and nonnormal data are presented as median and the interquartile range. Categorical data are described using relative frequencies. Comparisons between the two study groups are performed using Pearson chi-square or Student t test, according to variable type and distribution.
Lung cancer incidence densities per 1,000 person-years and lung cancer detection rates are calculated according to the baseline selection criteria used (P-IELCAP, PLuSS, and NLST) and the COPD-emphysema variants. The inverse of the detection rate estimates the number of people needed to be screened in a year to detect one lung cancer (NND) (23).
Incidence densities and detection rates were calculated with 95% confidence intervals (CI). A P value of less than 0.05 was considered statistically significant and all statistical analyses were performed using SPSS Statistics 20.0 for Windows (IBM, Chicago, IL).
Results
A total of 6,699 individuals were screened (3,061 in P-IELCAP, and 3,638 in PLuSS). The baseline characteristics and differences between both study groups are shown in Table 1. In general, there were significant differences in almost all of the characteristics recorded. When compared with PLuSS, the P-IELCAP population had more males, was significantly younger, and had a less intense smoking history. In comparison with PLuSS, lung function status at baseline was better in the P-IELCAP cohort (96 ± 18 vs. 82 ± 19 of FEV1% predicted; P < 0.001, respectively), with a 25% prevalence of AO, as compared with a 43% prevalence. The prevalence of emphysema, as detected by LDCT, was lower in the P-IELCAP cohort than in PLuSS (24% and 43%, respectively; P < 0.001).
Table 1.
Baseline Characteristics of Participants in Both Studies
| Characteristic | P-IELCAP (n = 3,061) | PLuSS (n = 3,638) | P Value |
|---|---|---|---|
| Age | 55 (49–62) | 58 (54–64) | <0.001 |
| Male, % | 73 | 51 | <0.001 |
| BMI | 28 ± 4 | 29 ± 5 | <0.001 |
| Pack-years | 32 (21–47) | 48 (33–63) | <0.001 |
| Current smoker, % | 64 | 65 | 0.26 |
| Time to last cigarette, yr | 6 (2–14) | 5 (2–8) | <0.001 |
| LDCT screening rounds | 2 (1–3) | 3 (2–4) | <0.001 |
| Lung cancer family history, % | 19 | 18 | 0.50 |
| Emphysema evidenced by LDCT, % | 24 | 43 | <0.001 |
| Number of lung cancers detected | 54 | 96 | 0.02 |
| Subjects meeting NLST criteria, n (%) | 1,112 (36) | 2,161 (59) | <0.001 |
Definition of abbreviations: BMI = body mass index; LDCT = low-dose chest computed tomography; NLST = National Lung Screening Trial; P-IELCAP = Pamplona International Early Lung Cancer Action Program; PLuSS = Pittsburgh Lung Screening Study.
Data are expressed as mean ± SD or median (interquartile range) if not otherwise indicated.
About one-third of the P-IELCAP cohort and 59% of PLuSS participants met NLST entry criteria. As expected, in comparison with individuals not meeting NLST criteria, those who did were older and had significantly greater tobacco exposure. Furthermore, in both cohorts individuals in the NLST-compliant subgroup had worse lung function and significantly more AO and emphysema (Table 2).
Table 2.
Baseline Characteristics of Study Participants According to Compliance of NLST Entry Criteria
| Characteristic | P-IELCAP |
PLuSS |
||||
|---|---|---|---|---|---|---|
| NLST Compliant (n = 1,112) | NLST Noncompliant (n = 1,949) | P Value | NLST Compliant (n = 2,161) | NLST Noncompliant (n = 1,477) | P Value | |
| Age | 61 (57–66) | 51 (46–55) | <0.001 | 61 (58–66) | 53 (51–56) | <0.001 |
| Male, % | 77 | 70 | <0.001 | 54 | 48 | 0.002 |
| BMI | 28 ± 4 | 27 ± 4 | <0.001 | 29 ± 5 | 29 ± 5 | 0.09 |
| Pack-years | 42 (33–60) | 26 (19–38) | <0.001 | 54 (43–68) | 36 (26–48) | <0.001 |
| Active smoker, % | 63 | 64 | 0.56 | 58 | 63 | 0.001 |
| Time to last cigarette, yr | 5 (1–8) | 9 (3–18) | <0.001 | 5 (2–8) | 5 (1–8) | 0.11 |
| LDCT screening rounds | 2 (1–4) | 2 (1–3) | 0.10 | 3 (2–5) | 2 (2–3) | <0.001 |
| Lung cancer family history, % | 16 | 20 | 0.02 | 17 | 19 | 0.16 |
| Emphysema by LDCT, % | 27 | 24 | <0.01 | 48 | 35 | <0.001 |
| No. of lung cancers | 33 | 21 | <0.001 | 77 | 19 | <0.001 |
Definition of abbreviations: BMI = body mass index; LDCT = low-dose chest computed tomography; NLST = National Lung Screening Trial; P-IELCAP = Pamplona International Early Lung Cancer Action Program; PLuSS = Pittsburgh Lung Screening Study.
Data are expressed as mean ± SD or median (interquartile range) if not otherwise indicated.
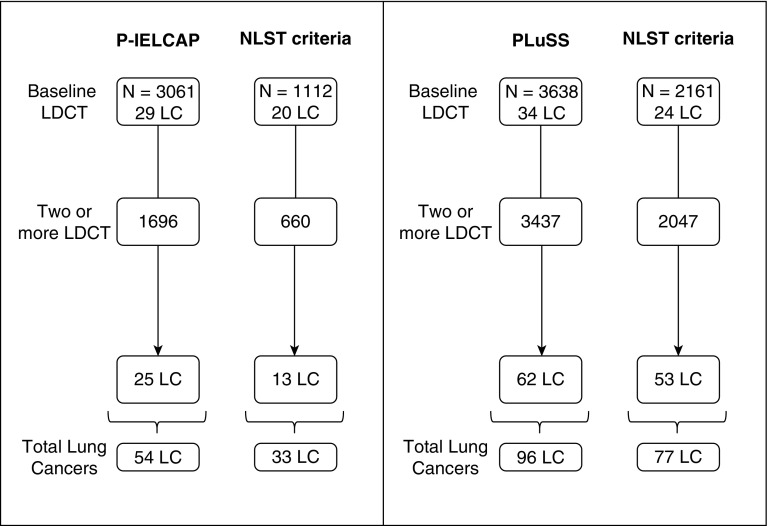
A total of 54 and 96 lung cancer diagnoses were made in P-IELCAP and PLuSS (1.8% and 2.6% overall detection rates, respectively). When limiting baseline and annual screening for individuals who met NLST criteria, total lung cancer diagnoses were 33 and 77 (i.e., 39% and 20% reduction in the diagnostic yield, respectively). This reduction was evident in both cohorts even after stratifying by prevalent (found on first LDCT) or incident cancers (all others) (Figure 1).
Figure 1.
Lung cancer distribution in each of the original cohorts (P-IELCAP [left] and PLuSS [right]), compared with the distribution after NLST criteria are applied. LC = lung cancer; LDCT = low-dose computed tomography; NLST = National Lung Screening Trial; P-IELCAP = Pamplona International Early Lung Cancer Detection Program; PLuSS = Pittsburgh Lung Screening Study.
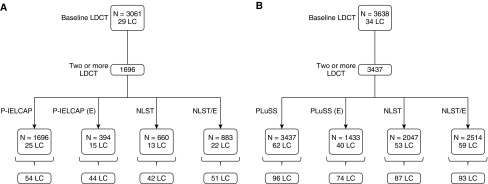
If screening was limited to annual screening of those who had emphysema on baseline CT imaging (P-IELCAP[E] and PLuSS[E]), independent of NLST compliance, 60% and 65% of the original incident lung cancers would have been detected in P-IELCAP and PLuSS, respectively (Figure 2). Annual screening performed on those who either met NLST criteria or had emphysema on baseline screening (NLST/E) resulted in the highest overall lung cancer diagnostic yield, detecting 94% and 97% of the original lung cancer cases of both cohorts (51 and 93 lung cancers in P-IELCAP and PLuSS, respectively) (Figure 2).
Figure 2.
(A and B) Lung cancer distribution after applying different intermediate selection criteria following the baseline screening round, limiting successive screening rounds to those with emphysema in the original cohorts (P-IELCAP[E] and PLuSS[E]), those who met NLST criteria only, and those who met NLST criteria or had emphysema, independent of NLST (NLST/E). (A) P-IELCAP; (B) PLuSS. LC = lung cancer; LDCT = low-dose computed tomography; NLST = National Lung Screening Trial; P-IELCAP = Pamplona International Early Lung Cancer Detection Program; PLuSS = Pittsburgh Lung Screening Study.
If annual screening was offered in the P-IELCAP cohort to the NLST/E subgroup, 88% of the incident lung cancers could have been detected by screening 48% fewer participants. Similarly, 95% of the incident lung cancers could have been detected by screening 27% fewer participants in the PLuSS cohort (Figure 3).
Figure 3.
Lung cancer ascertainment in P-IELCAP (solid squares) and PLuSS (open squares) in terms of number of participants screened according to the strategy used: NLST, NLST/E, P-IELCAP(E), or PLuSS(E). The data are normalized for each cohort; “1.00” represents 100% of the participants in each cohort (x-axis) or the total number of lung cancers (y-axis). (E) suffix = limitation of annual low-dose chest computed tomography in NLST, P-IELCAP, and PLuSS to individuals with emphysema; NLST = National Lung Screening Trial; NLST/E = extended NLST subcohort including individuals with emphysema independent of NLST; P-IELCAP = Pamplona International Early Lung Cancer Detection Program; PLuSS = Pittsburgh Lung Screening Study.
The lung cancer incidence densities for P-IELCAP and PLuSS were 7.27 (95% CI, 5.57–9.50) and 5.80 (95% CI, 4.75–7.08) lung cancers per 1,000 person-years, respectively. In both cohorts, the application of NLST criteria at baseline resulted in higher incidence densities (10.97; 95% CI, 7.80–15.43 and 7.47; 95% CI, 5.97–9.34, respectively) (Table 3). The highest incidence density was achieved when annual screening was limited to those individuals who had emphysema, independent of the NLST criteria (P-IELCAP[E] and PLuSS[E]) (Table 3).
Table 3.
Lung Cancer Incidence Density per 1,000 Person-Years
| n | No. LC | Person-Years of Follow Up | Incidence Density | 95% CI | |
|---|---|---|---|---|---|
| According to selection criteria at
baseline | |||||
| P-IELCAP | |||||
| P-IELCAP | 3,061 | 54 | 7,424 | 7.27 | 5.57–9.50 |
| NLST | 1,112 | 33 | 3,009 | 10.97 | 7.80–15.43 |
| PLuSS | |||||
| PLuSS | 3,638 | 96 | 16,552 | 5.80 | 4.75–7.08 |
| NLST | 2,161 | 77 | 10,311 | 7.47 | 5.97–9.34 |
| According to intermediate selection
criteria after the baseline screening round | |||||
| P-IELCAP | |||||
| P-IELCAP(E) | 3,061 | 43 | 1,573 | 27.33 | 20.27–36.86 |
| NLST/E | 3,061 | 51 | 3,909 | 13.05 | 9.91–17.17 |
| PLuSS | |||||
| PLuSS(E) | 3,638 | 74 | 7,286 | 10.16 | 8.09–12.76 |
| NLST/E | 3,638 | 93 | 12,567 | 7.40 | 6.04–9.07 |
Definition of abbreviations: CI = confidence interval; (E) suffix = limitation of annual low-dose chest computed tomography in P-IELCAP and PLuSS to individuals with emphysema; NLST = National Lung Screening Trial; NLST/E = extended NLST subcohort including individuals with emphysema independent of NLST; No. LC = number of lung cancers; P-IELCAP = Pamplona International Early Lung Cancer Action Program; PLuSS = Pittsburgh Lung Screening Study.
A similar pattern was observed when assessing lung cancer annual detection rates. In comparison with the original cohorts, the detection rates were higher when NLST criteria were applied: 0.52 (95% CI, 0.30–0.89) and 0.48 (95% CI, 0.37–0.63) lung cancers per year versus 0.43 (95% CI, 0.29–0.63) and 0.43 (95% CI, 0.34–0.55) in P-IELCAP and PLuSS, respectively. Annual screening of the NLST/E subgroup resulted in intermediate detection rates when compared with restrictive NLST criteria or broad P-IELCAP or PLuSS entry criteria, but had the highest overall lung cancer cases when compared with the other subgroups (Table 4).
Table 4.
Annual Lung Cancer Detection Rate
| N | No. LC | Duration (yr) | LCDR | 95% CI | NND | |
|---|---|---|---|---|---|---|
| P-IELCAP cohort* | ||||||
| P-IELCAP | 1,696 | 25 | 3.47 | 0.43 | 0.29–0.63 | 235 |
| NLST | 660 | 13 | 3.80 | 0.52 | 0.30–0.89 | 193 |
| NLST/E | 883 | 22 | 3.47 | 0.72 | 0.47–1.09 | 139 |
| P-IELCAP(E) | 394 | 15 | 3.07 | 1.24 | 0.75–2.06 | 81 |
| PLuSS cohort* | ||||||
| PLuSS | 3,437 | 62 | 4.20 | 0.43 | 0.34–0.55 | 233 |
| NLST | 2,047 | 53 | 5.41 | 0.48 | 0.37–0.63 | 209 |
| NLST/E | 2,514 | 59 | 5.31 | 0.44 | 0.34–0.57 | 226 |
| PLuSS(E) | 1,433 | 40 | 5.71 | 0.49 | 0.36–0.67 | 205 |
Definition of abbreviations: CI = confidence interval; (E) suffix = limitation of annual low-dose chest computed tomography in P-IELCAP and PLuSS to individuals with emphysema; LCDR = lung cancer detection rate; NLST = National Lung Screening Trial; NLST/E = extended NLST subcohort including individuals with emphysema independent of NLST; No. LC = number of lung cancers; NND = number of people needed to be screened in a year to detect one lung cancer; P-IELCAP = Pamplona International Early Lung Cancer Action Program; PLuSS = Pittsburgh Lung Screening Study.
Only lung cancers diagnosed at or after the second round of screening (incident cancers) are considered.
Differences in baseline characteristics of participants diagnosed with lung cancer between the NLST-compliant and -noncompliant groups are shown in Table E1 in the online supplement. A total of 21 and 19 participants with lung cancer were identified in the NLST-ineligible subgroups in P-IELCAP and PLuSS, respectively. They were significantly younger and had less tobacco exposure when compared with NLST-compliant patients with lung cancer. More than half of the P-IELCAP and PLuSS participants with lung cancer who would have been excluded from the NLST had spirometric-defined COPD, and as many as 84% had emphysema, as was the case in PLuSS. In the latter, significantly more adenocarcinomas and less squamous carcinomas were identified in the NLST-noncompliant group, whereas more large-cell carcinomas were found in P-IELCAP. Lung cancer stage distribution was similar in both groups. There were no significant differences in the number of deaths from lung cancer between the NLST-compliant and -noncompliant groups in each cohort.
Discussion
This study makes two important observations. Had the NLST selection criteria been used to identify screening candidates in P-IELCAP and PLuSS, a significant number of lung cancers would have been missed; and emphysema is shown to be a good criterion to refine the selection of candidates that may benefit most from annual screening.
In the wake of the NLST results, several guidelines have been published recommending lung cancer screening with LDCT (1, 4–8). All guidelines recommend using the NLST entry criteria for future screening (24). However, concerns about these criteria resulting in a significant proportion of lung cancers being missed have been raised (9). Although numerous screening studies using LDCT with alternative selection criteria are available in the literature (9, 21, 25–28), evidence on the effectiveness of NLST criteria outside the original trial is limited. In the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Tammemägi and coworkers (9) compared the accuracy of their prediction model (PLCOM2012) to detect lung cancer versus NLST criteria. Even though they used chest radiography rather than LDCT in their intervention group, this was compensated by their long follow-up time. However, the model’s predictive performance for individuals younger than 55 years of age was uncertain, because both trials focused on the same age range (9).
Inclusion criteria for P-IELCAP and PLuSS are broader and quite different than those used by the NLST. PLuSS used a slightly broader age range (50–79 yr) with a smoking history of at least 12.5 pack-years, whereas P-IELCAP set the minimum age limit at 40 years and the minimum tobacco exposure at 10 pack-years, well below NLST inclusion criteria (13, 14). Had PLuSS and P-IELCAP followed the latter, 20–39% of lung cancer diagnoses would have been missed (19 cases in PLuSS and 21 in P-IELCAP). Failure of the NLST criteria to include most subjects at risk has also been assessed by Pinsky and Berg (11) by reviewing the Surveillance, Epidemiology and End Results, the 2010 U.S. census, and the National Health Interview Survey. Only 27% of patients with lung cancer in those registries met NLST criteria, a proportion that improved to 68% when a broader age range was used and the smoking threshold was lowered (11). The same holds true for New Zealand. Young and Hopkins (29) found that among a representative sample of 446 lung cancer cases in New Zealand, 53% would have been missed by strict adherence to NLST screening inclusion criteria.
Wilson and coworkers (PLuSS) (14) and de Torres and coworkers (P-IELCAP) (13) independently described the importance of emphysema in a lung cancer screening setting using LDCT almost 10 years ago, an observation that has later been confirmed in a recent metaanalysis (30). Epidemiologic evidence from both publications coupled with biomolecular evidence that COPD and emphysema share common pathogenic pathways with lung cancer (31, 32) led to our present study hypothesis. We evaluated the impact emphysema might have as an intermediate criterion in refining eligibility for ongoing screening in the original P-IELCAP and PLuSS cohorts following baseline screening. We also explored the possibility of combining widely accepted NLST screening criteria with emphysema to consider all options (Figure 2).
The value of emphysema has been recently assessed by Kovalchik and colleagues (10) who included it in a lung cancer death risk prediction model of the original NLST population. In this model, the presence of emphysema was the factor associated with the highest risk of death from lung cancer. They demonstrated that emphysema coupled with other variables identifies high-risk individuals limiting false-positive results and potentially even screening costs (10). A study by Zulueta and coworkers (33) also found that emphysema was a significant predictor of death from lung cancer in a large screening cohort from New York. Considering the impact that emphysema has on lung cancer incidence and mortality, we chose to go one step further by exploring not only the impact emphysema has on NLST-eligible subjects, but on a broader lower-risk population. We found this approach appealing because up to 84% of subjects with lung cancer missed by NLST criteria in our cohorts had emphysema on baseline LDCT (see Table E1); thus, considering it outside the NLST range seemed clinically relevant.
Emphysema is an independent risk factor of death and as such, concerns might exist on whether a patient diagnosed with lung cancer and emphysema will benefit from treatment (34). We believe that including subjects with emphysema in a screening program could not only provide the benefit of increased lung cancer detection, but could also add the benefit from smoking cessation efforts and therapies to limit the progression of emphysema and/or COPD (35, 36). However, the severity of emphysema is an important point to consider. Beyond certain degrees of emphysema, the potential benefits of screening will be surpassed by the increased mortality associated with emphysema per se and existent comorbid illnesses. The implementation of a multidisciplinary approach and registry monitoring, as recommended by guidelines, will play an important role in the assessment of this kind of patient (6).
Limiting annual screening to individuals with emphysema found on a baseline LDCT in the P-IELCAP and PLuSS cohorts showed the highest lung cancer incidence densities and detection rates, and hence, the lowest NND. However, the highest absolute lung cancer counts were observed in subjects who either met NLST entry criteria and/or had emphysema on baseline LDCT (NLST/E). By using these criteria, 88% and 95% of incident lung cancers could be detected in P-IELCAP and PLuSS despite screening 48% and 27% fewer participants, respectively. Emphysema as a variable had less impact on PLuSS outcomes probably because this cohort had a higher proportion of participants who already met NLST criteria at baseline (1.5 times higher in PLuSS than in P-IELCAP), and the fact that a subgroup of already high-risk participants were singled out in PLuSS for ongoing screening (those with moderate to severe emphysema), in contrast to P-IELCAP, which made no such selection following the first round of screening.
The availability of two different LDCT screening cohorts with broader and more inclusive criteria than the NLST allowed us to test NLST entry criteria in the context of the added value of emphysema as a selection criterion. Although limiting screening to subjects eligible for the NLST results in a considerable reduction in the number of individuals screened, a significant number of lung cancers would have been missed in both P-IELCAP and PLuSS. Limiting ongoing screening to subjects who either meet NLST criteria or have emphysema on baseline LDCT might help in reducing the number needed to screen to optimize cancer detection rates. Our data suggest that the lung cancer cases identified in subjects not meeting NLST entry criteria (presumably a lower-risk population) are not different from those found in the NLST, because they share a similar proportion of cancer deaths (see Table E1). Missing lung cancers in younger patients may have a significant impact on the burden of this disease, because a recent study describing the economic burden of cancer across the European Union showed that lung cancer had the highest economic cost, at €18.8 billion, the highest productivity losses attributable to mortality, and the highest costs of informal care (37).
Second, it underscores the value of emphysema in refining selection criteria for screening. The fact that the results are reproducible in two geographically disparate cohorts with distinct baseline characteristics supports this observation. Furthermore, we noticed that by using radiographic emphysema as a selection criterion, we captured many of the participants who had COPD defined by spirometry. In the present study, only one additional lung cancer in each cohort would have been detected if COPD were added as a selection criterion beyond NLST eligibility and emphysema.
Our findings may not have immediate implications for the implementation of screening programs in the United States and it is beyond the scope of the study to suggest guideline policies. As newer evidence, such as that expected from the NELSON trial, with broader entry criteria than the NLST is presented in the future, guideline developers may or may not have to adapt entry criteria (38).
This study has potential limitations. Although the size of P-IELCAP and PLuSS and the number of lung cancer cases seem to be relatively small, they do not differ significantly from other major screening trials, excluding the NLST, NELSON, and I-ELCAP (24). Even though P-IELCAP’s participants were a much lower-risk population (younger, fewer pack-years, and had less emphysema than participants in PLuSS), lung cancer incidence densities and detection rates were similar to those found in PLuSS. Because these two parameters include time and the number of participants per group in their calculations, the longer cumulative follow up in the latter (almost three times longer than in P-IELCAP) together with larger subgroups in PLuSS may explain these differences. If follow-up periods and the subgroups’ sizes were comparable, P-IELCAP incidence densities and detection rates would be lower.
Demographic and methodologic differences, including variations in the LDCT imaging protocol and post hoc selection for continued screening in PLuSS could also explain some of these differences. In addition, NLST criteria were applied originally to a U.S. population and may not be applicable to a European cohort, such as P-IELCAP. Despite these differences, the impact of adding emphysema observed in P-IELCAP was externally validated in PLuSS, highlighting its role as a variable capable of improving the diagnostic yield of screening. Nonetheless, it is true that further validation in a much larger population is needed, which is not an easy task, considering that most of the currently existing trials either use similar criteria to NLST or are of similar size to P-IELCAP and PLuSS (24).
In conclusion, if the NLST entry criteria had been used in the lung cancer screening cohorts of P-IELCAP and PLuSS, a significant number of lung cancers would have been missed. Limiting annual screening to individuals who meet NLST criteria and/or those who have emphysema on baseline screening results in higher lung cancer detection rates and less cancers missed. Further studies are needed to evaluate the number of avoidable deaths and the cost-effectiveness of LDCT screening using these refined criteria.
Footnotes
Supported by grants for the Pamplona International Early Lung Cancer Detection Program from Red Temática de Investigación Cooperativa en Cáncer (RD12/0036/0062 and RD12/0036/0040), Fondo de Investigaciones Sanitarias (PI04/2404, PI04/2128, PI07/0792, PI10/01652, PI10/00166, PI11/01626, PI11/00618, and PI13/00806), Centro de Investigación Biomédica en Red de Enfermedades Respiratorias, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, European Regional Development Fund “Una manera de hacer Europa,” and the Ramon Areces Foundation. Pittsburgh Lung Screening Study was supported by the University of Pittsburgh Lung Cancer SPORE: NCI P50-CA90440, 1P50 HL084948, University of Pittsburgh Cancer Institute, and University of Pittsburgh Medical Center.
Author Contributions: Conception and design, P.S.-S., D.O.W., J.P.d.-T., J.L.W., J.B., A.C., A.B.A., J.P., G.B., L.M.S., M.J.P., R.P., L.M.M., and J.J.Z. Analysis and interpretation, P.S.-S., D.O.W., J.P.d.-T., J.L.W., and J.J.Z. Drafting the manuscript for important intellectual content, P.S.-S., D.O.W., J.P.d.-T., L.M.S., and J.J.Z.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201410-1848OC on February 10, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive services task force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]
- 3.Field JK, Oudkerk M, Pedersen JH, Duffy SW. Prospects for population screening and diagnosis of lung cancer. Lancet. 2013;382:732–741. doi: 10.1016/S0140-6736(13)61614-1. [DOI] [PubMed] [Google Scholar]
- 4.Wood DE, Eapen GA, Ettinger DS, Hou L, Jackman D, Kazerooni E, Klippenstein D, Lackner RP, Leard L, Leung AN, et al. Lung cancer screening. J Natl Compr Canc Netw. 2012;10:240–265. doi: 10.6004/jnccn.2012.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Lung AssociationProviding guidance on lung cancer screening to patients and physicians. 2012. Apr 23 [accessed 2013 Oct 17]. Available from: http://www.lung.org/lung-disease/lung-cancer/lung-cancer-screening- guidelines/lung-cancer-screening.pdf
- 6.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e78S–92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaklitsch MT, Jacobson FL, Austin JHM, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144:33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 8.Wender R, Fontham ETH, Barrera E, Jr, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, et al. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63:107–117. doi: 10.3322/caac.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tammemägi MC, Katki HA, Hocking WG, Church TR, Caporaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, Silvestri GA, Chaturvedi AK, Katki HA. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–156. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 12.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157:571–573. doi: 10.7326/0003-4819-157-8-201210160-00524. [DOI] [PubMed] [Google Scholar]
- 13.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 14.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138:1295–1302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 16.Sanchez PA, de-Torres JP, Wilson DO, Weissfeld J, Campo A, Alcaide AB, Zulueta JJ. Improving NLST criteria for lung cancer screening. Eur Respir J. 2014;44:352. [Google Scholar]
- 17.Bastarrika G, García-Velloso MJ, Lozano MD, Montes U, Torre W, Spiteri N, Campo A, Seijo L, Alcaide AB, Pueyo J, et al. Early lung cancer detection using spiral computed tomography and positron emission tomography. Am J Respir Crit Care Med. 2005;171:1378–1383. doi: 10.1164/rccm.200411-1479OC. [DOI] [PubMed] [Google Scholar]
- 18.International Early Lung Cancer Action Program protocol [accessed 2013 Oct 17]. Available from: http://www.IELCAP.org
- 19.Bastarrika G, Wisnivesky JP, Pueyo JC, Díaz L, Arraiza M, Villanueva A, Alcaide AB, Campo A, Seijo L, de Torres JP, et al. Low-dose volumetric computed tomography for quantification of emphysema in asymptomatic smokers participating in an early lung cancer detection trial. J Thorac Imaging. 2009;24:206–211. doi: 10.1097/RTI.0b013e3181a65263. [DOI] [PubMed] [Google Scholar]
- 20.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 21.Wilson DO, Weissfeld JL, Fuhrman CR, Fisher SN, Balogh P, Landreneau RJ, Luketich JD, Siegfried JM. The Pittsburgh Lung Screening Study (PLuSS): outcomes within 3 years of a first computed tomography scan. Am J Respir Crit Care Med. 2008;178:956–961. doi: 10.1164/rccm.200802-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, Hsieh LJ, Begg CB. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 23.Young RP, Hopkins RJ. Targeted CT image screening and its effect on lung cancer detection rate. Chest. 2013;144:1419–1420. doi: 10.1378/chest.13-1321. [DOI] [PubMed] [Google Scholar]
- 24.Shlomi D, Ben-Avi R, Balmor GR, Onn A, Peled N. Screening for lung cancer: time for large-scale screening by chest computed tomography. Eur Respir J. 2014;44:217–238. doi: 10.1183/09031936.00164513. [DOI] [PubMed] [Google Scholar]
- 25.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS International Early Lung Cancer Action Program Investigators. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355:1763–1771. doi: 10.1056/NEJMoa060476. [DOI] [PubMed] [Google Scholar]
- 26.Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, Passera E, Angeli E, Chiarenza M, Aranzulla G, et al. DANTE Study Group. A randomized study of lung cancer screening with spiral computed tomography: three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180:445–453. doi: 10.1164/rccm.200901-0076OC. [DOI] [PubMed] [Google Scholar]
- 27.Lopes Pegna A, Picozzi G, Falaschi F, Carrozzi L, Falchini M, Carozzi FM, Pistelli F, Comin C, Deliperi A, Grazzini M, et al. ITALUNG Study Research Group. Four-year results of low-dose CT screening and nodule management in the ITALUNG trial. J Thorac Oncol. 2013;8:866–875. doi: 10.1097/JTO.0b013e31828f68d6. [DOI] [PubMed] [Google Scholar]
- 28.Horeweg N, van der Aalst CM, Vliegenthart R, Zhao Y, Xie X, Scholten ET, Mali W, Thunnissen E, Weenink C, Groen HJ, et al. Volumetric computed tomography screening for lung cancer: three rounds of the NELSON trial. Eur Respir J. 2013;42:1659–1667. doi: 10.1183/09031936.00197712. [DOI] [PubMed] [Google Scholar]
- 29.Young RP, Hopkins RJ. Lung cancer risk prediction to select smokers for screening CT—letter. Cancer Prev Res (Phila) 2012;5:697–698, author reply 699. doi: 10.1158/1940-6207.CAPR-11-0531. [DOI] [PubMed] [Google Scholar]
- 30.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Ballaz S, Mulshine JL. The potential contributions of chronic inflammation to lung carcinogenesis. Clin Lung Cancer. 2003;5:46–62. doi: 10.3816/CLC.2003.n.021. [DOI] [PubMed] [Google Scholar]
- 32.Houghton AM, Mouded M, Shapiro SD. Common origins of lung cancer and COPD. Nat Med. 2008;14:1023–1024. doi: 10.1038/nm1008-1023. [DOI] [PubMed] [Google Scholar]
- 33.Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, Chen M, Libby DM, Smith JP, Pasmantier MW, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141:1216–1223. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould MK. Clinical practice. Lung-cancer screening with low-dose computed tomography. N Engl J Med. 2014;371:1813–1820. doi: 10.1056/NEJMcp1404071. [DOI] [PubMed] [Google Scholar]
- 35.Ashraf H, Saghir Z, Dirksen A, Pedersen JH, Thomsen LH, Døssing M, Tønnesen P. Smoking habits in the randomised Danish Lung Cancer Screening Trial with low-dose CT: final results after a 5-year screening programme. Thorax. 2014;69:574–579. doi: 10.1136/thoraxjnl-2013-203849. [DOI] [PubMed] [Google Scholar]
- 36.Gomez MM, LoBiondo-Wood G. Lung cancer screening with low-dose CT: its effect on smoking behavior. J Adv Pract Oncol. 2013;4:405–414. doi: 10.6004/jadpro.2013.4.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165–1174. doi: 10.1016/S1470-2045(13)70442-X. [DOI] [PubMed] [Google Scholar]
- 38.van Iersel CA, de Koning HJ, Draisma G, Mali WPTM, Scholten ET, Nackaerts K, Prokop M, Habbema JDF, Oudkerk M, van Klaveren RJ. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON) Int J Cancer. 2007;120:868–874. doi: 10.1002/ijc.22134. [DOI] [PubMed] [Google Scholar]