Abstract

Ionic liquids (ILs) have been proposed as promising media for the extraction and separation of bioactive compounds from the most diverse origins. This critical review offers a compilation on the main results achieved by the use of ionic-liquid-based processes in the extraction and separation/purification of a large range of bioactive compounds (including small organic extractable compounds from biomass, lipids, and other hydrophobic compounds, proteins, amino acids, nucleic acids, and pharmaceuticals). ILs have been studied as solvents, cosolvents, cosurfactants, electrolytes, and adjuvants, as well as used in the creation of IL-supported materials for separation purposes. The IL-based processes hitherto reported, such as IL-based solid–liquid extractions, IL-based liquid–liquid extractions, IL-modified materials, and IL-based crystallization approaches, are here reviewed and compared in terms of extraction and separation performance. The key accomplishments and future challenges to the field are discussed, with particular emphasis on the major lacunas found within the IL community dedicated to separation processes and by suggesting some steps to overcome the current limitations.
1. Introduction
Much attention is given today to the development of integrated and sustainable technologies to produce, extract, and purify a wide range of bioactive compounds and materials, not only those obtained from biomass conversion and processing but also those produced via fermentation and even by synthetic pathways.1 Over the years, separation techniques have been optimized and scaled up. Extraction methodologies used to carry out the extraction of target compounds from biomass and other raw matrices are usually grouped as mechanical-, high-pressure-, ultrasound-, and microwave-assisted (among others). Separation techniques are more complex in nature due to difficulties in isolating the target compounds at high yields and with high purity levels. In general, extraction and separation methods are connected, and both techniques should be ideally merged and conducted in a single step. However, current extraction-separation processes present several shortcomings, that in addition to low extraction efficiencies and poor selectivity could also add costs to the final product because of the high complexity of separation processes (e.g., by using chromatography) and high time and energy demands. Furthermore, harsh conditions and toxic volatile organic solvents are usually employed. Based on these shortcomings, researchers have been working on the development of alternative extraction and purification processes with “greener” and more sustainable credentials and for which the use of ionic liquids (ILs) has received attention.
ILs are liquid molten salts at temperatures below 100 °C2 and are typically composed of large and unsymmetrical organic cations and organic or inorganic anions. Beyond the excellent chemical, thermal, and electrochemical stability, nonflammability, and negligible volatility displayed by most aprotic ILs, ILs are also recurrently recognized by their excellent solvation ability for a wide range of compounds and materials, from synthetically produced to natural extracted ones, and as good stabilizing media for proteins, enzymes, nucleic acids, among others.3,4 Over the last two decades, ILs have evolved from potential solvents for the processing/complete dissolution of biomass5,6 to selective solvents for the extraction and purification of natural-derived compounds.7,8 In addition to their exceptional solvation ability, since ILs can swell or dissolve a wide range of biomass matrices, thus allowing an easier access to the target compounds, their aqueous solutions also display improved and unique solvation performance, as demonstrated by their outstanding hydrotropic nature9 and as surface-active ingredients,10 allowing enhanced extractions. Moreover, ILs have been recognized as tunable designer solvents, a result of the large number of ion combinations and the possibility of designing task-specific fluids. This feature overcomes the limited selectivity of common volatile organic solvents and thus allows the development of more effective purification platforms. The replacement of volatile organic solvents by nonvolatile ILs also eliminates solvent losses to the atmosphere, decreasing both the environmental footprint and the cost of the process.
The extractive performance and the purity level of the target compounds are crucial parameters to take into account when attempting the development of novel separation processes. Biomass, the matrix most investigated in this review, is a unique, ubiquitous, and sustainable renewable resource for the production of biofuels, heat, power, biomaterials, and biochemicals with commercial application.11 It should be highlighted that a survey of the literature regarding the ability of ILs to dissolve cellulose and other lignocellulosic fractions is outside the scope of this review and for which several valuable review manuscripts are already available.12−14
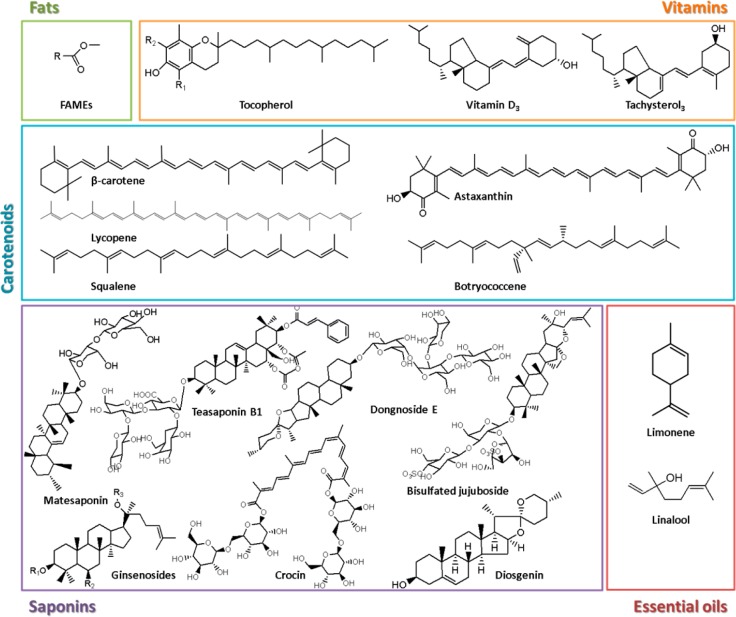
This critical review summarizes the use of ILs as solvents in the extraction and/or purification of bioactive compounds produced via fermentation or by synthetic routes, or derived from biomass matrices. A wide range of bioactive compounds is considered herein, ranging from small organic compounds, such as phenolic acids, alkaloids, fats, essential oils, carotenoids, vitamins, amino acids, among others, to more complex molecules, such as nucleic acids, proteins, enzymes, and antibodies. Within the range of appraised bioactive compounds and techniques, a large number of ILs have been investigated, their names and acronyms (divided by cation and anion) are provided in Table 1. Different IL-based processes are also addressed, ranging from liquid–liquid extractions (carried out either with hydrophobic ILs, ionic-liquid-based aqueous biphasic systems, or aqueous micellar biphasic systems), solid–liquid extractions (including microwave-assisted, ultrasound-assisted, among others), solid-phase extractions where IL-modified materials have been employed, and induced-precipitation techniques, such as three-phase partitioning and crystallization. Taking into account all of the IL-based techniques, and extraction and separation/purification processes proposed to date, a comprehensive overview on the improvements brought about by the use of ILs is presented. It should be stressed that only separation processes that could be scaled up while allowing the recovery of large amounts of target compounds at relatively low costs are considered. For instance, electrophoretic and chromatographic techniques are not considered in this review, for which several reviews are already available.15−18 Finally, the main challenges that still need to be addressed in the IL-based extraction and separation processes arena are discussed.
Table 1. Name and Acronym of the IL cations and Anions Considered in This Review.
| cation name | acronym | anion name | acronym |
|---|---|---|---|
| 1-(2-cyanoalkyl)-3-methylimidazolium | [(NC)CnC1im]+ | 2-(2-methoxyethoxy)ethylsulfate | [C1(OC2)2SO4]− |
| 1-(4-sulfonylbutyl)-3-methylimidazolium | [(HSO3)C4C1im]+ | 2-(N-morpholino)ethanesulfonate | [MES]− |
| 1,3-dihexyloxymethylimidazolium | [(C6H3OCH2)2im]+ | 2-[bis(2-hydroxyethyl)amino]ethanesulfonate | [BES]− |
| 1-alkyl-1-methylpiperidinium | [CnC1pip]+ | 2-hydroxy-3-morpholinopropanesulfonate | [MOPSO]− |
| 1-alkyl-1-methylpyrrolidinium | [CnC1pyrr]+ | 4-(2-hydroxyethyl)-1-piperazineethanesulfonate | [HEPES]− |
| 1-alkyl-2,3-dimethylimidazolium | [CnC1C1im]+ | 4-chlorophenoxyacetate | [CPA]− |
| 1-alkyl-3-methylimidazolium | [CnC1im]+ | acesulfamate | [Ace]− |
| 1-alkyl-3-methylpyridinium | [CnC1pyr]+ | acrylate | [Acr]− |
| 1-alkylimidazolium | [Cnim]+ | alalinate | [Ala]− |
| 1-alkylpyridinium | [Cnpyr]+ | alkylphosphonate | [CnPO3]− |
| 1-allyl-3-alkylimidazolium | [aCnim]+ | alkylsulfonate | [CnSO3]− |
| 1-benzyl-3-methylimidazolium | [C7H7C1im]+ | alkylsulfate | [CnSO4]− |
| 1-butyl-3-trimethylsilylimidazolium | [C4(C1C1C1Si)im]+ | aminoate | [AA]− |
| 1-carboxyethyl-3-methylimidazolium | [(HOOC)C2C1im]+ | asparatinate | [Asp]− |
| 1-hexyloxymethyl-3-methylimidazolium | [(C6H13OCH2)C1im]+ | benzoate | [Bz]− |
| 1-hydroxyalkyl-3-methylimidazolium | [(OH)CnC1im]+ | bicarbonate | [Bic]– |
| 1-methyl-3-(triethoxy)silypropyl imidazolium | [(C2H5O)3SiC3C1im]+ | bis(2,4,4-trimethylpentyl)phosphinate | [TMPP]− |
| 1-propylamine-3-methylimidazolium | [(NH2)C3C1im]+ | bis(2-ethylhexyl) phosphate | [BEP]− |
| 1-vinyl-3-(2-methoxy-2-oxylethyl)imidazolium | [VC1O(O)C2im]+ | bis(trifluoromethylsulfonyl)imide | [NTf2]− |
| 2-(alkyloxy)-N,N,N-trimethyl-2-oxoethanaminium (betain) | [N111[2O(O)n]]+ ([bet]+) | bitartrate | [Bit]− |
| 2-(hydroxyethyl)-N,N-dimethyl-3-(triethoxy) silypropyl ammonium | [N11[3Si(2O)(2O)(2O)](2OH)]+ | bromide | Br– |
| 3-(2-(butylamino)-2-oxoethyl)-1-ethylimidazolium | [(CH2CONHC4H9)C2im]+ | calkanoate | [Calc] |
| 3-(dimethylamino)-1-propylammonium | [N011(3N)] + | carboxylate | [CnCO2]− |
| 3-alkyl-1-vinyl-limidazolium | [VCnim]+ | chloride | Cl– |
| alkyl-1,8-diazabicyclo[5.4.0]undec-7-enium | [CnDBU]+ | cinnamate | [Cin]− |
| alkyl(tributyl)phosphonium | [Pn444]+ | citrate | [Cit]3− |
| alkyltropine | [Cntro]+ | cysteinate | [Cys]− |
| ammoeng 100 | [N114(2OmOH) (2OnOH)]+ | dialkylphosphate | [(Cn)2PO4]− |
| ammoeng 102 | [N218O(2OmOH) (2OnOH)]+ | dicyanamide | [N(CN)2]− |
| ammoeng 110 | [N221(O)nOH]+ | dihydrogencitrate | [DHCit]− |
| chirally functionalized methylimidazolium | [CwHxNyOz]+ | dihydrogenophosphate | [H2PO4]− |
| decyltris(3-hydroxypropyl)phosphonium | [P10(3OH)(3OH)(3OH)]+ | dimethylcarbamate | [N(C1)2CO2]− |
| ethyl l-phenylalaninium | [C2(l-Phe)]+ | glutarate | [Glut]− |
| hexaalkylguanidinium | [CnCnCnCnCnCnguan]+ | glycinate | [Gly]− |
| N,N,N,N-tetramethyl-3-(triethoxy)silylpropyl-guanidinium | [(C2H5O)3SiC3C1C1C1C1guan]+ | glycolate | [Glyc]− |
| N,N,N-trialkylammonium | [N0nnn]+ | Good’s buffers | [GB]− |
| N,N,N-trimethyl-N-(2-hydroxyethyl)ammonium (cholinium) | [N111(2OH)]+ | hexafluorophosphate | [PF6]− |
| N,N-dialkylammonium | [N00nn]+ | hydrogenosulfate | [HSO4]− |
| N,N-dialkyl-N-(2-hydroxyethyl)ammonium | [N0nn(2OH)]+ | hydroxide | [OH]− |
| N,N-didecyl-N-methyl-d-glucaminium | [C10C10C1gluc]+ | iodide | I– |
| N,N-dimethyl(2-methoxyethyl)ammonium | [N11(2(O)1)0]+ | itaconate | [Ita]− |
| N,N-dimethyl(cyanoethyl)ammonium | [N011(2CN)]+ | lactate | [Lac]− |
| N,N-dimethyl-N-(2- hydroxyethoxyethyl)ammonium | [N11(2(O)2OH)0]+ | levulinate | [Lev]− |
| N-Alkyl-N,N-dimethyl-N-(2-hydroxyethyl)ammonium | [N11n(2OH)]+ | lysinate | [Lys]− |
| N-benzyl-N,N-dimethyl-N-(2-hydroxyethyl)ammonium | [N11(2OH)(C7H7)]+ | malonate | [Mal]2– |
| N-butyl-N-methylmorpholinium | [C4C1mor]+ | methacrylate | [MAcr]− |
| N-ethyl-N-[3-(triethoxy) silypropyl] morpholinium | [(C2H5O)3SiC3C2mor]+ | N- [tris(hydroxymethyl)methyl]-3-amino-2-hydroxypropanesulfonate | [TAPSO]− |
| N-methyl-N,N,N-trioctylammonium | [N1888]+ | nitrate | [NO3]− |
| tetraalkylammonium | [Nnnnn]+ | N-trifluoromethanesulfonyl leucinate | [Tf-Leu]− |
| tetraalkylguanidinium | [CnCnCnCnguan]+ | N-tris(hydroxymethyl) methylglycinate | [Tricine]− |
| tetraalkylphosphonium | [Pnnnn]+ | N-tris(hydroxymethyl)methyl-2-aminoethanesulfonate | [TES]− |
| tetrakis(hydroxymethyl)phosphonium | [P(1OH)(1OH)(1OH)(1OH)]+ | O,O-diethyl dithiophosphate | [DTP]− |
| trihexyltetradecylphosphonium | [P66614]+ | oxalate | [Oxa]2– |
| triisobutyl(methyl)phosphonium | [Pi(444)1]+ | perchlorate | [ClO4]− |
| phenilalaninate | [Phe]− | ||
| phenylacetate | [PhAc]− | ||
| prolinate | [Pro]− | ||
| saccharinate | [Sac]− | ||
| salicylate | [Sal]− | ||
| serinate | [Ser]− | ||
| sorbate | [Sor]− | ||
| succinate | [Suc]− | ||
| sulfate | [SO4]2– | ||
| tetrachloroaluminate | [AlCl4]− | ||
| tetrafluoroborate | [BF4]− | ||
| thiocyanate | [SCN]− | ||
| tricyanomethanide | [C(CN)3]− | ||
| trifluoroacetate | [CF3CO2]− | ||
| trifluoromethanesulfonate | [CF3SO3]− | ||
| tris(pentafluoroethyl)trifluorophosphate | [FAP]− | ||
| valinate | [Val]− |
2. Small and Extractable Bioactive Compounds from Biomass
In the past few years, an increased demand for the use of natural compounds over their synthetic counterparts in nutraceutical, cosmetic, and pharmaceutical products has been experienced (e.g., biopharmaceuticals, natural-extracted antioxidants, among others).19,20 However, conventional extraction processes for the extraction of value-added compounds or fine chemicals from natural sources present several drawbacks, such as a low efficiency, nonselectivity, lengthy procedures, high energetic input, and generally involve the use of volatile and often toxic organic solvents, leading therefore to innate environmental and human concerns. Novel approaches have been proposed addressing the use of more sustainable extraction techniques and the use of safer alternative solvents (solvents produced from renewable resources, water, supercritical fluids, and ionic liquids (ILs)21). Among these, ILs are among the most studied alternative solvents for these purposes.8,22−25 IL-based extraction, separation, and purification technologies for bioactive ingredients from biomass have been extensively studied over the past two decades. ILs and their mixtures with water or organic solvents can be applied directly in the solid–liquid extraction (SLE) of value-added compounds from biomass. Due to their ionic character, ILs can interact with electromagnetic fields; thus, and in addition to more simple SLE approaches, IL-based microwave-assisted extraction (MAE) or ultrasonic-assisted extraction (UAE) may be preferred due to their shorter extraction times and higher extraction efficiencies. If the real matrix is liquid, such as a water-rich extract from biomass, IL-based liquid–liquid extractions (LLE) can be used. To minimize the IL consumption on the overall process, to improve the extraction performance, and to facilitate solvent/material recycling, ILs may also be confined into a solid matrix (IL-modified materials for solid-phase extractions, SPE).
This section addresses the use of ILs in the extraction, separation, and purification of biobased compounds and provides a comprehensive overview of the data hitherto reported on the development of IL-based SLE, including IL-based MAE and IL-based UAE, IL-based LLE (wherein IL-based aqueous biphasic systems are also covered), and IL-based SPE by the use of IL-modified materials. The distribution of the scientific works dealing with each IL-based technique for the extraction of natural compounds is depicted in Figure 1, where the families of natural compounds investigated are also highlighted. In these studies, aqueous solutions of ILs are the preferred choice for extraction/separation purposes, although the use of pure ILs or IL-ethanol/methanol mixtures was also addressed. The extraction of alkaloids, terpenoids, flavonoids, phenolic compounds, saponins, and other polycyclic aromatic compounds from natural sources has been investigated, with their incidence demonstrated in the radial graphs of Figure 1. Figure 2 shows the chemical structures of the compounds studied. The incidence of the use of distinct ILs, achieved by combinations of different ions shown in Figure 3, reveals that, as expected, 1-alkyl-3-methylimidazolium-based ILs are by far the most widely investigated and are mainly combined with the [BF4]−, Cl–, and Br– counterions. In this field, and particularly given the already large number of publications available, the application of more benign and biocompatible options, such as ammonium-based cations (including cholinium), still remains scarcely investigated.
Figure 1.
Distribution of the works dealing with each IL-based technique for the extraction and separation of small organic extractable compounds from biomass. The radial graphs display the number of scientific works addressing distinct families of natural compounds.
Figure 2.
Chemical structures of small organic compounds extracted and separated from biomass using IL-based techniques.
Figure 3.
ILs used for the extraction and separation of small organic extractable compounds from biomass as a function of cation–anion combinations. The usage incidence (number of articles) is represented by the circles’ size, which proportionally increases as follows: [0–5] < [5–10] < [10–15] < [15–30] < [30–40].
In this section, the issues that promote extraction efficiency are highlighted and a general overview on the advantages and disadvantages of the extraction methods employed is also provided. Moreover, the structure–property relationship between ILs and bioactive ingredients and their respective extraction mechanisms are discussed whenever applicable. Finally, the available methodologies for the back-extraction and recovery of bioactive compounds from IL solutions and simultaneous recovery and reuse of ILs is summarized. However, it can be anticipated that this aspect has been neglected in most of the published scientific studies.
2.1. IL-Based Solid–liquid Extractions
A large number of works exist on the use of IL-based SLE techniques with pure ILs, as well as with their aqueous solutions and IL-methanol/ethanol mixtures, for the extraction and separation of natural compounds, namely alkaloids, terpenoids, flavonoids, phenolics, saponins, lignans, among others.26−76 In addition to more simple SLE, where the biomass is placed in direct contact with the IL-based solvents and only the temperature, extraction time, and solid–liquid ratio are typically optimized, SLE techniques are often integrated with MAE and UAE to enhance the extraction efficiency while attempting to decrease the extraction time and amount of solvent used. This section is thus divided into three parts based on the most frequently employed extraction processes: (i) simple IL-based SLE; (ii) IL-based MAE; and (iii) IL-based UAE. The optimization of the extraction conditions (temperature, extraction time, pH, among others), selection of the IL and its concentration, and solid–liquid ratio are discussed. Table 2 summarizes the ILs employed and the bioactive compounds extracted from different natural sources.
Table 2. Extraction of Small and Extractable Natural Compounds from Biomass Using IL-Based SLE, Including IL-Based MAE and IL-Based UAE.
| bioactive compound | natural source | method | ILs (solvents) used |
|---|---|---|---|
| aesculetin and aesculin | Fraxinus rhynchophylla | IL-UAE | [C7H7C1im]Br, [C7H7C1im]Cl, [C10C1im]Br, [C12C1im]Br, [C2C1im][BF4], [C2C1im]Br, [C4C1im][BF4], [C4C1im][ClO4], [C4C1im][HSO4], [C4C1im][Tos], [C4C1im]Br, [C4C1im]Cl, [C4C1im]I, [C8C1im]Br, [C6C1im]Br, [(HSO3)C4C1im][HSO4], and [(OH)C2C1im]Cl (water)65 |
| artemisinin | Artemisia annua | IL-SLE | [N11(2(O)1)0][C2CO2], [N11(2OH)0][C7CO2] (pure IL)74,75 |
| baicalin, wogonoside, baicalein, and wogonin | Scutellaria baicalensis Georgi | IL-MAE | [C4C1im]Br, [C4C1im]Cl, [C4C1im][BF4], [C4C1im][Oac], [C4C1im][CF3SO3], [C2C1im]Br, [C6C1im]Br, [C8C1C1im]Br, [C10C1im]Br, and [C12C1C1im]Br (water)46 |
| caffeine | Paullinia cupana (guaraná) | IL-SLE | [C2C1im]Cl, [C2C1im][C1CO2], [C4C1im]Cl, [C4C1im][Tos], [C4C1pyrr]Cl, [(OH)C2C1im]Cl (water)29 |
| caffeoylquinic acids | Flos Lonicerae Japonicae | IL-UAE | [C4C1im]Br (water)60 |
| carnosic acid and rosmarinic acid | Rosmarinus officinalis | IL-MAE | [C10C1im]Br, [C2C1im]Br, [C4C1im][BF4], [C4C1im][NO3], [C4C1im]Br, [C4C1im]Br, [C4C1im]Cl, [C6C1im]Br, and [C8C1im]Br (water)47 |
| catharanthine, vinblastine, and vindoline | Catharanthus roseu | IL-UAE | [aC1im]Br, [C2C1im]Br, [C4C1im][BF4], [C4C1im][ClO4], [C4C1im][HSO4], [C4C1im][NO3], [C4C1im][Tos], [C4C1im]Br, [C4C1im]Cl, [C4C1im]I, [C6C1im]Br, and [C8C1im]Br (water)55 |
| cryptotanshinone, tanshinone I, and tanshinone II A | Salvia miltiorrhiza | IL-UAE | [C10C1im]Br, [C12C1im]Br, [C14C1im]Br, [C16C1im]Br, [C8C1im]Br, [C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, and [C8C1im]Cl (water)63,76 |
| (+)-catechin, ellagic acid, and pyrocatechol | Acacia catechu and Terminalia chebula | IL-SLE | [N1100][N(C1)2CO2] (pure IL)33 |
| fangchinoline and tetrandrine | Stephaniae tetrandrae | IL-UAE | [C4C1im][BF4] (water)56 |
| forskolin | Coleus forskohlii | IL-UAE | [C4C1im]Cl, [C4C1im]Br, [C4C1im][BF4], [C4pyr][BF4], [N000(2OH)][C0CO2], [C1C1C1C1guan][Lac](water)64 |
| galantamine, narwedine, N-desmethylgalantamine, and ungiminorine | L. aestivum | IL-SLE | [C4C1im]Cl, [C6C1im]Cl, [C8C1im]Cl, [C10C1im]Cl, [C4C1im]Br, [C4C1im][Sac], [C4C1im][Ace], [C4C1C1im]Cl, [C4C1im][C1CO2], [C4C1im][CF3CO2], [C4C1im][SCN], [C4C1im][N(CN)2], [C4C1im][C(CN)3], [C7H7C1im]Cl, [C4C1pyrr]Cl, [N11(2OH)(C7H7)]Cl, and [N221(O)nOH]Cl(water)32 |
| gallic acid | Suaeda glauca Bge. | IL-UAE | [C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, and [C8C1im]Cl (water)61 |
| glaucine | Glaucium flavum (papaveraceae) | IL-SLE | [C4C1im][Ace], [C10C1im][Ace], [C6C1im][Ace], [C8C1im][Ace], [C4C1im][Sac], [C4C1im]Br, and [C4C1im]Cl (water)27,28 |
| hydroxycamptothecin and camptothecin | Camptotheca acuminata | IL-UAE | [aC1im]Br, [C7H7C1im]Br, [C2C1im]Br, [C3C1im]Br, [C4C1im][BF4], [C4C1im][ClO4], [C4C1im][HSO4], [C4C1im][NO3], [C4C1im]Br, [C4C1im]Cl, [C6C1im]Br, [C8C1im]Br, and [C6H11C1im]Br (water)54 |
| isoliensinine, liensinine, and neferine | Nelumbo nucifera | IL-MAE | [C2C1im][BF4], [C4C1im][BF4], [C4C1im][PF6], [C4C1im]Br, [C4C1im]Cl, [C6C1im][BF4], and [C8C1im][BF4] (water)44 |
| iristectorin A, iristectorin B, and tectoridin | Iris tectorum | IL-UAE | [C4C1im][BF4], [C6C1im]Br, and [C8C1im]Br (water)58 |
| myricetin, quercetin, kaempferol | Bauhinia championii | IL-MAE | [C2C1im]Br, [C4C1im][BF4], [C4C1im][H2PO4], [C4C1im][HSO4], [C4C1im][PF6], [C4C1im]2[SO4], [C4C1im]Br, [C4C1im]Cl, [C6C1im]Br, and [(HOOC)C1C1im]Cl (water)41 |
| nuciferine, N-nornuciferine, O-nornuciferine | Nelumbo nucifera | IL-MAE | [C2C1im]Br, [C4C1im][BF4], [C4C1im][PF6], [C4C1im]Br, [C4C1im]Cl, [C6C1im]Br, and [C8C1im]Br (water)45 |
| piperine | Piper nigrum (white and black pepper) | IL-SLE | [C10C1im]Cl, [C12C1im]Cl, [C12C1im]Br, [C12C1im][CF3SO3], [C12C1im][C1CO2], [C12C1im][N(CN)2], [C14C1im]Cl, and [N111[2O(O)12]Cl (water)30 |
| piperine | Piper nigrum (white and black pepper) | IL-UAE | [C4C1im][BF4], [C4C1im][H2PO4], [C4C1im][PF6], [C4C1im]Br, [(HSO3)C4C1im]Br, and [C6C1im][BF4] (water)53 |
| polycyclic aromatic hydrocarbons | Petroleum Source Rock | IL-MAE | [C4C1im]Br, [C4C2C1im]Cl, and [C1C1C1im]2[SO4] (water)52 |
| quercetin, ellagic acid, gallic acid, pyrocatechol, trans-resveratrol | Psidium guajava (guava) and Smilax china | IL-MAE | [C2C1im][BF4], [C2C1im]Br, [C4C1im][BF4], [C4C1im][C1SO4], [C4C1im][H2PO4], [C4C1im][N(CN)2], [C4C1im]Br, [C4C1im]Cl, [C4py]Cl, and [C6C1im]Br (water)42 |
| rutin | Saururus chinensis and Flos sophorae | IL-MAE | [C4C1im][BF4], [C4C1im][Tos], [C4C1im]Br, and [C4C1im]Cl (water)43 |
| rutin, hyperoside, and hesperidin | Sorbus tianschanica leaves | IL-MAE | [C4C1im]Cl, [C4C1im]Br, [C4C1im][BF4], [C4C1im][NO3],[C4C1im][HSO4], [C4C1im][ClO4], [C2C1im][BF4], [C6C1im][BF4], and [C8C1im][BF4] (water)44 |
| saponins and polyphenols | Ilex paraguariensis (mate) and Camellia sinensis (tea) | IL-SLE | [aC1im]Cl, [C2C1im][Lac], [C2C1im][CF3SO3], [C2C1im][C2SO4], [C2C1im][C1CO2], [C2C1im][N(CN)2], [C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, [C7H7C1im]Cl, [C8C1im]Cl, [N111(2OH)][NTf2], [N111(2OH)]Cl, and [(OH)C2C1im]Cl (water)79 |
| saponins and polyphenols | Ginkgo biloba | IL-SLE | [C4C1im]Cl (pure IL)37 |
| shikimic acid | Illicium verum (star anise) | IL-MAE | [C2C1im][BF4], [C2C1im][CF3SO3], [C2C1im][C1CO2], [C2C1im][NTf2], [C2C1im][PF6], and [C2C1im]Cl (pure IL)51 |
| shikimic acid | Illicium verum (star anise) | IL-SLE | [C2im][HSO4], [C2C1im][HSO4], [(HSO3)C4C1im][H2PO4], [(HSO3)C4C1im][HSO4], [(HSO3)C4C1im][NTf2], [(HSO3)C4C1im]Br, and [(HSO3)C4C1im]Cl (ethanol)36 |
| senkyunolide H, senkyunolide I, Z-ligustilide | Ligusticum chuanxiong | IL-MAE | [N11(2(O)2OH)0][C2CO2] and [N011(2CN)][C2CO2] (pure IL)48 |
| shikimic acid | chinese conifer needles | IL-UAE | [C4C1im]Cl, [C4C1im]BF4, [C4C1im][NO3], [C2C1im]Br, [C3C1im]Br, [C4C1im]Br, [C5C1im]Br, [C6C1im]Br, [C8C1im]Br, [C10C1im]Br, [C7H7C1im]Br, (water)59 |
| shikonin β,β′-dimethylacrylshikonin | Arnebia euchroma | IL-UAE | [C2C1im][BF4], [C4C1im][BF4], [C6C1im][BF4], [C6C1im][PF6], [C8C1im][BF4], and [C8C1im][PF6] (pure IL)67 |
2.1.1. Simple Solid–Liquid Extractions
Recently, various works have been published addressing the use of IL aqueous solutions on the SLE of alkaloids (e.g., glaucine from Glausium flavum),27,28,31 caffeine from Paullinia cupana (guaraná seeds),29 galantamine, narwedine, N-desmethylgalantamine, and ungiminorine from the aerial parts of Leucojum aestivum,32 and piperine from Piper nigrum.30 Bogdanov et al.28 scanned a series of ILs with cations bearing different lengths of their alkyl side chains ([CnC1im]+ series) coupled to different anions (Cl–, Br–, [Ace]−, and [Sac]−) for the extraction of glaucine from Glausium flavum. The effect of the IL concentration in aqueous media, the extraction time, and the biomass-solvent ratio were also optimized. With aqueous solutions of [CnC1im][Ace] (n = 4, 6, and 8), an 85% extraction yield of glaucine was achieved at 80 °C for 1 h, a much higher extraction yield than that obtained with methanol, solutions of potassium acesulfamate, and water under similar conditions.28 The extraction yield of glaucine increased with the IL concentration in aqueous media, reaching a maximum of 99% at an IL concentration of 2 M. The extraction efficiencies obtained show that, besides the effect of the IL anion on the disruption of the matrix structure, there is a major role played by the imidazolium cation on the extraction process due to the aromatic π-cloud which allows strong interactions with polarizable and aromatic solutes.27,28 Similar results were obtained by Cláudio et al.29 and Svinyarov et al.,32 who investigated the extraction of caffeine from guaraná seeds and the extraction of galantamine, narwedine, N-desmethylgalantamine, and ungiminorine from the aerial parts of L. aestivum using aqueous solutions of a series of imidazolium-, pyrrolidinium-, and ammonium-based ILs. At the respective optimal conditions, [C4C1im]Cl was found to be the best IL in both studies.29,32 Extraction yields of caffeine of 9.4 wt % have been obtained, a value significantly higher than that obtained with dichloromethane under a Soxhlet extraction (4.30 wt %).29 An analytical procedure for the SLE of galantamine (widely used in Alzheimer’s disease, poliomyelitis, and other neurological diseases) from L. aestivum biomass, followed by HPLC quantification, was proposed by the other group of researchers.17 The role of the aromatic imidazolium cation of the IL was emphasized by the results obtained by all research groups,28,29,32 who attempted the extraction of aromatic compounds (alkaloids). Although scarcely investigated, Cláudio et al.29 proposed a back-extraction procedure for the target alkaloid followed by the reuse of the IL, a schematic representation of the proposed integrated process is sketched in Figure 4A.
Figure 4.
Schematic diagrams of integrated processes based on ILs comprising the extraction and separation of small organic extractable compounds from biomass and further IL recovery and reuse.29,33,93
Bica and co-workers30 reported the extraction of piperine from black pepper by SLE using aqueous solutions of surface-active ILs ([CnC1im]+, with n = 10, 12, and 14, combined with Cl–, Br–, [CF3SO3]−, [C1CO2]−, and [N(CN)2]− anions, as well as a long chain biodegradable and betain-derived IL, [N111[2O(O)12]]Cl). Below the critical micellar concentration (CMC) of the IL, the extraction yield of piperine was found to be <0.2 wt %, whereas a 4.0 wt % of extraction yield was recorded for IL concentrations higher than the CMC, while revealing a negligible influence of the IL anion.30 In this latter work the ability of the IL to self-aggregate in aqueous media seems to be the main favorable factor for the enhanced extraction yields observed and not the hydrotropic phenomenon9 or the ILs’ capacity to disrupt the cells. Although piperine is an alkaloid and an aromatic compound, it is of a more hydrophobic nature, as shown by its higher octanol–water partition coefficient [log(Kow) of piperine = 2.30 vs log(Kow) of caffeine = −0.13] (Chemspider database accessed on August 2016), thus requiring the creation of hydrophobic cores produced by surface-active ILs to increase its dispersion/solubility in aqueous media. Of particular interest, the biodegradable betain-based IL exhibited a better performance in the extraction of piperine30 and can be used as a prospective IL for greener and large-scale applications. The same group of researchers35 investigated the IL-based SLE of betulin (a pharmaceutically active triterpene from birch bark). In this work, imidazolium-based water-soluble ILs, such as [C2C1im]Cl, [C2C1im]Br, [C2C1im][N(CN)2], and [C2C1im][C1CO2], led to higher betulin yields (28–31 wt %) when compared with hydrophobic ILs based on [BF4]−, [PF6]−, and [NTf2]− anions (∼22 wt %).35 Overall, [C2C1im][C1CO2] was demonstrated to be the optimal solvent for dissolving birch bark and to subsequently extract betulin.35
Tamiflu is an important drug used for the treatment of influenza. Shikimic acid is extensively used as starting material for the synthesis of Tamiflu77 and is predominantly extracted from the Chinese herb Illicium verum (star anise). Ressmann et al.36 explored an alternative and effective method for the extraction of shikimic acid via dissolution of Illicium verum in the presence of Brønsted acidic IL solutions ([(HSO3)C4C1im][HSO4], [(HSO3)C4C1im][NTf2], [(HSO3)C4C1im]Br, and [(HSO3)C4C1im]Cl). Remarkably, the authors36 demonstrated that ILs act both as solvents and catalysts toward the in situ synthesis of shikimic acid ethyl ester and its ketal ester. Most ILs allowed a 81–99% conversion; however, a complete conversion was obtained in the presence of [(HSO3)C4C1im][NTf2], where the sulfonic acid side chain was demonstrated to be the main factor behind the efficient catalytic activity. Along the same lines, Usuki et al.37 further demonstrated the extraction of shikimic acid from Ginkgo biloba leaves using neat [C4C1im]Cl. At optimum conditions, the extraction yield of shikimic acid was 2.5 times higher than that obtained with methanol at 80 °C and 2 times higher than with dimethylformamide at 150 °C. Thus, neat ILs also appear as good alternatives to the commonly used volatile organic solvents in the extraction of shikimic acid.37 However, no attempts at shikimic acid conversion were carried out by Usuki et al.37
(+)-Catechin is an important flavonoid with benefits for human health and a tannin used in leather industries.78 As an alternative protocol, Chowdhury et al.33 proposed the SLE of tannins from Acacia catechu (catechu) and Terminalia chebula (myrobolan) using a protic IL ([N1100][N(C1)2CO2]): the process diagram is sketched in Figure 4B. Various tannins, such as (+)-catechin, gallic acid, ellagic acid, and pyrocatechol, were effectively extracted from the biomass samples using ([N1100][N(C1)2CO2]. High extraction efficiencies for both catechu (85%) and myrobolan (75%) were obtained, while with water as the main solvent, the extraction efficiency decreased to 64% and 52%, respectively.33 Whereas most studies discussed above focused on imidazolium-based ILs, the work reported by Chowdhury et al.33 is a notable example of good extraction yields achieved by other classes of ILs. Finally, and in contrast to aprotic imidazolium-based ILs, [N1100][N(C1)2CO2] can be distilled at around 45 °C under reduced pressure, allowing solvent recovery and reuse. Remaining within the use of protic and distillable ILs, their application in the SLE of artemisinin, a sesquiterpene lactone, from Artemisia annua was proposed by Bioniqs Ltd. in 2006.74 A protic and biodegradable IL, [N110(2OH)][C7CO2], was found to be the best IL investigated and, under the optimum conditions, high extraction yields were obtained when compared to those obtained by hexane at high temperatures. Later, in 2008, Bioniqs Ltd. used molecular simulation to design improved ILs for the SLE of artemisinin, and where [N11(2(O)1)0][C2CO2] leads to a significant improvement in the selectivity and extraction yield of artemisinin.75
Most bioactive compounds discussed above have a moderately high solubility in water; however, aqueous solutions of water-soluble ILs with low alkyl side chain length are not promising solvents when dealing with the extraction of more hydrophobic target biocompounds. To enhance the extraction and separation of such hydrophobic bioactive compounds in aqueous media, Jin et al.38 proposed a family of new water/IL mixtures with amphiphilic anionic functional long-chain carboxylate ILs (LCC-ILs) for the simultaneous dissolution of biomass and extraction of hydrophobic bioactive compounds. The LCC-ILs investigated possess weak polarity and strong hydrogen-bonding basicity simultaneously, thus displaying a high solubility for numerous hydrophobic natural compounds, such as tocopherol, perillyl alcohol, rutin, and ginkgolides. The water/LCC-IL mixtures investigated allowed extraction yields 2 to 12 times higher than that achieved with common organic solvents.38 The authors38 also studied the dissolution mechanism at a microscopic level and demonstrated the formation of nanomicelles when tocopherol is dissolved in water/LCC-IL mixtures, meaning that the formation of IL aggregates achieved by the use of surface-active ILs allows the incorporation of hydrophobic bioactive compounds into the micelle core, thereby enhancing the extraction yield, a similar result to that found by Bica and co-workers30 on the extraction of piperine using surface-active ILs.
2.1.2. Microwave-Assisted Extractions
The simpler SLE processes discussed above may require long extraction times and large volumes of solvent. Higher yields and faster extractions of biocompounds from biomass can be achieved through MAE processes. The pioneering work on the use of IL-MAE processes was reported by Li and co-workers in 2007,26 who demonstrated the effective utilization of aqueous IL solutions in MAE to extract trans-resveratrol from Rhizma polygoni. Through an orthogonal design methodology, the authors26 investigated the effect of the IL chemical structure ([C4C1im][BF4], [C4C1im]Br, and [C4C1im]Cl) and additional experimental conditions. Among the ILs studied, [C4C1im]Br was found to be the best solvent, with a 93% trans-resveratrol extraction yield obtained at the optimum conditions.26 In addition to this pioneering work, many more have followed; however, all have dealt with imidazolium-based ILs, and no studies were found on the use of other IL families in IL-based MAE processes. For instance, Pan and co-workers39,40 studied the extraction of alkaloids, such as isoliensinine, liensinine, neferine, nuciferine, n-nornuciferine, and o-nornuciferine from Nelumbo nucifera and claimed to have developed a rapid, effective, and more environmentally friendly method. In fact, the authors demonstrated that IL-based MAE enhances the extraction efficiencies by up to 50.0% while reducing the extraction time from 2 h to 90 s.39,40 After several optimization studies, including alteration of the chemical structure of the IL, the authors showed that [C4C1im][BF4] was the most effective IL for the extraction of isoliensinine, liensinine, and neferine, whereas [C4C1im]Br exhibited the best results on the extraction of nuciferine, n-nornuciferine, and o-nornuciferine.39,40 It was also concluded that increasing the IL alkyl side chain length (using [CnC1im]Br ILs) up to hexyl enhances the alkaloid extraction efficiency, while a further increase from hexyl to octyl drastically reduces the alkaloids extraction efficiency. These results suggest that surface-active ILs are not favorable for the extraction of these more water-soluble alkaloids and that the mechanism of extraction seems to be ruled by an hydrotropic effect9 and not by a micelle-mediated phenomenon, as discussed in the previous section when dealing with the extraction of more hydrophobic compounds, such as piperine using simpler SLE processes.30
The extraction of flavonoids from natural resources by IL-based MAE strategies was also demonstrated by several research groups,41−43 namely in the extraction of rutin from Saururus chinensis and Flos sophorae,43 of a series of polyphenolic compounds from Smilax china,42 and of kaempferol, myricetin, and quercetin from Bauhinia championii.41 The results obtained by all authors suggest that the extraction of flavonoids is mainly governed by the IL anion and where ILs with higher hydrogen bond acceptor ability, such as [C4C1im][H2PO4], [(HOOC)C1C1im]Cl, and [C4C1im]Br, provide the highest extraction yields.41−43 Du et al.42 additionally compared the quercetin extraction efficiency using pyridinium-based ILs and found that they afford higher extraction yields than their imidazolium counterparts. Thus, the effect of the IL cation, probably dominated by π–π interactions, cannot be ruled out in extractions of flavonoids. At this stage, it is still difficult to identify the most important characteristics of ILs, since only a limited matrix of ILs has been studied. Although some authors41−43 have claimed the pivotal role of the IL anion, the number of IL cations investigated is currently too small to draw general conclusions.
Aqueous solutions of ILs were also applied in the MAE of rutin, hyperoside, and hesperidin from S. tianschanica leaves,44 of ten flavonoid glycosides from Chrysanthemum morifolium Ramat,45 and of baicalin, wogonoside, baicalein, and wogonin from Scutellaria baicalensis Georgi.46 All authors demonstrated the superior performance of IL-based MAE when compared to more traditional methods and solvents. For instance, IL-based MAE resulted in the highest extraction yield (22.28%) of baicalin within 90 s, as compared to water-based MAE (9.77%, 90 s) and IL-SLE (16.94%, 30 min).46 In addition to the work discussed above on the SLE of shikimic acid from star anise, it was later demonstrated that the time of extraction (∼24 h) was drastically reduced to 10 min by using IL-based MAE.51 In this study, [C2C1im][C1CO2] led to the best results (10.7 wt %), whereas the poorer results were achieved with [C2C1im][PF6], with the observed degradation of shikimic acid.51 In this case, the hydrolysis of the hexafluorophosphate anion at high temperatures (100 °C), followed by the production of hydrofluoric acid80 cannot be ignored, highlighting the demand for more stable ILs in addition to the well-studied [BF4]- and [PF6]-based variants.
Lignans are compounds with a characteristic dibenzocyclooctadiene-type skeleton, present in various biomass matrices. IL-based MAE of four lignans from the fruits of Schisandra chinensis – schizandrin, schisantherin A, deoxyschizandrin and γ-schizandrin–was studied by Ma et al.50 Several combinations of imidazolium-based ILs with different anions and different alkyl side chain lengths (n = 2 to 12) were tested, with [C12C1im]Br providing the best extraction efficiencies (yield ≈9.9%).50 The application of IL-based MAE toward the extraction of carsonic acid (a terpenoid) and rosmarinic acid (a phenolic acid) from Rosmarinus officinalis (rosemary) was reported by Liu et al.47 The authors47 demonstrated an increase in the extraction yield of carnosic acid with the increase of the [C8C1im]Br concentration (above the CMC). These results reinforce the relevance of aqueous solutions of surface-active ILs in the extraction of more hydrophobic bioactive compounds, as discussed above.
Unlike the aforementioned studies that focused on imidazolium-based ILs, Yansheng et al.48 explored novel protic ILs composed of an ammonium-based cation and the propionate anion ([N11(2(O)2OH)0][C2CO2] and [N011(2CN)][C2CO2]) for the MAE of some benzofuranoids (senkyunolide H, senkyunolide I and Z-ligustilide) from Ligusticum chuanxiong Hort. The IL [N011(2CN)][C2CO2] led to high extraction yields of benzofuranoids within 1–5 min and at temperatures between 60 and 180 °C. Although not attempted by the authors, the use of protic ILs is advantageous since they can be recovered by distillation at reduced pressure and as attempted by Chowdhury et al.33
Summing up, almost all IL-based MAE processes reported to date are based on imidazolium-based ILs (with two exceptions: one evaluating the effect of the pyridinium cation and the second by employing protic ILs). The majority of ILs investigated comprise cations with short alkyl side chains length. Despite the lack of discussion on the mechanisms behind the improved extraction yields, a number of authors related the success of their extractions to the establishment of strong interactions, mainly hydrogen-bonding and π–π interactions, between the ILs and the target biocompounds. Various processing conditions were also addressed, such as irradiation power, solid–liquid ratio, time of extraction, chemical structure, and concentration of the ILs. In general, higher concentrations of the IL (up to a given limit, since at higher concentrations the viscosity of the solvent increases and leads to a decrease of the mass transfer of the solute as well as to a lower solubility of the target biocompounds) and lower solid–liquid ratios promoted higher extraction yields, while other parameters, such as the irradiation power, resulted in the lack of clear tendencies. Meanwhile, the use of tensioactive ILs promote either the increase or decrease of the extraction yield, depending mostly on the hydrophilic−lipophilic ratio of the target biocompound. However, we think that more studies need to be addressed in this field, since much more conditions need to be explored, namely the size and type of IL aggregates.
2.1.3. Ultrasound-Assisted Extractions
Although the MAE technology is rapid and effective toward the extraction of bioactive compounds from biomass, it is also energy intensive and difficult to scale up.8 To overcome these drawbacks, UAE can be used as a viable alternative to MAE. MAE allows rapid heating by irradiation, with further improvements on the solutes mass transfer, while UAE enhances the mass transfer mechanically.8 Moreover, when extracting thermosensitive biomolecules, UAE is a better choice since the extraction can be achieved without heating. The first demonstration of IL-based UAE for the extraction of natural compounds from biomass was carried out by Cao et al.53 (extraction of piperine from Piper nigru). Thereafter, several works were published demonstrating the use of IL-based UAE for the extraction of different alkaloids from biomass.54−57 For instance, Ma et al.49,54 studied the extraction of four biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill, 30- and 10-hydroxycamptothecin, and camptothecin from Camptotheca acuminate, while Yang et al.55 studied the extraction of vindoline, catharanthine, and vinblastine from Catharanthus roseu. Other researchers extracted fangchinoline and tetrandrine from Stephaniae tetrandrae(56) and berberine, jatrorrhizine, and palmatine from Phellodendron amurense Rupr.57 All authors53−57 investigated a large number of ILs with different anions and cation alkyl side chain lengths and found that the extraction of alkaloids is mainly governed by the IL anion, as previously observed by Pan and co-workers with IL-based MAE.39,40 In all these studies,53−57 more hydrophilic ILs, such as [C4C1im]Br and [C4C1im]Cl, display a better performance in the extraction of alkaloids. Nevertheless, considering the observations made by Ma et al.49,54 and Yang et al.,55 wherein the extraction efficiency of alkaloids increases with the cation alkyl side chain length, it is clear that the contribution of the IL cation cannot be neglected. The performance of UAE assisted by ILs was compared against that obtained by other solvents and techniques, including UAE with pure water,49,54,55,57 aqueous solutions of sodium chloride or sodium bromide,49,54,55 heat reflux extraction with pure water57 or methanol,53 regular ultrasound extraction without the presence of IL,53,56 and refluent extraction with ethanol.56 Taking into account the general results reported, it seems that the use of IL-based UAE is advantageous because not only the extraction efficiencies obtained are higher, but they are also achieved within shorter extraction times53−57 and at lower temperatures.55 Some authors reported that the use of ILs contributes to the development of more benign/pollution-free methodologies, justified by the elimination of environmentally harmful organic solvents,55,57 while avoiding the degradation of alkaloids57 that occurs at higher temperatures. Although a relevant aspect for guaranteeing the sustainability of the developed processes, the recyclability of the solvent was only addressed by Ma et al. and Yang et al.49,54,55 They reported no losses in the extraction yields over four cycles of extraction-reuse of the solvent.49,54,55
In addition to alkaloids, IL-based UAE was successfully applied in the extraction of flavonoids (tectoridin, iristectorin A, and iristectorin B) from Iris tectorum.58 The authors58 screened the effect of different aqueous IL solutions and compared their results with those obtained by methanol and aqueous solutions of sodium chloride, finding that the best results were obtained with 0.5 M of [C8C1im]Br.58 Recently, IL-based UAE was also applied in the extraction of shikimic acid from conifer needles,59 caffeoylquinic acid from Flos Lonicerae Japonicae,60 and gallic acid from Suaeda glauca leaves.61 All authors evaluated the extraction yields achieved by several ILs with different cations and anions, and optimized various extraction conditions, including the IL concentration, ultrasonic power, extraction time, solid–liquid ratio, and temperature.59−61 The extraction efficiency generally increases by the application of lower solid–liquid ratios and higher temperatures.58−66,76 In general, all authors reported a dominant impact of the IL cation regarding the extraction yield of flavonoids. Although scarcely investigated and as carried out by Ma et al. and Yang et al.49,54,55 when dealing with the extraction of alkaloids, Chen and co-workers59 have additionally shown that the extraction yield of shikimic acid is reproducible over five cycles of solvent reuse, representing therefore a step toward the development of more sustainable IL-based processes.
The use of IL-based UAE was also explored for the extraction of terpenoids, such as ginsenosides, cryptotanshinone, tanshinone I, and tanshinone II A, from various natural sources.62,63,76 Lin et al.62 used this method for the extraction of ginsenoside derivatives from Ginseng roots, finding [C3C1im]Br to be the best IL with an extraction yield of ∼17 mg g–1, a higher yield than that obtained with water (∼13 mg.g–1) or ethanol (∼6 mg g–1).62 Wu et al.63 and Bi et al.76 reported a comprehensive study on the extraction of tanshinones from Salvia miltiorrhiza using long alkyl chain ILs, [CnC1im]Br (n = 8, 10, 12, 14, 16). The best extractions were obtained with the longest alkyl chain IL investigated ([C16C1im]Br), with yields of 0.6, 1.2, and 1.4 mg g–1 for cryptotanshinone, tanshinone I, and tanshinone IIA, respectively.63 As discussed in the two previous sections, these terpenoids are highly lipophilic and are thus better extracted by micelle-mediated processes.63,76 Harde et al.64 extracted forskolin from Coleus forskohlii roots using six ILs with different cations and anions, from which tetramethyl guanidinium lactate led to the best extraction efficiency (87.4%). The shorter extraction times (4 h) offered by IL-based UAE, as compared to Soxhlet extraction (12 h), show that IL-based UAE is a faster and more efficient approach for the extraction of terpenoids.64 This work64 is also a relevant contribution toward the field of IL-based extraction processes by using ILs other than the well-studied imidazolium-based examples.
Yang et al.65 demonstrated the application of IL-based UAE for the extraction of two benzopyranoids (aesculetin and aesculin) from Fraxinus rhynchophylla. They studied the effects of the IL anion and cation, achieving extraction efficiencies in the range of 60–100%, with [C4C1im]Br appearing to be the best solvent. The authors65 also conducted a comparison study with conventional UAE using several molecular solvents, ethanol-based heating reflux and a simple stirring extraction, and were able to demonstrate65 that IL-based UAE leads to higher extraction yields. On the other hand, Xiao et al.67 employed [CnC1im][BF4] ILs and demonstrated that the extraction yield of β,β′-dimethylacrylshikonin (more hydrophobic than shikonin) significantly increases with the IL cation alkyl chain length.67
Overall, IL-based UAE has been effectively applied to the extraction of a wide variety of natural products from a large number of plant species, although the scalability of the processes and commercial exploitation of the extracted products lagged behind. Compared to IL-based MAE, IL-based UAE offers the advantage of conducting the extractions at lower temperatures, and thus it is more appropriate for the extraction of thermolabile biocompounds. However, the number of ILs tested is still very limited, and the definition of heuristic rules regarding the use of different cations and anions is still lacking. For instance, it has been shown that the IL anion plays a major role in the extraction of alkaloids, while the IL cation alkyl side chain length plays a major role on the extraction of flavonoids. In our opinion, the absence of these rules is related to the absence of more complete studies evaluating both the IL cation and anion in the extraction of similar classes of bioactive compounds. A more complete evaluation is thus required, while attempting the correlation of the extraction yields with the IL properties, namely, their hydrophilic/lipophilic nature and hydrogen bond acidity or basicity. Moreover, the use of ILs that form aggregates, either by a micelle-mediated phenomenon or by a hydrotropic effect,9 needs to be comprehensively addressed to better understand the mechanisms that control the extraction of target biocompounds. Additional extraction conditions, such as the pH, still need to be ascertained, since some extractions may be improved by variation of the speciation of the target molecules, a factor not considered by most of the authors.
2.1.4. More Complex Solid–Liquid Extractions
In view of the advantages of IL-based UAE and MAE discussed above in the extraction of natural bioactive compounds, an integrated approach combining UAE and MAE (UMAE) was developed by Lu et al. for the extraction of gallotannins from Galla chinensis(68) and anthraquinones from Rheum spp. (rhubarb).69 The authors68 studied the replacement of organic solvents in UMAE by [C4C1im][BF4], [C4C1im]Br, and [C4C1im]Cl, whereby [C4C1im]Br was found to be the best solvent in terms of extraction efficiency. Moreover, IL-based UMAE considerably shortened the extraction time from 6 h (attained with IL-based UAE) to 1 min. A comparative analysis of IL-UAME with other methods, such as SLE under heat and reflux conditions, UAE and MAE, was also carried out.69 IL-UMAE led to higher extraction yields (24% improvement) within a shorter extraction time (2 min).69 In addition to IL-UMAE, Liu et al.70 applied IL-based ultrahigh pressure extraction (UPE) for the extraction of tanshinones from Salvia miltiorrhiza. The IL-based UPE approach, using 0.5 M of [C8C1im][PF6] in an ethanol solution, provided higher extraction yields with lower processing times and energy and solvent consumptions.70 This high efficiency of the IL-UPE method was ascribed to an improved disruption of the plants’ tissue cells.70
All the IL-based extraction methodologies discussed above (IL-based SLE, IL-based MAE, IL-based UAE, IL-based UMAE, and IL-based UPE) have limitations in terms of the extraction of some bioactive compounds, since some of them are unstable, labile, thermosensitive, and susceptible to oxidation when in contact with air. To overcome such limitations, recently, a new extraction approach called negative-pressure cavitation extraction (NPCE) has been proposed.72 Compared to the other extraction techniques, NPCE is carried out at low temperatures under an inert atmosphere. Duan et al.72 studied the IL-based NPCE method for the extraction of flavonoids, such as genistin, genistein, and apigenin from the roots of Cajanus cajan (pigeon pea), using [C8C1im]Br. The authors72 initially performed the lab-scale extraction and after optimizing the extraction conditions, the process was scaled-up. Similar extraction yields in lab and pilot scales were obtained, suggesting that IL-based NPCE is an appropriate option for the extraction of natural compounds on an industrial scale. In fact, and to the best of our knowledge, this is the only report on IL-based extraction approaches attempting the scale-up viability of the process.
Recently, Wang et al.73 demonstrated a simultaneous extraction and detection of anthraquinones from the root of Rheum palmatum L., using IL-based microwave homogeneous liquid–liquid microextraction (IL-based MA-HLLME). Enhanced extractions of four anthraquinones, namely aloe-emodin, emodin, chrysophanol, and physcion, were obtained within a short time period.73 Moreover, the proposed IL-based MA-HLLME protocol does not require the use of volatile organic solvents, and it only requires low amounts of solvent compared to IL-based UAE and IL-based heat reflux extractions.
In summary, a wide variety of SLE approaches (simple IL-based SLE, IL-based MAE, IL-based UAE, IL-based UMAE, IL-based UPE, and IL-based NPCE) have been investigated over the past few years for the extraction of bioactive compounds from natural sources. All these techniques have their particular requirements and offer certain benefits compared to others. Although the MAE is a faster technique, it still presents some drawbacks regarding the possible degradation of thermosensitive molecules; for these situations, the UAE seems a better approach to be applied. Nevertheless, whatever the methodology applied, ILs have a definitive role toward an enhanced extraction performance when compared to conventional molecular solvents. Owing to the distinct properties of ILs and their ability to interact with bioactive compounds, via hydrogen-bonding, dispersive, π···π and n···π interactions, they could be realized as tailored solvents, thus surpassing the performance of traditional molecular solvents. Moreover, the role of ILs in SLE processes from biomass is not limited to the improved solute–solvent interactions but also due to the effect of ILs in disrupting the biomass organized structure. The combination of ILs with different SLE methods favors the disruption of plant cells and acts synergistically by improving the mass transfer and solubility of bioactive compounds in IL-based solvents. On the other hand, the use of aqueous solutions of ILs stands out, since it is avoiding the dissolution of the lignocellulosic fraction of most biomass samples considered.
It is clear, however, that more studies need to be carried out regarding the different solid–liquid extraction approaches discussed herein. Other cation families (phosphonium, quaternary ammonium, and cholinium derivatives) need to be evaluated, in particular those with higher hydrogen bond abilities, which seem promising for the extraction of natural compounds. The aromaticity of ILs also plays an important role in the extraction of different compounds, since imidazolium-based ILs are often referred to as the most promising solvents. Nevertheless, there is still a need for further investigation of nonaromatic cations and aromatic anions in order to fully understand their role. When using ILs in aqueous solution, the authors also need to be careful in the selection of the anion structures, and to avoid the use of [PF6]− and [BF4]−, due to their poor stability in water and consequent formation of fluoridric acid.80 Even though we can assume that for some natural compounds the presence of acids is not harmful, up to a certain extent, it, however, represents a loss of IL if its reuse is envisaged.
The use of surface-active ILs, in turn, led either to an increase or to a decrease of the extraction yield, a phenomenon that mainly depends on the hydrophilic–lipophilic ratio of the target biocompound. However, more studies need to be performed, since the size and type of IL aggregates should also be evaluated. For more hydrophobic biocompounds, it has been demonstrated that ILs with the ability to self-aggregate are the most promising solvents, while more hydrophilic compounds are better extracted with ILs with a higher ability to establish hydrogen bonds or to create solute-IL aggregates by a hydrotropic phenomenon.9 It seems that a threshold on the hydrophobic nature of biocompounds exists, and this should be used to a priori identify the most promising classes of ILs to be applied.
It is our belief that more efficient processes will be developed in the near future and properly scaled up. One work72 addressing the SLE technology scale-up was found in the open literature, while successfully demonstrating its potential. Despite the near absence of studies resorting to the scale-up of these extraction processes, their implementation still needs to follow some criteria, namely the market price of the biomolecules being extracted and their purity level, the costs associated with the process and ILs used, and the suitability of recovering and reusing the employed solvents. Everything considered, it will be much easier to define and optimize IL-based methodologies to make the process of solid–liquid extractions more selective and cost-effective and if IL-based methodologies are indeed the best option to replace the conventional processes. Although a large number of reports exists on the use of IL-based SLE of small organic bioactive compounds, most of these were focused on optimizing the extraction yield, and while some attempted the recovery of solutes and reuse of solvents, no indications of the purity of the extracts were provided (an important feature which defines the current price of biocompounds when foreseeing their commercialization).
2.2. IL-Based Liquid–Liquid Extractions
After the extraction steps mainly carried out by the SLE techniques described above, IL-based LLE approaches have been studied as a subsequent stage for the separation and purification of target biocompounds, namely by (i) the application of hydrophobic ILs; and (ii) by the use of IL-based aqueous biphasic systems (ABS). Since most biomass extracts are water-rich, hydrophobic water-immiscible ILs are used initially, defined here as IL-LLE, while in the second option a salting-out agent is added to the water-miscible ILs to create a second liquid phase, defined here as IL-ABS. Although not extensively investigated, IL-based LLE may, in some cases, allow the combination of extraction, purification, and concentration in a single step.81 An overview of IL-based LLE processes for the separation and purification of bioactive compounds is provided in Table 3.
Table 3. Extraction and Separation of Small and Extractable Organic Compounds from Biomass Using IL-Based LLE.
| bioactive compound | natural source | method | ILs (solvents) used |
|---|---|---|---|
| aloe anthraquinones | aloe powder | IL-ABS | [C2C1im]Br + salt + water, [C4C1im]Br + salt + water, [C6C1im]Br + salt + water, [C4C1im][BF4] + salt + water, [C2C1im][BF4] + salt + water [C4C1im][N(CN)2] + salt + water; salt = NaH2PO4, (NH4)2SO4, Na2SO4, and MgSO482 |
| caffeic acid, vanillic acid, gallic acid, and vanillin | lignin depolymerization | IL-ABS | PEG 8000 + NaPA 8000 + [C12C1im]Cl, PEG 8000 + NaPA 8000 + [C14C1im]Cl83 |
| caffeine | – | IL-ABS | [C4C1im][CF3SO3] + sugars + water, [C4C1im][BF4] + sugars + water, [C4C1im] [N(CN)2] + amino acids + water; amino acids = l-Pro, l-Lys84,85 |
| caffeine and nicotine | mixture of alkaloids | IL-LLE | [P66614][NTf2] + K3PO4 + water, [P66614]Br + K3PO4 + water, [P66614]Cl + K3PO4 + water, [P66614][C1SO3] + K3PO4 + water, [P66614][N(CN)2] + K3PO4 + water, [P66614][TMPP] + K3PO4 + water86 |
| caffeine and nicotine | synthetic urine | IL-ABS | [C2C1im]Cl + K3PO4 + water, [C4C1im]Cl + K3PO4 + water, [C6C1im]Cl + K3PO4 + water, [C4C1C1im]Cl + K3PO4 + water, [C7C1im]Cl + K3PO4 + water, [C8C1im]Cl + K3PO4 + water [aC1im]Cl + K3PO4 + water, [C10C1im]Cl + K3PO4 + water, [C12C1im]Cl + K3PO4 + water, [OHC2C1im]Cl + K3PO4 + water, [C7H7C1im]Cl + K3PO4 + water, [C2C1im][CF3SO3] + K3PO4 + water, [C2C1im][CF3SO3] + K3PO4 + water87 |
| capsaicin | Capsicum frutescens | IL-ABS | [N111(2OH)]Cl + acetonitrile + water, [N111(2OH)][Bit] + acetonitrile + water, [N111(2OH)][DHCit] + acetonitrile + water88 |
| codeine and papaverine | Pericarpium papaveris | IL-ABS | [C4C1im]Cl + K2HPO4 + water89 |
| ellagic acid | Acacia catechu and Terminalia chebula | IL-LLE | [N1100][N(C1)2CO2] (pure)33 |
| eugenol and propyl gallate | – | IL-ABS | [C2C1im]Cl + C6H5K3O7/C6H8O7 + water, [C4C1im]Cl + C6H5K3O7/C6H8O7 + water, [C6C1im]Cl + C6H5K3O7/C6H8O7 + water, [C8C1im]Cl + C6H5K3O7/C6H8O7 + water, [C4C1pip]Cl + C6H5K3O7/C6H8O7 + water, [C4C1pyrr]Cl + C6H5K3O7/C6H8O7 + water, PEG + K2HPO4/KH2PO4 + [CnC1im]Cl (n = 2–8)90 |
| flavonoids and pectin | Ponkan peels | IL-ABS | [N111(2OH)][Ala] + K3PO4 + water, [N111(2OH)][Ser] + K3PO4 + water, [N111(2OH)][Cys] + K3PO4 + water, [N111(2OH)][Pro] + K3PO4 + water, [N111(2OH)][Asp] + K3PO4 + water, [N111(2OH)][Val] + K3PO4 + water, [N111(2OH)][Leu] + K3PO4 + water, [N111(2OH)][Phe] + K3PO4 + water,91 |
| gallic acid | – | IL-ABS | [C7C1im]Cl + salt + water, [C8C1im]Cl + salt + water, [C2C1im][CF3SO3] + salt + water, [C4C1im][CF3SO3] + salt + water, [C4C1im][N(CN)2] + salt + water, [C4C1im][C1SO4] + salt + water, [C4C1im][C8SO4] + salt + water, [C4C1im][C2SO4] + salt + water, [C4C1im]Br + salt + water; salt = K3PO4, Na2SO4, and K2HPO4/KH2PO492 |
| gallic, vanillic, and syringic acids | – | IL-ABS | [C4C1im][CF3SO3] + Na2CO3 + water, [C4C1im][SCN] + Na2CO3 + water, [C4C1im][C1SO3] + Na2CO3 + water, [C4C1im][C2SO4] + Na2CO3 + water, [C4C1im][C1SO4] + Na2CO3 + water, [C4C1im][Tos] + Na2CO3 + water, [C4C1im]Br + Na2CO3 + water [C4C1im][N(CN)2] + Na2CO3 + water, [C4C1im][(C1)2PO4] + Na2CO3 + water, [C4C1im]Cl+ Na2CO3 + water, [C4C1im][C1CO2] + Na2CO3 + water93 |
| gallic, vanillic, and syringic acids | mixture of phenolic acids | IL-ABS | PEG 300 + Na2SO4 + 5 wt % [C4C1im][Tos] + water, PEG 300 + Na2SO4 + 5 wt % [C4C1im][SCN] + water, PEG 300 + Na2SO4 + 5 wt % [C4C1im][N(CN)2] + water, PEG 300 + Na2SO4 + 5 wt % [C4C1im][C1CO2] + water, PEG 300 + Na2SO4 + 5 wt % [C4C1im]Cl + water, PEG 300 + Na2SO4 + 5 wt % [C4C1pyrr]Cl + water, PEG 300 + Na2SO4 + 5 wt % [C4C1pip]Cl + water94 |
| glaucine | Glaucium flavum Cr. | IL-ABS | [C4C1im][Ace] + Na2CO3 + water, [C4C1im][Ace] + (NH4)2SO4 + water, [C4C1im][Ace] + MgSO4 + water, [C4C1im][Ace] + NaH2PO4 + water95 |
| glycine | pharmaceutical sample | IL-LLE | [C4C1im][PF6] + dicyclohexano-18-crown-6 + water96 |
| indole-3-butyric acid | pea plants | IL-LLE | [C4C1im][PF6], [C6C1im][PF6], [C8C1im][PF6], [C6C1im][BF4], and [C8C1im][BF4] (water)81 |
| L-tryptophan, caffeine, and β-carotene | – | IL-ABS | [C4C1im][CF3SO3] + sugars + water, [C4C1im][BF4] + sugars + water84 |
| nicotine, caffeine, theophylline, and theobromine | mixture of alkaloids | IL-ABS | [C4C1im]Cl + C6H5K3O7/C6H8O7 + water, [C6C1im]Cl + C6H5K3O7/C6H8O7 + water, [C7C1im]Cl + C6H5K3O7/C6H8O7 + water, [C8C1im]Cl + C6H5K3O7/C6H8O7 + water, [C10C1im]Cl + C6H5K3O7/C6H8O7 + water, [C4C1im]Cl + C6H5K3O7 + water, [C6C1im]Cl + C6H5K3O7 + water, [C8C1im]Cl + C6H5K3O7 + water, [C10C1im]Cl + C6H5K3O7 + water85 |
| para red and Sudan dyes | chili powder | IL-LLE | [C4C1im][PF6] and [C8C1im][PF6] (water)97 |
| trans-zeatin, indole-3-acetic acid | Kappaphycus alvarezii sap | IL-LLE | [C4C1im][PF6], [C8C1im][BF4], and [C4C1C1im][NTf2] (water)98 |
| tyrosol | olive mill wastewater | IL-LLE | [P4441][NTf2], [N4441][NTf2], and [N1888][NTf2] (water)99 |
| vanillin | – | IL-ABS | [C2C1im]Cl + K3PO4 + water, [C4C1im]Cl + K3PO4 + water, [C6C1im]Cl + K3PO4 + water, [C4C1C1im]Cl + K3PO4 + water, [C7C1im]Cl + K3PO4 + water, [aC1im]Cl + K3PO4 + water, [C10C1im]Cl + K3PO4 + water, [C12C1im]Cl + K3PO4 + water, [OHC2C1im]Cl + K3PO4 + water, [C7H7C1im]Cl + K3PO4 + water, [C4C1im][CF3SO3] + K3PO4 + water, [C4C1im][N(CN)2] + K3PO4 + water, [C4C1im][C1SO4] + K3PO4 + water, [C4C1im][C1SO3] + K3PO4 + water, [C4C1im][C1CO2] + K3PO4 + water, [C4C1im]Br + K3PO4 + water100 |
2.1.6. Liquid–Liquid Extraction with Hydrophobic ILs
Hydrophobic ILs ([PF6]-based) were effectively applied by Absalan et al.81 in the extraction of a plant growth regulator, 3-indole-butyric acid (IBA), from its aqueous extracts. The higher extraction efficiency was observed with [C4C1im][PF6], with a preconcentration factor of 100. After the back-extraction of IBA from the IL-rich phase, the IL was successfully reused five times.81 Along the same lines, Prasad and co-workers98 studied three imidazolium-based ILs ([C4C1im][PF6], [C8C1im][BF4], and [C4C1mim][NTf2]) to extract plant growth regulators present in the sap from fresh Kappaphycus alvarezii seaweed. The authors98 showed that [C4C1im][PF6] was able to extract 65% of the total trans-zeatin and 18% of the total indole-3-acetic acid present in the sap, whereas [C4C1mim][NTf2] was not able to extract any compound and in agreement with the findings of Absalan et al.81 who also identified [C4C1im][PF6] as the best IL. Fan et al.97 studied IL-based LLE approaches for red and Sudan dyes from chili powder, using [C4C1im][PF6] and [C8C1im][PF6], the latter IL being the most effective. In another study, glycine was successfully extracted from pharmaceutical wastes by LLE using [C4C1im][PF6] and dicyclohexano-18-crown-6.96
As alternatives to imidazolium-based fluids, Larriba et al.99 proposed the use of [P4441][NTf2], [N4441][NTf2], and [N1888][NTf2] for the extraction of tyrosol, a naturally occurring antioxidant, from olive mill wastewaters. The authors99 showed that at optimum conditions, >94% of tyrosol extraction was achieved with [P4441][NTf2] and [N4441][NTf2], corresponding to higher extraction yields than that obtained with ethyl acetate. The addition of 20 wt % of NaCl to the water-rich medium further increased the extraction efficiency to the IL phase and ascribed by the authors99 as a result of a salting-out effect. In this context, stronger salting-out species can be chosen among the large plethora of salts available. Finally, Larriba et al.99 demonstrated the back-extraction of tyrosol from ILs using an aqueous solution of 0.1 M of NaOH followed by their recycling (after their neutralization with phosphoric acid).
Although six studies have been found resorting to the use of hydrophobic ILs for LLE purposes, this is a significantly low amount when compared, for instance, with the use of IL-based ABS discussed in the next section. In fact, the number of available hydrophobic water-immiscible ILs is much more limited when compared to water-miscible ones; this imposes severe limitations in terms of variability and tuning of the IL chemical structures aiming at optimizing the extraction performance of these systems and which seems to be the main justification for the reduced number of works presented in this section. Furthermore, most of these studies deal with IL anions with poor stability in water, such as [PF6]− and [BF4]−.80 With regard to the potential scale-up of these works, and despite the lack of deeper analyses, it needs to be highlighted that fluorinated-based anions tend to be very costly, having thus an additional relevant impact on the economic viability of the developed processes.
2.1.7. IL-Based Aqueous Biphasic Systems
As highlighted above, IL-based LLE approaches using hydrophobic ILs have several drawbacks. Moreover, most of the successful extractions of natural compounds from biomass were carried out with aqueous solutions of water-miscible imidazolium-based ILs, combined with Cl–, Br–, [C1CO2]−, and [BF4]− anions.8,22−24 Unless total selectivity is reached, after the extraction, a second step for the fractionation of the extracts is usually required, and where ABS can be used.101 The pioneering work of Rogers and co-workers102 established that ABS could be prepared by mixing hydrophilic ILs and inorganic salts in aqueous solution. Typically, inorganic salts induce the salting-out of ILs in aqueous media, thus leading to the creation of two liquid aqueous-rich phases. A recent review of IL-based ABS, comprising both the fundamentals behind their formation and possible application, can be found elsewhere.7 Due to the large plethora of available water-miscible ILs and second phase-forming components of ABS (salts, polymers, carbohydrates, and amino acids), it is possible to tailor the polarities of the phases and IL-based ABS have indeed allowed selective and enhanced separations.7 IL-based ABS has been extensively studied to purify crude extracts from biomass,86,97 for the fractionation of alkaloids87,89,103,104 and antioxidants,90,92,94,100 among others.
A number of alkaloids, such as codeine, papaverine, caffeine, nicotine, theophylline, and theobromine were purified by IL-based ABS.87,89,103 In 2005, Li et al.89 first demonstrated the application of the [C4C1im]Cl/K2HPO4-based ABS for the separation of opium alkaloids (codeine and papaverine), extracted from Pericarpium papaveris. After a previous IL-SLE step with aqueous solutions of [C4C1im]Cl, K2HPO4 was then added to create an ABS. The extraction yields obtained were comparable with those obtained with conventional LLE but with reduced extraction times and without using volatile organic solvents. In contrast to this work, where a single IL was studied, Freire et al.87 explored the extraction of caffeine and nicotine using several imidazolium ILs and K3PO4-based ABS. The authors87 showed that both solutes preferentially partition into the more hydrophobic phase (i.e., the IL-rich phase). The complete extraction of caffeine and nicotine into the IL-rich phase was achieved in a single step after a proper optimization of the IL chemical structure and mixture compositions.87 The capability of ABS composed of phosphonium-based ILs and inorganic salts,104 imidazolium-based ILs and carbohydrates,84 and imidazolium-based ILs and amino acids85 to separate caffeine was also demonstrated. In most cases,84,85,104 caffeine preferentially migrates to the IL-rich phase, although higher extraction efficiencies were obtained in IL-salt ABS due to stronger salting-out effects exerted by salts.87 Passos et al.103 investigated the effect of the IL cation alkyl side chain length ([CnC1im]Cl, n = 4–10) on the partition of a series of alkaloids (nicotine, caffeine, theophylline, and theobromine) of variable hydrophobicity. The authors demonstrated that the partition coefficients of alkaloids increase with the cation alkyl chain length up to n = 6, whereas a further increase in the cation side chain was deleterious to the extraction, explained by the self-aggregation of ILs with long alkyl chains,103 which seems not to be favorable for the extraction of more hydrophilic alkaloids, as discussed above in the SLE section.
IL-based ABS were also investigated for the separation of phenolic compounds, such as vanillin, gallic acid, vanillic acid, syringic acid, eugenol, and propyl gallate.83,90,92,94,100 Cláudio et al.92,100 studied IL-based ABS formed by a wide variety of imidazolium ILs and K3PO4 for the extraction of vanillin and formed by imidazolium ILs + phosphate and sulfate salts for the separation of gallic acid. It was shown that vanillin preferentially migrates to the IL-rich phase,100 while at low pH values the neutral form of gallic acid favorably migrates to the IL-rich phase and at high pH values its anionic form preferentially concentrates in the salt-rich phase.92 This pH-driven phenomenon was later demonstrated to be of high relevance to the fractionation of mixtures of phenolic compounds and to proceed with back-extraction studies and solvent reuse.93
ILs can also be successfully used as adjuvants in conventional polymer-salt-based ABS. Almeida et al.94 investigated ABS composed of polyethylene glycol (PEG) of different molecular weights and Na2SO4, using 5–10 wt % of ILs as adjuvants, for the separation of gallic, vanillic, and syringic acids from aqueous media. The partition extent of phenolic acids into the PEG-rich phase was shown to be dependent on the IL employed, which also preferentially partitions to the polymer-rich phase. The addition of only 5 wt % of IL led to 80–99% extraction efficiencies of all phenolic acids, thus confirming the capability of the IL to tune the polarity of the PEG-rich phase, even in low amounts.94 Santos et al.90 studied ABS composed of various ILs and a citrate (C6H5K3O7/C6H8O7) buffer at pH 7 for the extraction of eugenol and propyl gallate and compared their extraction ability to systems formed by PEG and C6H5K3O7/C6H8O7 (at pH 7), using imidazolium-based ILs as adjuvants. The complete extraction (100%) of the two antioxidants was obtained using both IL-based and PEG-based (with the IL as adjuvant) ABS.90 These results support the enhanced potential of ILs as adjuvants to tailor the polarities of coexisting ABS phases, while minimizing the cost and the environmental impact of the extraction/purification processes. More recently, Santos et al.83 developed an integrated approach for the fractionation of five phenolic compounds from lignin depolymerization using polymeric ABS (composed of PEG and sodium polyacrylate (NaPA) containing ionic surfactants as electrolytes). Two ILs ([CnC1im]Cl, n = 12 and 14) were used among the several surfactants investigated. Simple and fast methods to isolate phenolic compounds from the coexisting phases were successfully implemented by the authors.83
Even though a large number of IL-based ABS have been evaluated for the separation of small natural-derived organic compounds, these have been mainly applied to standard mixtures of biocompounds. Reports on integrated processes comprising both the extraction of biocompounds from biomass and their further purification using IL-based ABS are indeed scarce.79,82,88,91,93,95,105−107 Among these, the extraction of anthraquinone derivatives from Aloe vera using aqueous solutions of ILs, followed by the formation of IL-based ABS (with imidazolium ILs and Na2SO4) for the purification of the extracts, was recently demonstrated.82 Under optimized conditions, the extraction efficiencies of anthraquinones, namely aloe-emodin and chrysophanol, were 92.34% and 90.46%, respectively.82 The authors82 thereafter studied the back-extraction of the target compounds, followed by IL recovery by the formation of a new ABS with the addition of an alkaline salt.82 Bogdanov and co-workers95 also developed an approach to recover ILs after extracting glaucine from crude plant extracts of Glaucium flavum Cr. (Papaveraceae) using IL-based ABS formed by [C4C1im][Ace] and distinct salts. Recently, Wang et al.91 reported the use of biobased ILs comprising the cholinium cation and different amino-acid-derived anions ([N111(2OH)][Ala], [N111(2OH)][Ser], [N111(2OH)][Cys], [N111(2OH)][Pro], [N111(2OH)][Asp], [N111(2OH)][Val], [N111(2OH)][Leu], and [N111(2OH)][Phe]) for the simultaneous extraction of flavonoids and pectin from ponkan peels, which were then isolated by ABS formation with K3PO4. Yang et al.106 and Tan et al.107 demonstrated that an effective extraction and purification of chlorogenic acid from ramie leaves and flavonoids from Apocynum venetum L. leaves could be achieved by coupling IL-based UAE with IL-based ABS. Improved results were obtained by Tan et al.107 since flavonoids mainly partition to the IL-rich top phase, while impurities tend to concentrate in the salt-rich layer. Nevertheless, none of these works completely characterized the extracts and provided purification factors.
In general, IL-based ABS for the extraction and purification of bioactive compounds were mainly carried out with imidazolium-based ILs, combined with chloride, bromide, acetate, dicyanimide, and tetrafluoroborate anions. Only two works79,91 highlighted the potential of more biocompatible and biodegradable cholinium-based ILs as potential alternatives to imidazolium counterparts. Nowadays, there is a large number of more benign ILs available, such as those composed of cholinium-, glycine-, and glycine-betaine-based cations, combined with anions derived from carboxylic acids,108,109 biological buffers,110 and amino acids,111 among others, which deserve to be explored for the extraction of bioactive compounds from biomass. Since the concept behind the use of ABS comprises the separation and purification of target molecules from the main contaminants present in biomass extracts, in future works attention should also be given to the latter, because in some cases it is easier to manipulate the partition behavior of contaminants among the coexisting phases.112,113 Moreover, and as shown in this section, integrated strategies combining IL aqueous solutions used in the extraction of natural compounds from biomass combined with their direct use in the formation of ABS for the purification step are still very scarce. However, only by developing integrated strategies and attempting IL reuse and recovery will it be possible to develop cost-effective techniques while contributing to a lower environmental footprint of real and large-scale applications.
2.3. Solid-Phase Extractions Using IL-Modified Materials
Solid-phase extractions (SPE) are also purification processes and are based on solid materials employed as affinity stationary phases to induce the adsorption of target molecules from liquid extracts. Although the liquid nature of ILs disappears upon their immobilization on solid supports, their chemical features, including the possibility of multiple interaction sites and types of interactions, are still maintained and seen as a way of tailoring the materials’ performance. Most studies with supported ILs employed silica or polymers as solid phases.114−127 The pioneering study by Tian et al.114 reported the use of methylimidazolium-modified silica, with Cl– as the counterion, to isolate tanshinone I, tanshinone IIA, and cryptotanshinone from Salvia miltiorrhiza Bunge extracts,115 followed by the works of Row and co-workers116,117 who prepared a similar material aiming at increasing the selectivity for tanshinones from Salvia miltiorrhiza Bunge and to extract liquiritin and glycyrrhizic acid from licorice. All works demonstrated higher recovery yields and selectivity for tanshinones with IL-supported silica, as an alternative to nonmodified commercial silica cartridges. The same group of researchers118 also synthesized similar IL-modified silica materials for the isolation of protocatechuic, ferulic, and caffeic acids from the extracts of Salicornia herbacea L., with recovery yields of 94.69%, 79.09%, and 87.32%, respectively. This study showed that electrostatic interactions exert a major effect on the material adsorption capacity for phenolic acids.118 Still, the regeneration of the IL-modified materials was not addressed in any of the previously described works, being however an important step to guarantee the potential applicability of these materials.
In addition to the previous works where the optimization of the IL-supported materials was more restricted, Bi et al.119 studied IL-confined silica adsorbents for a two-step extraction-separation of the alkaloid oxymatrine from the extracts of Sophora flavescens Ait. The authors119 optimized various process variables, such as the IL alkyl side chain length and anion nature, temperature, contact time, and solid–liquid ratio. Short chain imidazolium ILs were shown to be the most effective. Finally, and contrarily to the previously described works, the authors119 also evaluated the recyclability of the IL-supported material, achieving oxymatrine recovery yields in the range of 89.7–93.4% over four cycles. Along the same lines, Yao and co-workers120 demonstrated the use of a SiO2·Im+·PF6– column to separate phenolic acids from aqueous extracts of Salvia militiorrhiza Bunge, namely protocatechuic aldehyde, sodium danshensu, rosmarinic acid, lithospermic acid, and salvianolic acid B. The SiO2·Im+·PF6– column was shown to be effective for adsorption, desorption, and reusability, revealing that IL-supported silica materials are good candidates for the separation of phenolic compounds and other natural products.120 An overview of the application of IL-modified silica in the extraction and separation of small organic extractable compounds from biomass is provided in Table 4.
Table 4. Extraction and Separation of Small and Extractable Organic Compounds from Biomass Using IL-Modified Materials in SPE.
| bioactive compounds | natural source | method | IL-based material used |
|---|---|---|---|
| caffeine and theophylline | green tea | IL-SPE | [C1im]-modified polymer122 |
| liquiritin and glycyrrhizic acid | licorice | IL-SPE | [C2C1im]Cl-based silica and poly(butylpyridine chloride)divinylbenzene117,124 |
| matrine, oxymatrine, sophocarpin, and sophoridine | Sophora flavescens Ait | IL-SPE | poly(3-aminopropyl imidazole bromide hydrobromide) 4-(chloromethyl) styrene121 |
| oxymatrine | S. flavescens Ait | IL-SPE | imidazolium [BF4]-, [PF6]- and [NTf2]-based silica119 |
| phenolic acids | S. herbacea | IL-SPE | poly([aC2im]Br) ethylene123 |
| protocatechuic aldehyde, sodium danshensu, rosmarinic acid, lithospermic acid, and salvianolic acid B | S. miltiorrhiza Bunge | IL-SPE | SiO2·Im+·PF6–120 |
| tanshinones | S. miltiorrhiza Bunge | IL-SPE | imidazolium chloride-based silica; [C1im]Cl-based silica; and poly([R1im]Cl) imprinted on 4-(chloromethyl) styrene114,116,126 |
| three phenolic acids | Saliconia herbacea L. | IL-SPE | imidazolium chloride-based silica118 |
Although IL-supported silica materials were shown to be promising toward the extraction and selective separation of natural compounds, SPE using silica still poses some disadvantages, such as a high cost, fewer functional groups available per contact area, long synthesis protocols, and restricted pH stability, which limits its widespread application.22 Some of these limitations can be overcome by polymers displaying a wider range of pH stability. The published studies in this direction are listed in Table 4.
The use of polyaminopropylimidazolium as adsorbent for the extraction of two alkaloids, matrine and oxymatrine, from the aqueous extract of Sophora flavescens Ait.121 was recently reported; when compared to traditional polymers, the IL-based polymer exhibits a better selectivity. The regeneration of the IL-based polymer was also attempted without a significant decrease of the extraction performance. The same group of authors122 further prepared a methylimidazolium-modified polymer for the extraction of caffeine and theophylline from green tea extracts, which was shown to be more efficient than the typical C18 adsorbent. All of these imidazolium-based polymers are more efficient and selective than traditional polymers due to the establishment of stronger and more specific interactions with the target biocompounds, which are not possible with the nonmodified materials.
IL-confined polymers synthesized by a molecular imprinting technique were also used as effective adsorbents of phenolic acids from Salicornia herbacea L. extracts.123 Higher recovery yields of protocatechuic acid (90.1%), ferulic acid (95.5%), and caffeic acid (96.6%) from the aqueous plant extract were obtained, when compared to those previously discussed by the use of IL-silica particles.119 In addition to the well investigated imidazolium-based materials, an alkylpyridinium-modified polymer was used for the isolation of liquiritin and glycyrrhizin from liquorice extract,124 which also displayed a higher selectivity than a C18 column. The amounts of recovered liquiritin and glycyrrhizin were 2.75 mg g–1 and 4.5 mg g–1, respectively, which are considerably higher than the amounts obtained using an IL-based silica material (0.18 mg g–1 and 1.0 mg g–1 for liquiritin and glycyrrhizic acid, respectively).117 In summary, it has been demonstrated that IL-modified polymers are better candidates than IL-silica-based ones for the extraction and separation of small organic compounds from biomass extracts.
Generally, imidazolium-based ILs have been the preferred choice for the modification of silica or polymers in SPE,114−127 with only one exception reporting a polymer modified with a pyridinium-based IL.124 Most of the bioactive compounds extracted from biomass are aromatic in nature and contain a large number of hydroxyl groups; thus, it is likely that the aromatic imidazolium/pyridinium rings may be responsible for some specific interactions, including π···π and additional hydrogen-bonding interactions, with the target compounds, resulting therefore in higher selectivities and extraction efficiencies. However, other cations, such as tetraalkylphosphonium and tetraalkylammonium, should be additionally explored in the synthesis of IL-supported task-specific materials to confirm this hypothesis. On the other hand, the counteranions of IL-modified materials mostly comprise Cl–, Br–, [PF6]−, and [BF4]−. Under a similar analogy, the identification of tailored materials can only be achieved after exploring other anions. There are nowadays a wide range of anions available, including some with aromatic rings, such as tosylates and salicylates, that deserve to be investigated. Furthermore, and given that the anion is not covalently attached to the solid material, it is crucial to guarantee the lack of ionic exchange during the separation of the target biocompounds, which can be in a charged state if working at pH values higher than their pKa values. In addition, the functionalization of the IL (in the cation core, anion, and/or alkyl side chain) could be attempted to increase selectivity and recovery. This means that the optimization of performance of these processes should be carried out case-by-case, taking into account the specific chemical characteristics of the target molecules being extracted and of the main contaminants present, so that IL-modified materials can be properly designed. Finally, and from the few works reported in this field, there is a clear lack of tests available to evaluate the chemical integrity of the extracted natural compounds and the regeneration of the modified materials, crucial steps regarding the potential application of these IL-modified materials.
2.4. Back-Extraction Steps and IL Recovery
Most examples of the extraction of biocompounds from biomass were carried out on a lab scale, and pilot scale studies using IL-based extraction approaches are rare.72 Moreover, most authors focused on the extraction of natural compounds and respective extraction yields without paying attention to their separation from the IL-rich phase, although the recovery of the products and recycling of the solvent are mandatory issues for large scale application and to guarantee the sustainability of the process. Among all the published manuscripts concerning IL-based extractions of biocompounds, only 18% considered the isolation of the solutes from the IL solution and the recovery/regeneration of the ILs used. Different approaches for the separation of biocompounds from the IL-rich phase and solvent recovery have been reviewed recently by a number of authors.8,23−25,128Figure 4 depicts examples of integrated processes comprising the extraction, purification, and recovery of bioactive compounds (e.g., caffeine, hydrolyzable tannins, and gallic acid), followed by the recycling and reuse of ILs.29,33,93
The most common applied methods comprise back-extraction approaches and precipitation of the active ingredients with antisolvents. Other approaches include the use of ion-exchange resins, macroporous resins, distillable ILs,33 and thermoresponsive polymeric ILs.129 For instance, Lu et al.129 developed thermoresponsive polymeric ILs as reusable extractants for the extraction and recovery of tocopherol analogues. The synthesized ILs display thermoresponsive behavior in acetonitrile with upper critical solution temperatures varying from 25.7 to 34.8 °C, allowing their complete thermo-separation by a decrease in temperature.
An overview of the articles published so far shows that ILs are not only appropriate but frequently a high-performance media for the extraction of bioactive compounds from biomass. Valuable fine chemicals of different classes, such as alkaloids, terpenoids, flavonoids, phenolic acids, among others, have been successfully extracted with pure ILs or with IL/water or IL/alcohol mixtures. It has been shown that mainly H-bonding, π···π, and electrostatic interactions occurring between ILs and natural compounds are responsible for the enhanced extraction performance and high selectivity observed. ILs may also contribute to the rupture of the plants’ cell walls, in some cases with the partial dissolution of biopolymers, thus allowing improved access to the active ingredients. The efficiency of IL-based extraction methods can also be enhanced through their integration with MAE and UAE. On the other hand, the actual isolation/recovery of bioactive compounds, the IL recycling, and the scale-up of the process still remain challenging tasks. Very few studies exist in the literature describing integrated and complete processes. Finally, and even after a decade on studies on the use of ILs for the extraction of small organic extractable compounds from biomass, imidazolium-based ILs are still the most well-investigated for such a purpose. At this stage, and given the large number of ILs already available, it is crucial to move to naturally derived, more benign, nontoxic, and low-cost ILs.
3. Lipids and Other Hydrophobic Compounds
Large chemical and biological diversity exists in lipids, with their highly hydrophobic nature being a common characteristic. Noteworthy categories include fats and oils, phospholipids, and steroids.130 Of significant importance for both human diet and industry, fats and oils have seen their global market forecasted to achieve a value of USD 170 billion by 2019.131 The industrial applications of lipids are quite broad, covering the food, nutraceutical, cosmetic, pharmaceutical, and chemical fields.132 Furthermore, the variety of lipid sources is immense, comprising plant seeds, food, and animal processing wastes and high lipid content microorganisms.132,133 Xu et al.134 recently summarized the unique opportunities that ILs bring to lipid processing.
This section focuses on the analysis of more efficient extraction and separation approaches based on ILs for lipid processing. To this end, four main approaches were found in the literature, their frequency being depicted in Figure 5: (i) LLE, where ILs are generally combined with organic solvents, mainly hexane; (ii) SLE, using either pure ILs or cosolvent systems wherein simpler extractions or microwave- and ultrasound-assisted processes are used; (iii) ABS composed of hydrophilic ILs and salts or carbohydrates; and (iv) SPE systems where ILs are immobilized onto silica. Comprising more than half of the works published so far, SLE is the most widely studied technique, followed by LLE. The use of ABS is however limited due to the poor solubility of this class of compounds in water or aqueous phases. Tables 5, 6, 7, and 8 overview the works under discussion in the current section. As shown in the radial graphs depicted in Figure 5, distinct compounds were separated and purified with various techniques, with essential oils and microbial lipids (mainly from microalgae) representing the more appealing classes. The chemical structures of the most representative compounds studied with ILs as main extraction and purification solvents are presented in Figure 6.
Figure 5.
Distribution of the works dealing with each IL-based technique for the extraction and separation of lipids and related compounds. The radial graphs display the number of scientific works addressing distinct classes of lipids and related compounds.
Table 5. Extraction and Separation of Lipids and Related Compounds Using IL-Based LLE.
| bioactive compound | IL (+ other solvents) used | isolation strategy |
|---|---|---|
| essential oils | [C2C1im][C1SO3] + LiOH,167 [C2C1im][C2SO4],168 [C2C1im][C1(OC2)2SO4],169 [C2C1im][NTf2],170 [C4C1im][NTf2],173 [C6C1im][NTf2],170,173 [C10C1im][NTf2],170 [C1pyr][C1SO4],171 [C2pyr][C2SO4],171 [C2C1im][C1CO2],172 [C4C1im][C1CO2],172 [C4C1im]Cl,174[C4C1im][C1SO3],174 [C4C1im][(C1)2PO4],174 and [C4C1im][CF3SO3]174 | |
| FAMEs | [C2C1im][BF4] + AgBF4 + hexane,135,136 [C4C1im][BF4] + AgBF4 + hexane,135,136 [C6C1im][BF4] + AgBF4 + hexane,135,136 [C8C1im][BF4] + AgBF4 + hexane,136 [C4C1im][NO3] + AgBF4 + hexane,135 [C4C1im][CF3CO2] + AgBF4 + hexane,135 [C4C1im][PF6] + AgBF4 + hexane,135,136 [C6C1im][PF6] + AgBF4 + hexane,136 [C6C1im][PF6] + AgBF4 + octane,136 [C6C1im][PF6] + AgBF4 + isooctane,136 [C6C1im][PF6] + AgBF4 + decane,136 [C6C1im][PF6] + AgBF4 + dodecane,136 [C8C1im][PF6] + AgBF4 + hexane,136 [C4C1im][NTf2] + AgBF4 + hexane,135 [C4C1pyr][BF4] + AgBF4 + hexane,135 [C4pyr][BF4] + AgBF4 + hexane,135 [C6C1im][PF6] + hexane,135 [C4C1im][PF6] + hexane,139 [C8C1im][PF6] + hexane,139 [C4C1im][BF4] + hexane,139 [C8C1im][BF4] + hexane,139 [C4C1im][CF3SO3] + hexane,139 [C4C1pyr][CF3SO3] + hexane,139 [C8C1pyr][BF4] + hexane,139 [C4C1pyr][N(CN)2] + hexane,139 [C4C1pyrr][FAP] + hexane,139 [N114(2OmOH) (2OnOH)][C1SO4] (Ammoeng 100) + hexane,139 [N218O(2OmOH) (2OnOH)][C2SO4] (Ammoeng 102) + hexane139 | 1-hexene135,139 |
| lipids | [C4C1im]Cl + water + hexane,145 [C4C1im][BF4] + water + hexane,145 [C4C1im]Cl + water + CO2 + hexane,145 [C4C1im][BF4] + water + CO2 + hexane145 | |
| tocopherols | [C4C1im]Cl + hexane,185 [C4C1im]Cl + methanol + hexane,185 [C4C1im][CF3SO3] + methanol + hexane,185 [C4C1im][BF4] + methanol + hexane,185 [C4C1im]Cl + acetonitrile + hexane,186 [C4C1im]Cl + dimethyl sulfoxide + hexane,186 [C4C1im]Cl + N,N-dimethylformamide + hexane,186 [C4C1im][PF6] + acetonitrile + hexane,186 [C4C1im][BF4] + acetonitrile + hexane,186 [C4C1im][CF3SO3] + acetonitrile + hexane,186 [C4C1im][C1SO4] + acetonitrile + hexane,186 [C4C1im]Br + acetonitrile + hexane,186 [C6C1im]Cl + acetonitrile + hexane,186 [C8C1im]Cl + acetonitrile + hexane,186 [C2C1im][Ala] + acetonitrile + hexane,187 [C2C1im][Gly] + acetonitrile + hexane,187 and [C2C1im][C1CO2] + acetonitrile + hexane187 | water addition185 |
| vitamin D3 and tachysterol3 | [C8C1im][BF4] + hexane,188 [C6C1im][BF4] + hexane,188 [C4C1im][BF4] + hexane,188 [C4C1im][PF6] + hexane,188 [C4C1im][CF3SO3] + hexane,188 [C4C1im][NTf2] + hexane,188 [(NC)C3C1im][NTf2] + hexane,188 [C4pyr][NTf2] + hexane,188 [C4C1pyrr][NTf2] + hexane,188 [C2C1im][NTf2] + hexane,188 [OHC2C1im][NTf2] + hexane,188 and [C4C1pyrr][NTf2] + N,N-dimethylformamide + hexane188 | back-extraction188 |
Table 6. Extraction and Separation of Lipids and Related Compounds Using IL-based SLE.
| bioactive compounds | IL (+ other solvents) used | isolation strategy |
|---|---|---|
| astaxanthin | [C4C1im]Br + ethanol,176 [C4C1im]Cl + ethanol,176 [C4C1im][C1SO3] + ethanol,176 [C4C1im][BF4] + ethanol,176 [C2C1im][BF4] + ethanol,176 [C6C1im][BF4] + ethanol,176 [(NH2)C3C1im]Br + ethanol,176 [C2C1im]Br + dichloromethane/methanol,177 [C4C1im]Br + dichloromethane/methanol,177 [C6C1im]Br + dichloromethane/methanol,177 [C8C1im]Br + dichloromethane/methanol,177 [C10C1im]Br + dichloromethane/methanol,177 [C14C1im]Br + dichloromethane/methanol,177 [C2C1im][BF4] + dichloromethane/methanol,177 [C2C1im][PF6] + dichloromethane/methanol,177 [C2C1im][NTf2] + dichloromethane/methanol,177 [C2C1im][C1CO2] (pure),178 [C2C1im][BF4] (pure),178 [C2C1im][C2SO4] (pure),178 [C2C1im][C1SO3] (pure),178 [C4C1pyr][C1SO4] (pure),178 and [C4C1im][C1SO3] (pure),178 [C2C1im][(C4)2PO4],179 [C4C1im][(C4)2PO4],179 [C4C1im][C1CO2],179 [C4C1im][N(CN)2],179 [N221(O)nOH]Cl,179 [P4441][C1SO4],179 and [Pi(444)1][Tos]179 | SPE,176 centrifugation,179 and LLE with ethyl acetate178 |
| bio-oils | [C2C1im][C1SO4] + methanol148,149 and [C2C1im][C1CO2] + methanol148,149 | |
| essential oils | [C6C1im][PF6] (pure),159,160 [C4C1im]Cl + water,47,50,161−163 [C4C1im]Br + water,47,50,161−163 [C4C1im][BF4] + water,47,50,161 [C4C1im][NO3] + water,47,50,161 [C4C1im][HSO4] + water,50,161 [C4C1im][ClO4] + water,50,161 [C4C1im][C1CO2] + water,50,161 [C4C1im][OH] + water,50 [C2C1im]Br + water,47,50,161 [C6C1im]Br+ water,47,50,161 [C8C1im]Br + water,47,50,161 [C10C1im]Br + water,47,50,161 [C12C1im]Br + water,50 [aC1im]Cl + water,162,163 [C2C1im][C1CO2] + water,162,163 [aC1im]Cl (pure),164 [C4C1im]Cl (pure),164 [C2C1im][C1CO2] (pure),164 [C2C1im]Br + water,165 [C2C1im]Br + LiCl + water,165 [C4C1im]Br + water,165 [C4C1im]Br + LiCl + water,165 [C2C1im]Br + water,165 [C2C1im]Br + LiCl + water,165 [C2C1im][C1CO2] + water,165 [C2C1im][C1CO2] + LiCl + water,165 [C4C1im][C1CO2] + water,165 [C4C1im][C1CO2] + LiCl + water,165 [C4C1im][H2PO4] + water,165 [C4C1im][H2PO4] + LiCl + water,165 [OHC2C1im]Cl + water,166 [C4C1mor]Cl + water,166 [C0C1im]Cl + water166 and [N111(2OH)]Cl + water166 | addition of ethanol followed by azeotropic distillation of ethanol/water,162,163 direct distillation,164 and LLE with ethyl acetate164 |
| FAMEs | [C2C1im]Cl·2AlCl3 + methanol + dichloromethane,141 [C4C1im]Cl146 and [C4C1im][PF6]146 | crystallization of the IL with hexane141 |
| fats | [C6pyr]Br (pure),140 [(NC)C2C1im]Br (pure),140 [C4C1im]Cl (pure),140 and [C3C1im]Cl (pure)140 | |
| lipids | [C4C1im][C1SO4] + methanol,142 [P(1OH)(1OH)(1OH)(1OH)]Cl + water + methanol,142,153 [C2C1im][C1SO4] + methanol,147 [C2C1im][C1CO2] + methanol,150 [C4C1im][CF3SO3] + methanol,151 [C4C1im][C1SO4] + methanol,151 [C4C1im][C1SO3] + methanol,151 [C4C1im][BF4] + methanol,151 [C4C1im][PF6] + methanol,151 [C4C1im][NTf2] + methanol,151 [C4C1im]Cl + methanol,151 [C2C1im][C1SO4] + methanol,151 [C2C1im]Cl + methanol,151 [C2C1im]Br + methanol,151 [C2C1im][C1CO2] + methanol,151 [N0222][HSO4] + supercritical water,152 [C4pyr][HSO4] + supercritical water,152 [C4pyr][H2PO4] + supercritical water152 and [(NH2)C3C1im]Br + supercritical water,152 [C2C1im][C1CO2],154−156 [C2C1im][HSO4],154,156 [C2C1im][(C2)2PO4],154,156 [C2C1im][SCN],154,156 [C2C1im][NTf2],154,156 [C2C1im][C1CO2] + FeCl3·6H2O,154,155 [C2C1im][C1CO2] + FeCl3·6H2O,154 [C2C1im][HSO4] + FeCl3·6H2O,154 [C2C1im][(C2)2PO4] + FeCl3·6H2O,154 [C2C1im][SCN] + FeCl3·6H2O,154 [C2C1im][NTf2] + FeCl3·6H2O,154 [C2C1im][BF4],156 [aC1im]Cl,156 [C2C1im][AlCl4],156 [C2C1im][C2SO4],156 [C2C1im][C1SO3],156 [C2C1im]Cl + methanol,156 [C4C1im]Cl + hexane,156 [C2C1im][C1CO2] + methanol,156 [C2C1im][C1CO2] + chloroform,156 [C2C1im][C1CO2] + hexane,156 [C2C1im][C1CO2] + [C2C1im][NTf2],156 [C2C1im][C1CO2] + [C2C1im][BF4],156 [C2C1im][C1CO2] + [C2C1im]Cl,156 [C2C1im][C1CO2] + [C2C1im][(C2)2PO4],156 [C2C1im]Cl + [C2C1im][NTf2],156 [C2C1im]Cl + [C2C1im][BF4],156 [C2C1im]Cl + [C2C1im][(C2)2PO4],156 [C2C1im][NTf2] + [C2C1im][BF4],156 [C2C1im][C2SO4] + [C2C1im][SCN],156 [C2C1im][C2SO4] + [C2C1im][SCN],156 [C2C1im][C2SO4] + [C2C1im][C1SO3],156 [C2C1im][HSO4] + [C2C1im][(C2)2PO4],156 [C2C1im][HSO4] + [C2C1im][SCN],156 [C2C1im][HSO4] + [C2C1im][C2SO4],156 [C2C1im][HSO4] + [C2C1im][C1SO3],156 [C2C1im][(C2)2PO4] + [C2C1im][C2SO4],156 [C2C1im][(C2)2PO4] + [C2C1im][SCN],156 [C2C1im][(C2)2PO4] + [C2C1im][C1SO3],156 [C2C1im][SCN] + [C2C1im][C1SO3]156 and [C4C1im][C1SO4] (pure)157 | centrifugation + evaporation of methanol and residual water,153 back-extraction with hexane followed by solvent evaporation,155 and water addition157 |
| lycopene | [C4C1im][BF4] + ethanol,180 [C6C1im]Cl + ethanol,180 [C4C1im]Cl + ethanol,180 [C4C1im][PF6] + ethanol,180 and [C4C1im][PF6] (pure)180 | |
| saponins | [N111(2OH)]Cl + water,79 [C2C1im]Cl + water,79 [C4C1im]Cl + water,79 [C6C1im]Cl + water,79 [C8C1im]Cl + water,79 [aC1im]Cl + water,79 [C7H7C1im]Cl + water,79 [OHC2C1im]Cl + water,79 [N111(2OH)][C1CO2] + water,79 [N111(2OH)][C5CO2] + water,79 [C2C1im][N(CN)2] + water,79 [C2C1im][C2SO4] + water,79 [C2C1im][CF3SO3] + water,79 [C2C1im][Lac] + water,79 [C2C1im][C1CO2] + water,79 [N111(2OH)]Cl + water + ethanol,183 [N111(2OH)][C1CO2] + water + ethanol,183 [N111(2OH)][C2CO2] + water + ethanol,183 [N111(2OH)][C3CO2] + water + ethanol,183 [N111(2OH)][C5CO2] + water + ethanol,183 [N111(2OH)][Suc] + water + ethanol,183 [N111(2OH)][Lac] + water + ethanol,183 [N111(2OH)][Cit] + water + ethanol,183 [N111(2OH)][Oxa] + water + ethanol,183 [N111(2OH)][Mal] + water + ethanol,183 [N111(2OH)][Bz] + water + ethanol,183 [N111(2OH)][Sal] + water + ethanol,183 [N111(2OH)][PhAc] + water + ethanol,183 [N111(2OH)]Cl:Glycer + water + ethanol,183 [N111(2OH)]Cl:EG + water + ethanol,183 [N111(2OH)]Cl:U + water + ethanol,183 [N111(2OH)]Cl:HAc + water + ethanol,183 [N111(2OH)]Cl:HProp + water + ethanol,183 [N111(2OH)]Cl:HLac + water + ethanol,183 [N111(2OH)]Cl:HOx + water + ethanol,183 [N111(2OH)]Cl:HMal + water + ethanol,183 [N111(2OH)]Cl:HPhAc + water + ethanol,183 [C3C1im]Br + water,62 [C3C1im][BF4] + water,62,184 [C3C1im]I + water,62 [C2C1im]Br + water,62,184 [C4C1im]Br + water,62,184 [C6C1im]Br + water,62 [C2C1im]Cl + water,184 [C2C1im][BF4] + water,184 and [C4C1im][BF4] + water184 | IL-based ABS79 |
| β-carotene (and other isoprenoids) | [P66614]Cl,181 [P66614][NTf2],181 [C4C1pyr][NTf2],181 [P4444]Cl,181 [P10(3OH)(3OH)(3OH)]Br,181 [N111(2OH)]Cl,181 and [N111(2OH)][NTf2]181 | vacuum distillation181 |
Table 7. Extraction of Lipids and Related Compounds Using IL-Based ABS.
| bioactive compound | IL-based ABS |
|---|---|
| β-carotene | [Pi(444)1][Tos] + K3PO4 + water,104 [C4C1im][CF3SO3] + sucrose + water,84 [C4C1im][CF3SO3] + D-(+)-glucose + water,84 [C4C1im][CF3SO3] + D-(−)-fructose + water,84 [C4C1im][CF3SO3] + D-(+)-mannose + water,84 [C4C1im][CF3SO3] + D-(+)-xylose + water,84 [C4C1im][CF3SO3] + D-(+)-maltitol + water,84 [C4C1im][CF3SO3] + xylitol + water84 and [C4C1im][CF3SO3] + D-sorbitol + water84 |
| Crocins | [C4C1im][BF4] + phosphate + water182 and [C2C1im][C1CO2] + phosphate + water182 |
Table 8. Extraction of FAMES Using IL-Based SPE.
Figure 6.
Chemical structures of lipids and related compounds extracted and separated with IL-based separation techniques.
The usage incidence of distinct IL ions combinations is depicted in Figure 7, allowing the inspection of the most commonly adopted ILs. The 1-alkyl-3-methylimidazolium cations, [CnC1im]+, are by far the most studied cations, while [BF4]−, [PF6]− (both water-unstable80), Cl–, [NTf2]− (poorly biodegradable and toxic), and [CnCO2]− represent the most frequently studied anions. More recently, acyclic core ILs, such as phosphonium-based derivatives, and generally less toxic cations, such as those belonging to the quaternary ammonium and cholinium families, have started to attract some attention. This last aspect, together with the increasing trend of using natural organic-acid-derived anions, has contributed toward the enhancement of the benign character and sustainability of the technologies applied in lipid processing. Although the remaining sections are organized according to the type of the IL-based technique, this section is instead organized according to the class of compounds as a way of improving and facilitating the critical revision of the data available.
Figure 7.
ILs used for the extraction and separation of lipids and related compounds as a function of cation–anion combinations. The usage incidence (number of articles) is represented by the size of the circles, which proportionally increases as follows: [0–3] < [3–6] < [6–9] < [9–12] < [12–20].
3.1. Fats
In 2008, Li and Li135 disclosed their pioneering work on how to recover omega-3 polyunsaturated fatty acid methyl esters (FAMEs) from fish oil, combining ILs and silver salts. The IL cation family, alkyl chain length, and functionalization, and the anion nature were screened, along with six silver salts. In initial tests, hexane was used as the main solvent in standard mixtures of five FAMEs, with the ILs and the silver salts acting as the extraction phases. Hydrophobic ILs with large anions of low lattice energy were identified as the best.135 The [C6C1im][BF4] + AgBF4 system was shown to be far more effective than [C6C1im][BF4] alone or with AgBF4 in water or ethylene glycol. Moreover, the higher the unsaturation degree, the easier was the removal of lipids with the IL/silver salt mixture. In this work, the selectivity of the process was increased by 1 order of magnitude.135 The industrial applicability of the process was ascertained by successfully addressing two important aspects: (i) the recyclability of the IL/silver salt phase using n-hexene as a stripping agent and (ii) its successful application to real fish oil. As follow-up studies, the authors136−138 studied the extraction equilibrium parameters and novel routes based on silica-supported ILs to facilitate the recycling stage. In an additional work,139 it was shown that π···π stacking governs the selective adsorption of polyunsaturated fatty acids and ethyl esters from fish oil. Among the eleven ILs studied, those containing aromatic rings provided enhanced selectivities for polyunsaturated compounds. Major achievements were indeed gauged by these authors,139 since the purity of the compounds extracted was boosted by performing a multiple-step reverse extraction and because of the short processing time, recyclability, and operational simplicity of the process.
Aiming at minimizing food disposal to landfill, Lateef et al.140 developed an approach to selectively extract fats from chocolate. Among the four ILs screened, [C3C1im]Br, and more notably [(NC)C2C1im]Br, were capable of separating sugars from cocoa butter fats. The enhanced ability of ILs to form hydrogen bonds with sugars, attributed to the cyano group at the cation, was highlighted as a crucial feature. Bollin and Viamajala141 reported the in situ transesterification of soy flake lipids using Lewis acidic chloroaluminate ILs as catalysts. The reactive extraction of triglycerides as FAMEs was conducted in a mixture of [C2C1im]Cl·2AlCl3, methanol, and dichloromethane, followed by the recovery of solubilized FAMEs and glycerides by LLE with hexane. After stipulating the optimal conditions of temperature, time of extraction, cosolvent, and reactant ratio, the authors were able to recover >90% of soy flour lipids as FAMEs.141
The extraction of lipids from primary sewage sludge for biodiesel production using ILs was also attempted.142 Both dry and raw sludge were tested along with two imidazolium-based ILs and a phosphonium-based IL, and these were compared to the traditional Soxhlet method carried out with volatile molecular solvents. The advantage of using [P(1OH)(1OH)(1OH)(1OH)]Cl relies on its lower cost and commercial availability. The lipid extraction yields were consistently higher for raw than for dried sludge, thus avoiding the additional cost of drying. Although both ILs extracted lipids from sludge, only the performance of [P(1OH)(1OH)(1OH)(1OH)]Cl was comparable to that of the conventional method. Remarkably, this IL also allowed the recovery of cellulose-based materials through precipitation, further boosting the development of intergrated biorefinery approaches.142
Algal biomass is seen today as a promising source of lipids, usually studied for the production of biodiesel. The replacement of volatile organic solvents is not the only driver for using ILs for this application; their remarkable biomass dissolution ability and cellular disruption potential are also essential. Several works in the literature addressed algal biomass dissolution for lipid extraction. For instance, Teixeira143 reported the dissolution of algal biomass belonging to Chlorella, Chlamydomonas, Chlorococcum, Scenedesmus, Selenastrum and Neochloris genera with ILs and further conceptualized an IL-based process to recover lipids, sugars, and proteins. However, high temperatures (100–140 °C) were necessary to achieve the complete dissolution and cell lysis. Ohno’s group144 proposed a novel method for treating wet and saliferous marine microalgae based on polar ILs. The proposed method allows: (i) the use of milder temperature conditions, (ii) operational simplicity, and (iii) IL recyclability.144 Fuel extraction was also coupled with CO2 capture as a way of compensating its energy consumption, by using [C4C1im]Cl and [C4C1im][BF4] to hydrolyze the wet algae Chlorella vulgaris.145 Although significant efforts are still needed to ensure its feasibility, the promising status of this extraction technology may be gauged by the quality of the biodiesel produced, which meets the standards imposed by legislation.145 Kilulya et al.146 studied the extraction of fatty acids from cyanobacteria. From the two ILs studied, only [C4C1im]Cl was able to dissolve this marine biomass, particularly at high temperatures.
Cooney and co-workers147 used cosolvent mixtures of [C2C1im][C1SO4] and methanol to extract lipids from diverse types of biomass, such as microalgae and oil seeds. They found that neither the IL nor methanol as pure solvents are suitable for the lipids extraction, as opposed to their mixtures.147 As the IL-methanol mixture is immiscible with the lipids extracted, the extracted lipids can be easily recovered as an immiscible phase.147 Other works from the same research group addressed the coextraction of compounds based on a similar approach, namely (i) a simultaneous extraction of bio-oil and fermentable sugars from jatropha and safflower biomass using [C2C1im][C1CO2] and methanol as cosolvent;148 (ii) bio-oil and phorbol ester corecovery from jatropha biomass using [C2C1im][C1SO4] and methanol as cosolvent;149 and (iii) lipid and fermentable sugar separation from Rhodosporidium toruloides with [C2C1im][C1CO2]-methanol mixtures wherein the carbohydrates were recycled for yeast consumption.150 Following a similar line of research, Kim et al.151 were able to boost the yield of lipids extracted from Chlorella vulgaris using [C4C1im][CF3SO3]-methanol as the best mixture (12.5%–19.0% of lipids extracted), which compares with the conventional method of Bligh and Dyer (10.6%–11.1% of lipids extracted). The results obtained showed that hydrophilic ILs are the best for these applications,151 although other mixtures of interest can also be found in the literature (e.g., IL-water,152,153 IL-molten salts,154,155 and IL-IL).156 A [N0222][HSO4]-subcritical water mixture extracted 73.63% of the total lipids, as compared to 60.44% with the conventional method of Bligh and Dyer,152 while synergetic effects between two ILs or an IL and a molten salt lead to enhanced yields and good purity levels when compared to the corresponding pure compounds.154−156 Of particular interest is the approach of Olkiewicz et al.,153 wherein lipids from two microalgae species, namely Chlorella vulgaris and Nannochloropsis oculata, were extracted using a [P(1OH)(1OH)(1OH)(1OH)]Cl aqueous solution (at 80% w/w) at 100 °C for 24 h. The IL-based method proposed performs better than both the Bligh and Dyer method and Soxhlet extractions, in particular at extracting lipids from Chlorella vulgaris and for recovering a larger saponifiable fraction from Nannochloropsis oculata.153 These results suggest that the type of biomass (cell wall resistance and polar/apolar lipid content) may influence the effectiveness of the IL-based extraction processes. Ultimately, the energetic efficiency of the proposed IL-based process was assured through: (i) the reduction of both temperature and extraction time and (ii) the recyclability and reusability of the IL.153
Only Kim et al.157 reported the use of ultrasound-assisted techniques for the extraction of lipids. The [C4C1im][C1SO4] used yielded higher amounts of lipids extracted from Chlorella vulgaris (47 mg g–1 of dry cell weight and 75 mg g–1 of dry cell weight, without and with ultrasound irradiation) than the two conventional methods, Soxhlet (21 mg g–1 of dry cell weight) and Bligh & Dyer’s method (29 mg g–1 of dry cell weight).157
Despite all of the authors’ efforts to evaluate the effect of various ILs, the imidazolium-based fluids are by far the most well-studied. However, a lack of understanding of the potential mechanisms of extraction and the mode of action of the ILs is still evident. While some works assume that ILs act as cell disruptors, on the other hand, they may also increase the solubility of the target compounds in aqueous or organic media. The understanding of the molecular-level-mediated mechanisms is thus mandatory to define rules for the design of ILs. Some of these studies were carried out at high temperatures, which seems to be advantageous to extract fats from biomass but detrimental for other (thermolabile) bioactive compounds present, like carotenoids, chlorophylls, and proteins. In this sense, these works showed a lack of commitment on the part of the authors in developing an integrated biorefinery approach, which is often the only way to make these processes of extraction economically viable and ensure their industrial potential.
3.2. Essential Oils
Essential oils are complex mixtures of fragrance components (e.g., terpenes and terpenoids) that can be isolated from natural matrices. Their wide industrial applications in domains such as perfumery, cosmetics, nutrition, and pharmaceuticals, along with the increasing demand for natural and organic personal care products, has led to a forecast of a global market value of USD 11.67 billion by 2022.158 Zhai et al.159 reported, for the first time, the IL-based MAE of essential oils from the fruits of Illicium verum and Cuminum cyminum. Similar works have followed.47,50,160−163 Although distinct sources of essential oils were considered (Cinnamomum cassia, Forsythia suspensa, Rosmarinus officinalis, Schisandra chinensis, Cinnamomum verum, Dryopteris fragrans, and Fructus forsythiae), these works all have in common the optimization of the operational conditions, namely the irradiation power, temperature, time, solid–liquid ratio and IL structure, as well as the chemical characterization of the essential oil extracted.47,50,159−163 Compared to other methods (e.g., hydrodistillation, microwave hydrodistillation, steam-distillation, solvent-free MAE, and heat reflux extraction), time, operational complexity, energy consumption, and the use of hazardous organic solvents, were all minimized in IL-based MAE processes, while the quality of the essential oils recovered was maintained or even improved.47,50,159−163 Ma et al.50 and Liu et al.47 brought an additional edge to this technique, since the simultaneous extraction of essential oils, lignans, carnosic, and rosmarinic acids was successfully achieved. Jiao et al.162,163 introduced an innovative approach wherein the microwave-assisted IL treatment was coupled to hydrodistillation. Among the four ILs tested, [C2C1im][C1CO2] was found to be the best. Further optimization regarding extraction parameters was conducted by a surface response methodology, being the optimal conditions for essential oil extraction (0.91% over 14.2 min from Dryopteris fragrans and 9.58% over 29.3 min from Fructus forsythiae) comparable to other techniques (0.33% over 94 min from Dryopteris fragrans by solvent-free MAE, 4.08% over 100 min from Fructus forsythia by hydrodistillation, and 5.43% over 45 min from Fructus forsythia by microwave-assisted aqueous IL hydrodistillation).162,163 By characterization using gas chromatography/mass spectrometry (GC/MS), the essential oils obtained were shown to be of similar or even better quality than those obtained by conventional methods.162,163 Remarkably, the reusability of [C2C1im][C1CO2] was further demonstrated in five cycles.162,163
Some authors have instead resorted to simple solvent extraction approaches based on ILs.164−166 Bica et al.164 showed that ILs could dissolve fresh biomass containing fragrances. The strategy outlined by the authors,164 depicted in Figure 8 A, was inspired by the low volatility of ILs, which allows the direct distillation and separation of essential oils at lower temperatures (60–65 °C). Hence, this approach guarantees energetic efficiency and is advantageous for thermolabile fragrances. Citrus oil, having limonene as its major component, was selected as model matrix, and with [C2C1im][C1CO2] a yield of 0.74 g of limonene was achieved from a total mass of 10 g of fresh orange peels. Nuclear magnetic resonance (NMR) spectroscopy and GC/MS results were used to demonstrate the oil quality. Aiming at the isolation of the essential oils, the authors164 suggested an LLE step with ethyl acetate instead of distillation; however, the purity of the essential oil was compromised.
Figure 8.
Schematic diagrams of integrated processes based on ILs comprising the extraction and purification of lipids and related compounds.79,164
In addition to pure ILs, IL aqueous solutions were recently investigated by Li et al.,165 who performed a systematic study of the impact of lithium salts on the IL-based extraction of essential oils from Tussilago farfara. The authors165 optimized the IL concentration and chemical structure ([CnC1im], with n = 2 and 4, combined with [C1CO2]−, [H2PO4]−, and Br– anions) along with the extraction time and lithium chloride concentration. The highest essential oil yield was obtained with [C4C1im][C1CO2] and lithium chloride after 2 h of water distillation, improving both the efficiency and speed of the conventional system distillation. Flamini et al.166 also resorted to aqueous solutions of ILs, nevertheless using cheaper and more environmentally friendly ILs. Chloride-based ILs were used as additives in hydrodistillation, including a functionalized imidazolium IL, [OHC2C1im]Cl, a morpholinium IL, [C4C1mor]Cl, a protic IL, [C0C1im]Cl, and an ammonium salt, [N111(2OH)]Cl. Rosmarinus officinalis was adopted as the essential oil source. The [OHC2C1im]Cl produced the best yield, improving the value achieved with conventional hydrodistillation from 1.58% (w/w) up to 1.93% (w/w). Given the good results obtained with the cheaper [C0C1im]Cl [yield of 1.77% (w/w)], the authors underlined its contribution toward the sustainability and greenness of the process developed.166 It is worth noting that a chemical composition of the essential oil similar to that obtained by traditional hydrodistillation was obtained.165,166
Essential oil deterpenation processes are imperative for obtaining high quality oils. Oxygenated terpene derivatives are more appealing considering their organoleptic properties, making the separation of terpenes using ILs a topic of interest. Arce and collaborators167 were pioneers in this field, with their first work published in 2006. The authors added [C2C1im][C1SO3] to ethylene glycol and 2-butene-1,4-diol for the deterpenation of citrus oil, which was simulated by means of a synthetic mixture of limonene and linalool. The liquid–liquid equilibria data for the ternary systems were reported and the solvent capacity scrutinized regarding the solute distribution ratio and selectivity. In spite of the lower extraction ability of [C2C1im][C1SO3] when compared with organic solvents, 100% of extracted product was achieved. After this preliminary study, the authors continued to search for more efficient ILs and published five further articles on the subject.168−172 They have enlarged the number of ILs suitable for deterpenation processes and improved deterpenation yields, providing important insights for task-specific IL design. Various structurally distinct ILs were studied: [C2C1im][C2SO4], [C2C1im][C1(OC2)2SO4], [CnC1im][NTf2] (n = 2, 6 and 10), [C1pyr][C1SO4], [C2pyr][C2SO4], and [CnC1im][C1CO2] (n = 2 and 4). Altogether, the results obtained suggest that ILs with acetate anions and shorter alkyl substituents are the most advantageous structural features.168−172 An additional work by different authors173 focused on the determination of the liquid–liquid equilibria data of binary mixtures formed by [CnC1im][NTf2] (n = 4 and 6) and linalool (as the only citrus essential oil representative component) for deterpenation purposes, yielding similar conclusions to those gathered by Arce and co-workers.170 More recently, Martins et al.174 proposed a novel and quicker way to evaluate the aptitude of an IL for deterpenation processes. The authors174 demonstrated that a large number of liquid–liquid experiments can be avoided by using infinite dilution activity coefficient data predicted by COSMO-RS (conductor-like screening model for real solvents). The authors174 studied seventeen terpenic compounds in four imidazolium-based ILs as a basis for the creation of a model to screen the different structural features of ILs for terpene and terpenoid separation. Polar anions, such as acetate-based examples, led to enhanced separation abilities, in good agreement with the experimental findings of Arce and collaborators.172 Although rarely applied, the use of predictive models to test and identify the potential interactions between the target compounds and ILs appears to be a valuable strategy before carrying out case-by-case experimental studies aimed at screening appropriate ILs.
3.3. Carotenoids
Carotenoids are fat-soluble pigments with several health benefits and broad industrial applications.175 ILs have also been considered as solvents for their efficient and sustainable recovery from diverse sources. The first work available aimed at this application, by Bi et al.,176 dates from 2010. The background of this work is related to the environmental issues associated with shrimp waste and to the possibility of recovering bioactive compounds from such a matrix, namely astaxanthin, which is a highly valuable carotenoid. UAE was the technique studied, in a first attempt using molecular solvents (e.g., methanol, ethanol, n-hexane, ethyl acetate, acetone, dichloromethane, and water) and then using the best molecular solvent (i.e., ethanol) in combination with ILs. Among the seven ILs investigated, [(NH2)C3C1im]Br provided the highest recovery of astaxanthin. At optimal conditions of IL concentration, ultrasonic power, time and solid–liquid ratio, 92 μg of astaxanthin per gram of waste was obtained compared to ca. 50 μg of astaxanthin per gram of waste yielded by the conventional UAE with pure ethanol.176 Lee et al.177 also used ILs as additives in dichloromethane/methanol solutions to extract astaxanthin from Portunus trituberculatus, achieving 45.81 μg per gram of waste under the optimal conditions. Recently, Praveenkumar et al.178 studied the extraction of the same carotenoid from the microalgae Haematococcus pluvialis. The authors178 developed a novel strategy based on the germination of aplanospores, allowing the cells to lose their hard structure and the ILs to penetrate the cells. In a first stage, the authors178 inspected the IL impact upon the cells before and after germination, while in a second stage, the IL chemical structure influence in the astaxanthin extraction was addressed. Of the five imidazolium-based ILs screened, those containing sulfate-based anions were the most effective. Also, longer alkyl side chain lengths at both the cation ([C2C1im]+ vs [C4C1im]+) and the anion ([C1SO4]− vs [C2SO4]−) enhanced the extraction ability. Ultimately, and even with a limited number of ILs tested regarding the alkyl side chain length effect, [C2C1im][C2SO4] was the one leading to the highest astaxanthin yield, 19.5 pg per germinated cell, over a small extraction time at ambient temperature.178 It should be stressed that this result is almost as high as that obtained by the conventional extraction with ethyl acetate, which requires higher energetic inputs. The possibility of reusing the IL was also addressed by carrying out a back-extraction of the pigment with ethyl acetate.178 In this work, a brief discussion about the advantages of using [C2C1im][C2SO4] should have been included, since not only improved results are obtained with the conventional process using ethyl acetate but also the same organic solvent was then applied in the back-extraction to recover the target carotenoid, leaving thus an open question regarding the real advantage of an intermediate step using ILs.
The cell wall robustness of Haematococcus pluvialis, a natural source of astaxanthin, motivated Desai and co-workers179 to develop an alternative approach for astaxanthin extraction. Traditionally, mechanical disruption (with high energy consumption) is the preferred method, thus the novel proposed technology affords milder conditions. This was accomplished by a cell pretreatment step using ILs as permeabilizing agents.179 In the subsequent step of extraction with ethyl acetate, the aqueous solution of [C2C1im][(C4)2PO4] exhibited the best permeabilization aptitude under the optimal conditions (40 wt % in water and 40 °C), yielding 77.04% of astaxanthin in micrograms per milligrams of dry biomass. The large scale implementation of this technology was reinforced by establishing the reusability of the IL over three cycles.179
ILs were further applied in either pure form or as ethanol solutions for the extraction of lycopene from tomato-based matrices.180 Pure [C4C1im][PF6] yielded the highest amount of recovered lycopene (5.56 μg of lycopene per gram of tomato) that favorably compares with the results achieved with acetone (3.65 μg of lycopene per gram of tomato), ethanol (0.34 μg of lycopene per gram of tomato), or IL-ethanol solutions (1.23–2.37 μg of lycopene per gram of tomato).180 Also supporting the solvation power of ILs for carotenoids (β-carotene) and other isoprenoids (squalene and botryococcene), Lovejoy et al.181 proposed a novel approach combining IL-based extraction with biocatalytic processes. The panel of ILs comprises [P66614]Cl, [P66614][NTf2], [C4C1pyrr][NTf2], [P4444]Cl, [P10(3OH)(3OH)(3OH)]Br, [N111(2OH)]Cl, and [N111(2OH)][NTf2], while the reference solvents are hexane and hexadecane. The algal species adopted were the blue-green algae Synechocystis sp. PCC6803 and the colonial green algae Botryococcus braunii. Prior to assessing the solvation potential of ILs, their cytotoxicity was assessed toward both algae, demonstrating a high dependency on both the IL chemical structure and the algae species under study.181 With regard to the solubility of the three isoprenoids, distinct patterns were observed, as for β-carotene its solubilization in two of the ILs, [P4444]Cl and [C4C1pyrr][NTf2], greatly exceeded that obtained in hexane (1.0 and 0.2–0.3 wt % vs 0.14), while for squalene (miscible with hexane), [P66614]-based ILs were the best candidates (9.5–11.3 wt %). Isoprenoids were recovered from [P66614]-based ILs and [C4C1pyrr][NTf2] by vacuum distillation with no decomposition effects observed. As a proof of concept, immiscible ILs were further exploited as biocompatible routes for the extraction of botryococcenes from Botryococcus braunii cultures. Similar levels of extraction to those accomplished by organic solvents were achieved, although the complete disintegration of the cells was noted for some ILs. Altogether, these results suggest that a careful balance between the cytotoxicity and solvation ability of ILs should be considered.181
ABS formed by ILs were also applied for the separation of carotenoids.84,104,182 Coutinho and collaborators reported two articles wherein the β-carotene separation was evaluated in ABS composed of (i) phosphonium-based ILs + K3PO4104 and (ii) ILs + carbohydrates.84 In both studies, β-carotene extensively partitioned toward the IL-rich phase. A remaining work was devoted to the extraction of crocins (i.e., carotenoid derivatives) from Crocus sativus.182 The ABS investigated ranged from polymer–polymer to polymer-salt, alcohol-salt, and IL-salt, the latter including [C4C1im][BF4] and [C2C1im][C1CO2]. During the optimization studies, the recoveries of crocins ranged from 38 to 66% for polymer–polymer, 72 to 100% for polymer-salt, 91 to 98% for alcohol-salt, and 74 to 97% for IL-salt ABS.182 Unfortunately, none of these works using ABS showed the isolation of the compounds being extracted nor the recycling of the ILs.
3.4. Saponins
Saponins are interesting compounds due to their versatility, from both chemical structure and biological activity perspectives. Their structures, consisting of a nonpolar aglycone of triterpenic or steroidal nature, coupled to polar sugar moieties, is responsible for their surface-active properties. Studies dealing with the extraction and purification of saponins were published between 2013 and 2014, and they follow distinct lines of research.62,79,183,184 Marrucho and co-workers reported two works using ILs: one wherein aqueous solutions of imidazolium and cholinium ILs were used to extract saponins and polyphenols from Ilex paraguariensis and Camellia sinensis followed by saponin recovery by ABS,79 and another wherein the focus was the utilization of cholinium-based ILs and deep eutectic solvents in water or water/ethanol mixtures to extract saponins from Agave sisalana and Ziziphus joazeiro.(183) In the first work,79 a systematic study of the IL structure, solid–liquid ratio, temperature, and contact time was performed. Under the optimal conditions established from central composite experimental designs, [N111(2OH)]Cl (at 30 wt %) was selected to extract saponins and polyphenols from the two matrices and further used to pursue the purification of saponins using ABS. At the end, it was possible to recover the saponins in a water-phase almost free of [N111(2OH)]Cl, by using [N111(2OH)][NTf2],79 as represented in Figure 8 B. In their second work,183 the authors comprehensively studied thirteen ILs and nine deep eutectic solvents in terms of their ability to extract saponins from two plant sources. Again, depending on the matrix type, distinct results of extraction and selectivity (over polyphenolics) were obtained, independently of the use of water or water ethanol/mixtures. From the results obtained, two deep eutectic solvents, [N111(2OH)]Cl:[N111(2OH)][C1CO2] and [N111(2OH)]Cl:[N111(2OH)][C2CO2] in water/ethanol mixtures, were selected as the optimal solvents for the extraction of saponins from Ziziphus joazeiro and Agave sisalana, respectively. The rationale behind this choice was the extraction efficiency, selectivity, and price. These were further used in statistical experimental designs to optimize the extraction conditions.183
The two remaining works resorted to UAE or combined UMAE methods. Lin et al.62 developed an aqueous IL-based UAE approach for ginsenosides (dammarane-type triterpene saponins) from ginseng roots to diminish the long times of extraction and large quantities of solvents conventionally required. The selection of the best operational conditions was based on different IL cations ([CnC1im]Br, n = 2, 3, 4, and 6), anions ([C3C1im]X, X = I–, [BF4]− and Br–), their concentration (0 to 0.9 M), solid–liquid ratio (1:5, 1:10, 1:20, and 1:30), and extraction time (10–50 min). The best results were accomplished with [C3C1im]Br at 0.3 M in water, with a yield of 17.81 ± 0.47 mg per gram of ginsenosides −3.16 times higher than that afforded by the conventional UAE method with methanol.62 Wang et al.184 combined both ultrasound and microwave to develop an extraction approach for steroidal saponins from Discorea zingiberensis based on ILs. Six ILs were screened and compared with the performance of water in relation to their aptitude to extract diosgenin. Notably, all ILs performed better than water, [C2C1im][BF4] being responsible for the higher diosgenin yield. Under the optimal conditions, 0.5 M of [C2C1im][BF4] allowed a yield of diosgenin of 10.24 mg per gram, a value slightly lower than those obtained with heat reflux extraction (11.17 mg per gram) and UAE (11.13 mg per gram). Despite this fact, the method was able to surpass the conventional approaches in terms of extraction time.184
3.5. Vitamins
Tocopherols are fat-soluble antioxidants that constitute vitamin E. Upon their extraction from natural sources, tocopherols are obtained as a complex mixture of four homologues, namely α-, β-, γ-, and δ-tocopherol. Despite their similar structure, they differ in their biological activity, highlighting the importance of finding fractionation approaches (α-tocopherol possesses the strongest biological activity). The techniques available for such a purpose are efficient, although very difficult to scale up. To overcome this limitation, Ren and collaborators185−187 attempted the development of a novel platform based on ILs for the selective separation of tocopherol isomers. Having as background the set of intermolecular interactions that ILs can establish with organic molecules, the authors postulated that tocopherol homologues with different hydrogen-bond acidities could be selectively separated via hydrogen-bonding interactions. A number of reports were published based on (i) the use of hexane plus IL and methanol mixtures,185 (ii) the use of hexane plus IL-co-solvent mixtures186 in selective LLE, and (iii) theoretical studies envisaging a deeper understanding of the underlying molecular mechanisms in order to find the optimum solvents.187 Using [C4C1im]Cl as the extractive agent, a maximum selectivity ratio of 21.3 of δ- to α-tocopherol was observed with a 1:1.3 mixture of [C4C1im]Cl:methanol, followed by 18.8 obtained with the pure IL, a result ascribed to favorable interactions of the OH groups with the chloride anion.185 The role of the IL anion in the separation of these homologues was also inspected, which varied in the order: [BF4]− (6.7) < [CF3SO3]− (7.8) < Cl– (21.3), following the hydrogen bond basicity of the ILs.185 As a way of reducing the amount as well as the viscosity of the IL-based solvent, the use of acetonitrile, N,N-dimethylformamide, and dimethyl sulfoxide (DMSO) as cosolvents with [C4C1im]Cl was further addressed.186 The selectivity significantly depends on the cosolvent employed, acetonitrile and DMSO being responsible for the higher and lower values attained, respectively. Other ILs were screened, whereby those with Cl– anions led to the best selectivities and those with larger cation alkyl side chains to improved distribution coefficients. Again, hydrogen-bond basicity was shown to determine the selective extraction,186 as further stressed in their theoretical studies.187 Ren and collaborators188 published another work of particular interest in this framework, wherein the selective LLE of vitamin D3 and tachysterol3 (structurally differing in the position of the double bonds) was attempted. Among the seven organic solvents and eleven ILs investigated, the former were responsible for higher distribution coefficients, while the latter induced higher selectivities. In particular, ILs containing [NTf2]− and [CF3SO3]− anions, [C4C1pyr]+ or [C4C1pyrr]+ cations, and −CN or OH functionalized alkyl chains, were the best candidates for this application. In this case, π···π stacking was suggested to be the dominant type of interaction, allowing the selective distribution of the two homologues. A continuous multistage extraction was also conceptualized with achievable vitamin D3 purities of >98%.188
As with most biocompounds reported in this review, the limited variety of IL structures is also shown in the extraction of lipids and related compounds, as well as the absence of studies on their recovery and further recycling of the ILs and remaining solvents.
4. Amino Acids
In recent years, ILs have been extensively investigated for the extraction and purification of proteins. This interest in protein purification is also extended to amino acids, the “building blocks” of proteins. Amino acids play an important role in physological phenomena where proteins are critical and also participate in a wide variety of biochemical reactions. Given their importance, IL-based recovery approaches for amino acids are reviewed in this section. Four main techniques, around which this section is organized, were identified in twenty-six published manuscripts, with their relative distribution graphically shown in Figure 9. Clearly, the application of IL-based ABS is the most investigated technique, followed by LLE using hydrophobic ILs, and finally by both IL-based three-phase partitioning (TPP) and SPE approaches. Although some works deal with distinct types of amino acids either as model compounds or aimed at developing specific purification processes, most articles used Trp as a model amino acid (Figure 9). The chemical structures of the fourteen amino acids extracted are depicted in Figure 10, which also comprises enantiomeric pairs for the cases wherein chiral separation was envisaged. The usage frequency of several cation–anion combinations is displayed in Figure 11, wherein a preference for hydrophobic (e.g., [NTf2]−, [PF6]−, and [BF4]−) or hydrophilic (e.g., Cl– and Br–) anions and imidazolium-based cations is clear. The optimization of the extraction conditions was cautiously analyzed in terms of several parameters (e.g., the concentration and chemical structure of IL or target amino acids, pH, and temperature). Table 9 provides a survey of the works reviewed according to the amino acid extracted, technique adopted, and IL used.
Figure 9.
Distribution of the works dealing with each IL-based technique for the extraction and separation of amino acids. The radial graphs display the number of scientific works addressing distinct types of amino acids.
Figure 10.
Chemical structures, names, and abbreviations of all amino acids extracted and separated with IL-based techniques.
Figure 11.
ILs used for the extraction and separation of amino acids as a function of cation–anion combinations. The usage incidence (number of articles) is represented by the size of the circles, which proportionally increases as follows: [0–2] < [2–4] < [4–6] < [6–8] < [8–9].
Table 9. Extraction and Separation of Amino Acids Using IL-Based Processes.
| amino acid | method | IL used |
|---|---|---|
| Abu | IL-ABS | ([C4C1im]Br, [C6C1im]Br, and [C8C1im]Br)192 |
| Ala | IL-ABS and IL-LLE | ([C4C1im]Br, [C6C1im]Br, [C8C1im]Br),192 [C4C1im][PF6],96,204,207 ([C6C1im][PF6], [C8C1im][PF6], [C6C1im][BF4], [C4C1im][BF4], and [C8C1im][BF4]207 |
| Arg | IL-LLE | [C4C1im][PF6]204 |
| Asp | IL-LLE | [(CH2CONHC4H9)C2im][NTf2]210 |
| enantiomers of Phe | IL-ABS, IL-TPP, and IL-SLE | ([C10H18N3O2][PF6], [C11H21N4O2][PF6]),203 ([C2tro][l-Pro], [C3tro][l-Pro], [C4tro][l-Pro], [C5tro][l-Pro], [C6tro][l-Pro], [C7tro][l-Pro], [C8tro][l-Pro]),212 and [C2(l-Phe)][NTf2]213 |
| Glu | IL-ABS | ([C4C1im][PF6], [C6C1im][PF6], [C8C1im][PF6], [C6C1im][BF4], [C8C1im][BF4])207 |
| Gly | IL-ABS and IL-LLE | ([C4C1im]Br, [C6C1im]Br, [C8C1im]Br),192 and [C4C1im][PF6]96,204 |
| His | IL-LLE | [C4C1im][PF6],204 [(CH2CONHC4H9)C2im][NTf2],210 and [C4C1im][Phe]214 |
| Leu | IL-ABS and IL- LLE | [C4C1im]Br,192,196 ([C6C1im]Br, [C8C1im]Br),192 [C4C1im][PF6],96,204,205 ([C6C1im][BF4], [C8C1im][BF4], and [C6C1im][PF6])205 |
| Met | IL-ABS | ([C4C1im]Br, [C6C1im]Br, and [C8C1im]Br)192 |
| Phe | IL-ABS, IL-LLE, and IL-SLE | [C4C1im][PF6],194,204,205 ([C6C1im][PF6], [C6C1im][BF4),205 [C4C1im]Br,194,196 ([C4C1im][NTf2], [C4C1im]Cl, [C2C1im][CF3SO3], [N111(2OH)][C3CO2], [N111(2OH)][C5CO2], [N111(2OH)][C7CO2], [P4444][C11CO2], [N111(2OH)][C7CO2], [N111(2OH)][C11CO2]),194 ([C8C1im][BF4]),200,205 [(CH2CONHC4H9)C2im][NTf2],210 [C4C1im][Phe],214 and [C4C1im][CF3SO3]200 |
| Thr | IL-ABS | ([C4C1im]Br, [C6C1im]Br, and [C8C1im]Br)192 |
| Trp | IL-ABS and IL-LLE | [C7H7C1im]Cl,189 ([aC1im]Cl, [OHC2C1im]Cl, [C2C1im]Cl, [im]Cl),189,201 [C4C1im]Cl,189,194,201 [C1im]Cl,189,200 [C2C1im][CF3SO3],190,194 ([C2C1im]Cl, [C2C1im][C1CO2], [C2C1im][C2SO4], [C2C1im][C1SO4],190 [C4C1im][N(CN)2],190,197 [C4C1im]Cl,190,194,197 [C2C1im]Br,198 [C4C1im]Br,190,192,196,202 ([C6C1im]Br, [C8C1im]Br),192 [C4C1im][CF3SO3],84,190,197,200 ([C4C1im][C1SO3], [C4C1im][CF3CO2]),190,197 [C4C1im][PF6],96,194,204−206,208,210 [C6C1im][PF6],205,210 [C8C1im][PF6],206,208,210 [C4C1im][NTf2],194,206 [C4C1im][BF4],200 [C4C1im][C1CO2],201 [C6C1im][BF4],205,206 [C8C1im][BF4],205,206,208 ([N111(2OH)][C3CO2], [N111(2OH)][C5CO2], [N111(2OH)][C7CO2], [P4444][C11CO2], [N111(2OH)][C7CO2], [N111(2OH)][C11CO2]),194 [P4441][C1SO4],193,194 ([P4444]Cl, [N4444]Cl, [C4C1pip]Cl, [C4C1pyrr]Cl, [C4C1im][SCN]),197 ([aC1im]Cl, [C4C1im]Cl, [C7H7C1im]Cl, [C4C1im][C1SO4], [C4C1im][HSO4]),201 ([C2C1im][NTf2], [C3C1im][NTf2], [C5C1im][NTf2], [C6C1im][NTf2], [C7C1im][NTf2], [C8C1im][NTf2]), [C3C1pyrr][NTf2], [C3C1pyrr][NTf2], [C3C1pip][NTf2], [C2C2im][NTf2], [C4C1pyrr][NTf2], [C4C1im][NTf2]),206 ([C4C1im][NTf2], [(CH2CONHC4H9)C2im][NTf2], [C4C1pyr][NTf2], [C4C1pyr][NTf2]),210 ([C6C1im][BF4], [C4C1im][BF4], [C5C1im][BF4], [C5C1im]Br),199 and [C4C1im][Phe]214 |
| Tyr | IL-ABS and IL-LLE | [C2C1im]Br,198 [C4C1im]Br,192,196 ([C6C1im]Br, [C8C1im]Br),192 ([C6C1im][BF4], [C8C1im][BF4], [C4C1im][PF6]), and [C6C1im][PF6])205 |
| Val | IL-ABS and IL-LLE | [C4C1im]Br,192,196 ([C6C1im]Br, [C8C1im]Br),192 ([C6C1im][BF4], [C8C1im][BF4], [C4C1im][PF6], [C6C1im][PF6]),205 and [(CH2CONHC4H9)C2im][Tf2N]210 |
4.1. IL-Based Aqueous Biphasic System Extractions
In 2009, IL-based ABS were used for the first time for the extraction of the essential amino acid l-Trp, leading to two seminal works.189,190 In both works, l-Trp presented a preferential migration to the IL-rich phase. When studying the IL anion effect, the authors observed that the l-Trp partitioned preferentially for IL-rich phases, while closely following the Hofmeister series. However, and compared to the cation effect, the influence of the IL anion on the amino acid partitioning was revealed to be less relevant.189,190 Both works demonstrated that IL-based ABS led to substantially more extensive extraction efficiencies than those obtained with conventional PEG-based ABS, reinforcing the suitability of IL-based ABS to recover l-Trp and other amino acids. The partitioning behavior of l-Trp, as well as of Gly, Ala, Abu, Val, Leu, Thr, Met, and Tyr with acetate-based ILs, [CnC1im][C1CO2] (n = 4, 6, 8) and [CnC1im]Br (n = 4, 6, 8), was further explored.191,192 As reported earlier, the more hydrophobic cations displayed the best extraction results.191,192 Nevertheless, when Louros et al.193 applied phosphonium-based ILs to the extraction of amino acids, this trend was not confirmed. The authors193 observed that although containing a more hydrophobic cation, this IL family leads to a significantly lower partition coefficient (KTrp ≈ 9) when compared to ABS formed by imidazolium-based ILs (KTrp ≈ 37). The extraction of l-Trp and dl-Phe was further studied by Xie et al.194 with a series of novel ABS using biocompatible ILs composed of long chain carboxylate anions and a cholinium cation. Partition coefficients of l-Trp of 58.5 and of dl-Phe of 120 were reported. In accordance with previous studies,195 and as discussed in previous sections, long alkyl chains on imidazolium cations cause IL self-aggregation in ABS. This encouraged the authors to investigate the aggregation behavior of the IL in the phase of the studied ABS using polarizing optical microscopy (POM),194 observing Iiquid crystal structures in the IL-rich phase of two of the studied systems ([N111(2OH)][C7CO2] and [N111(2OH)][C11CO2]). The good performance of [N111(2OH)][C7CO2] suggested that the IL self-aggregation phenomenon may contribute to the partitioning of the target biomolecules into the IL-rich phase, but different results were obtained with the IL [N111(2OH)][C11CO2]. Its higher aggregation tendency decreased the interaction between the IL anion and the solute, impairing the extraction performance.194
Further studies on amino acid partitioning in IL-based ABS were performed by Zafarani-Moattar and Hamzehzadeh,196 who introduced an organic salt (potassium citrate) to form IL-based ABS for the extraction of l-Trp, l-Phe, l-Tyr, l-Leu, and l-Val. Various pH values and phase-forming component compositions were investigated. Hydrophobic interactions emerged as the main driving force; however, other parameters, including amino acid size, accessible surface area, and polarizability, were also revealed to be important. Based also on the role of electrostatic interactions and salting-out effects, the authors196 proposed a successful model to describe the partition coefficient of all the investigated amino acids for the set of ABS studied. This study was later extended, using the same organic salt conjugated with imidazolium-, pyrrolidinium-, phosphonium- and ammonium-based ILs for l-Trp extraction.197 Good extraction efficiencies were achieved, between 72% and 99%, by the IL-rich phase in a single step.197 The authors demonstrated that the IL-rich phases exibited a pH between the pKa1 and pKa2 (2.38 and 9.39, respectively) of l-Trp, although lower partitioning coefficients were observed for lower pH values. The authors197 also observed that the IL anion has a stronger effect on the partition coefficients of l-Trp when in acidic environment.
With a goal of designing more environmentally benign and biocompatible ABS, Zafarani-Moattar and Hamzehzadeh198 employed PPG 400 and hydrophilic ILs, [CnC1im]Br (n = 2, 4), for the extraction of l-Trp and l-Tyr. Curiously, in polymer-IL-based ABS, l-Tyr displays a preferential partition toward the polymer-rich phase while l-Trp kept the preference for the IL-rich phase. This difference was attributed to the lack of one pyrrole ring in l-Tyr when compared to l-Trp.198 However, the salt replacement by the polymer in IL-based ABS resulted in significantly lower partition coefficient values, which highlights the need to strike a balance between the properties of the phase-forming components, the nature of the target compounds, and the processing conditions, in order to achieve the highest efficiency. Even so, this opposite partition of the two amino acids may be seen as a promising trend when envisaging the development of fractionation platforms of amino acids from complex and raw mixtures. Along these lines, Freire et al.84 replaced the typically used inorganic/organic salts by a large range of mono- and disaccharides combined with [C4C1im][CF3SO3] to form ABS. Despite the advantages of using saccharides as phase-forming components (carbohydrates are noncharged, biodegradable and nontoxic), the extraction efficiencies obtained in this work were significantly lower (∼50%) than those previously observed with ABS formed by ILs and salts.84 Later, an amino acid extraction was also attempted using the cationic surfactant 3-p-nonylphenoxy-2-hydroxypropyl trimethylammonium bromide as a phase-forming component in ABS with two distinct series of ILs ([CnC1im][BF4], n = 2, 3, 4, 6, and [CnC1im]Br, n = 3, 4, 6).199 The best extraction efficiency to the IL-rich phase was achieved for [BF4]-based ILs. Furthermore, an increase in the alkyl side chain of the cation leads to a decrease of the ABS extraction performance.199
In the search for more biocompatible and less toxic IL-based ABS for amino acid extractions, recently, organic biological buffers, namely Good’s Buffers (GBs), were used in combination with ILs to form ABS. Single-step extraction efficiencies of l-Trp and l-Phe for the GB-rich phase ranging between 22.4% and 100.0%, were observed. In contrast to the IL-salt ABS previously discussed, in most of the GB-based ABS studied, the amino acid preferentially migrated to the more hydrophilic (GB-rich) phase, which was justified by the role of H-bonding and dispersive forces promoted by the predominant zwitterionic form of both amino acids at the system’s pH. The most exciting result from the work of Luís et al.200 was observed in two of the studied ABS, in which l-Phe completely partitioned to the GB-rich phase, while l-Trp showed a preferential affinity to the opposite phase. Together with the data collected by Zafarani-Moattar and Hamzehzadeh,198 such results highlight the fact that these systems can be employed in the fractionation of complex mixtures of amino acids, as for instance from a fermentation broth or from hydrolyzed peptide mixtures, as illustrated in Figure 12.
Figure 12.

Schematic diagram of integrated processes based on IL-based ABS comprising the extraction and purification of amino acids from complex mixtures.198,200
In an attempt to develop an alternative technique for PEG functionalization, Pereira et al.201 proposed a new approach using ILs as adjuvants in typical polymer-based ABS for the separation and purification of amino acids. Several imidazolium-based ILs were added at 5 wt % to PEG600 + NaSO4 ABS, which were able to enhance the extraction performance of conventional systems. The authors201 demonstrated that salting-in ILs enhance the partition coefficient of l-Trp for the PEG-rich phase while salting-out ILs lead to a decrease, which stresses the need to carefully select the type of IL used. It was additionally demonstrated that ILs also preferentially partition to the PEG-rich phase, making them able to tailor the properties of the polymer phase and affinities for amino acids.201 Despite these promising results published in 2010,201 it was only in 2014 that ILs were again employed as adjuvants in the creation of ABS for the extraction of amino acids.202 The authors202 used [C4C1im]Br as an adjuvant in PEG 400 + tripotassium citrate ABS, and demonstrated that a small addition of [C4C1im]Br doubles the extraction ability of amino acids toward the polymer-rich phase.
A recent study revealed that imidazolium-based chiral ILs can be used to separate racemic mixtures of amino acids (d-Phe and l-Phe), whereby a maximum extraction efficiency of 53% was reported.203 The d-enantiomer interacts with ILs, remaining in the bottom IL-rich phase, while the l-enantiomer migrates to the Na2SO4-rich phase. 1H NMR spectropscopy and density functional theory (DFT) calculations showed that hydrogen-bonding interactions between the carboxylate and amide groups, and resonance-assisted hydrogen-bonding interactions between amino and hydroxyl groups, play a pivotal role.203 Given the potential for forming ABS combining ILs with amino acids,7 and although not attempted thus far, a much more simpler approach can be anticipated, namely on the use of chiral amino acids as phase promoters of ABS and on their use for the separation of racemic mixtures of amino acids.
Summing up, the extraction of a wide range of amino acids was investigated using IL-based ABS, and the diversity of results obtained is quite significant. Besides the large number of amino acids and process conditions analyzed, the variety of extraction platforms applied is also appreciable, ranging from ABS based on ILs + (organic or inorganic) salts, ILs + GBs, ILs + polymer, ILs + (mono and di)saccharides, ILs + cationic surfactants to ILs (adjuvants) + PEG + salt combinations. The results here described suggest that the most effective systems for extracting amino acids are those using ILs as adjuvants (not only because of the high extraction efficiencies obtained, but also due to the reduced costs of the process associated with the low amount of ILs used), followed by the IL + salt, IL + polymer, and IL + saccharides. High extraction efficiencies for ABS formed by GBs were also found, however their influence is quite diverse and depends on the properties of GBs and amino acids. The same conclusion was found for the definition of the best pH to be applied due to the inherent speciation of amino acids, and consequently on the main interactions taking place. Again, a more applied industrial perspective seems to be missing. Despite their crucial role in the development of sustainable and economically viable purification processes, in the works overviewed in this section, the isolation and recovery of amino acids from the ABS phases where they are enriched has been neglected, as well as the recovery and reuse of the ILs. Also scaled-up strategies for the best-performing systems are absent. Moreover, most of the reported studies employed amino acids as model compounds to mimic the behavior of proteins in ABS, their purification not being a main focus of the studies.
4.2. Liquid–liquid extractions with hydrophobic ILs
Amino acid extractions using IL-based LLE approaches employing hydrophobic ILs were first described in 2003 by Carda-Broch et al.,204 using a crown ether (dibenzo-18-crown-6)-modified IL, obtaining distribution values about 2 orders of magnitude higher than those garnered without the crown ether. After this pioneer study, a more detailed work on the application of a similar modified IL was published,96 in which the IL dicyclohexano-18-crown-6 was used to extract both hydrophilic and hydrophobic amino acids (Trp, Gly, Ala, Leu, Arg and Lys). The partitioning behavior was shown to be pH-dependent, but still allowed high extraction efficiencies in all situations (92–96%).96 Futher studies were conducted205,206 in which different imidazolium-based ILs ([C4C1im][PF6], [C6C1im][PF6], [C6C1im][BF4] and [C8C1im][BF4]) were tested for the recovery of l-Trp, l-Phe, l-Tyr, l-Leu and d-Val. By obtaining a close correlation between the logarithm function of the partition coefficient and the hydrophobicity of amino acids, the authors205 proposed hydrophobic interactions as the driving forces in the preferential partitioning of the amino acids to the IL-phase. Higher extraction degrees were observed at low pH values, a result of strong electrostatic interactions occurring between the cationic form of the amino acids and the anion of the ILs, and with [BF4]− considered as the most effective IL anion.205 Later, the same imidazolium-based ILs were applied to the extraction of Lys, Ala and Glu from aqueous media.207 Similarly to results reported previously, the partition coefficients of the amino acids depend on the pH of the aqueous solution, and on the amino acids and IL chemical structures. On the basis of this pH dependence, the authors finally reported a back-extraction step using a phosphate buffer solution to recover the amino acids from the IL phase,207 an approach depicted in Figure 13.
Figure 13.
Schematic diagram of an integrated process on the extraction and recovery of amino acids using IL-based LLE, with a pH-aided back-extraction step.207
In a different approach, Seduraman et al.208 described, by molecular dynamic simulations, that the ability of ILs to extract amino acids depends on the water amount at the IL-phase, a condition that may help other authors to understand some of their experimental results. Further studies on the solubility and stability of several amino acids (Ala, Val, Leu, His, Trp, and Tyr) in aqueous solutions of ILs were conducted by Vasantha et al.209 In general, a decrease of the amino acid solubility was observed with an increase of the IL concentration, which according to the authors,209 is dictated by unfavorable interactions of the ILs with the amino acid surface.
Inspired by the remarkable results obtained with functionalized ILs on the extraction of metal ions, Huaxi et al.210 synthesized a new hydrophobic amide-based functionalized IL, [(CH2CONHC4H9)C2im][NTf2], to extract amino acids. Depending on the pH values, this new IL allows a higher partition coefficient and higher selectivity for l-Trp than conventional ILs, explained by the favorable hydrogen bonds established between the acetyl group of [(CH2CONHC4H9)C2im][NTf2] and the NH2 group of l-Trp. The authors210 also observed that the increase of the IL volume ratio and the initial concentration of l-Trp culminate in a reduction of the partition coefficient. By a pH swing effect and using a similar strategy to the one described above,207 the authors210 were able to recycle and reuse the [(CH2CONHC4H9)C2im][NTf2] without compromising the extraction efficiency, even after four cycles.
Tang and coauthors211 employed functional amino-acid-based ILs (AAILs) as solvents and selectors for the LLE of racemic mixtures of amino acids. With these ILs as an acceptor phase and ethyl acetate as a donor phase, it was possible to extract the l-enantiomer into the IL phase with higher efficiencies. This enantioselective enrichment was driven by a chiral ligand-exchange mechanism, since a minimum concentraction of the target amino acid and chelant (Cu2+) is necessary. AAILs proved to be excellent solvents since they not only display chiral recognition ability but also extract more than 99% of the amino acids from the donor phase.211
Overall, the aforementioned works show that under appropriate pH conditions and with the adequate IL chemical structures, hydrophobic ILs can be used as successful solvents to recover amino acids from aqueous solutions or organic media, with optimized extraction efficiencies similar to those provided by IL-based ABS described in the previous section. The range of hydrophobic ILs is however more limited than hydrophilic ones typically used in the formation of IL-based ABS. Despite the efforts to extract amino acids with hydrophobic ILs, unfortunately only ILs based on the imidazolium cation, some of them functionalized, and using different anion structures, were investigated. Although some attempts have been carried out, there is a general lack of results considering the isolation of amino acids from the IL-rich phase, as well as the description of adequate strategies to recover and reuse the ILs.
4.3. IL-Based Three-Phase Partitioning
IL-based three-phase partitioning (IL-TPP) is usually achieved by the creation of an additional phase in the two-phase systems described above (IL-based ABS), which corresponds to the desired precipitated product. ABS based on chiral tropine ILs and inorganic salts were prepared for the enantiomeric separation of a racemic mixture of Phe,212 as depicted in Figure 14. In this study, the phase behavior of IL-based ABS was comprehensively investigated along with the factors that influence the separation efficiency. When the amount of d-Phe and l-Phe reached approximately the range of 15–20 mg g–1 (the concentration required for the enantioselectivity to occur), a TPP system was created: the top IL-rich phase, the middle phase with precipitated amino acids, and the bottom salt-rich phase. In general, more hydrophobic ILs allow improved selectivities for the separation of racemic mixtures of Phe. On the other hand, large amounts of salt and water compromise the IL enantioselectivity. Under the optimum conditions, the enantiomeric excess value of l-Phe in the middle phase of the IL-TPP was 65%, while the d-enantiomer remains in the IL-rich phase.212 The obtained results prompted the authors212 to conclude that this system could be a promising approach for the racemic resolution of amino acids. Although other conditions such as temperature and pH could be additionally evaluated to improve the selectivity, IL-TPP appears as a promising strategy for the separation of other enantiomers of high commercial interest. In this sense, and given the single report found for the separation of amino acids,212 we would like to recommend researchers to further explore this technique since, in addition to the good results obtained, the recovery of the target compounds and recycling of the ILs is also much easier to accomplish, allowing the development of more cost-effective purification strategies.
Figure 14.

Schematic representation of IL-based TPP processes for the chiral resolution of racemic mixtures of amino acids.212
4.4. Solid-Phase Extractions Using IL-Modified Materials
IL-based SPE methods aimed at extracting and separating amino acids, although scarce, cover two distinct approaches: (i) ILs immobilized on silica and (ii) ILs on the preparation of molecularly imprinted polymers (MIPs). The first approach was studied by Marwani et al.,213 wherein a new chiral IL, [C2(l-Phe)][NTf2], was immobilized on silica for the enantioselective separation of d-Phe from aqueous media. Data on adsorption isotherms revealed that the adsorption capacity of the solid support for d-Phe was of 97.35% at pH 3.0. The feasibility of the methodology was ultimately validated by implementing it to real samples with satisfactory results.213 Yang et al.,214 on the other hand, turned their attention to the second approach by applying the oil-soluble 1-butyl-3-methylimidazolium α-aminohydrocinnamic acid ([C4C1im][Phe]) to prepare surfaces of MIPs in acetonitrile for the selective recognition of l-Phe. This approach is schematically displayed in Figure 15. Binding studies, such as adsorption kinetics, adsorption thermodynamics, SPE application, and the chiral resolution of racemic phenylalanine mixtures were performed. This IL-based copolymerizing process in acetonitrile, when compared with the traditional imprinting process with acetonitrile/H2O, created more binding sites and allowed a higher adsorption of l-Phe, resulting in the selective separation of l-Phe from other amino acids (l-Trp and l-His), with a recovery above 90.6%. With these results, the authors suggested that [C4C1im][Phe] imprinting polymers provide a new pathway for separating amino acids.214 Nevertheless, no further studies using this approach are so far available.
Figure 15.

Schematic representation of IL-based SPE processes for the selective separation of enantiomeric mixtures of amino acids.214
5. Proteins
Proteins are essential components of living organisms, being responsible for critical physiological functions, including gene expression, signal transduction, metabolism, and immunity. They comprise several physiologically relevant groups, including enzymes and antibodies. Proteins may also have diagnostic and prognostic significance in a large set of diseases. An abnormal increase or decrease of protein levels in serum and/or urine, for example, can be used as a disease biomarker. In fact, these biomarkers not only improve the early stage diagnosis of several pathologies (cancer included) but also allow to evaluate the response to therapy and to predict possible relapses.215,216 As a result of their hugely diverse functions in organisms, and variety of potential applications, proteins have a major impact in several industries, such as the textile, cosmetic, food, and pharmaceutical industries. Therefore, the development of alternative and cost-effective techniques for their recovery, purification, and concentration is of high relevance. IL-based extraction and purification processes for proteins are discussed in this section.
Figure 16 illustrates the six principal techniques reported in the literature for IL-assisted proteins separation processes: (i) IL-based ABS, (ii) hydrophobic IL-based LLE, (iii) IL-based TPP, (iv) surfactant-based systems containing ILs (comprising microemulsion systems and AMBS), (v) IL-modified materials for SPE, and (vi) SLE of proteins using ILs. The radial graphs in Figure 16 provide a summary of the various types of proteins investigated as well as of the different techniques addressed. The optimization of the extraction conditions, selection, and concentration of the phase-forming compounds, pH, and temperature are some of the variables emphasized and discussed. Finally, the results obtained, in what concerns the extraction efficiencies and stability of biomolecules, are also outlined and discussed.
Figure 16.
Distribution of the works dealing with each IL-based technique for the extraction and separation of proteins. The radial graphs display the number of scientific works which have addressed distinct types of proteins.
The stability of proteins in IL milieu has been the subject of a large amount of research, as mirrored by the considerable number of reviews on the topic.3,217,218 Such in-depth revisions point out the importance of understanding the IL-protein interactions for any protein-related application (e.g., biocatalysis, preservation, separation, and purification processes).3,217,218 Although general facts are difficult to gather at present due to the odd effects that distinct proteins suffer in the presence of different ILs, it is known that both cation and anion properties and the lypophilic-hydrophilic nature of the IL play crucial roles in their stability.3,217,218 IL anions of enhanced kosmotropicity and cations of high chaotropicity afford advantageous features in the field.3,217,218 Although scattered patterns exist across the literature, the body of results available is quite promising in what concerns the applications of ILs: it has been shown that certain ILs improve the thermal stability and half-lives of various proteins and enzymes217 and allow some enzymes to display superactivity.219 There are many techniques that yield important information on protein stability, including infrared spectroscopy,217 NMR spectroscopy, dynamic light scattering, and circular dichroism.217,220 These techniques have been used, sometimes combined with molecular docking or other computational approaches, as suitable and primordial strategies to better understand the IL-aided extraction of proteins.221
All of the ILs used for protein extraction and separation are depicted in Figure 17, according to their cation–anion combinations. 1-Alkyl-3-methylimidazolium, [CnC1im]+, is the most investigated cation, especially when combined with halogenated anions. Contrarily to the other classes of compounds discussed earlier, the poor incidence of the use of hydrophobic anions, namely those based on [NTf2]−, [BF4]−, and [PF6]− anions, is conspicuous, particularly due to the low solubility of proteins in these ILs, as discussed below. More recently, naturally derived ILs (from cholinium, carboxylic acids, amino acids, and Good’s buffers) are being increasingly adopted for the extraction and separation of proteins. In Table 10, it is possible to find a summary of these articles, organized according to the protein extracted, technique adopted, and IL used.
Figure 17.
ILs used for the extraction and separation of proteins as a function of cation–anion combinations. The usage incidence (number of articles) is represented by the size of the circles, which increases proportionally as follows: [0–3] < [3–6] < [6–9] < [9–19].
Table 10. Extraction and Separation of Proteins Using IL-Based Processes.
| protein | method | IL used |
|---|---|---|
| alcohol dehydrogenases | IL-ABS | [N221(O)nOH]Cl (ammoeng 110)231 |
| aloe proteins | IL-ABS | [C4C1im][BF4]248 |
| azocasein | IL-ABS | [N11[2(N11)]0][C1CO2]245 |
| bacteriorhodopsin | IL-LLE | [C6C1im][PF6]271 |
| bovine serum albumine | IL-ABS, IL-TPP, and IL-SPE | [C2C1im]Cl, [C4C1im]Cl222,232,290 [C6C1im]Cl,232 [C8C1im]Cl,232,235,290 [N221(O)nOH]Cl (ammoeng 110),224 [C4C1im]Br,225,232,233,235 [C6C1im]Br,225,232,235 [C8C1im]Br,225,229,232 [C2C1im]Br,235 [C6C1im]Br, [C4C1im][N(CN)2],234,235 ([C6C6C1C1C1C1guan]Br, [C4C4C1C1C1C1guan]Br, [C1C1C1C1guan][C0CO2], [C1C1C1C1guan][C1CO2], [C1C1C1C1guan][C2CO2], [(OH)C3(OH)C3C1C1C1C1guan]Cl, [(OH)C5(OH)C5C1C1C1C1guan]Cl, [C1C1C1C1guan][Lac]),235 ([C1C1C1C1guan][C1CO2], [C1C1C1C1guan][MAcr], [C1C1C1C1guan][Acr], [C1C1C1C1guan][Lac], [C1C1C1C1guan][Mal], [C1C1C1C1guan][Ita], [C1C1C1C1guan][Sor], [C1C1C1C1guan][Cinn]),236 [N4444][Tricine], [N4444][HEPES], [N4444][TES],110 ([N111(2OH)][Tricine], [N111(2OH)][MES], [N111(2OH)][TES], [N111(2OH)][HEPES]),221 [N111(2OH)]Cl,221,242 ([N111(2OH)][C1CO2], [N111(2OH)][C2CO2], [N111(2OH)][C3CO2], [N111(2OH)][OHC1CO2], [N111(2OH)][Lac]),240,242 ([N111(2OH)]2[Oxa], [N111(2OH)]3[Cit]),240 ([N111(2OH)][H2PO4], [N111(2OH)][Bit], [N111(2OH)][DHCit]),242 ([N111(2OH)][Lys], [N111(2OH)][β-Ala], [N111(2OH)][Gly], [N111(2OH)][Ser]),243 [C4C1im][CF3SO3],273 ([aC4im]Cl, [VC8im]Br),288 ([(C2H5O3)3SiC3C1im]Cl, [N11[3Si(2O)(2O)(2O)](2OH)]Cl, [(C2H5O)3SiC3C2mor]Cl, [(C2H5O)3SiC3C1C1C1C1guan]Cl, [(C2H5O3)3SiC3C1pyrr]Cl),289 ([N011(2OH)][C2CO2], [N011(2OH)][C3CO2], [N011(2OH)][C4CO2], [N011(2OH)][C5CO2], [N022(2OH)][C2CO2], [N022(2OH)][C3CO2], [N022(2OH)][C4CO2], [N022(2OH)][C5CO2]),237 [N11(2OH)(3OH)]Cl, [C4C1im]Cl, [C4C1im]Br, [(OH)C3C1im]Br,241 and [C4C1im][BF4]262 |
| bromelain | IL-AMBS | [C10C1im]Cl, [C12C1im]Cl, [C14C1im]Cl, [P666 14]Br, [P666 14][C9CO2], and [P666 14][TMPP]279 |
| Candida antarctica lipase B/A | IL-ABS | [C2C1im][C4SO4],252 ([C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, [C7C1im]Cl, [C8C1im]Cl, [C7H7C1im]Cl, [C4C1im][CF3SO3], [C4C1im][C1SO3] [C4C1im][N(CN)2], [C4C1pyrr]Cl, [C4C1pyr]Cl, [C4C1pip]Cl, and [C8pyr][N(CN)2])253 |
| Chlorella pyrenoidosa proteins | IL-SLE | ([N011(3N)][C0CO2], [N0444][C0CO2], [N0222][C0CO2], [N0022][C0CO2], [N011(3N)][C1CO2], [N0444][C1CO2], [N0222][C1CO2], [N0022][C1CO2], [N011(3N)][CF3CO2], [N0444][CF3CO2], [N0222][C1CO2], and [N0022][CF3CO2])282 |
| Cordyceps sinensis proteins | IL-ABS | [C4C1im]Cl249 |
| Cytochrome c | IL-ABS, IL-AMBS, IL-LLE, and IL-SLE | ([C2C1im]Br, [C4C1im]Br,225 [C6C1im]Br),226 [N111(2OH)][C1CO2], [N111(2OH)][C2CO2], [N111(2OH)][C3CO2], [N111(2OH)][Glyc], [N111(2OH)][Lac], [N111(2OH)]2[Oxa], [N111(2OH)]3[Cit]),240 ([N5555][Gly], [P4444][Gly], [N4444][Gly], [N2222][Gly]),239 ([C2C1im][(C1)2PO4], [C2C1im][C1SO3], [C2C1im][Tos], [C2C1im][C1CO2], [C2C1im][C1CO2], [C2C1im][N(CN)2], [C2C1im][CF3SO3], [OHC2C1im]Cl),34 ([C10C1im]Cl, [C12C1im]Cl, [C14C1im]Cl, [P66614]Cl, [P66614]Br,[P66614][N(CN)2], [P66614][TMPP], [P66614][C9CO2], [P8888]Br, [N8888]Br),278 [HOC2C1im][NTf2],263 [C4(C1C1C1Si)im][PF6],270 [C4C1im][NTf2],266 [C1im]Cl,286 ([aC4im]Cl, [VC8im]Br),288 and [N11[2(N11)]0][C1CO2]245 |
| hemoglobin | IL-ABS, IL-LLE, and IL-SLE | ([C4C1im]Cl, [C4C1im]Br, [C6C1im]Br, [C8C1im]Cl),232 [C6C1im]Cl,232,235 [C8C1im]Br,229,232 [C4(C1C1C1Si)im][PF6],263,269,278 [C4 C1im][PF6],276,277 [C10C1im]Br,277 [C1im]Cl,286,287 ([aC4im]Cl, [VC8im]Br),288 ([(C2H5O3)3SiC3C1im]Cl, [N11[3Si(2O)(2O)(2O)](2OH)]Cl, [(C2H5O)3SiC3C2mor]Cl, [(C2H5O)3SiC3C1C1C1C1guan]Cl, [(C2H5O3)3SiC3C1pyrr]Cl),289 ([C1C1C1C1guan][C1CO2], [C1C1C1C1guan][MAcr], [C1C1C1C1guan][Acr], [C1C1C1C1guan][Lac], [C1C1C1C1guan][Mal], [C1C1C1C1guan][Ita], [C1C1C1C1guan][Sor], [C1C1C1C1guan][Cinn]),236 ([N011(2OH)][C2CO2], [N011(2OH)][C3CO2], [N011(2OH)][C4CO2], [N011(2OH)][C5CO2], [N022(2OH)][C2CO2], [N022(2OH)][C3CO2], [N022(2OH)][C4CO2], and [N022(2OH)][C5CO2])289 |
| hexahistidine-tagged recombinant proteins | IL-LLE | [C4C1im][NTf2]267,268 |
| Horseradish peroxidase | IL-ABS and IL-LLE | [C4C1im]Cl258 and [P4444][Tf-Leu]265 |
| immunoglobulin Y | IL-ABS | ([N111(2OH)][HEPES], [N111(2OH)][MES], [N111(2OH)][TES], and [N111(2OH)][Tricine])246 |
| lactoferrin | IL-TPP | ([C4C1im][N(CN)2], [C4C1im][CF3SO3], [C4C1im][C1CO2]),273 and [C4C1im][BF4]272 |
| lipase from Bacillus sp. | IL-ABS | ([N111(2OH)]Cl, [N111(2OH)][Bit], [N111(2OH)][DHCit]),254 ([C2C1im]Cl*, [C4C1im]Cl*, [C6C1im]Cl*, [C8C1im]Cl*),113 ([C4C1im][N(CN)2] [C4C1pyr]Cl, [C4C1im]Cl, and [C8C1im]Cl)112 |
| lipase from Burkholderia cepacia | IL-ABS | [N4444][MOPSO], [P4444][MOPSO], [N4444][BES], [P4444][BES], [N4444][TAPSO], and [P4444][TAPSO]256 |
| lysozyme | IL-ABS, IL-LLE, and IL-SLE | [N221(O)nOH]Cl (ammoeng 110)224 [C2C1im]Cl,290 [C4C1im]Cl,232,264,290 [C8C1im]Cl,290 [C4C1im]Br,232,233 [C6C1im]Br,232 [C8C1im]Br,229,232,235 ([C1C1C1C1guan][C0CO2], [C1C1C1C1guan][Lac])235 ([N111(2OH)][C1CO2], [N111(2OH)][C2CO2], [N111(2OH)][C3CO2], [N111(2OH)][Glyc], [N111(2OH)][Lac], [N111(2OH)]2[Oxa], [N111(2OH)]3[Cit]),240 [P4444][Tf-Leu],265 [C1im]Cl,286 ([aC4im]Cl, and [VC8im]Br)288 |
| myoglobin | IL-ABS | [N221(O)nOH]Cl (ammoeng 110),224 [C4 C1im]Br,233 ([C2C1im]Cl, [C4C1im]Cl, and [C8C1im]Cl)290 |
| ovalbumine | IL-ABS and IL-IL-SLE | [C1C1C1C1guan][C0CO2], [C1C1C1C1guan][C2CO2]),236 [C1C1C1C1guan][Acr], [C1C1C1C1guan][MAcr], [C1C1C1C1guan][C1CO2], [C1C1C1C1guan][Lac], [C1C1C1C1guan][Mal], [C1C1C1C1guan][Ita], [C1C1C1C1guan][Sor], [C1C1C1C1guan][Cinn]),236 ([(C2H5O3)3SiC3C1im]Cl, [N11[3Si(2O)(2O)(2O)](2OH)]Cl, [(C2H5O)3SiC3C2mor]Cl, [(C2H5O)3SiC3C1C1C1C1guan]Cl, [(C2H5O3)3SiC3C1pyrrolium]Cl),289 ([N011(2OH)][C2CO2], [N011(2OH)][C3CO2], [N011(2OH)][C4CO2], [N011(2OH)][C5CO2], [N022(2OH)][C2CO2], [N022(2OH)][C3CO2], [N022(2OH)][C4CO2], and [N022(2OH)][C5CO2])237 |
| papain | IL-ABS | (([N111(2OH)][C1CO2], [N111(2OH)][C2CO2], [N111(2OH)][C3CO2], [N111(2OH)][OHC1CO2], [N111(2OH)][Lac], [N111(2OH)]2[Oxa], [N111(2OH)]3[Cit]),240 ([C4C1im]Cl, and [C4C1im]Br)257 |
| pepsin | IL-ABS | [N221(O)nOH]Cl (ammoeng 110)224 |
| phycobiliproteins | IL-SLE | [C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, [C8C1im]Cl, [C10C1im]Cl, [C12C1im]Cl, [C2C1im][C1CO2], [C4C1im][N(CN)2], [C4C1im][CF3SO3], [C4C1im][C1CO2], [C4C1im][(C1)2PO4], [C4C1im][Tos], [C4C1im][C1SO3], [C4C1im][SCN], [C4C1im][CF3CO2], [C2im][C1CO2], [C4C1pip]Cl, [C4C1pyrr][C1CO2], [C4C1pyrr]Cl, [C4C1pyr]Cl, [N111(2OH)][C1CO2], [N111(2OH)]Cl, [N4444]Cl, and [P4444]Cl285 |
| rubisco | IL-ABS | [N221(O)nOH]Cl (ammoeng 110)247 |
| superoxide dismutase | IL-ABS | [C2C1im][C1CO2]259 |
| Thermomyces lanuginosus lipase | IL-ABS | ([C2C1im]Cl, [C3C1im]Cl,[C4C1im]Cl, [C5C1im]Cl, [C2C1im][C2SO4], [C2C1im][C2SO3], [C2C1im][C4SO3], and [C2C1im][C1CO2])251 |
| tranferrin | IL-ABS | [C4C1im]Cl222 |
| trypsin | IL-ABS | [N221(O)nOH]Cl (ammoeng 110)224 ([C4C1im]Br, [C6C1im]Br),225 [C8C1im]Br,225,235 ([C1C1C1C1guan][C0CO2], [C1C1C1C1guan][Lac]),235 and [N111(2OH)][Gly]243 |
| wheat-esterase | IL-ABS | [C4C1im][BF4]260 |
| wool keratin proteins | [C4C1im]Cl284 | |
| yeast proteins | IL-SLE | ([N011(3N)][C0CO2], [N011(2N)][C0CO2], [N0022][C0CO2], [N0222][C0CO2], [N0333][C0CO2], [N0444][C0CO2], [N02(i3)(i3)][ C0CO2], [N011(3N)][CF3CO2], [N011(2N)][CF3CO2], [N0022][CF3CO2], [N0222][CF3CO2], [N0333][CF3CO2], [N0444][CF3CO2], [N02(i3)(i3)][CF3CO2], [N011(3N)][C1CO2], [N011(2N)][C1CO2], [N0022][C1CO2], [N0222][C1CO2], [N0333][C1CO2], [N0444][C1CO2], and [N02(i3)(i3)][C1CO2])280 |
| Y-globulins | IL-ABS | ([C4C1im]Br, [C6C1im]Br, and [C8C1im]Br)225 |
5.1. IL-Based Aqueous Biphasic Systems
Due to the labile nature of proteins, they are easily denaturated by organic solvents, which results in a loss of their biological activity. To overcome these drawbacks, ABS-based extraction processes have been recognized as efficient and more biocompatible extraction alternatives.7 IL-based ABS, being mainly composed of water, offer many relevant advantages when dealing with proteins, such as short equilibration time, mild operating conditions, and a biocompatible environment.7 The pioneering work on the use of IL-based ABS ([C4C1im]Cl + K2HPO4) for the extraction of proteins was reported by Du et al.222 The determination of protein levels in human urine might be used as a biomarker of urological pathologies and for which the authors222 proposed the use of IL-based ABS as alternative strategies for their extraction from real samples. Upon phase separation, the proteins were mainly concentrated in the IL-rich phase, while most contaminant proteins remained in the salt-rich layer. By increasing the amount of inorganic salt, the authors222 additionally observed an increase in the extraction efficiency (90% to 100%) of BSA [structural homologue of human serum albumin (HSA)] to the IL-rich phase, as well as a maximum enrichment factor of 20 (attained by a second phase separation).222 By spectroscopic techniques, the authors222 finally demonstrated that the studied proteins maintain their structural integrity and biological properties when extracted into the IL-rich phase.
The purification of cytochrome C, myoglobin, ovalbumin, and hemoglobin was explored and further compared with traditional PEG-based systems by Ruiz-Angel et al.223 The results obtained revealed larger partition coefficients in IL-ABS (2–3 orders of magnitude), reinforcing the usefulness of these systems for protein extraction and purification. Because proteins can easily lose their native structure by slight changes in the surrounding environment, properties such as pH, temperature, IL, and salt concentration need to be carefully controlled. Taking into account this feature, Du et al.222 studied the extraction of proteins by IL-based ABS and showed that at temperatures below 60 °C the extraction efficiency does not decrease, remaining close to 90%. The partition of proteins with IL-based ABS is however governed by complex phenomena, as concluded by three additional works published in 2009224,225 and 2011,226 which were mainly focused on addressing the protein partitioning driving forces in IL-based ABS. The influence of pH, ILs concentration, temperature, protein size, conformation, and surface structure were some of the variables studied. To this end, a large number of proteins [pepsin, hemoglobin (Hb), lysozyme (lyz), myoglobin (myo), bovine serum albumin (BSA), trypsin (try), cytochrome C (cyt-c) and y-globulins] were investigated. Despite their different molecular weights and isoelectric point (pI) values, all proteins partitioned preferentially into the IL-rich phase (with extraction efficiencies ranging between 60 and 100%).224−226 The first two works224,225 stated that hydrophobic interactions are the driving force in the partition of proteins in IL-based ABS. The work by Shu et al.227 further demonstrated that the extraction of BSA into ILs is an entropically driven phenomenon resulting from predominant hydrophobic and electrostatic interactions. Similar conclusions were provided by Yan et al.228 and Lin et al.229 When dealing with proteins or enzymes, the pH is one of the most important parameters to take into account. In fact, some studies showed that the closer the pH of the system is to the pI of each protein, the more significant the hydrophobic interactions are and the easier it is to manipulate the migration of proteins between the phases. It is important to note that all of these works rely on imidazolium-based ILs; it thus seems that the electron-rich aromatic π system on the cationic moiety of these ILs may also allow strong interactions with proteins and govern the partitioning trend. However, studies at variable pH values also showed that the extraction efficiency of proteins (cyt-c) decreases as the pH increases, indicating that electrostatic interactions can influence the partitioning.226 In addition to the pH, the amount of water molecules in each phase was also suggested to influence the partitioning of proteins.224 The water content of the IL-phase was also suggested as a key driver of proteins’ extraction with liquid−liquid extraction using hydrophobic ILs, as further stressed.230 These outcomes indicate the difficulties of predicting the partitioning trend of proteins in IL-based ABS.
In addition to the well-studied imidazolium-based ILs, the BSA, myo, try, and lyz partition behavior were further studied with ABS formed by Ammoeng ILs and K2HPO4/KH2PO4.224 These ILs are acyclic ammonium salts with an amphiphilic structure, comprising both hydrophobic (long alkyl side chain) and hydrophilic (hydroxyl) groups. The authors224 demonstrated that in the investigated systems hydrophobic interactions do not control the partitioning; instead, electrostatic interactions were proven to play the primary role as demonstrated by the good correlation found between the charge of the proteins and their partition coefficient.224,225 The use of similar ammonium-based ILs for the extraction of two distinct alcohol dehydrogenases was reported.231 Besides the good extraction efficiencies (90%), a concomitant increase in the specific activity of the two enzymes was observed.231 All these results highlight that the IL used (cation and anion chemical structures) has a strong influence on the proteins partition and stability. Summing up, hydrophobic and electrostatic interactions, as well as salting-out effects, were all suggested to be dominant factors in the partition of proteins in IL-ABS. However, their incidence depends on the type of protein and phase-forming components used.
On the basis of the gathered knowledge, later works have appeared on the use of IL + salt ABS for the extraction of proteins by addressing other parameters, such as tie-line length (TLL) or phase-forming components composition, temperature, and presence of salt additives. Pei et al.232 explored the potential of IL-based ABS to recover proteins from wastewater, using BSA, lysozyme, and hemoglobin as models. Again, hydrophobic interactions were identified as the driving forces, since the complete extraction into the IL-rich phase was achieved when the pH of the systems was close to the proteins pI.232 An increase of the TLL resulted in an improved partition coefficient for lyz but a decrease for BSA and Hb, the most hydrophilic proteins investigated. The authors232 also observed that higher temperatures favor the protein extraction, thus confirming the endothermic nature of the partition process. However, in a previously highlighted work regarding the extraction of BSA in [C10C1im]Cl-based ABS,228 it was shown that higher temperatures led to a decrease in the extraction efficiency into the IL-rich phase (exothermic process). Such contradictory results reveal a crucial need to better understand the dominant interactions occurring in protein-IL systems.
An additional study on the partitioning of several model proteins with different charges (lyz, myo, and BSA) was performed in IL + salt ABS, this time using a dye (Reactive Red-120) as a ligand, in order to allow affinity-induced partitions.233 All of the observations gathered in this work are in agreement with those mentioned above, with the nature of the proteins, the pH of the aqueous phase, the temperature, and the composition of the ABS influencing the partition coefficients. Albeit investigated only to a limited extent, in this work, a novel and interesting step for the recovery of the hydrophilic IL using the hydrophobic [C4C1im][PF6] as extractant was proposed.233 Lin et al.229 identified the importance of the formation of IL-protein complexes on the extraction performance of IL-based ABS, a trend first proposed by Pei and coauthors.234 By dynamic light scattering (DLS) and transmission electron microscopy (TEM), the presence of spherical IL-protein clusters was confirmed.229 This aggregation phenomenon was also evaluated by other authors,235,236 using ABS formed by functionalized guanidinium ILs toward BSA, lyz, try, and ovalbumin (ova).
The extraction of model proteins (BSA, ova, and Hb) was further studied using a series of hydroxyl-ammonium ILs.237 Under optimum conditions, N,N-dimethylethanolamine propionate ([N011(2OH)][C2CO2]) allowed extraction efficiency up to 99.50%.237 However, Bisht et al.,238 when analyzing the stability of lysozyme in ammonium-based ILs, found that its thermal stability gradually decreased with increasing concentration of ILs. In a search for alternative and more biocompatible ILs, Wu et al.239 used a new class of biocompatible amino-acid-based ILs combined with K2HPO4 to separate cyt-c. A preferential partitioning of cyt-c toward the IL-rich phase was found, with partition coefficients ranging from 2.83 to 20.7. Hydrophobic interactions were again considered as the main driving force dictating the partitioning behavior of the target protein.239 The extraction of cyt-c was further investigated by Santos et al.,34 using a quaternary system formed by PEG 8000, sodium poly(acrylate) (NaPA8000), and water with ILs as electrolytes. After characterizing the systems in terms of pH and the IL distribution between the phases, the authors34 demonstrated that cyt-c is enriched in the NaPA8000-rich phase with an extraction efficiency higher than 96.13%.
In order to maintain the pH of the systems and to avoid the use of additional buffer solutions, the use of alternative ILs with buffering characteristics can be seen as a feasible approach. Such ILs, named Good’s buffer ILs (GB-IL), were first developed in 2014110 and comprise anions derived from biological buffers (Good’s buffers, GBs). The authors110 demonstrated the self-buffering characteristics of these new ILs and their ability to form ABS with salts and used them in the extraction of BSA. Remarkably, an extraction efficiency of 100% in a single step for the GB-IL-rich phase was obtained in most systems. The authors110 also performed a series of studies to ascertain the impact of GB-ILs on the stability of proteins. In general, it was found that these GB-ILs display a higher stabilizing effect over the studied protein when compared to conventional ILs, being also reported as less toxic.110
Aiming at finding more biocompatible purification processes, [N111(2OH)]Cl and several other derivatives were considered in the design of ABS to extract and purify several proteins (BSA, try, papain, and lyz).240,241 When combining [N11(2OH)(3OH)]Cl with KH2PO4, BSA was extracted in a larger extent toward the IL-rich phase (extraction yield of 84.32%) than when applying imidazolium-like ILs (extraction yields of 59.74–68.03%).241 Along the same line of research, ILs formed by cholinium and anions derived from carboxylic acids ([N111(2OH)][C1CO2], [N111(2OH)][C2CO2], [N111(2OH)][C3CO2], [N111(2OH)][Glyc], [N111(2OH)][Lac], [N111(2OH)]2[Oxa], or [N111(2OH)]3[Cit]) were combined with polypropylene glycol (PPG400) (a nontoxic, biodegradable and thermosensitive polymer) to form ABS.240 Similar to what was observed in IL + salt ABS,241 as the protein size increases, the excluded-volume effect also increases, leading to a decrease of the extraction efficiency.240 Additionally, the partition coefficients of lyz decrease with increasing amounts of phase-forming components of ABS (which inherently represents a decrease of the water content). The authors240 claimed that a more structured IL-rich phase, resulting from adding IL, requires the energy needed to destroy the IL-water network, thus hindering the protein migration to the IL-rich phase. Also, and unlike to that observed in IL + salt systems, changes in pH values do not interfere with the protein partition trend in a pH range between 5 and 11. ABS formed by cholinium-based ILs + PPG400 not only allowed an efficient extraction performance (86.4–100%) but also worked as good stabilizers and promoters of the trypsin activity (except with [N111(2OH)][C1CO2]). Cholinium-based ILs and PPG400 were further used in three other works to extract BSA.221,242,243 The preferential partition of BSA to the IL-rich phase was always observed, with two of these works reporting the complete extraction of BSA into the IL phase and without compromising the protein’s native conformation.221,242 In both works, the partition of BSA seems not to be dictated by the relative hydrophobicity of the phases or salting-out effects, as in most IL + salt systems, but by specific interactions, such as hydrogen bonding and dispersive interactions occurring between the protein and the IL ions. Curiously, in both works, cholinium chloride was considered the worst salt, since a significant amount of precipitated protein was observed.221,242 The BSA partition behavior in ABS formed by ILs composed of cholinium as cation and amino-acid-derived anions was later studied by Song et al.243 BSA showed a higher affinity to the IL-rich phase formed by less hydrophobic anions. Moreover, by using amino acids as anions, the authors243 were able to manipulate the interactions between the phase-forming components and the proteins by changing their surface charge through pH variations. When the pH of the system was higher than the pI of the proteins and the amino acid anions, proteins mainly partition to the IL phase. On the other hand, at lower pH values, the partitioning toward the polymer-rich phase is favored.243
Protic ILs represent another way of moving toward the implementation of more benign and low-cost ILs. Compared to the majority of their aprotic counterparts, protic ILs have simpler synthetic routes, lower cost, and are more environmentally friendly.244 Within a separation and purification framework, protic ILs exhibit a remarkable advantage when combined with PPG400 to form ABS (i.e., they display a large dependence on temperature and thermoreversible behavior).245 Small temperature changes at operationally convenient values promote reversible phase transitions, as opposed to aprotic IL/salt or aprotic IL/polymer ABS. The complete extraction of cyt-c and azocasein was accomplished in a single step using these systems and maintained along three cooling–heating cycles, with no stability losses.245
Most studies reported and discussed up to this point were carried out with pure proteins and only the extraction efficiency of IL-based ABS was addressed. However, value-added proteins with biological, clinical, pharmaceutical, and industrial relevance are usually present in complex media. Therefore, further investigations envisaging the purification of proteins from complex sources using IL-based ABS are strongly recommended, while simultaneously evaluating the extraction efficiency, recovery yield, and purification factor. Recently, Taha et al.246 prepared novel ABS formed by cholinium-GB-based ILs and PPG400 for the extraction of immunoglobulin Y (IgY) from chicken egg yolk. Although being considered a biopharmaceutical, IgY is a glycoprotein and is thus discussed in this section. The combination of GB-ILs with PPG400 to form ABS allowed the preferential partition of IgY to the IL-rich phase.246 Despite the good extraction efficiencies obtained (ranging between 79 and 94%), the greatest challenge remains on the complete separation of IgY from the major contaminant proteins.246 Taken together, the remarkable results achieved with ABS formed by cholinium-based ILs and polymers reveal that these systems can be considered as alternatives to traditional extraction methods, mostly due to the flexibility displayed in directing the target protein to a desired phase through the proper selection of the ILs based on their hydrophobicity. IL-polymer-based ABS seems thus more amenable to be tuned when compared with IL-salt-systems, where the salting-out effect exerted by the salt plays the primary role and leads to the migration of all proteins present in the medium to the IL-rich phase. Still dealing with more complex matrices, Desai and co-workers247 perfomed the purification of rubisco from plant extracts using ABS formed by ammonium-based ILs (Ammoeng 110) and sodium–potassium phosphate. The authors247 showed that rubisco migrates to the IL-rich phase with partition coefficients 3–4 times higher than those obtained with PEG-based systems. However, at high concentrations of ILs (>50% w/w), the studied proteins start to aggregate. Such results suggest that there is a limited range of IL concentrations that can be used in protein extraction.247
Pei and co-workers234 introduced, for the first time, the application of IL + salt ABS ([C4C1im][N(CN)2] + K2HPO4) as a selective fractionation strategy to separate proteins from polyssacharides, under the biorefinery concept. In their work, BSA was successively separated (82.7–100.7%) into the IL-rich phase, with saccharides being preferentially concentrated into the opposite phase. The formation of IL aggregate-protein complexes was for the first time suggested as the driving force in the selective separation of proteins.234 Later, other studies followed involving attempts to fractionate sugars and proteins. Chen and coauthors237 used ABS formed by ether-functionalized ILs and demonstrated that 76.1–94.3% of BSA migrates to the IL-rich phase, while all sugars are extracted into the salt-rich phase in an one-step separation process.237 Tan et al.248 applied [C4C1im][BF4] + NaH2PO4 ABS to simultaneously separate crude polysaccharides (APS) and proteins from Aloe leaves. After establishing the optimal conditions, 93.12% of APS were extracted into the salt-rich phase, whereas 95.85% of the proteins were extracted into the IL-rich phase.248 A similar work was reported later regarding Cordyceps sinensis polysaccharides (CSPS).249 The ABS investigated led to 89.4% of CSPS extracted into the salt-rich phase and 88.2% of proteins extracted into the IL-rich phase.249 Further attempts were carried out by the authors to purify the extracted polysaccharides (APS or CSPS) by dialysis248 or by combining dialysis with ethanol precipitation.249 Finally, the IL-rich phases were reused in order to decrease the costs and the environmental impact associated with the current extraction procedures.248,249
In order to decrease the environmental and economic impact of IL-based ABS processes, some (still scarce) studies attempted to recover the ABS phase-forming components after the extraction of proteins. In addition to the previously described works,248,249 Freire and co-workers250 studied the recyclability and reusability of phosphonium- and ammonium-based ILs after the extraction of BSA into the IL-rich phase, where the protein was recovered by dialysis. The extraction efficiencies were maintained at 100% in three-step sequential extractions comprising both the BSA recovery and the IL reusability. Li et al.240 used the PPG400 thermosensitive polymer in the formulation of ABS, which allows simple recovery of the polymer by a temperature increase. These more integrated processes are depicted in Figure 18 (panels A and B), as a way of highlighting the possibility of designing integrated strategies for the use of IL-based ABS for the extraction and separation of proteins.
Figure 18.
Schematic representation of an integrated process for the recovery of proteins, comprising the production and separation/purification steps, as well as the recycling of the phase-forming components of IL-based ABS. (A) Process highlighting the recovery of the target protein by dialysis.250 (B) Process including a thermoseparating polymer that facilitates the recycling and reuse of the ABS phase forming agents.240
Lipases are a class of enzymes with relevant industrial use, in sectors ranging from the petrochemical, pharmaceutical, food, paper, and waste management industries. IL-based ABS were also investigated to extract and purify lipases,112,113,251−254 recently reviewed by Ventura et al.255 The first study was reported by Deive et al.251 who explored the partition behavior of Thermomyces lanuginosus lipase (TlL) in IL-based ABS. The authors251 started by selecting the appropriate IL, concluding that the enzyme native conformation and the lipolytic activity are preserved when exposed to an aqueous solution comprising a short alkyl chain cation IL, [C2C1im][C2SO4]. Under the optimized conditions, it was possible to recover 99% of TlL from aqueous solutions, while preserving its biocatalytic activity.251 Moved by this finding, the same research group252 proceeded to the optimization of the Candida antarctica lipase A (CaLA) extraction from aqueous solutions, using ABS formed by different combinations of ILs ([C2C1im]+ cation paired with [CnSOm]− anions (n = 2, 4, and 6 and m = 3 and 4)) and inorganic salts. The results obtained revealed that [C2C1im][C4SO4] was the best candidate investigated, with extraction efficiencies higher than 99% obtained in a single step. From the ABS tested in both studies,251,252 the combination of [C2C1im][C4SO4] and (NH4)2SO4 for ABS creation was found to be the most successful system for lipase extraction. Further studies on lipase partitioning were conducted by Ventura et al.,253 this time with Candida antarctica lipase B (CaLB). Various ILs comprising pyridinium, pyrrolidinium, piperidinium, and imidazolium cations, the latest conjugated with different anions, were used.253 The enzyme recovery efficiencies obtained for all systems were above 97%, being the highest purification factor (PF) obtained with [C8C1im]Cl (2.6). It should be however highlighted that a commercially pure enzyme was used, which justifies the low PF obtained in this study.253 Given the good results obtained, the same group of researchers112 moved to the use of IL-ABS as downstream processes to purify an extracellular lipolytic enzyme produced by Bacillus sp. ITP-001 from the fermentation broth. In this work, even better results were obtained, namely with both high purification factors and enzyme recovery efficiencies toward the salt-rich phase. The best results were obtained with ABS formed by [C8C1im]Cl (recovery =92.2% and PF = 51). The authors112 showed that the optimized IL-ABS are more efficient than conventional polymer-based ABS and proposed a way to isolate the lipolytic enzyme and to recycle the solvents. Seeking more efficient methods for lipase extraction, the same research group254 further studied ABS formed by cholinium-based ILs and tetrahydrofuran (THF) for the purification of lipase from Bacillus sp. ITP-001. A significant increase of the PF, from 12.7 to 136.8, was observed when compared with the traditional prepurification technique.254 Finally, the same authors113 applied ABS formed by polymers and salts, while using ILs as adjuvants, in the purification of CaLB from real systems (fermentation broth). The most relevant result of this work was the increase of the PF with the application of ILs as adjuvants (PF = 245.0), when compared with IL-based ABS (PF ≈ 51–137)253,254 and with polymer-based ABS (PF = 201.5).112 A synergetic effect arising from the IL and the polymer was mentioned by the authors as the main reason behind this increase in the PF.113 This last type of ABS also allows a decrease of the concentration of the IL employed and thus renders the system more biocompatible and inexpensive.113 Thus, and as discussed before with other classes of biocompounds, the strategy of using ILs as adjuvants in ABS, instead of using them as main phase-forming components, emerges as an efficient alternative for protein purification and deserves future attention. Still dealing with lipases, a recent study has demonstrated the potential of using GB-based ILs for the purification of lipase from Burkholderia cepacia.256 It was found that the enzymatic activity of the enzyme was 1.7–3.0-fold higher in IL-rich phases than in a phosphate buffer and a selectivity of 3.57 was achieved when considering its purification from the fermentation broth.256
In addition to lipases, other enzymes have been successfully extracted using IL-based ABS. Papain, for example, is, along with lipase, one of the most widely used industrial enzymes, being highly relevant to the cosmetic, food, textile, and pharmaceutical industries. Despite the large number of polymer-based ABS reported for papain extraction, only one work considered the use of ILs.257 In this work, the papain partitioning was studied in ABS formed by [C4C1im]Cl or [C4C1im]Br + K2HPO4.257 The increase of [C4C1im]Br and K2HPO4 concentrations, in a pH close to the protein pI, allowed a maximum extraction of 98.33%, while allowing the stability of the protein.257 A similar IL-based ABS has also been applied to the extraction of horseradish peroxidase (HRP).258 This system allowed the recovery of 80% of HRP into the IL-rich phase and the preservation of more than 90% of the enzyme activity.258
Superoxide dismutases (SOD) are antioxidant defense systems against superoxide radicals and have major applications in the medical and pharmaceutical fields. Despite the extensive amount of SOD purification protocols available (dialysis, ultrafiltration, acetone precipitation, extraction with organic solvents, and chromatographic techniques), all of them are laborious, time- and energy-consuming, and result in low recovery yields. To overcome these drawbacks, IL-based ABS are considered as an alternative path for a faster and more effective purification method. A variety of ABS (polymer–polymer, polymer-salt, alcohol-salt, and IL-salt) were studied for the SOD recovery by Simental-Martinez et al.259 These authors studied the SOD partition behavior in ABS using a standard solution and also a complex extract obtained from the yeast Kluyveromyces marxianus. Their results indicated that the enzyme always partititioned into the IL-rich phase.259 Satisfactory purification results were achieved with an activity recovery yield of 91%, with 60% of the total protein being found in the same phase. Although IL-based ABS were among the two systems that allowed better enzyme recovery, the authors concluded that the PEG3350-potassium phosphate ABS was better in terms of SOD recovery, enzyme specific activity maintenance, and purification.259
Further applications of IL-based ABS in the purification of enzymes include the extraction of wheat esterase from wheat extracts.260 This enzyme can substitute the traditional acetylcholinesterase (AChE) extracted from animal blood or tissues. Jiang and coauthors260 evaluated the performance of an IL-based ABS for the extraction and purification of wheat esterase. It was demonstrated that the enzyme preferentially partitions into the IL-rich phase with an extraction yield of 88.93% and a PF of 4.23 under the optimum conditions,260 results further justified by the electrostatic interactions occurring between the amino acids present on the protein surface and the IL cation, as described elsewhere.224
The results attained with IL-based ABS to extract and purify proteins, along with the simple routes that these systems bring in terms of recycling and reusing of the phase-forming components, are per se encouraging aspects for large scale applications. Still, the creation of highly efficient interfaces that enable a shift from batch to continuous mode operations is missing. Having also a great processing capacity which spans from kilograms to tonnes, microfluidic devices represent a viable route for such a turning point.261 These miniaturized devices provide improved yields and purities within shorter times, provide an amazing operational flexibility related with the wide array of possible configurations, and offer additional economic and environmental benefits.261 Currently, the implementation of microfluidic devices within the field of ILs is mostly focused on applications wherein the ILs themselves are synthesized or serve as solvents in biocatalysis, while their use in extraction and purification processes remains largely neglected.261 Novak et al.262 delivered a major report addressing the intensification of BSA purification by IL-based ABS resorting to microfluidic devices. An ABS composed of [C4C1im][BF4] + D-fructose was investigated and further integrated within a parallel flow microfluidic device.262 After adjusting the fluid flow pattern, the BSA extraction within the microchannels was successfully carried out, yielding partition coefficients of 14.6, 20-fold higher than those achieved with conventional ABS composed of polymers and salts.262 Productivities of 17.4 Kgh–1 m–3 for this IL-aided microfluidic separation technique were predicted, far greater than those estimated for the conventional system (1.48 Kgh–1 m–3). Among the benefits that IL-based ABS display over conventional ones, the authors emphasized the lower viscosities of the phases as one of the most significant advantages within the microfluidic separation domain.262
From the works reviewed in this section, some conclusions arise. The number of systems using hydrophobic ILs is very low, as described in the next section, which is again explained by the lower variability of the chemical structures of the hydrophobic ILs. However, the set of hydrophilic ILs available for the preparation and application of IL-based ABS is much more diverse, thus allowing the tailoring of both the extraction and purification routes. Within IL-based ABS, two distinct types of works were found, the first (representing the majority of the published works) showing the application of IL-based ABS in the partition of model proteins and enzymes and the second in which ABS were used to extract and purify proteins from real and complex matrices. The use of model proteins and enzymes is not very helpful to draw conclusions about the applicability or success of ABS, since for these systems to be used as downstream processes with industrial application, the partition behavior of both the target proteins and remaining contaminants should be addressed. Even when looking to the purification capacity of IL-based ABS from real matrices, most authors focused on tailoring the IL chemical structure and mixture compositions for the target proteins/enzymes; however, sometimes, it is easier to optimize IL-based ABS toward removing the main contaminants from the complex and original medium. From the entire set of strategies discussed in this section, the use of ILs as adjuvants in typical ABS seems to be the most advantageous, not only because higher PFs were achieved but also due to the lower cost of these systems. The use of GB-ILs seems also to be very promising since there is no need to add extra buffer solutions, although many more optimization studies are required. Finally, not only more optimization investigations are mandatory for the application of IL-based ABS as downstream processes but also new strategies that can compensate the worst results in terms of purification, namely pretreatment steps and the use of sequential purification steps (by liquid–liquid chromatographic devices, for instance).
5.2. Liquid–Liquid Extractions with Hydrophobic ILs
As shown in the previous sections, hydrophobic IL-water biphasic systems have been broadly studied and applied for the extraction and purification of several compounds. However, only a limited number of work on protein extraction has been reported, mostly due to the fact that protein dissolution requires the presence of an “hydrated IL”. Research on protein extraction by LLE-related methods comprised the modification of proteins with amphiphilic polymers, the addition of crown ethers, and the formation of aqueous microemulsion droplets using hydrophobic ILs. In 2006, Shimojo et al.263 investigated the extraction of heme proteins using ILs from an aqueous phase through the addition of dicyclohexano-18-crown-6 (DCH18C6). The enhanced solubility of cyt-c in the hydrophobic IL phase was explained as a result of the coordination between DCH18C6 and the protein lysine residues. Interestingly, the cyt-c-DCH18C6 complex in the IL-phase provided remarkably high peroxidase activity when compared with native cyt-c.263 Along the same lines, Tzeng et al.264 modified an IL for the extraction of lysozyme. An imidazolium-based IL ([C4C1im]Cl) was merged with a dye [silver salt Cibacron Blue 3GA (CB)] (Figure 19 A), and this system was then applied to the extraction of lyz. After the addition of the IL to the medium, an increase of the partitioning of lyz into the IL phase from 4.4% to 81% was observed.264 Similarly to the works previously discussed in the section on IL-based ABS, this extraction process was pH-dependent, where an increase in the pH results in lower extraction efficiencies. At pH 4, the system reached its maximum extraction capacity (90%).264 According to the authors, the electrostatic interactions between the three negatively charged sulfonate groups on CB and the positive surface charge of lyz are responsible for the observed pH dependency. Although three other proteins were tested (cyt-c, ova, and BSA), the developed process only displays specificity for lyz.264 Finally, the authors264 addressed the recyclability of the ILs used and concluded that the IL phase could be reused for at least eight cycles without compromising the extraction performance.
Figure 19.
Schematic representation of the integrated process for the recovery of proteins, comprising their production, separation/purification, recovery, and recycling of the phase-forming components based on the use of hydrophobic ILs for LLE. (A) Process with the recovery of the target protein by back-extraction and recycling of the IL.264 (B) Process including a thermoseparating polymer that facilitates the recycling and reuse of the phase-forming agents.265
Kohno et al.265 performed the functionalization of ILs with leucine by synthesizing the IL [P4444][Tf-Leu] (trifluoromethanesulfonyl leucine anion). The prepared IL displays a lower critical solution temperature (LCST) behavior with water. With a water content of 21 wt %, cyt-c was fully extracted into the IL-rich phase. Moreover, the distribution ratio (D) of other proteins (lys, cyt C, chymotrypsin (Cht), laccase (Lac), Hb, horseradish peroxidase (HRP), and BSA) were assessed, and only Lac and HRP remained in the aqueous phase, not having migrated to the IL-hydrated phase. In a subsequent work,230 the same research team proposed the hydrophilicity index (HI) as an indicator of the phase behavior of IL/water mixtures. In this work, the authors230 exploited the LCST-type phase transition for extracting proteins from the aqueous to the IL phase. A scheme of the strategy outlined by the authors is provided in Figure 19B. Ito and coauthors266 also applied IL-based LLE to perform the cyt-c extraction using a phosphonium-type zwitterion to control the water content at the IL layer. By adding the N,N,N-tripentyl-4-sulfonyl-1-butanephosphonium-type zwitterion (P555C4S), the authors266 were able to increase the water content from 0.4 to 62.7% in the IL phase, while facilitating the dissolution of cyt-c, which reached a distribution value of 94%. In addition, the authors266 demonstrated that a decrease in the water content in the IL-phase could be induced by the addition of an inorganic salt. On the basis of this, it was possible to re-extract cyt-c from the IL phase to an aqueous phase by controlling the amount of added inorganic salt, without significant changes in the structure of the protein.266
Recently, two works regarding the extraction of hexahistidine-tagged (His-tagged) recombinant proteins by LLE using triazacyclononane-IL-sorbent were published.267,268 In both works, it was concluded that the selective partitioning of the target proteins between the IL and aqueous phases is governed by the proteins’ affinity to the IL, the presence and nature of coordinated metal ions, and by the ionic strength. The addition of triazacyclononane-IL-sorbent to water resulted in purity values of 90%268 and 95%.267 In both works,267,268 it was claimed that the sorbents used are easily regenerated in situ by the addition of EDTA followed by the reimmobilization of metal ions. It should be noted that all of the previously described works highlight the pivotal role of the IL water content in the IL phase for protein extraction, a crucial feature when dealing with hydrophobic ILs.
As previously mentioned, the separation and purification of specific proteins from real matrices are among the most complex procedures. In addition to the studies carried out with pure and model proteins discussed above, Cheng et al.269 reported the direct extraction of hemoglobin (Hb) by [C4(C1C1C1Si)im][PF6], without using any additional reagent or extractant. The authors269 suggested that the interaction/coordination occurring between the iron atom in the heme group of Hb and the cationic IL moiety is responsible for the Hb transfer into the IL phase. The system was then successfully used to extract Hb from human whole blood.269 The same hydrophobic IL was applied in the extraction of cyt-c from an aqueous solution.270 It was demonstrated that the protein concentration, pH, and extraction time affect the extent of cyt-c partitioning to the IL-rich layer (85% of extraction efficiency at pH 1).270 In both works,269,270 a back-extraction process to recover the protein and the IL was proposed by the addition of deionized water at pH 6.7 as a stripping reagent.
Envisaging intensification processes to support large-scale implementations of IL-based LLE, Huh et al.271 attempted the purification of bacteriorhodopsin from Halobacterium salinarium by an hydrophobic IL [C6C1im][PF6]-K2HPO4/KH2PO4 buffer system and a conventional ABS composed of a polymer and the same salt within a microfluidic device. During the optimization studies, the pH and flow-rate of the salt phase were manipulated in order to achieve high recovery yields. Although conventional ABS was more efficient at extracting bacteriorhodopsin (90.23% vs 84.32%), the IL-based LLE technique performed better in its purification due to the enhanced capacity of removing the main contaminants (lipids, proteins, and sugars) (1.16 vs 1.41 of purification fold).271 An integrated purification method for bacteriorhodopsin was finally outlined by the authors,271 coupling a dialysis step within the microfluidic channel and where the purification factor was increased up to 1.71.
As discussed previously, the number of hydrophobic ILs is much more limited than the set of hydrophilic structures that can be used in the design of ABS, which clearly imposes relevant limitations to these processes, not to mention the lower biodegradability of hydrophobic ILs, their higher cost, and their higher toxicity. Neverthless, one of the main advantages of applying hydrophobic ILs solutions is that their recycling is facilitated due to their lower solubility in water. Moreover, it was also shown that the use of thermosensitive polymers conjugated with the use of hydrophobic ILs can also facilitate the design and development of much more simple and efficient processes. However, all processes need to be carefully selected since all the reported works show that the efficiency of the purification processes largely depends on the characteristics of the proteins. However, the protein isolation is one of the simplest tasks when compared to the remaining biomolecules addressed in this review, since due to their larger size they can be easily separated by dialysis or by induced-precipitation.
5.3. IL-Based Three-Phase Partitioning
As discussed above in the separation of other value-added compounds, IL-based TPP combines the advantages of IL-based ABS with the advantages of TPP when envisaging the separation of proteins. With IL-based TPP, it is possible to neglect the back-extraction step for the recovery of proteins from the IL-rich phase since proteins concentrate and form an isolated “solid” layer. Despite these advantages, few studies resorting to the use of ILs in TPP processes for the separation of proteins have been reported.272−274Figure 20 schematizes IL-based TPP processes for the recovery of proteins.
Figure 20.
Schematic representation of the integrated process for the recovery of LF from whey based on an IL-based TPP strategy and direct recycling and reuse of the phases.275
The first study in this field used [C4C1im][BF4]/NaH2PO4 ABS-TPP for the recovery of lactoferrin (LF), whereby 74% to 99% of LF was recovered as an isolated and middle interphase.272 It was shown that the temperature, IL content, and salt concentration are important parameters to tailor the protein recovery yield.272 However, despite the good results obtained, [C4C1im][BF4] may suffer from hydrolysis in aqueous media, as discussed previously. This prompted the authors273 to perform a second study in the search for alternative ILs that may display similar lactoferrin recovery results, yet without compromising the IL stability. To this end, the authors investigated different hydrophilic ILs ([C4C1im][N(CN)2], [C4C1im][CF3SO3], and [C4C1im][C1CO2]).273 The [C4C1im][N(CN)2] was immediately discarded because it induced the migration of almost all LF to the IL-rich phase (97%), thus making the protein recovery process more complex. After several optimization steps, the authors273 concluded that the [C4C1im][CF3SO3]/NaH2PO4 system was the best candidate for LF recovery using IL-TPP, with the best results obtained at low pH values (98% of LF recovery). In this work, BSA was also investigated and an opposite behavior was observed, which allowed the authors273 to suggest the establishment of a selective recovery mechanism of proteins. Again, these results show that the performance of each technique or IL-based system largely depends on the characteristics of the individual proteins, which hampers the creation of heuristic rules to the a priori selection of improved systems and conditions. Overall, and based on these two works, IL-based TTP appear as promising alternatives for the recovery of proteins, as it induces satisfactory recovery values, while eliminating the need to perform a back-extraction step from the IL-rich phase to recover the target proteins.
In order to reduce the amount of IL required, the same group of authors274 finally evaluated the possibility of recycling the IL present in the IL-rich phase. Two alternatives were investigated: (i) the addition of higher amounts of inorganic salt to induce a stronger salting-out of the IL and (ii) the evaporation of water and concentration of the IL-rich phase under vacuum.274 The authors274 found that the use of vacuum enabled the reuse of all the IL and the salt required to create a new IL-based TPP process. This approach does not require additional reagent consumption and allows the recovery of the costly ILs employed, thus reinforcing the sustainability and viability of IL-TPP systems. However, one of the disadvantages that this technology displays when compared to IL-based ABS is the extensive experimental work previously required aimed at promoting the formation of the third phase containing the precipitated target protein. In fact, the number of IL-based systems promoting the formation of a third phase, in which the proteins can be concentrated although not degraded, is limited, which imposes additional limits on the industrial interest in this approach.
5.4. Surfactant-Based Systems Containing ILs
Within surfactant-based systems for the separation and recovery of proteins, two major strategies were found in the open literature: (i) the use of microemulsions276,277 and (ii) the use of aqueous micellar biphasic systems (AMBS).278,279 In microemulsion systems, the formation of an aqueous domain in the IL continuous phase enables protein dissolution, as demonstrated by Shu et al.,276 who proposed a bis-2-ethylhexyl-sulfosuccinate (AOT)/water/[C4C1im][PF6] microemulsion for the selective isolation of hemoglobin from biological sample matrices; the overall process is depicted in Figure 21 A. An extraction efficiency of ca. 96% was achieved for a 100 ng L–1 Hb solution by using an equal volume of the microemulsion.276 This remarkable result was explained by the interaction/coordination occurring between the iron atoms in Hb and the cationic imidazolium ring in the IL. Furthermore, a back-extraction efficiency of 73% was achieved, applying an aqueous urea solution as a stripping agent.276 Similarly, Mao et al.277 isolated hemoglobin from human blood using novel IL-based microemulsion systems, where the organic surfactants/cosurfactants normally involved were substituted by ILs. The IL [C10C1im]Br was used as the surfactant and the IL [C4C1im][PF6] as substitute for the organic solvent.277 The dual-IL microemulsion system proved to be more effective for the extraction of proteins in comparison with the isolated [C4C1im][PF6] system. Lower pH values were found to be favorable for the selective extraction of Hb, as a result of the coordination interaction between the heme group of Hb and the imidazolium cationic moiety,277 and in agreement with the results of Shu et al.276 By adding an alkaline Britton–Robinson buffer at pH 12, the authors also performed the back-extraction of the protein with a recovery yield of 55.6%.277 In summary, and although only briefly investigated, both works273,274 demonstrated the potential applicability of IL-based microemulsions for the isolation of Hb from human whole blood samples, while opening the path to explore this type of system for the purification of other proteins from real and complex matrices.
Figure 21.
Schematic representation of the recovery of proteins from complex mixtures using surfactant-based systems: (A) microemulsion system comprising the pretreatment of the human samples, separation/purification of the target protein, and a back-extraction step276 and (B) IL-based AMBS.278,279
Aqueous micellar biphasic systems (AMBS) are surfactant-based ABS. The incorporation of ILs as cosurfactants in such systems was first proposed by Vicente et al.,278 where three families of ILs, viz. imidazolium-, quaternary phosphonium-, and ammonium-based ILs, were combined with the nonionic surfactant Triton X-114 to form AMBS for the selective extraction of cyt-c and the dye Rhodamine 6G (R6G). A representative scheme of the selective separation approach is given in Figure 21B. The results obtained demonstrate that the presence of ILs as cosurfactants not only enhance the partition coefficients of cyt-c but also lead to an improved selectivity. The opposite migrations (cyt-c preferentially migrates to the nonmicellar phase and R6G to the micellar phase) were explained by a balance between hydrophobic and electrostatic interactions, as well as by excluded-volume effects.278 Given the obtained results with the tested AMBS, the authors progressed toward real applications (i.e., to the valorization of pineapple stem by bromelain recovery).279 The authors were able to selectively extract bromelain from the contaminant proteins, while a stabilizing effect over the target enzyme was also noticed in the presence of the AMBS containing [P66614][C9CO2].279 Albeit not extensively addressed, the use of ILs as cosurfactants in AMBS seems to be very promising, since outstanding purification results have been described. Furthermore, the use of IL-based AMBS allows a decrease both in the environmental impact and cost of the process (low amounts of phase-forming components when compared to IL-based ABS). However, more studies in this field are required and need to be taken into account to better understand the real viability of this purification platform. The formed micelles/aggregates need to be comprehensively characterized for the identification of rules that could correlate the purification and recovery yields of proteins with the size, form, and type of the micelles. Then, the development of strategies to isolate the proteins after the purification step and the recycling of the surfactants and ILs still need to be addressed. On the other hand, AMBS formed only by ILs have not yet been found and could be interesting in future studies to take main advantages of their “designer solvent” characteristic.
5.5. IL-Based Solid–Liquid Extractions
Regarding the application of ILs and IL solutions to extract and recover target proteins from solid matrices and/or suspensions, Ge et al.280 proposed a method for direct protein extraction from yeast cells using ILs. After testing several ILs, it was shown that the extraction efficiency is inversely related to the hydrophobicity of the cations, which, according to the authors,280 is probably due to the established hydrophobic interactions between the proteins and the IL that has a suppressive effect on the protein solubility. On the other hand, for the anions effect, the extraction efficiency generally increases with their hydrogen-bond-accepting ability. The IL [N011(3N)][C0CO2] was selected as the best performing candidate.280 The alkaline pH provided by an [N011(3N)][C0CO2] IL solution (∼9.0) was also highlighted as contributing to the cell wall breakage by a weakening and/or disruption of inter- and intramolecular hydrogen bonds in the polysaccharide chains.281 When compared to conventional salts, the protic IL used allowed its removal from the protein solution by the application of vacuum at room temperature.280 With high-resolution techniques of proteome analysis, the authors280 finally confirmed that the protein chemical structure as well as the immunoreactivity are maintained. IL solutions were also used to extract intracellular proteins (and subsequently to break cell walls) from the microalgae Chlorella pyrenoidosa.282 However, this time, the extraction efficiency was lower (12%) than that obtained with traditional processes (∼16–23%).282
Inspired by the fact that [C4C1im]Cl was reported as an excellent solvent for wool,283 Plowman et al.284 investigated the extraction of wool proteins using the same IL. When wool was incubated with the IL at high temperatures, the partial breakdown of the fibers into smaller fragments that included cells, subcellular structures, and individual macro fibrils was observed. In general, it was found that the IL aids in the extraction of cytokeratins. The wool treated with the IL was also treated with urea/thiourea, and it was possible to identify more proteins than when using each extraction method individually.284 On the basis of the results obtained, the authors284 suggested that in order to study the complete wool proteome, a combination of both methods (ILs and the traditional urea/thiourea process) should be used.
Ventura and co-workers285 attempted the extraction of phycobiliproteins from the red macroalgae Gracilaria sp. This phycobiliprotein-rich macroalgae has a large water content, which limits the economic viability of the current extraction methods (e.g., SLE with sodium phosphate buffer). After optimizing the extraction time and solid–liquid ratio using the conventional method, the aptitude of several ILs to extract the proteins was disclosed. The structural features of the ILs were optimized, demonstrating that more hydrophilic ILs better extract phicobiliproteins, while those of lower hydrophilicity are better extraction solvents for chlorophylls and carotenoids.285
In summary, only four works have thus far been published concerning the use of ILs for the extraction of proteins from raw solid materials. Despite the lack of results on the reuse of these IL-based aqueous solutions, and the potential of these systems to be applied at an industrial level (e.g., through life cycle assessment studies), in all cases, promising results were obtained when compared with traditional processes and solvents, thus opening the door for more studies in this field.
5.6. Solid-Phase Extractions Using IL-Modified Materials
As mentioned before with other solutes, SPE has been widely applied as preconcentration and purification techniques and where ILs were recently introduced as modifiers of the chemical and physical characteristics of adsorbent materials.23 In the field of proteins, Shu and co-workers286 developed a new method for immobilizing [C1im]+ moieties onto polyvinyl chloride (PVC) chains, forming [C1im]Cl–PVC hybrids. The characterization of [C1im]Cl–PVC hybrids revealed that the immobilization of the IL strongly depends on the variation of the [C1im]/PVC molar ratio. With a maximum immobilization ratio of 15.1% (obtained with a 4:1 molar ratio of [C1im]/PVC), it was possible to adsorb Lys, cyt-c, and Hb with efficiencies of 97%, 98%, and 94%, respectively, while the retention of acidic protein species [BSA, transferring (Trf) and immunoglobulin G (IgG)] remained negligible.286 This adsorption phenomenon proved to be highly dependent on the pH and ionic strength of the sample solution. Moreover, when compared to pure PVC, [C1im]Cl–PVC facilitated the elution of the retained proteins, and no protein denaturation was observed.286 The selective isolation of Hb was further studied using imidazolium-modified polystyrene materials.287 In this work, imidazolium cations were grafted onto the surface of a chloromethyl polystyrene, forming PS-CH2-[C1im]+Cl–.287 This process was different from that previously reported by Shu et al.,286 since it was not necessary to introduce any solid substrate (e.g., silica beads). This is a result of the cross-linked rigid polymer that can act as a support. The adsorption efficiency of Hb reached values up to 91%, with almost no protein denaturation observed.287 Remarkably, in both works,286,287 the authors validated their results by successfully isolating Hb from human whole blood samples (i.e., from real and complex samples). Two more polymer materials were synthesized and further used for protein separation, where [aC4im]Cl and [VC8im]Br were used as functional monomers and acrylamide as a cofunctional monomer.288 It was found that the [aC4im]Cl-based polymer material has a high binding capacity for Hb, while the [VC8im]Br-based polymer possesses a high binding capacity for BSA. Although suggestions regarding the importance of electrostatic, dispersive, and hydrogen-bonding interactions were given,288 no clear conclusions were provided on the molecular-level mechanisms ruling the protein adsorption.
IL-modified magnetic nanoparticles (ILs-MNPs) were recently developed as a new sorbent material by attaching hydroxy functional groups to the surface of silica-coated magnetic Fe3O4 (Figure 22).289 Under optimal conditions, this process led to a BSA extraction efficiency of 86.9%. The regeneration of the protein was also addressed, and the researchers reported that with concentrations of NaCl greater than 1.1 mol L–1, the desorption ratios of BSA reached 95.3%. Moreover, almost 95% of [N11[3Si(2O)(2O)(2O)](2OH)]Cl-MNPs were recovered with no significant losses on their extraction efficiency over four cycles.289 Therefore, the high adsorption capacity, selective adsorption, reusability, and ease of recovering MNPs by applying a magnetic field, make IL-MNPs promising materials for a wide range of applications. Unfortunately, there are no indications regarding the selective nature of these materials when applied to real and more complex matrices with a large number of proteins present. Moreover, the costs associated with the synthesis of these materials should also be taken into account, since for the purification of low cost proteins, such as BSA, these task-specific materials are probably not the most promising. Finally, and although not hitherto reported, the magnetic nature of the materials and supports could also be investigated by their modification with magnetic ILs.
Figure 22.
Schematic representation of an integrated process for the recovery of proteins using IL-MNPs, including the recycling and reuse of the material.289
6. Nucleic Acids
Nucleic acids, viz. deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), are biopolymers of nucleotides that play important roles in many chemical reactions and gene expression. From aquaculture to forensic science, medicine, and biotechnology, there are multiple areas in which these biomolecules are of high relevance.291−294 However, and apart from the type of application, the purity and integrity of the nucleic acids are essential parameters that depend upon the conditions under which the purification process is conducted. Efficient purification approaches can be developed if some criteria are fulfilled, namely: (i) the matrix disruption for complete release of the intracellular material, (ii) enzyme inactivation to avoid the degradation of the nucleic acids, and (iii) the recovery of high-purity nucleic acids free of contaminants.295 Traditionally, the purification of these biopolymers is based on liquid–liquid extraction techniques involving phenol and chloroform, but more recently, it has evolved toward more biocompatible and efficient processes.295 Inspired by the “designer solvent” status of ILs and the aptitude that these neoteric solvents have in preserving the integrity and structure of nucleic acids296 and at promoting PCR amplification,297 IL researchers have been successful at developing pioneering extractive technologies comprising nucleic acids. Within this section, the three main kinds of IL-based extraction approaches will be reviewed, namely: (i) LLE involving hydrophobic and hydrophilic ILs, and one work based on IL-based ABS, (ii) solid–liquid extractions from real matrices, and (iii) solid-phase extractions with IL-modified materials (Figure 23 and Table 11). Envisioning the substitution of the well-established protocols based on the use of two harmful organic solvents (phenol and chloroform), 60% of the available literature relies on the use of LLE techniques with alternative solvents. Moreover, by observation of the radial graphs depicted in Figure 23, the limited attention given to RNA is noticed, which may be due to the poor stability of RNA and inherently complex manipulation required. Finally, the IL ion combinations studied thus far are represented in Figure 24. A major usage incidence of [CnC1im]+ cations is apparent, whereas Cl–, [CnCO2]−, and [PF6]− have been the most frequently selected anions. Yet, it should be remarked that the literature available on the use of ILs for the extraction and purification of nucleic acids is still scarce.
Figure 23.

Distribution of the works dealing with each IL-based technique for the extraction and separation of nucleic acids. The radial graphs display the number of scientific works addressing DNA and RNA.
Table 11. Extraction and Separation of Nucleic Acids Using IL-Based Processes.
| nucleic acid | IL used + system/medium | isolation strategy |
|---|---|---|
| liquid–liquid extraction | ||
| DNA | [C4C1im][PF6] + water,299,301 [C2C1im][PF6] + water, [C6C1im][PF6] + water,301 [C4C1im][BF4] + water,301 [P66614][NTf2] + virus suspension,300 [N04(2OH)(2OH)][NTf2] + virus suspension,300 [N1888][Calc] + virus suspension,300 [N1888][CPA] + virus suspension,300 [N011(2OH)][NTf2] + virus suspension,300 [C6C1im][FAP] + virus suspension,300 [P66614][FAP] + virus suspension,300 [C4C1pyrr][NTf2] + virus suspension,300 [N011(2OH)][C2CO2] + virus suspension,300 [C1C1im][C1PO3] + virus suspension,300 [N(C7H7)888][FeCl3Br] + water,302,303 [P666 14][FeCl4] + water,302,303 [(C7H7)C16im-C12-(C7H7)C16im][NTf2][FeCl3Br] + water,302 [C4C1im]Cl + water + LiNTf2,304 [C10C1im]Br + water + LiNTf2,304 [C16C1im]Br + water + LiNTf2,304 [(OH)C3C10im]Br + water + LiNTf2,304 [(OH)C3C16im]Br + water + LiNTf2,304 [C10C10C1gluca]Br + water + LiNTf2,304 [C4C1im][BF4] + KH2PO4 + water305 | back extraction,299,301 precipitation,300 and immersion in buffer302 |
| RNA | [P66614][NTf2] + virus suspension,300 [N04(2OH)(2OH)][NTf2] + virus suspension,300 [N1888][Calc] + virus suspension,300 [C6C1im][FAP] + virus suspension,300 [P66614][FAP] + virus suspension,300 [C4C1pyrr][NTf2] + virus suspension,300 [N011(2OH)][C2CO2] + virus suspension,300 [C1C1im][C1PO3] + virus suspension300 | precipitation300 |
| solid–liquid extraction | ||
| DNA | [C2C1im][C1CO2] + buffer,306,307 [C2C1im][(C1)2PO4] + buffer,306,307 [C2C1im][C0CO2] + buffer,306 [C2C1im][N(CN)2] + buffer,306 [C2C1im][SCN] + buffer,306 [C2C1im][(C4)2PO4] + buffer,306 [amim]Cl + buffer,306 [DBU][C0CO2] + buffer,306 [N111(2OH)][C0CO2] + buffer,306,307 [N111(2OH)][C1CO2] + buffer,306,307 [N111(2OH)][C3CO2] + buffer,306,307 [N111(2OH)][C5CO2] + buffer,306,307 [N111(2OH)][C7CO2] + buffer,306,307 [N111(2OH)][C9CO2] + buffer,306,307 [N111(2OH)][C11CO2] + buffer,306,307 [N111(2OH)][Lac] + buffer,306,307 [N111(2OH)][DTP] + buffer,306 [N111(2OH)][H2PO4] + buffer,306,307 [N111(2OH)][(C4)2PO4] + buffer,306,307 [N111(2OH)][TMPP] + buffer,306,307 [N111(2OH)][BEP] + buffer,306 [N111(2OH)][Bic] (pure),306 [C4DBU]Cl (pure),306 [C12bet]Cl (pure),306 [OHC2C1im]Cl (pure),306 [C4C1im]Cl (pure),306 [C4C1im][NTf2] (pure),306 [C4C1im][PF6] (pure),306 [C4C1im]Br (pure),306 [C6C1im]Cl (pure),306 [C8C1im]Cl (pure),306 [C10C1im]Cl (pure),306 [C12C1im]Cl (pure),306 [C14C1im]Cl (pure),306 [C4pyr]Cl (pure),306 [DBU][C5CO2] (pure),306 [N000(2OH)][C0CO2] (pure),306 [N000(2OH)][C1CO2] (pure),306 [N000(2OH)][Lac] (pure),306 [N000(2OH)][C5CO2] (pure),306 [N00(2OH)(2OH)][C5CO2] (pure),306 [N0(2OH)(2OH)(2OH)][C5CO2] (pure),306 [C2C1im]Cl + buffer,307 [C4C1im]Cl + buffer,307 [C6C1im]Cl + buffer,307 [guan][C0CO2] + buffer,307 [guan][C1CO2] + buffer,307 [guan][C3CO2] + buffer,307 [guan][C5CO2] + buffer,307 [guan][C7CO2] + buffer,307 [guan][C9CO2] + buffer307 | |
| IL-modified materials for solid-phase extraction | ||
| DNA | poly([VC1O(O)C2im][PF6]) microspheres308 | stripping with NaCl308 |
Figure 24.
ILs used for the separation and purification of nucleic acids as a function of cation–anion combinations. The usage incidence (number of articles) is represented by the size of the circles, which proportionally increases as follows: 1 < 2 < 3.
6.1. IL-Based Liquid–Liquid Extractions
In 2007, a patent by the group of Ohno298 and an article by Wang and co-workers299 were released regarding the use of ILs for the extraction and separation of nucleic acids. The former covers the use of ILs as powerful solvents and preservation milieu for DNA and RNA,298 whereas the latter discloses the direct extraction of double-stranded DNA within a two-phase [C4C1im][PF6]-water system.299 The IL-aqueous phase ratio, DNA concentrations, extraction time, and temperature were first addressed by the second group of authors.299 Higher IL-aqueous phase ratios, lower DNA concentrations allied to short extraction times, and mild temperatures boosted the DNA extraction into the [C4C1im][PF6] phase. Since proteins (contaminants) did not migrate into the IL-phase, and in accordance with the trends discussed previously on the partitioning of proteins to hydrophobic ILs, which require the presence of water at the IL-phase to be more effective, the authors299 demonstrated the high selectivity of their technology for the separation and preconcentration of DNA from biological matrices. The back-extraction of DNA was successfully accomplished using a phosphate-citrate buffer.299 Rossmanith and co-workers300 further disclosed the capacity of the ILs for disintegrating virus particles for nucleic acid isolation. Feline calicivirus and phage P100 were adopted as model RNA and DNA viruses.300 Sixteen ILs were screened regarding their aptitude to induce phase separation, being further applied for virus cracking. In addition to hydrophobic ILs, some water-miscible examples were also tested by the same authors and for convenience they will be also discussed in this section. The proposed experimental procedure consists of two major steps: (i) nucleic acid isolation with ILs and with a commercial kit for comparison purposes and (ii) precipitation and recovery of the target nucleic acids, since most ILs constrain the polymerase phase reaction (PCR) used for their quantification in the last step.300 The results attained disclose outstanding disintegration and nucleic acid isolation abilities of ILs, comparable to those delivered by the commercial kit. For model DNA extraction, while [P66614][NTf2] and [N04(2OH)(2OH)][NTf2] performed similarly to the commercial kit (recoveries of 80 and 107%, respectively), [N1888][Calc], [N1888][CPA], [N011(2OH)][NTf2], [C6C1im][FAP], [P66614][FAP], and [C1C1im][C1PO3] (recoveries of 143–169%) led to much higher recoveries.300 Not all ILs were as favorable as previously exposed, with most ILs having the [N1888]+ cation (with [Oro]−, [H2NSO3]−, [IO3]−, and [FeCl4]− as the anion moieties) and the IL [C8C1im][IO4] failing at extracting DNA. When attempting the RNA isolation from Feline calicivirus, the recoveries were generally below those of the market kit, excepted for [C1C1im][C1PO3], which performed twice as well. Temperature was found to be the main factor responsible for the low yields obtained with several ILs. Hence, it is possible to surmise that it is the nucleic acid degradation that hampers the isolation approach and not the aptitude of the IL for virus cracking.300
In the field of analytical chemistry, one of the techniques available to detect DNA relies on staining dyes which bind to the nucleic acid and emit a strong fluorescence signal. However, DNA samples free of dyes may find application in several industries, and this was the rationale behind the highly innovative work of Khimji et al.301 The authors301 found that when adding a certain amount of [C4C1im][PF6] to a buffered solution of single-stranded DNA and a dye, the strong green fluorescence vanished. On one hand, the authors hypothesized that such a finding was a result of the selective partitioning of the dye and DNA toward opposite phases, while on the other hand they could not abandon the possibility of their simultaneous migration into the IL-phase coupled to a loss of fluorescence.301 Both hypotheses were cautiously investigated and major conclusions were ultimately made: (i) the proposed method is suitable to isolate DNA from staining dyes with no loss of integrity, (ii) as DNA remains in the aqueous phase it can be separated from the dyes (whose migration is to the opposite phase, i.e., the IL-phase), and (iii) the proposed method is more appropriate for staining dye extraction than DNA.301
Since tedious centrifugation steps are a common practice in DNA extraction protocols, the utilization of magnetic ILs is helpful due to the possibility of using an external magnetic-field-aided separation. Under this scenario, Clark et al.302 tested first a triad of hydrophobic magnetic ILs ([N(C7H7)888][FeCl3Br], [P666 14][FeCl4], and [(C7H7)C16im-C12-(C7H7)C16im][NTf2][FeCl3Br]) to induce phase separation in aqueous solution.302 Further optimization studies, performed with standard salmon DNA, led to the conclusion that the dispersive droplet extraction is more efficient (79.3% vs 63.1%) and faster (5 s vs 120 min) than the single droplet mode and that only very low amounts of IL (ca. 10 μL) and mild pH conditions are needed.302 In order to assess the real applicability of this technique, two major challenges were embraced, namely the extraction of smaller DNA molecules and their purification from a complex matrix containing metal ions and albumin.302 In the former, this technique was shown to be promising at selectively extracting DNA of distinct sizes if the magnetic IL was well-designed; in the latter, the most significant achievement relied on an insignificant coextraction of protein of only 5% using [N(C7H7)888][FeCl3Br].302 This IL was then selected to extract DNA from Escherichia coli cell lysates, which was then simply isolated from the magnetic IL by a 2 min immersion in a Tris·HCl solution at pH 8.302 As a follow-up,303 the same group of authors developed a method which allowed the direct amplification of DNA by PCR. For this study, a PCR buffer was designed to constrain the interference of magnetic ILs within the reaction.303
Rapid DNA extraction and preconcentration by in situ dispersive liquid–liquid microextraction was reported in 2013 by Anderson and co-workers for the first time.304 In this study,304 six water-miscible ILs, [C4C1im]Cl, [C10C1im]Br, [C16C1im]Br, [(OH)C3C10im]Br, [(OH)C3C16im]Br, and [C10C10C1gluca]Br, were employed, which are more structurally flexible than the water-immiscible examples mentioned above. The developed method implies the addition of a water-miscible IL to the DNA-containing sample, followed by the addition of LiNTf2 as a metathesis reagent to promote the in situ formation of a water-immiscible IL, which will undergo phase separation.304 The resulting IL yielding the best extraction efficiency result (97%) was [(OH)C3C16im][NTf2]. The nucleic acid extraction from a complex mixture formed by metals and proteins suggested that while the former do not affect the extraction performance, the latter are more challenging, and a judicious manipulation of the pH under which the extraction is conducted is vital to avoid the coextraction of undesired species.304 As demonstrated by 31P NMR spectroscopy, electrostatic interactions play a fundamental role in the extraction of DNA.304
IL-based ABS can overcome some disadvantages displayed by LLE based on hydrophobic ILs, as already exposed in this critical review. Yet, the use of these aqueous-rich systems to extract and purify nucleic acids is rare and more comprehensive knowledge must be gathered. The only report on IL-based ABS was released in 2012 by Huang and Huang.305 The novelty and good performance claimed notwithstanding, this study focused on the combined use of the water-unstable [C4C1im][BF4]80 with the inorganic salt KH2PO4.305
6.2. IL-Based Solid–Liquid Extractions
Two works by the group of Bica306,307 reported the use of IL-based SLE to extract genetic material from food matrices, attempting the creation of less laborious, time saving, and cost-effective routes for food quality control. These works included maize- and meat-derived products, and extended screenings of the chemical structures of the ILs (e.g., imidazolium-, cholinium-, and guanidinium-based, among others) were cautiously conducted.306,307 After the optimization of the SLE conditions (IL structure; concentration, pure or in aqueous/buffered-solution; and temperature), the created protocols allowed the extraction of DNA with aqueous solutions of ILs within short periods of time (5 min for maize and 25 min for meats) at ambient temperature.306,307 ILs of higher hydrophobic character, such as those containing hydrophobic anions such as [NTf2]− and [PF6]−, or those incorporating large alkyl side chains ([CnC1im]+, n = 8–14), were shown to be ineffective for DNA isolation.306 Although the efficiency was shown to depend upon the type of matrix, either maize or meat-derived products, in general, [C2C1im][(C1)2PO4] and two environment-friendly ILs, [N111(2OH)][C0CO2] and [N111(2OH)][C5CO2], were those yielding the best compromise between DNA quality, efficiency, and environmentally friendly nature.306,307
6.3. Solid-Phase Extractions with IL-Modified Materials
A single entry in the literature is dedicated to the development of IL-modified materials for the SPE of nucleic acids,308 in which polymeric ILs were used. The major benefit of these modified materials is claimed to be the conjugation of the properties of both ILs and polymers, namely high stability, outstanding structural flexibility, and enhanced robustness. After the detailed characterization of the poly([VC1O(O)C2im][PF6]) microspheres prepared, the adsorption of DNA from E. coli cultures was investigated. In a first attempt, the authors have optimized the adsorption phenomenon, considering two essential parameters: time and ionic strength. Ion-exchange was proposed as the major driving force contributing to such a phenomenon.308 An improved adsorption capacity of about 191 μg mg–1 was exhibited by the prepared material, which was at least 1 order of magnitude higher than those displayed by other materials reported in the literature. DNA recovery from the polymeric IL microspheres was also a matter of study, as a way of supporting their reusability.308 The stripping reagents screened spanned from buffers to electrolyte solutions, and NaCl was the preferred choice with a maximum elution efficiency of around 81%. The picture emerging from these results is that by simply playing the salt concentration, enhanced adsorptions and stripping solvents, high yields can be attained.308 Ultimately, the isolation of plasmid DNA from real samples (i.e., E. coli cultures), using the prepared microspheres was carried out, its performance being further compared with that yielded by a commercialized kit. Poly([VC1O(O)C2im][PF6]) microspheres performed better than the commercial kit regarding the amounts of plasmid DNA processed (ca. 33 μg vs 10 μg), while both DNA purity and integrity were maintained.308
At this stage, there is still a lot to be done in the field of IL-based extractions and separations of nucleic acids. The stability of nucleic acids, particularly DNA, is already well-understood, as recently reviewed by the groups of Zhao and Sugimoto.296,309 Accordingly, major achievements were made regarding the use of ILs as stabilizing and preservation media,296 particularly the long-term stability of DNA over 6 months disclosed by the group of MacFarlane4 and the maintenance of the DNA G-quadruplex structure revealed by Fujita and Ohno.310 Prasad and co-workers also demonstrated that it is possible to dissolve high concentrations of DNA in bioderived ILs without affecting the structural integrity of the biomolecule.311,312 All these works resort to cholinium-based ILs, suggesting that their use could be the key for creating highly efficient extraction platforms for DNA and RNA. Curiously, and as described before, only very few attempts at the extraction and separation steps were carried out with cholinium-based ILs. These should be strategically used, since these can be designed to be hydrophobic (by combining, for instance, fluorinated anions), thus creating two-phase systems with water, or hydrophilic, easily creating IL-based ABS, either with polymers or with salts.
7. Pharmaceuticals and Drugs
Pharmaceuticals (i.e., any drug used with medicinal purposes) have faced an increase in their consumption in an epoch where the improvement of the mean age and quality of life is a demand. In this framework, major challenges related to the sustainability of pharmaceutical industries and their products are targets of special attention.313−317 The production processes in pharma industries often rely on the use of organic solvents, which are toxic and environmentally hazardous, with a major impact on the life cycle analysis of their products.318,319 Recently, Roschangar et al.315 suggested a novel concept, the “green aspiration level”, which measures the environmental impact of producing a given pharmaceutical, taking into account the complexity of its ideal synthetic process. On the basis of this concept, several production processes have been modified across the pharmaceutical industry: Pfizer transformed the manufacturing processes of two of its top selling drugs, sertraline hydrochloride (Zoloft) and sildenafil citrate (Viagra), using safer and greener approaches;320 Merck and Codexis developed an enzymatic process for the synthesis of sitagliptin (Januvia);321 and Roche excluded the highly toxic thionyl chloride from the synthetic route to oseltamivir phosphate (Tamiflu).322 This last example was also the object of study by Bica and co-workers,36 who in 2011 proposed the application of ILs to isolate a precursor of this active pharmaceutical ingredient from natural sources. In fact, the use of hazardous solvents is declining and alternative solvents have been highlighted as more desirable options within the pharmaceutical industry.319,323 ILs are one of these alternative classes of solvents that have been studied for the production, separation, and purification of drugs, as shown by the number of works dealing with this topic, as discussed in this section.
Numerous authors have devoted their attention to the determination of the solubility of active pharmaceutical ingredients in ILs as the basis for the development of IL-based separation and purification processes. Currently, the spectrum of compounds investigated is broad, ranging from cardioactive prototype drugs324 to antibiotics,325−329 nonsteroidal anti-inflammatory drugs,326,327,330 analgesic,330,331 anthelmintic,331 and androgen331 compounds. Most of these systematic studies focus on the use of hydrophobic ILs composed of nitrogen-324,325,328−331 or phosphorus-based326,327 cations and anions such as [NTf2]−324−326,328,329 and [PF6]−,330,331 while only few works have reported the use of hydrophilic ILs.324,331 Rogers and co-workers,332 in a study of drug delivery, recently shown that ILs, if cautiously designed, can boost the water solubility of poorly soluble active pharmaceutical ingredients. This concept can be thus extended to the extraction and purification of drugs using aqueous IL solutions, as also discussed in this section.
This section overviews more efficient separation routes for pharmaceutical drugs by taking advantage of the unique characteristics of the ILs. Figure 25 depicts the three main approaches found in the literature: (i) LLE, where hydrophobic ILs are generally adopted as substitutes for conventional organic solvents, (ii) ABS composed of ILs and salts, polymers, or amino acids, sometimes combined with previous SLE steps, and (iii) crystallization in ILs or IL-enriched media. Separation and purification processes in aqueous media are the most widely adopted approaches, representing 50% of the articles reviewed in this section. Distinct types of compounds were the target of separation and purification by each of these techniques, as suggested by the radial graphs depicted in Figure 25, with antibiotics standing out as the compounds attracting the most attention. Besides antibiotics, nonsteroidal anti-inflammatory drugs, analgesics, vasodilators, antidepressants, fibrates, hypnotics, anticonvulsants, immunosuppressants, and enantiomers with pharmacological activity are within the drugs studied. Overall, 38 structurally distinct compounds were investigated; their chemical structures are shown in Figure 26.
Figure 25.
Distribution of the works dealing with each IL-based technique for the extraction and separation of pharmaceuticals. The radial graphs display the number of scientific works addressing distinct types of pharmaceuticals.
Figure 26.
Chemical structures of the pharmaceuticals extracted and separated with IL-based separation processes.
Figure 27 displays the utilization incidence of distinct combinations of IL ions. As expected, and as with the use of ILs for the separation and purification of other value-added compounds discussed above, 1-alkyl-3-methylimidazolium-based ILs are the most well-investigated, although an appreciable usage of more benign ammonium-based cations (i.e., [Nwxyz]+ and [N111(2OH)]+) began over the past few years. [BF4]−, Cl–, and [PF6]− are the anions most frequently paired with [CnC1im]+. The more recent use of organic-acid-derived anions, namely [Ac]−, [Glut]−, [Lev]−, and [Suc]−, should however be noted, indicative of a promising trend toward the use of more biocompatible ILs in separation processes for drug production.
Figure 27.
ILs used for the separation and purification of pharmaceuticals as a function of cation–anion combinations. The usage incidence (number of articles) is represented by the size of the circles, which proportionally increases as follows: [0–2] < [2–4] < [4–6] < [6–9].
The review of the literature related with this section will be presented following the most frequently used separation processes. Within each part, the IL choice and the optimization of the separation conditions (e.g., temperature, pH, and phase volume ratio) that lead to the best extraction efficiencies, as well as the strategies outlined to successfully isolate the target compound from the IL matrix (when applicable), will be discussed.
7.1. Liquid–Liquid Extractions with Hydrophobic ILs
Cull et al.333 were the first to report the use of ILs in the LLE of pharmaceuticals aiming at overcoming the potential hazards of organic solvents. The authors333 used a [C4C1im][PF6] + water biphasic system to extract erythromycin A, a macrolide antibiotic industrially produced by aerobic fermentation, showing that the use of ILs could be as efficient as butyl acetate. This pioneering work triggered a new trend of seeking novel IL + water biphasic extraction systems for antibiotics. Table 12 presents the systems reported in the literature for such a goal. On the basis of the data reviewed, a scheme of a general process based on these LLE systems is outlined in Figure 28.
Table 12. Extraction and Separation of Pharmaceuticals Using LLE with Hydrophobic ILs.
| pharmaceutical | system used | isolation strategy |
|---|---|---|
| amide (intermediate) and ammonium salt (contaminant): aliskiren synthesis | [C2C1im][NTf2] + water343 and [C2C1im][C1CO2] + ethyl acetate343 | washing with water and precipitation343 |
| amoxicillin | [C8C1im][BF4] + water334 | |
| ampicillin | [C8C1im][BF4] + water334 | |
| erythromicin A | [C4C1im][PF6] + water333 and [C4C1pyrr][NTf2] + water335 | high pressure CO2335 |
| ibuprofen | [N114(2OH)][NTf2] + water,339 [N116(2OH)][NTf2] + water,339 [N118(2OH)][NTf2] + water,339 and [N11 10(2OH)][NTf2] + water339 | |
| indomethacin | [N114(2OH)][NTf2] + water,339 [N116(2OH)][NTf2] + water,339 [N118(2OH)][NTf2] + water,339 and [N11 10(2OH)][NTf2] + water339 | back extraction with NaOH339 |
| lidocaine | [N114(2OH)][NTf2] + water,339 [N116(2OH)][NTf2] + water,339 [N118(2OH)][NTf2] + water,339 and [N11 10(2OH)][NTf2] + water339 | |
| mandelic acid enantiomers | [C8C1im][BF4] + water + β-cyclodextrin derivatives341 and [C4C1im][PF6] + water + β-cyclodextrin derivatives341 | |
| nitrofurantoin | [(C6H13OCH2)C1im][BF4] + water336 and [(C6H13OCH2)2im] [NTf2] + water336 | |
| penicillin G | [C4C1im][PF6] + water,337,338 [C6C1im][PF6] + water,337,338 [C8C1im][PF6] + water,337,338 and [N1888]Cl + water337 | back extraction with potassium bicarbonate338 |
| phenacetin | [N114(2OH)][NTf2] + water,339 [N116(2OH)][NTf2] + water,339 [N118(2OH)][NTf2] + water,339 and [N11 10(2OH)][NTf2] + water339 | |
| progesterone and pregnenolone | [C4C1im][BF4] + tert-butyl methyl ether340 |
Figure 28.
Schematic representation of the integrated process comprising the production, separation/purification, recovery of the target molecule, and recycling of solvents in two-phase LLE comprising ILs. A and B correspond to processes where an induced precipitation with CO2335 and back-extraction338,339 approaches were used to recover the pharmaceuticals, while C represents the process of purification of an intermediate of aliskiren synthesis.343
In 2005, Soto et al.334 proposed the application of biphasic [C8C1im][BF4] + water systems for the extraction of two other antibiotics, amoxicillin and ampicillin. The partition coefficient results achieved (from 0.17 to 20.34) indicated a clear dependency of the antibiotics partition on the pH, due to their anionic (at pH 8) or zwitterionic (at pH 4) forms. Manic et al.335 successfully extracted erythromycin A from an aqueous solution using [C4C1pyrr][NTf2]. The major achievement of this work was that 40 times less volume of the IL than that of an aqueous solution was used in ten successive cycles to achieve an overall yield higher than 80%. High pressure CO2 was used to isolate ca. of 76% of erythromycin,335 this being one of the few examples where the recovery of pharmaceuticals from the IL-rich phase was attempted. After proving the chemical stability of the extracted antibiotic with ILs, the authors designed a valuable extraction process (represented in Figure 28A) with potential for industrial applications.
Biphasic IL + water systems composed of two hydrophobic imidazolium-based ILs ([(C6H13OCH2)2im][NTf2] and [(C6H13OCH2)C1im][BF4]), at different pH values, were investigated by Domańska and collaborators336 for the extraction of nitrofurantoin, an antibiotic prescribed for the treatment of infections of the urinary tract. The nitrofurantoin exhibited preferential partitioning toward the IL phase, except when [(C6H13OCH2)C1im][BF4] was employed at pH ≥ 3.13. The best conditions were obtained with the IL [(C6H13OCH2)2im][NTf2] and low pH (partition coefficient of 19.7), where the partitioning was explained based on a balanced contribution of π···π stacking, lone pair electrons, permanent dipoles, and electrostatic interactions.336
Penicillin G, a microbially produced antibiotic, was also the target of extraction by [CnC1im][PF6] (n = 4, 6, and 8) in two works by Matsumoto et al.337 and Liu et al.338 Matsumoto et al.337 also included [N1888]Cl in their study, whereby this IL, at pH 6, led to the largest quantities of penicillin G extracted and where the authors337 suggested an anion exchange mechanism between the Cl– and the antibiotic (which possesses a dissociation constant of 2.76). However, no real support for this assumption was provided by the authors,337 since the tests carried out to prove their hypothesis led to inconclusive results. The isolation of antibiotics from [N1888]Cl was also attempted but with no success.337 However, inconsistent results were reported by Liu and collaborators338 using [CnC1im][PF6] (n = 4, 6, and 8) ILs. In this work, [C4C1im][PF6] at pH 2 led to higher extraction performances (partition coefficient of ca. 10 and extraction efficiency >80%). In this work, a simple isolation of the antibiotic (>95%) from the IL phase was achieved using a weak base (potassium bicarbonate). Notably, this system was successfully employed for the antibiotic extraction from its fermentation broth, with enhanced selectivity for contaminant removal than that achieved with the conventional process employing butyl acetate.338 This last step is of high relevance, given that most authors carry out extraction studies with aqueous solutions spiked with pharmaceuticals and do not prove the feasibility of the developed processes with real matrices. Figure 28B shows a schematic representation of the integrated process proposed by the authors.338
A later study by Wang and co-workers339 focused on the development of nontoxic IL-based extraction systems. For this purpose, the naturally occurring cholinium cation was used for the preparation of hydrophobic ILs of increasing alkyl chain length [i.e., [N11n(2OH)]+ (n = 4, 6, 8 and 10)] combined with the [NTf2]− anion.339 Four distinct drugs were investigated, namely the nonsteroidal anti-inflammatory drugs ibuprofen and indomethacin, the analgesic drug phenacetin, and the anesthetic and analgesic agent lidocaine. After the optimization of the extraction volume phase ratio and the equilibrium time, the impact of pH, the chemical structure of the ILs, and temperature upon the partitioning of the drugs was assessed. With dependence on the drug under investigation, distinct effects were noticed: the indomethacin migration to the IL phase was significantly limited by higher pH conditions; enhanced performances were obtained for both ibuprofen and indomethacin by increasing the IL alkyl side chain length, which is in contrast to the pattern observed for lidocaine; and finally, the extraction mechanism of ibuprofen is endothermic. Finally, the authors339 highlighted the importance of isolating the pharmaceuticals from the ILs by removing more than 65% of indomethacin through pH changes (with 0.1 mol L–1 NaOH), allowing the authors to envisage an integrated process similar to that represented in Figure 28B.
In a recent work, Vitasari et al.340 have successfully separated the similar drugs progesterone and pregnenolone by IL-based liquid–liquid extractions. The number of solvents able to solubilize these two steroids is limited, making ILs excellent candidates for such an application. The search for suitable systems was performed in three steps: (i) selection of suitable organic solvents by COSMO-RS, (ii) experimental determination of organic solvent-fluorinated IL combinations able to form two liquid phases, and (iii) determination of the IL concentration in the organic solvent phase.340 The tert-butyl methyl ether-[C4C1im][BF4] mixture was elected as the ideal system to pursue studies on the partitioning of progesterone and pregnenolone. A selectivity of 2.1 was reached, and the purification of progesterone was successfully conducted by simulating a countercurrent extraction process.340
Enantioseparation processes are vital in the pharmaceutical industry due to the dramatic discrepancy of pharmacologic effects between enantiomers. Despite their relevance, there is, however, only one work dealing with the separation of chiral drugs, namely mandelic acid, using hydrophobic ILs + water systems.341 This hydrocarboxylic acid is used as an intermediate for the synthesis of antibiotics, antitumor agents, and nonsteroidal anti-inflammatory drugs.342 [C8C1im][BF4] and [C4C1im][PF6] were used as the IL phases with β-cyclodextrin derivatives as chiral selectors. The study suggested that [C4C1im][PF6] as extraction solvent and hydroxypropyl-β-cyclodextrin as chiral selector was the optimal combination. By decreasing the temperature, pH, and the concentration of enantiomers, and by increasing the chiral selector concentrations, improved enantioselectivities were obtained.341
During the manufacturing process in any pharmaceutical industry, the final produced drugs cannot contain impurities and should obey the standards imposed by legal guidelines. Encouraged by such a necessity, Rogers and co-workers343 proposed an IL-based separation strategy for an intermediate of the aliskiren synthesis from an interfering ammonium salt formed during the reaction. Aliskiren is a direct renin inhibitor used to treat high blood pressure. By investigating hydrophobic versus hydrophilic ILs, distinct biphasic systems were created: [C2C1im][C1CO2] + ethyl acetate, [C2C1im][C1CO2] + n-heptane, [C2C1im][NTf2] + n-heptane, and [C2C1im][NTf2] + water. The solubilities of the reactants (a lactone and 3-amino-2,2-dimethylpropanamide), amide products, and ammonium salts in both ILs and the three solvents were measured, and based on the results obtained, the [C2C1im][NTf2] + water biphasic system was selected to separate the standard mixture. At the end of the process, the purities of both lactone and amide products were not as high as desired due to contamination issues with the hydrophobic IL. Hence, the authors were forced to adopt the [C2C1im][C1CO2] + ethyl acetate biphasic system, given the possibility of being able to remove [C2C1im][C1CO2] from the lactone and amide product (both hydrophobic) by a simple washing step with water, as sketched in Figure 28C. Both regenerated lactone and amide were separated with high purity and the remaining reactant and ammonium salt were precipitated and recovered by washing the standard mixture with water. When applying this procedure to an actual reaction mixture (composed of reactants, products, and some byproducts), an additional step consisting of washing the regenerated amide with n-heptane was needed to remove the residual byproducts.343
Also for the intermediate R-phenylacetylcarbinol, the replacement of toluene in liquid–liquid extraction by the use of ILs was investigated. Computer-aided molecular design was used, due to its time- and money-saving advantages over common systematic experimental studies.344
To summarize, two-phase systems with hydrophobic ILs + water are by far the most studied for the separation of drugs, as only one work343 addressing hydrophilic ILs in combination with organic solvents for separation purposes exists. It is well-documented that hydrophobic ILs are more toxic than the hydrophilic ones and some of them are water-unstable (e.g., with the [BF4]− anion). Even so, a recent trend toward the use of water-stable and more benign ILs has been observed in recent years.
Isolation strategies, with vital relevance for future industrial applications, although not conducted in most of the works herein reviewed, were however contemplated by some researchers. This more complex strategy allows researchers to obtain the target product free of IL (step 3 of Figure 28) and the recycled IL for further use (step 4 of Figure 28). Also missing in most reported works is an assessment of the chemical stability and pharmacological activities of the drugs extracted as a way of reinforcing the promising status of IL-based technologies for the purification of pharmaceuticals.
7.2. Aqueous Biphasic Systems and Aqueous Solutions of Hydrophilic ILs
More environmentally friendly routes for the purification of pharmaceuticals appeared with the use of IL-based ABS or IL aqueous solutions. The first report on the extraction of drugs using IL-based ABS dates from 2005,345 in which the authors successfully extracted two opium drugs, the analgesic morphine (maximum extraction efficiency achievable of 67%), and the vasodilator papaverine (maximum extraction efficiency achievable of 96%) using ABS formed by [C4C1im]Cl and K2HPO4. A summary of the ABS and IL aqueous solutions studied is reported in Table 13 and some representative processes are depicted in Figure 29.
Table 13. Extraction and Separation of Pharmaceuticals Using IL-Based ABS or Aqueous Solutions of ILs.
| pharmaceutical | system used | isolation strategy |
|---|---|---|
| amitriptyline hydrochloride | [Pi(444)1][Tos] + K2HPO4/KH2PO4 pH 7,358 [P4441][C1SO4] + K2HPO4/KH2PO4 pH 7,358 [P4444]Br + K2HPO4/KH2PO4 pH 7,358 [N4444]Br + K2HPO4/KH2PO4 pH 7,358 [N4444]Cl + K2HPO4/KH2PO4 pH 7,358 [N4444]Br + K2HPO4,358 [N4444]Br + K3PO4,358 [N4444]Cl + K2HPO4,358 and [N4444]Cl + K3PO4358 | precipitation with water or KOH aqueous solution as antisolvents358 |
| cephalexin | [C4C1im][BF4] + ZnSO4354 | |
| chloramphenicol | [C4C1im]Cl + K2C4H4O6,355 [C6C1im]Cl + K2C4H4O6,355 [C7H7C1im]Cl + K2C4H4O6,355 [HOC6C1im]Cl + K3PO4,356 [HOC6C1im]Cl + K2HPO4,356 and [HOC6C1im]Cl + K2CO3356 | |
| ciprofloxacin (or its hydrochloride salt form) | [N111(2OH)][Glut] + K3PO4,349 [N111(2OH)][Suc] + K3PO4,349 [N111(2OH)][Lev] + K3PO4,349 [N111(2OH)][C1CO2] + K3PO4,349 [N111(2OH)]Cl + K3PO4,349 and [C4C1im][CF3SO3] + Lysine85 | |
| ibuprofen | [C4C1im]Cl + H2O,359 [C4C1im]Cl + C6H5K3O7/C6H8O7 pH 7 + H2O,359 [N11(C7H7)(2OH)]Cl + H2O,359 [N11(C7H7)(2OH)]Cl + C6H5K3O7/C6H8O7 pH 7 + H2O,359 [N4444]Cl + H2O,359 and [N4444]Cl + C6H5K3O7/C6H8O7 pH 7 + H2O359 | precipitation with water or KCl aqueous solution as antisolvents359 |
| morphine | [C4C1im]Cl + K2HPO4345 | |
| papaverine | [C4C1im]Cl + K2HPO4345 | |
| paracetamol | [N4444]Cl + C6H5K3O7/C6H8O7 pH 7,317 [N3333]Cl + C6H5K3O7/C6H8O7 pH 7,317 [N2222]Cl + C6H5K3O7/C6H8O7 pH 7,317 [N4444]Br + C6H5K3O7/C6H8O7 pH 7,317 [N3333]Br + C6H5K3O7/C6H8O7 pH 7,317 [N2222]Br + C6H5K3O7/C6H8O7 pH 7,317 [N2222]Br + K2HPO4/KH2PO4 pH 7,317 [N2222]Br + K2CO3,317 [N2222]Br + C6H5K3O7/C6H8O7 pH 5,317 [N2222]Br + C6H5K3O7/C6H8O7 pH 6,317 and [N2222]Br + C6H5K3O7/C6H8O7 pH 8317 | |
| penicillin G | [C4C1im]Cl + NaH2PO4,351 [C4C1im][BF4] + NaH2PO4,352 and [C4C1im]Br + NaH2PO4353 | hydrophobic IL + water LLE352 |
| tetracycline (or its hydrochloride salt form) | [C4C1im][BF4] + NaH2PO4,346 [C4C1im]Cl + K2HPO4,347 [C2C1im]Cl + Na2CO3,348 [C4C1im]Cl + Na2CO3,348 [C6C1im]Cl + Na2CO3,348 [aC1im]Cl + Na2CO3,348 [C4C1pyrr]Cl + Na2CO3,348 [P4444]Cl + Na2CO3,348 [N111(2OH)][Glut] + K3PO4,349 [N111(2OH)][Suc] + K3PO4,349 [N111(2OH)][Lev] + K3PO4,349 [N111(2OH)][C1CO2] + K3PO4,349 [N111(2OH)]Cl + K3PO4,349 [N111(2OH)]Cl + PEG 600,350 [N111(2OH)][C1CO2] + PEG 600,350 [N111(2OH)][Bic] + PEG 600,350 [N111(2OH)][DHCit] + PEG 600,350 and [N111(2OH)][H2PO4] + PEG 600350 | back extraction with serial combination of distinct cholinium-PEG-based ABS350 |
| α-cyclohexylmandelic acid enantiomers | [C4C1im][BF4] + (NH4)2SO4 + hydroxypropyl-β-cyclodextrin,357 [C2C1im][BF4] + (NH4)2SO4 + hydroxypropyl-β-cyclodextrin357 and [C4C1im][N(CN)2] + (NH4)2SO4 + hydroxypropyl-β-cyclodextrin357 |
Figure 29.
Schematic representation of integrated processes for the recovery of drugs, comprising the production, separation/purification of the drug and contaminants/excipients, isolation of the drug, and recycling of the phase-forming components in IL-based ABS. (A) Process with ABS with both separation/purification and back-extraction steps,350 (B) process where both hydrophilic and hydrophobic ILs are used for the separation/purification and isolation of target pharmaceuticals,352 and (C) process for the valorization of pharmaceutical wastes using aqueous solutions of ILs.359
It is probably not surprising that most works deal with antibiotics, tetracycline being the most studied. Ma et al.346 first applied ABS constituted by [C4C1im][BF4] plus NaH2PO4 to the purification of tetracycline. Since then, other systems were investigated aiming at using water-stable347,348 and more benign ILs,349,350 as well as other phase-forming agents besides salts,347−349 in particular polymers.350 Extraction efficiencies consistently higher than 80% were achieved when either K2HPO4347 or Na2CO3348 were used as the salting-out agents in several IL-based ABS. However, there were systems formed by more benign cholinium-based ILs that led to a distinct behavior. Shahriari et al.349 reported for the first time ABS comprised of this type of IL with K3PO4. They showed that both tetracycline and its hydrochloride salt present distinct partition trends between the two phases. Although observing a preferable partition of antibiotics toward the IL-rich phase, when using [N111(2OH)][Glut] the opposite behavior was observed. The partition was explained in light of the aptitude of K3PO4 for salting-out, with [N111(2OH)][Glut] being the exception.349 Another work350 employed PEG 600 and cholinium-based ILs to generate ABS for the prepurification of tetracycline from the fermentation broth of Streptomyces aureofaciens. While Shahriari et al.349 reported a preferential partition of tetracycline toward the IL-rich phase,349 on the other hand, Pereira et al.350 demonstrated that in polymer-IL-based ABS the antibiotic partitions preferentially toward the polymer-rich phase. Again, it was observed that the IL structure has a significant impact on the partition behavior. Even though conventional ABS composed of a polymer and a salt (PEG + Na2SO4) and of two salts ([N111(2OH)]Cl + K3PO4) revealed better performance in extracting tetracycline, the main advantage afforded by using PEG and cholinium-based ILs relies on their boosted biocompatibility and biodegradability. The most relevant results reported by Pereira et al.350 comprise the evaluation of the applicability of these systems for the prepurification of tetracycline from its production medium (i.e., a fermentation broth), highlighting therefore the potential of IL-based ABS to be applied to real systems. The authors350 finally discussed the possibility of varying the partition tendencies for either salt- or PEG-rich phases to create purification and back-extraction approaches, anticipating an integrated process similar to that represented in Figure 29A.
Penicillin G has been studied as a target compound in three published works comprising the use of IL-based ABS. In these studies, [C4C1im]Cl,351 [C4C1im][BF4],352 and [C4C1im]Br353 were used, with NaH2PO4 as the salting-out agent and two distinct lines of research were adopted: while Liu and co-workers351 extracted penicillin G from a filtered fermentation broth with efficiencies higher than 90%, Jiang and collaborators352 addressed the approaches applied to isolate this antibiotic by adding an hydrophobic IL, as described in Figure 29B. In addition to tetracycline and penicillin G, other antibiotics were also studied, namely ciprofloxacin and its hydrochloride salt,85,349 cephalexin,354 and chloramphenicol.355,356 Of particular interest is the work of Han et al.,355 which followed an emergent green tendency of applying a less toxic and more biodegradable organic salt, such as K2C4H4O6, as a replacement for the typically used inorganic salts.
In the field of enantioseparations, Chen et al.357 have recently addressed the use of IL-based ABS formed by [C4C1im][BF4], [C2C1im][BF4], and [C4C1im][N(CN)2] and the salt (NH4)2SO4, adding hydroxypropyl-β-cyclodextrin as a chiral selector for enantiomers, for the separation of the mandelic acid derivative α-cyclohexylmandelic acid. Despite the lack of chiral recognition of some systems, under optimal conditions (salt amount, temperature, pH, and content of the chiral selector), the system composed of [C4C1im][BF4], (NH4)2SO4, and hydroxypropyl-β-cyclodextrin granted the best separation factor (ratio of the partition coefficients of the two enantiomers) of 1.59.357
In 2014, Coutinho’s research group317 published a pioneering work where ABS composed of ILs were applied in the valorization of pharmaceutical wastes. This work looked at these residues as a rich source of active pharmaceutical ingredients, which are currently disposed of by incineration. The authors317 attempted the extraction of paracetamol directly from expired pills to further serve as starting materials or standards in several industries. For this purpose, novel ABS composed of tetraalkylammonium halides and three salts, namely C6H5K3O7/C6H8O7 and K2HPO4/KH2PO4 buffers and K2CO3, were investigated. After an optimization study comprising the ammonium IL chemical structure, salt, tie-line length, and pH, carried out with the pure compound, the best conditions were then used to extract paracetamol from Ben-u-ron 500 pills, yielding complete extraction. Another two works followed with the same goal; the first, in which an integrated multistep purification process was proposed based on IL-based ABS for the recovery of the antidepressant drug amitriptyline in its hydrochloride salt form from ADT 25 pills;358 and the second, wherein a simpler route was designed to purify ibuprofen using IL aqueous solutions.359 In the first work, the amitriptyline hydrochloride was successfully separated from its main contaminants (excipients present in ADT 25 pills) in three steps: a solid–liquid extraction using water as the extracting agent, a purification step using ABS composed of ammonium, and phosphonium-based ILs and phosphate salts (extraction efficiencies ranging from 92 to 100%) and the isolation of the antidepressant through precipitation with water or alkaline aqueous solutions as antisolvents (isolation efficiencies ranging from 95 to 99%).358 In the second study, the ibuprofen was extracted with ca. 80% purity from the Brufen 200 pills, by varying the relative compositions of aqueous solutions of ILs ([C4C1im]Cl, [N11(C7H7)(2OH)]Cl, and [N4444]Cl) and citrate (an industrially used hydrotrope), with a maximum extraction efficiency of 98% attained. More than 90% of ibuprofen was isolated from the IL aqueous solution, with water or saline solutions used as antisolvents, as described in Figure 29C.359
While significant progress was made in the recovery of pharmaceuticals by implementing IL-based ABS, as described above, both favorable trends and failures similar to those observed at the level of the hydrophobic IL + water two-phase systems were observed. A trend toward the creation of more benign systems is already noticeable in both IL and phase-forming agents. Although inorganic salts remain the first choice as phase-forming agents of ABS, organic salts and polymers are gaining favor in the IL-based ABS community as greener and more sustainable options. In some works, the isolation of the target pharmaceuticals and drugs was evaluated and different strategies were presented. However, the development of strategies to recover and reuse the ILs and other expensive phase-forming components is still infrequent. The stability of target pharmaceuticals, and their crystalline structure and polymorph formation when recovered from IL matrices, are additional factors that deserve more attention. Finally, none of the discussed studies evaluated the potential scale-up of the developed technologies, which remains a minor or unaddressed topic.
7.3. Crystallization in IL Media
Crystallization is vital in several processes within the pharmaceutical industry and ILs have been also investigated for this purpose. This technique was often selected by the authors addressing the isolation of the target compounds from the IL matrix resultant from the processes described above. Table 14 provides an overview of all crystallization strategies conducted in IL media. In this field, Kroon et al.360 demonstrated the possibility of using supercritical CO2 as antisolvent, by lowering the solubility in [C4C1im][BF4] of N-acetyl-(S)-phenylalanine methyl ester, the product resulting from the asymmetric hydrogenation of methyl-(Z)-α-acetamido cinnamate. This work360 further opened the way to testing the conditions of crystallization of methyl-(Z)-α-acetamido cinnamate, an intermediate in the production of Levodopa, a drug used against Parkinson’s disease, from [C4C1im][BF4].361 The authors measured the phase behavior of the ternary system composed of [C4C1im][BF4], CO2, and methyl-(Z)-α-acetamido cinnamate. It was concluded that CO2 can act as either cosolvent or antisolvent in distinct concentration regions. Low concentrations of CO2 (30 mol %) yielded a higher solubility of methyl-(Z)-α-acetamido cinnamate in [C4C1im][BF4] + CO2 than in pure IL, while at high CO2 concentrations (40 mol % and 50 mol %) the opposite behavior is observed. With the use of these results, two possible strategies to crystallize this Levodopa intermediate from the IL were proposed: (i) by a thermal shift or (ii) by a crystallization phenomenon induced by CO2.362 After testing the CO2 solubility in systems containing [C4mim][BF4] and three organic solutes of pharmaceutical relevance and showing that these affect the phase behavior of the initial binary system (i.e., [C4C1im][BF4] + CO2),362 Kühne et al.363 presented another study wherein improvements on the naproxen synthetic route, a broadly used nonsteroidal anti-inflammatory drug, were the main target of research. The phase behavior (solid–liquid and liquid–vapor transitions) of the ternary system formed by [C4C1im][BF4], CO2, and naproxen suggests that the CO2 presence (in the range of 10 to 50 mol %) in combination with increasing pressures prompts the complete dissolution of naproxen in the pure IL. Moreover, when the CO2 concentration is further increased within the aforementioned regime, lower temperatures are needed to dissolve the drug in [C4C1im][BF4].363 Unfortunately, due to experimental limitations, the antisolvent phenomenon was not observed; nevertheless, an expectation of its occurrence at CO2 concentrations of 60 mol % was suggested. Finally, it was envisaged that by tuning the amount of CO2 dissolved in the system, it is possible to obtain either homogeneous or heterogeneous solid + liquid systems that are operationally convenient for naproxen reactions or separations, respectively.363
Table 14. Separation and Isolation of Pharmaceuticals by Crystallization Methods in IL Media.
| pharmaceutical | IL | crystallization approach |
|---|---|---|
| 4-aminophenol, 4-nitrophenol, and 4′-chloroacetanilide (contaminants): production of paracetamol | [C2C1im][C1CO2]x[NTf2]1–x (IL mixture)364 and [C2C1im][NTf2]369 | |
| acetylsalicylic acid | [C2C1im][NTf2]369 | cooling crystallization369 |
| adefovir dipivoxil | [aC2im][BF4]366,367 | precipitation with water366 and with ILs [C4C1C1im][BF4],367 [aaim][BF4],367 [C2C1im][C2SO4],367 [aC2im]Br,367 and [aaim]Br367 as antisolvents |
| cyclosporine | [C2C1im][NTf2]369 | cooling crystallization369 |
| etomidate | [C2C1im][NTf2]369 | cooling crystallization369 |
| fenofibrate | [C2C1im][NTf2]369 | cooling crystallization369 |
| griseofulvin | [C2C1im][NTf2]369 | cooling crystallization369 |
| itraconazole | [C2C1im][NTf2]369 | cooling crystallization369 |
| methyl-(Z)-α-acetamido cinnamate (intermediate): production of Levodopa | [C4C1im][BF4]361 | thermal shift,361 precipitation with CO2 as antisolvent361 |
| naproxen | [C4C1im][BF4],363 [C2C1im][NTf2]369 | precipitation with CO2 as antisolvent,363 cooling crystallization369 |
| paracetamol | [C2C1im][C1CO2]x[NTf2]1-x (IL mixture),364 [C4C1im][PF6],368 [C6C1im][PF6],368 and [C2C1im][NTf2]369 | precipitation with 1,1,1,3,3,3-hexafluoroisopropanol as antisolvent,364 cooling crystallization368,369 |
| rifampicin (ultrafine particles) | [C2C1im][C1PO3]365 | precipitation with KH2PO4/NaOH pH 6.8 as antisolvent365 |
| rufinamide | [C2C1im][NTf2]369 | cooling crystallization369 |
| salicylic acid | [C2C1im][NTf2]369 | cooling crystallization369 |
Myerson’s group364 published an innovative work focused on the purification of paracetamol by crystallization. The main idea consisted of the manipulation of the hydrogen bonding interactions for tailoring the solubility of paracetamol and its main impurities (4-aminophenol, 4-nitrophenol, and 4′-chloroacetanilide) in IL media. ILs composed of ions of increasing hydrogen bond basicity ([NTf2]−, [BF4]−, and [C1CO2]−) and hydrogen bond acidity ([C4pyr]+, [C4C1im]+, [C2C1im]+, and [OHC2C1im]+) were tested, whereby it was found that the hydrogen bond basicity of the anion plays the dominant role in the crystallization of paracetamol. [C2C1im][C1CO2] showed the best ability to solubilize paracetamol. Due to its high viscosity, IL mixtures formed by [C2C1im][C1CO2] and the less viscous [C2C1im][NTf2] were also investigated.364 The ability of [C2C1im][C1CO2]x[NTf2]1–x to solubilize paracetamol and 4-aminophenol linearly correlates with the [C1CO2]− concentration. Spectroscopic studies demonstrated that paracetamol shields the [C1CO2]− anion, while proving the importance of hydrogen bonding in the dissolution phenomenon. Three strong hydrogen-bond-donating compounds (ethanol, acetic acid, and 1,1,1,3,3,3-hexafluoroisopropanol) were studied as antisolvents. The latter provided the most promising results, inducing a strong decrease of the solubility of paracetamol. With its use, the coprecipitation of only one impurity, the weakest hydrogen bonding impurity 4-aminophenol, was observed. This study provided novel insights on the importance of understanding the molecular interactions acting in IL media to design efficient crystallization processes and represents the only report available on IL mixtures for processing drugs.364
Two distinct perspectives of antisolvent precipitation strategies in IL media were presented by Viçosa et al.,365 in the preparation of ultrafine particles, and by An and Kim,366,367 in polymorphic design. Indeed, in addition to the separation and purification of the desired drugs, these works addressed other important questions occurring during the formulation and processing of pharmaceuticals. Rifampicin, being a sparingly water-soluble antibiotic, has its bioavailability restricted, and the preparation of ultrafine particles may be promising.365 Preliminary tests proved that raw rifampicin was more soluble in [C2mim][C1PO3] than in other solvents, while in mixtures of this IL and phosphate buffer (KH2PO4 + NaOH at pH 6.8), the solubility drastically decreases. These results support the choice of phosphate buffer as the antisolvent in the preparation of ultrafine rifampicin particles. Notably, the particles were prepared with great purity (93 to 108%) and improved dissolution rate.365
The polymorphic design of active pharmaceutical ingredients plays a key role in the pharmaceutical domain and it often depends on the crystallization conditions. As ILs can establish a wider range of interactions when compared to traditional solvents, they have been studied for this application by An and Kim.366,367 Currently used to treat chronic hepatitis B, adefovir dipivoxil was the object of these two works. In a first attempt,366 the combination of [aC2im][BF4] and water as the solvent and antisolvent, respectively, was able to produce novel polymorphs of the target antiviral drug that are unachievable with conventional organic solvents. In a second study,367 the authors used pairs of distinct ILs, one of which working as the solvent ([aC2im][BF4]) and the other ([C4C1C1im][BF4], [aaim][BF4], [C2C1im][EtSO4], [aC2im]Br, and [aaim]Br) as the antisolvent. Despite the fact that some combinations did not induce crystallization or only produced the usual polymorph, [aC2im][BF4] + [C4C1mim][BF4] generated exceptional interactions with adefovir dipivoxil and led to the formation of a new polymorph.367
The antisolvent crystallization methods reviewed herein are summarized in Figure 30A. These processes may run into some operational obstacles related to the presence of IL, soluble contaminants, and antisolvent, which hamper the recycling and reuse of the IL. Cooling crystallization is thus foreseen by some authors as a favorable method of processing active pharmaceutical ingredients, as sketched in Figure 30B. Smith et al.368 studied paracetamol cooling crystallization in two IL media, namely [C4C1im][PF6] and [C6C1im][PF6]. By the proper manipulation of three variables (i.e., type of solvent, paracetamol concentration, and crystal growth method), new crystal habits different from those commonly obtained with organic solvents were observed. Aiming at developing purification processes, Myerson’s group369 selected [C2C1im][NTf2], a thermally stable and low-viscosity IL, as the ideal solvent to perform cooling crystallization of active pharmaceutical ingredients. Twelve pharmaceuticals divided into the following classes were studied: analgesics (paracetamol), fibrates (fenofibrate), nonsteroidal anti-inflammatory drugs (ibuprofen, acetylsalicylic acid, salicylic acid, and naproxen), antibiotics (itraconazole, griseofulvin, and amoxicillin), hypnotics (etomidate), anticonvulsants (rufinamide), and immunosuppressants (cyclosporine). Ten of these drugs were miscible with the IL, the exceptions being represented by ibuprofen and amoxicillin, the latter being thermally unstable. Distinct solubility profiles were observed, even for compounds with close melting points, suggesting the occurrence of specific interactions between the IL and the active pharmaceutical ingredient. From the results collected, the solubility of many of these drugs varies from low (at room temperature) to extremely high (at higher temperatures), highlighting the promising capability of [C2C1im][NTf2] as a solvent for cooling crystallization processes. To provide a proof of this concept, this approach was applied to the purification of paracetamol in the presence of its most common impurities. Ultimately, and comparing the data obtained with those obtained through the antisolvent approach,364 pharmaceuticals with higher yields and purity levels were obtained.369
Figure 30.
Schematic representation of the integrated processes proposed, comprising production, extraction, and purification through crystallization using (A) antisolvents361,363−367 or (B) cooling crystallization361,368,369 and the recycling of the IL.
From all works reviewed in this section, there are two main approaches to induce the crystallization of pharmaceuticals: precipitation with antisolvents and cooling crystallization. The supremacy of hydrophobic ILs is transversal to these articles and only one article assessed the use of IL mixtures (to tailor the viscosity of the solvent). In this sense, more studies need to be carried out, not only by using different ILs as solvents but also considering conditions other than temperature, for example the pH (to manipulate the speciation of the drugs) and pressure (to control the solubility of the drugs), without neglecting the understanding of the specific interactions taking place in the IL media, which are crucial to the identification of task-specific solvents. The characterization of the crystals also needs to be taken into account, principally regarding the crystal size distribution, crystal shape, and polymorphic forms produced, since these are crucial parameters to attest the quality and industrial potential of the crystallization process. Crystallization is itself important for drug purification but when integrated with the remaining techniques described in previous sections can lead to even more outstanding results. For instance, in most of the LLE approaches discussed above, one of the major drawbacks identified was the lack of attempts at the recovery of the target pharmaceuticals from the IL-rich phase, which, when combined with crystallization-induced approaches, can allow the design of integrated and effective purification processes for pharmaceuticals.
Having the active pharmaceutical ingredients produced, purified, and isolated, they could then be directed toward commercialization for human consumption. Although outside the scope of the present review, the fate of active pharmaceutical ingredients in the environment is a matter of concern and needs to be addressed. The presence of several classes of pharmaceuticals in the environment has been detected316,370 and, under this scenario, ILs and their use in several techniques offer several advantages. IL-based ABS371−376 and liquid–liquid microextractions377−382 are the preconcentration approaches recurrently used for several classes of drugs from the most diverse matrices, such as water371,372,374,377,378,380,382 and food.373,375−377,379,381 Besides that, the use of IL-based ABS to remove pharmaceutical contaminants from wastewater has also been proposed.383,384
8. Conclusions and Future Perspectives
This work aimed to provide an overview of the application of ILs for the extraction and separation/purification of different classes of bioactive compounds, including small organic extractable compounds from biomass (e.g., alkaloids, flavonoids, terpenoids, terpenes, antioxidants, phenolic compounds, among others), lipids (including saponins, carotenoids, and some vitamins), amino acids, proteins, nucleic acids, and synthetic and biobased drugs/pharmaceuticals. The ILs were either applied as the main solvents, as cosurfactants, electrolytes or adjuvants, or as supported materials to tune the adsoption/affinity capacity of silica and polymers. In general, it was demonstrated that, if properly selected, IL-based solvents and materials are able to afford higher extraction yields and purification factors when compared to traditional solvents and materials.
In addition to the nature of the ILs, their sustainable character and costs associated with separation IL-based processes here reviewed also depend on the source of bioactive compounds, which can be divided into the following categories: biomass (for the extraction of bioactive compounds and the most widely investigated), fermentation broths (for the production of proteins and biopharmaceuticals), other biological matrices (e.g., plant- and animal-derived food matrices for the extraction of nucleic acids), and crude synthetic-derived broths (for the production of synthetic drugs/pharmaceuticals). With the use of fermentation broths, the process to be developed will be of high cost. On the other hand, using biomass, the costs will be variable, depending on the biomass type and source. Processes being developed using biomass with applications in animal feed or human food will be competing with these established markets, making the biomass less attractive, unless the product or compound to be purified has a very high commercial value, or if the biomass itself has no value at all for human food and animal feed. Other remarkable sources of biomass were also reviewed in this work, namely those that are currently considered byproducts (residues and wastes) of the agro-forestry sector, poorly valorized native plants, and microalgae and marine raw materials, such as macroalgae. Included in this topic of highly relevant biomass sources are also various invasive species (halophyte plants) and residues obtained from different industries (pulp and paper, fish, seafood, among others) which should be the focus of attention in the coming years.
Despite the efforts made by different authors to screen different ILs and to evaluate various process conditions, most studies are based on ILs comprising imidazolium cations and fluorinated anions, such as [NTf2]−, [PF6]−, and [BF4]−, which are not only more expensive but also moderately toxic, of low biodegradability, and (in some cases) water-unstable.80 However, the use of hydrophilic ILs (including imidazolium, pyridinium, phosphonium, and quaternary ammonium cations combined with anions derived from carboxylic acids, amino acids, Good’s buffers, and halogens, as well as some more scarcely studied protic ILs) appears to be a more promising option due to their larger chemical structure diversity, allowing them to be more task-specific, as well as due to to their more biocompatible nature. In fact, the potential application of this class of ILs has been demonstrated in the extraction and purification of bioactive compounds from biomass, of amino acids, and proteins from aqueous media and raw materials, and as stabilizing media for proteins, enzymes, and nucleic acids. Within the hydrophilic ILs considered, the use of IL aqueous solutions as major solvents was shown to be the preferred choice. This is a particularly good strategy since aqueous solutions of ILs allow the high viscosity of some ILs to be overcome, while contributing to an increase of the biocompatible nature of the solvent and to a decrease of the overall solvent cost. Moreover, ILs have been shown to be successful in the extraction of small extractable compounds by favoring their solubility in aqueous solutions, either by a hydrotropic or micelle-mediated effect, while avoiding the dissolution and extraction of the lignocellulosic fraction, as well as in the extraction of proteins, which, according to the works here reviewed, display limited solubility in hydrophobic ILs and where “hydrated” ILs have shown to lead to improved results.
With regard to the use of IL-based separation processes, and despite the importance attributed in most studies to the partition or adsorption behavior of the target compound into the IL-rich phase or onto the IL-modified material, the most important information, although much less frequently addressed, falls within the separation and purification performance of these systems when applied to real matrices. While for agriculture and animal feed, the purity required for biocompounds or refined extracts is low, in the cosmetic, food, and mainly pharmaceutical and medicinal sectors, the purity levels required are much higher. This means that the requirements of industry and markets are crucial when attempting the design and development of separation and purification processes based on ILs. Unfortunately, the studies reviewed here show in most cases a complete lack of connection between the industry requirements and the process being developed. This is apparent from a general analysis of the works reviewed herein, and where most of the studies were carried out with commercial and already purified target compounds, without attempting the evaluation of the optimized IL-based processes performance when dealing with raw and biological matrices. Moreover, and although usually claimed as model compounds, most of the compounds investigated are of very low commercial value, for example, l-tryptophan and BSA. It is our contention that, in the next few years, definitively value-added compounds with a high market place will be the main focus of research within the IL community dealing with separation processes. An additional indicator of the poor relationship between the interests of industry and academic research in this field is the absence of scaled-up studies, of utmost importance to ascertain on the industrial viability of the studied techniques. The economic evaluation of the developed processes has also lagged behind. For instance, and when dealing with the use of ILs for the extraction of small organic extractable compounds from biomass, it was recently demonstrated, by an economical analysis assessment, that the application of ILs as extraction solvents is only viable when the concentration of extracted compounds is considerably high (>5 wt %) or when they are truly high value-added compounds.8
In summary, the IL community dealing with separation processes needs to take into account the following items in the near future: (i) to address the life cycle analysis of their processes and products, (ii) to develop purification processes in continuous and/or intensified regimes able to be scaled-up and that are feasible for solvent and material recycling, (iii) to conduct economic analyses of the separation process, solvent, and material costs, and cost and purity level required of the target compound, and (iv) to attempt the decrease of energy and solvent consumption, effluent discharge and material disposal, while foreseeing the development of more sustainable technologies. Remarkably, some authors have already developed pioneering work under these guidelines. In more recent years, research has been directed to the use of more benign IL alternatives, such as those composed of cholinium cations and anions derived from carboxylic acids; the intensification of some purification processes was already demonstrated, such as by the use of microfluidic devices; the ILs and additional solvents have been a target of recycling and further reuse; and more remarkably, authors are starting to leave their comfort zone of evaluating the performance of their separation techniques with model compounds and are finally directing their investigation to real matrices and real application problems.385 However, and despite all these efforts, further work needs to be carried out so that IL-based separation processes can take their place in industrial applications.
The development of cost-effective and more sustainable extraction and separation processes is the critical step toward the recovery and commercialization of new and low-cost bioactive products for the nutraceutical, cosmetic, and pharmaceutical sectors, while envisaging their widespread use in the near future to boost the quality of modern society, and in which ILs could play a remarkable role as alternative solvents and materials.
Acknowledgments
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, POCI-01-0145-FEDER-007679 (FCT ref. UID/CTM/50011/2013), financed by national funds through the FCT/MEC and when appropriate cofinanced by FEDER under the PT2020 Partnership Agreement. The authors acknowledge FCT for the IF contract IF/00402/2015 of S.P.M.V. and the doctoral grants SFRH/BD/94901/2013 and SFRH/BD/100155/2014 of F.A.e.S. and M.V.Q., respectively. M.G.F. acknowledges the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant 337753.
Glossary
Abbreviations
- Abu
2-aminobutyric acid
- AChE
acetylcholinesterase
- Ala
alanine
- AAILs
amino-acid-based ILs
- ABS
aqueous biphasic systems
- AMBS
aqueous micellar biphasic systems
- Arg
arginine
- Asp
aspartic acid
- AOT
bis-2-ethylhexylsulfosucciante
- BSA
bovine serum albumin
- CaLA
Candida antarctica lipase A
- CaLB
Candida antarctica lipase B
- UMAE
combination of the ultrasonic- and microwave-assisted extractions
- CSPS
Cordyceps sinensis polysaccharides
- CMC
critical micellar concentration
- APS
crude polysaccharides
- cyt-c
cytochrome C
- DFT
density functional theory
- DNA
deoxyribonucleic acid
- DCH18C6
dicyclohexano-18-crown-6
- d-Phe
d-phenylalanine
- DLS
dynamic light scattering
- FAMEs
fatty acid methyl esters
- GC-MS
gas chromatography/mass spectrometry
- Glu
glutamic acid
- Gly
glycine
- GB-IL
Good’s buffer ILs
- GBs
Good’s buffers
- Hb
hemoglobin
- His
histidine
- HRP
horseradish peroxidase
- HSA
human serum albumin
- ILs-MNPs
IL-modified magnetic nanoparticles
- IgG
immunoglobulin G
- IgY
immunoglobulin Y
- ILs
ionic liquids
- LF
lactoferrin
- Leu
leucine
- LLE
liquid–liquid extraction
- LCC-ILs
long-chain carboxylate ILs
- LCST
lower critical solution temperature
- l-Phe
l-phenylalanine
- l-Trp
L-tryptophan
- Lyz
lysozyme
- Met
methionine
- MA-HLLME
microwave homogeneous liquid–liquid microextraction
- MAE
microwave-assisted extraction
- MIPs
molecularly imprinted polymers
- Myo
myoglobin
- NPCE
negative-pressure cavitation extraction
- NMR
nuclear magnetic resonance
- Ova
ovalbumin nuclear magnetic resonance
- K
partition coefficient
- POM
polarizing optical microscopy
- PEG
polyethylene glycol
- PPG
polypropylene glycol
- PVC
polyvinyl chloride
- PF
purification factor
- RNA
ribonucleic acid
- NaPA
sodium polyacrylate
- SLE
solid–liquid extraction
- SOD
superoxide dismutases
- THF
tetrahydrofuran
- TlL
Thermomyces lanuginosus lipase
- TPP
three-phase partitioning
- Thr
threonine
- TLL
tie-line length
- TEM
transmission electron microscopy
- Try
trypsin
- Try
tyrosine (tyr)
- UPE
ultrahigh pressure extraction
- UAE
ultrasonic-assisted extraction
- Val
valine
Biographies
Sónia P. M. Ventura was born in 1983 in Albergaria-a-Velha, Portugal. She was an Invited Assistant Professor at the Chemistry Department of University of Aveiro, Portugal, from 2014–2015. Currently, she is an Assistant Researcher at the Aveiro Institute of Materials at the Chemistry Department of University of Aveiro. She graduated, did her MSc studies (2007), and obtained her Ph.D. (2012) in Chemical Engineering at the University of Aveiro. Her main research lines include the development of aqueous biphasic systems comprising the use of alternative solvents (ionic liquids, copolymers, and surfactants) and their application in the purification of natural compounds. Currently, she is dedicated to the development of task-specific liquid–liquid extraction platforms to be applied as marine biorefinery strategies.
Francisca A. e Silva was born in 1989 in Aveiro, Portugal. She studied at the University of Aveiro, where she received her MSc in Biotechnology in 2012, with one year abroad at La Sapienza University of Rome. Presently, she is a Ph.D. student in Chemical Engineering at the University of Aveiro, contributing toward the creation of sustainable approaches for the separation and purification of pharmaceutical compounds by implementing ionic-liquid-based technologies.
Maria V. Quental was born in 1989 in Aveiro, Portugal. She finished her MsC in Clinical Biochemistry at the University of Aveiro, Portugal, in 2014. Currently, she is a Ph.D. student in Biochemistry at the same university, and her research interests are mainly focused on the development of purification platforms for biomolecules resorting to ionic liquids.
Dibyendu Mondal was born in 1989 in West Bengal, India. He completed his MSc in Chemistry from the Indian Institute of Technology, Guwahati, in 2011. Thereafter, he joined at Central Salt & Marine Chemicals Research Institute, Bhavnagar, India, and finished his Ph.D. in Chemical Science in 2015. Since January 2016, he has been a postdoctoral researcher under an ERC project at CICECO, University of Aveiro, Portugal. His main areas of research address sustainable energy, biomass conversion, dissolution, and processing of biomacromolecules (nucleic acids, proteins, polysaccharides, and other bioactive polymer) using ionic liquids and deep eutectic solvents.
Mara G. Freire is a Coordinator Researcher at the Chemistry Department of University of Aveiro, Portugal, and the Director of Group 5 within CICECO, Biomedic and Biomimetic Materials. In 2014, she was awarded with a European Research Council (ERC) Starting Grant regarding the development of cost-effective purification platforms for antibodies using ionic liquids. She received her Ph.D. degree in 2007 in Chemical Engineering from University of Aveiro, Portugal. From 2008–2013, she was a postdoctoral researcher at Instituto de Tecnologia Química e Biológica, New University of Lisbon, Portugal. Her main research interests comprise the development of alternative purification platforms for value-added biopharmaceuticals and pretreatment strategies of human fluids for the improved detection of cancer biomarkers.
João A. P. Coutinho is a Full Professor at the Chemistry Department of University of Aveiro, Portugal, where he is the vice-director of CICECO, one of the leading European Laboratories in Materials Science. He studied Thermodynamics and Petroleum Technology at Technical University of Denmark where he got his Ph.D. in Chemical Engineering in 1995 and was then a researcher at IFP, France. Since 1997, he leads a multidisciplinary research team that focuses on a range of subjects from flow assurance and petroleum production from nonconventional reservoirs, to the production and formulation of biofuels, and the development of novel separation processes for the biorefinery. Currently, he strives to apply ionic liquids to these processes and is trying to better understand their physic-chemical behavior for that purpose.
The authors declare no competing financial interest.
References
- Kates R. W.; Clark W. C.; Corell R.; Hall J. M.; Jaeger C. C.; Lowe I.; McCarthy J. J.; Schellnhuber H. J.; Bolin B.; Dickson N. M.; et al. Sustainability Science. Science 2001, 292, 641–642. 10.2139/ssrn.257359. [DOI] [PubMed] [Google Scholar]
- Seddon K. R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. . [DOI] [Google Scholar]
- Naushad M.; Alothman Z. A.; Khan A. B.; Ali M. Effect of Ionic Liquid on Activity, Stability, and Structure of Enzymes: A Review. Int. J. Biol. Macromol. 2012, 51, 555–560. 10.1016/j.ijbiomac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan R.; Izgorodin A.; Ganesh V.; Surianarayanan M.; MacFarlane D. R. Long-Term Structural and Chemical Stability of DNA in Hydrated Ionic Liquids. Angew. Chem., Int. Ed. 2010, 49, 1631–1633. 10.1002/anie.200906610. [DOI] [PubMed] [Google Scholar]
- Swatloski R. P.; Spear S. K.; Holbrey J. D.; Rogers R. D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. 10.1021/ja025790m. [DOI] [PubMed] [Google Scholar]
- Fort D. A.; Remsing R. C.; Swatloski R. P.; Moyna P.; Moyna G.; Rogers R. D. Can Ionic Liquids Dissolve Wood? Processing and Analysis of Lignocellulosic Materials with 1-n-butyl-3-methylimidazolium Chloride. Green Chem. 2007, 9, 63–69. 10.1039/B607614A. [DOI] [Google Scholar]
- Freire M. G.; Claudio A. F. M.; Araujo J. M. M.; Coutinho J. A. P.; Marrucho I. M.; Lopes J. N. C.; Rebelo L. P. N. Aqueous Biphasic Systems: A Boost Brought about by using Ionic Liquids. Chem. Soc. Rev. 2012, 41, 4966–4995. 10.1039/c2cs35151j. [DOI] [PubMed] [Google Scholar]
- Passos H.; Freire M. G.; Coutinho J. A. P. Ionic Liquid Solutions as Extractive Solvents for Value-Added Compounds from Biomass. Green Chem. 2014, 16, 4786–4815. 10.1039/C4GC00236A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio A. F. M.; Neves M. C.; Shimizu K.; Canongia Lopes J. N.; Freire M. G.; Coutinho J. A. P. The Magic of Aqueous Solutions of Ionic Liquids: Ionic Liquids as a Powerful Class of Catanionic Hydrotropes. Green Chem. 2015, 17, 3948–3963. 10.1039/C5GC00712G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesic M.; Marques M. H.; Plechkova N. V.; Seddon K. R.; Rebelo L. P. N.; Lopes A. Self-aggregation of Ionic Liquids: Micelle Formation in Aqueous Solution. Green Chem. 2007, 9, 481–490. 10.1039/b615406a. [DOI] [Google Scholar]
- Agrimi G.; Alexopoulou E.; Bartzoka E. D.; Buonerba A.; Cavani F.; Christou M.; Colucci A.; Couturier J.-L.; Crestini C.; Dubois J.-L.. Biorefineries: An Introduction; Walter de Gruyter GmbH & Co KG, 2015. [Google Scholar]
- Pinkert A.; Marsh K. N.; Pang S.; Staiger M. P. Ionic Liquids and their Interaction with Cellulose. Chem. Rev. 2009, 109, 6712–6728. 10.1021/cr9001947. [DOI] [PubMed] [Google Scholar]
- Wang H.; Gurau G.; Rogers R. D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. 10.1039/c2cs15311d. [DOI] [PubMed] [Google Scholar]
- Zhu S.; Wu Y.; Chen Q.; Yu Z.; Wang C.; Jin S.; Ding Y.; Wu G. Dissolution of Cellulose with Ionic Liquids and its Application: A Mini-review. Green Chem. 2006, 8, 325–327. 10.1039/b601395c. [DOI] [Google Scholar]
- Soares B.; Passos H.; Freire C. S. R.; Coutinho J. A. P.; Silvestre A. J. D.; Freire M. G. Ionic Liquids in Chromatographic and Electrophoretic Techniques: Toward Additional Improvements in the Separation of Natural Compounds. Green Chem. 2016, 18, 4582–4604. 10.1039/C6GC01778A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T. D.; Zhang C.; Hantao L. W.; Anderson J. L. Ionic Liquids in Analytical Chemistry: Fundamentals, Advances, and Perspectives. Anal. Chem. 2014, 86, 262–285. 10.1021/ac4035554. [DOI] [PubMed] [Google Scholar]
- Koel M. Ionic Liquids in Chemical Analysis. Crit. Rev. Anal. Chem. 2005, 35, 177–192. 10.1080/10408340500304016. [DOI] [Google Scholar]
- Sun P.; Armstrong D. W. Ionic Liquids in Analytical Chemistry. Anal. Chim. Acta 2010, 661, 1–16. 10.1016/j.aca.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Pokorný J. Are Natural Antioxidants Better - and Safer - than Synthetic Antioxidants?. Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. 10.1002/ejlt.200700064. [DOI] [Google Scholar]
- Zhang L.; Kujawinski D. M.; Federherr E.; Schmidt T. C.; Jochmann M. A. Caffeine in your Drink: Natural or Synthetic?. Anal. Chem. 2012, 84, 2805–2810. 10.1021/ac203197d. [DOI] [PubMed] [Google Scholar]
- Capello C.; Fischer U.; Hungerbuhler K. What is a Green Solvent? A Comprehensive Framework for the Environmental Assessment of Solvents. Green Chem. 2007, 9, 927–934. 10.1039/b617536h. [DOI] [Google Scholar]
- Tang B.; Bi W.; Tian M.; Row K. H. Application of Ionic Liquid for Extraction and Separation of Bioactive Compounds from Plants. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2012, 904, 1–21. 10.1016/j.jchromb.2012.07.020. [DOI] [PubMed] [Google Scholar]
- Pereira M. M.; Coutinho J. A. P.; Freire M. G. In Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives; The Royal Society of Chemistry, 2016. [Google Scholar]
- Bogdanov M. G. In Alternative Solvents for Natural Products Extraction; Chemat F., Vian A. M., Eds.; Springer: Berlin, 2014. [Google Scholar]
- Ressmann A. K.; Bica K. In Ionic Liquids for Better Separation Processes; Rodríguez H., Ed.; Springer: Berlin, 2016. [Google Scholar]
- Du F.-Y.; Xiao X.-H.; Li G.-K. Application of Ionic Liquids in the Microwave-assisted Extraction of Trans-Resveratrol from Rhizma Polygoni Cuspidati. J. Chromatogr. A 2007, 1140, 56–62. 10.1016/j.chroma.2006.11.049. [DOI] [PubMed] [Google Scholar]
- Bogdanov M. G.; Svinyarov I. Ionic Liquid-supported Solid–liquid Extraction of Bioactive Alkaloids. II. Kinetics, Modeling and Mechanism of Glaucine Extraction from Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2013, 103, 279–288. 10.1016/j.seppur.2012.10.035. [DOI] [Google Scholar]
- Bogdanov M. G.; Svinyarov I.; Keremedchieva R.; Sidjimov A. Ionic Liquid-supported Solid–liquid Extraction of Bioactive Alkaloids. I. New HPLC Method for Quantitative Determination of Glaucine in Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2012, 97, 221–227. 10.1016/j.seppur.2012.02.001. [DOI] [Google Scholar]
- Claudio A. F. M.; Ferreira A. M.; Freire M. G.; Coutinho J. A. P. Enhanced Extraction of Caffeine from Guarana Seeds using Aqueous Solutions of Ionic Liquids. Green Chem. 2013, 15, 2002–2010. 10.1039/c3gc40437d. [DOI] [Google Scholar]
- Ressmann A. K.; Zirbs R.; Pressler M.; Gaertner P.; Bica K. Surface-active Ionic Liquids for Micellar Extraction of Piperine from Black Pepper. Z. Naturforsch., B: J. Chem. Sci. 2013, 68, 1129–1137. 10.5560/znb.2013-3196. [DOI] [Google Scholar]
- Bogdanov M. G.; Keremedchieva R.; Svinyarov I. Ionic Liquid-supported Solid–liquid Extraction of Bioactive alkaloids. III. Ionic Liquid Regeneration and Glaucine Recovery from Ionic Liquid-aqueous Crude Extract of Glaucium flavum Cr. (Papaveraceae). Sep. Purif. Technol. 2015, 155, 13–19. 10.1016/j.seppur.2015.02.003. [DOI] [Google Scholar]
- Svinyarov I.; Keremedchieva R.; Bogdanov M. G. Ionic Liquid-supported Solid–liquid Extraction of Bioactive Alkaloids. IV. New HPLC Method for Quantitative Determination of Galantamine in Leucojum Aestivum L. (Amaryllidaceae). Sep. Sci. Technol. 2016, 51, 2691–2699. 10.1080/01496395.2016.1171783. [DOI] [Google Scholar]
- Chowdhury S. A.; Vijayaraghavan R.; MacFarlane D. R. Distillable Ionic Liquid Extraction of Tannins from Plant Materials. Green Chem. 2010, 12, 1023–1028. 10.1039/b923248f. [DOI] [Google Scholar]
- Santos J. H. P. M.; e Silva F. A.; Coutinho J. A. P.; Ventura S. P. M.; Pessoa A. Jr Ionic Liquids as a Novel Class of Electrolytes in Polymeric Aqueous Biphasic Systems. Process Biochem. 2015, 50, 661–668. 10.1016/j.procbio.2015.02.001. [DOI] [Google Scholar]
- Ressmann A. K.; Strassl K.; Gaertner P.; Zhao B.; Greiner L.; Bica K. New Aspects for Biomass Processing with Ionic Liquids: Towards the Isolation of Pharmaceutically Active Betulin. Green Chem. 2012, 14, 940–944. 10.1039/c2gc16389f. [DOI] [Google Scholar]
- Ressmann A. K.; Gaertner P.; Bica K. From Plant to Drug: Ionic Liquids for the Reactive Dissolution of Biomass. Green Chem. 2011, 13, 1442–1447. 10.1039/c1gc15058h. [DOI] [Google Scholar]
- Usuki T.; Yasuda N.; Yoshizawa-Fujita M.; Rikukawa M. Extraction and Isolation of Shikimic Acid from Ginkgo Biloba Leaves Utilizing an Ionic Liquid that Dissolves Cellulose. Chem. Commun. 2011, 47, 10560–10562. 10.1039/c1cc13306c. [DOI] [PubMed] [Google Scholar]
- Jin W.; Yang Q.; Huang B.; Bao Z.; Su B.; Ren Q.; Yang Y.; Xing H. Enhanced Solubilization and Extraction of Hydrophobic Bioactive Compounds using Water/Ionic Liquid Mixtures. Green Chem. 2016, 18, 3549–3557. 10.1039/C6GC00584E. [DOI] [Google Scholar]
- Lu Y.; Ma W.; Hu R.; Dai X.; Pan Y. Ionic Liquid-based Microwave-assisted Extraction of Phenolic Alkaloids from the Medicinal Plant Nelumbo Nucifera Gaertn. J. Chromatogr. A 2008, 1208, 42–46. 10.1016/j.chroma.2008.08.070. [DOI] [PubMed] [Google Scholar]
- Ma W.; Lu Y.; Hu R.; Chen J.; Zhang Z.; Pan Y. Application of Ionic Liquids based Microwave-assisted Extraction of Three Alkaloids N-nornuciferine, O-nornuciferine, and Nuciferine from Lotus Leaf. Talanta 2010, 80, 1292–1297. 10.1016/j.talanta.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Xu W.; Chu K.; Li H.; Zhang Y.; Zheng H.; Chen R.; Chen L. Ionic Liquid-based Microwave-assisted Extraction of Flavonoids from Bauhinia championii (Benth.) Benth. Molecules 2012, 17, 14323. 10.3390/molecules171214323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F.-Y.; Xiao X.-H.; Luo X.-J.; Li G.-K. Application of Ionic Liquids in the Microwave-assisted Extraction of Polyphenolic Compounds from Medicinal Plants. Talanta 2009, 78, 1177–1184. 10.1016/j.talanta.2009.01.040. [DOI] [PubMed] [Google Scholar]
- Zeng H.; Wang Y.; Kong J.; Nie C.; Yuan Y. Ionic Liquid-based Microwave-assisted Extraction of Rutin from Chinese Medicinal Plants. Talanta 2010, 83, 582–590. 10.1016/j.talanta.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Gu H.; Chen F.; Zhang Q.; Zang J. Application of Ionic Liquids in Vacuum Microwave-assisted Extraction followed by Macroporous Resin Isolation of Three Flavonoids Rutin, Hyperoside and Hesperidin from Sorbus Tianschanica Leaves. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2016, 1014, 45–55. 10.1016/j.jchromb.2016.01.045. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Wu D.; Cai P.; Cheng G.; Huang C.; Pan Y. Special Effect of Ionic Liquids on the Extraction of Flavonoid Glycosides from Chrysanthemum Morifolium Ramat by Microwave Assistance. Molecules 2015, 20, 7683. 10.3390/molecules20057683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Zhao S.-h.; Chen J.; Zhang L.-w. Application of Ionic Liquid-based Microwave-assisted Extraction of Flavonoids from Scutellaria Baicalensis Georgi. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2015, 1002, 411–417. 10.1016/j.jchromb.2015.08.021. [DOI] [PubMed] [Google Scholar]
- Liu T.; Sui X.; Zhang R.; Yang L.; Zu Y.; Zhang L.; Zhang Y.; Zhang Z. Application of Ionic Liquids based Microwave-assisted Simultaneous Extraction of Carnosic Acid, Rosmarinic Acid and Essential Oil from Rosmarinus Officinalis. J. Chromatogr. A 2011, 1218, 8480–8489. 10.1016/j.chroma.2011.09.073. [DOI] [PubMed] [Google Scholar]
- Yansheng C.; Zhida Z.; Changping L.; Qingshan L.; Peifang Y.; Welz-Biermann U. Microwave-assisted Extraction of Lactones from Ligusticum Chuanxiong Hort. Using Protic Ionic Liquids. Green Chem. 2011, 13, 666–670. 10.1039/c0gc00864h. [DOI] [Google Scholar]
- Ma C.-H.; Liu T.-T.; Yang L.; Zu Y.-G.; Wang S.-Y.; Zhang R.-R. Study on Ionic Liquid-based Ultrasonic-assisted Extraction of Biphenyl Cyclooctene lignans from the Fruit of Schisandra Chinensis Baill. Anal. Chim. Acta 2011, 689, 110–116. 10.1016/j.aca.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Ma C.-H.; Liu T.-T.; Yang L.; Zu Y.-g.; Chen X.; Zhang L.; Zhang Y.; Zhao C. Ionic Liquid-based Microwave-assisted Extraction of Essential Oil and Biphenyl Cyclooctene Lignans from Schisandra Chinensis Baill Fruits. J. Chromatogr. A 2011, 1218, 8573–8580. 10.1016/j.chroma.2011.09.075. [DOI] [PubMed] [Google Scholar]
- Zirbs R.; Strassl K.; Gaertner P.; Schroder C.; Bica K. Exploring Ionic Liquid-biomass Interactions: Towards the Improved Isolation of Shikimic Acid from Star Anise Pods. RSC Adv. 2013, 3, 26010–26016. 10.1039/c3ra45572f. [DOI] [Google Scholar]
- Akinlua A.; Jochmann M. A.; Schmidt T. C. Ionic Liquid as Green Solvent for Leaching of Polycyclic Aromatic Hydrocarbons from Petroleum Source Rock. Ind. Eng. Chem. Res. 2015, 54, 12960–12965. 10.1021/acs.iecr.5b03367. [DOI] [Google Scholar]
- Cao X.; Ye X.; Lu Y.; Yu Y.; Mo W. Ionic Liquid-based Ultrasonic-assisted Extraction of Piperine from White Pepper. Anal. Chim. Acta 2009, 640, 47–51. 10.1016/j.aca.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Ma C.-h.; Wang S.-y.; Yang L.; Zu Y.-g.; Yang F.-j.; Zhao C.-j.; Zhang L.; Zhang Z.-h. Ionic Liquid-aqueous Solution Ultrasonic-assisted extraction of Camptothecin and 10-hydroxycamptothecin from Camptotheca Acuminata Samara. Chem. Eng. Process. 2012, 57–58, 59–64. 10.1016/j.cep.2012.03.008. [DOI] [Google Scholar]
- Yang L.; Wang H.; Zu Y.-g.; Zhao C.; Zhang L.; Chen X.; Zhang Z. Ultrasound-assisted Extraction of the Three Terpenoid Indole Alkaloids Vindoline, Catharanthine and Vinblastine from Catharanthus Roseus using Ionic Liquid Aqueous Solutions. Chem. Eng. J. 2011, 172, 705–712. 10.1016/j.cej.2011.06.039. [DOI] [Google Scholar]
- Zhang L.; Geng Y.; Duan W.; Wang D.; Fu M.; Wang X. Ionic Liquid-based Ultrasound-assisted Extraction of Fangchinoline and Tetrandrine from Stephaniae Tetrandrae. J. Sep. Sci. 2009, 32, 3550–3554. 10.1002/jssc.200900413. [DOI] [PubMed] [Google Scholar]
- Wang W.; Li Q.; Liu Y.; Chen B. Ionic Liquid-aqueous Solution Ultrasonic-assisted Extraction of Three kinds of Alkaloids from Phellodendron Amurense Rupr and Optimize Conditions use Response Surface. Ultrason. Sonochem. 2015, 24, 13–18. 10.1016/j.ultsonch.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Li W.; Wang J. Ionic Liquid Based Ultrasonic Assisted Extraction of Isoflavones from Iris Tectorum Maxim and Subsequently Separation and Purification by High-speed Counter-current Chromatography. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2011, 879, 975–980. 10.1016/j.jchromb.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.; Hou K.; Li S.; Zu Y.; Yang L. Extraction and Chromatographic Determination of Shikimic Acid in Chinese Conifer Needles with 1-benzyl-3-methylimidazolium Bromide Ionic Liquid Aqueous Solutions. J. Anal. Methods Chem. 2014, 2014, 1–12. 10.1155/2014/256473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T.; Lai C.-J.-S.; OuYang H.; He M.-Z.; Feng Y. Ionic Liquid-based Ultrasound-assisted Extraction and Aqueous Two-phase System for Analysis of Caffeoylquinic Acids from Flos Lonicerae Japonicae. J. Pharm. Biomed. Anal. 2016, 120, 134–141. 10.1016/j.jpba.2015.12.019. [DOI] [PubMed] [Google Scholar]
- Wang X.-H.; Cai C.; Li X.-M. Optimal Extraction of Gallic Acid from Suaeda Glauca Bge. Leaves and Enhanced Efficiency by Ionic Liquids. Int. J. Chem. Eng. 2016, 2016, 1–9. 10.1155/2016/5217802. [DOI] [Google Scholar]
- Lin H.; Zhang Y.; Han M.; Yang L. Aqueous Ionic Liquid Based Ultrasonic Assisted Extraction of Eight Ginsenosides from Ginseng Root. Ultrason. Sonochem. 2013, 20, 680–684. 10.1016/j.ultsonch.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Wu K.; Zhang Q.; Liu Q.; Tang F.; Long Y.; Yao S. Ionic Liquid Surfactant-mediated Ultrasonic-assisted Extraction Coupled to HPLC: Application to Analysis of Tanshinones in Salvia Miltiorrhiza Bunge. J. Sep. Sci. 2009, 32, 4220–4226. 10.1002/jssc.200900398. [DOI] [PubMed] [Google Scholar]
- Harde S. M.; Lonkar S. L.; Degani M. S.; Singhal R. S. Ionic Liquid Based Ultrasonic-assisted Extraction of Forskolin from Coleus Forskohlii Roots. Ind. Crops Prod. 2014, 61, 258–264. 10.1016/j.indcrop.2014.07.016. [DOI] [Google Scholar]
- Yang L.; Liu Y.; Zu Y.-G.; Zhao C.-J.; Zhang L.; Chen X.-Q.; Zhang Z.-H. Optimize the Process of Ionic Liquid-based Ultrasonic-assisted Extraction of Aesculin and Aesculetin from Cortex Fraxini by Response Surface Methodology. Chem. Eng. J. 2011, 175, 539–547. 10.1016/j.cej.2011.09.110. [DOI] [Google Scholar]
- Lu H.-T.; Jiang Y.; Chen F. Preparative High-speed Counter-current Chromatography for Purification of Shikonin from the Chinese Medicinal Plant Lithospermum Erythrorhizon. J. Chromatogr. A 2004, 1023, 159–163. 10.1016/j.chroma.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Xiao Y.; Wang Y.; Gao S.; Zhang R.; Ren R.; Li N.; Zhang H. Determination of the Active Constituents in Arnebia Euchroma (Royle) Johnst. by Ionic Liquid-based Ultrasonic-assisted Extraction High-performance Liquid Chromatography. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2011, 879, 1833–1838. 10.1016/j.jchromb.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Lu C.; Wang H.; Lv W.; Ma C.; Lou Z.; Xie J.; Liu B. Ionic Liquid-based Ultrasonic/microwave-assisted Extraction Combined with UPLC–MS–MS for the Determination of Tannins in Galla Chinensis. Nat. Prod. Res. 2012, 26, 1842–1847. 10.1080/14786419.2011.607454. [DOI] [PubMed] [Google Scholar]
- Lu C.; Wang H.; Lv W.; Ma C.; Xu P.; Zhu J.; Xie J.; liu B.; Zhou Q. Ionic Liquid-based Ultrasonic/Microwave-assisted Extraction combined with UPLC for the Determination of Anthraquinones in Rhubarb. Chromatographia 2011, 74, 139–144. 10.1007/s10337-011-2023-5. [DOI] [Google Scholar]
- Liu F.; Wang D.; Liu W.; Wang X.; Bai A.; Huang L. Ionic Liquid-based Ultrahigh Pressure Extraction of Five Tanshinones from Salvia Miltiorrhiza Bunge. Sep. Purif. Technol. 2013, 110, 86–92. 10.1016/j.seppur.2013.03.012. [DOI] [Google Scholar]
- Dong L. L.; Fu Y. J.; Zu Y. G.; Li J.; Li X. J.; Efferth T. Negative Pressure Cavitation Accelerated Processing for Extraction of Main Bioactive Flavonoids from Radix Scutellariae. Chem. Eng. Process. 2011, 50, 780–789. 10.1016/j.cep.2011.05.014. [DOI] [Google Scholar]
- Duan M.-H.; Luo M.; Zhao C.-J.; Wang W.; Zu Y.-G.; Zhang D.-Y.; Yao X.-h.; Fu Y.-J. Ionic Liquid-based Negative Pressure Cavitation-assisted Extraction of Three Main Flavonoids from the Pigeonpea Roots and its Pilot-scale Application. Sep. Purif. Technol. 2013, 107, 26–36. 10.1016/j.seppur.2013.01.003. [DOI] [Google Scholar]
- Wang Z.; Hu J.; Du H.; He S.; Li Q.; Zhang H. Microwave-assisted Ionic Liquid Homogeneous Liquid–liquid Microextraction Coupled with High Performance Liquid Chromatography for the Determination of Anthraquinones in Rheum palmatum L. J. Pharm. Biomed. Anal. 2016, 125, 178–185. 10.1016/j.jpba.2016.03.046. [DOI] [PubMed] [Google Scholar]
- Extraction of Artemisinin using Ionic Liquids; Project Report 003–001/1; Bioniqus Ltd: York, U.K., 2006.
- Extraction of Artemisinin using Ionic Liquids; Project Report 003–003/3; Bioniqus Ltd: York, U.K., 2008.
- Bi W.; Tian M.; Row K. H. Ultrasonication-assisted Extraction and Preconcentration of Medicinal Products from Herb by Ionic Liquids. Talanta 2011, 85, 701–706. 10.1016/j.talanta.2011.04.054. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Antiviral Agents Active Against Influenza A Viruses. Nat. Rev. Drug Discovery 2006, 5, 1015–1025. 10.1038/nrd2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao T.; Komine Y.; Soga S.; Meguro S.; Hase T.; Tanaka Y.; Tokimitsu I. Ingestion of a Tea Rich in Catechins Leads to a Reduction in Body Fat and Malondialdehyde-Modified LDL in Men. Am. J. Clin. Nutr. 2005, 81, 122–129. [DOI] [PubMed] [Google Scholar]
- Ribeiro B. D.; Coelho M. A. Z.; Rebelo L. P. N.; Marrucho I. M. Ionic Liquids as Additives for Extraction of Saponins and Polyphenols from Mate (Ilex paraguariensis) and Tea (Camellia sinensis). Ind. Eng. Chem. Res. 2013, 52, 12146–12153. 10.1021/ie400529h. [DOI] [Google Scholar]
- Freire M. G.; Neves C. M. S. S.; Marrucho I. M.; Coutinho J. A. P.; Fernandes A. M. Hydrolysis of Tetrafluoroborate and Hexafluorophosphate Counter Ions in Imidazolium-based Ionic Liquids. J. Phys. Chem. A 2010, 114, 3744–3749. 10.1021/jp903292n. [DOI] [PubMed] [Google Scholar]
- Absalan G.; Akhond M.; Sheikhian L. Extraction and High Performance Liquid Chromatographic Determination of 3-indole Butyric Acid in Pea Plants by using Imidazolium-based Ionic Liquids as Extractant. Talanta 2008, 77, 407–411. 10.1016/j.talanta.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Tan Z.; Li F.; Xu X. Isolation and Purification of Aloe Anthraquinones based on an Ionic Liquid/Salt Aqueous Two-phase System. Sep. Purif. Technol. 2012, 98, 150–157. 10.1016/j.seppur.2012.06.021. [DOI] [Google Scholar]
- Santos J.; Martins M.; Silvestre A. J. D.; Coutinho J. A. P.; Ventura S. P. M. Fractionation of Phenolic Compounds from Lignin Depolymerisation using Polymeric Aqueous Biphasic Systems with Ionic Surfactants as Electrolytes. Green Chem. 2016, 18, 5569–5579. 10.1039/C6GC01440B. [DOI] [Google Scholar]
- Freire M. G.; Louros C. L. S.; Rebelo L. P. N.; Coutinho J. A. P. Aqueous Biphasic Systems Composed of a Water-stable Ionic Liquid + Carbohydrates and their Applications. Green Chem. 2011, 13, 1536–1545. 10.1039/c1gc15110j. [DOI] [Google Scholar]
- Domínguez-Pérez M.; Tomé L. I. N.; Freire M. G.; Marrucho I. M.; Cabeza O.; Coutinho J. A. P. (Extraction of Biomolecules using) Aqueous Biphasic Systems formed by Ionic Liquids and Aminoacids. Sep. Purif. Technol. 2010, 72, 85–91. 10.1016/j.seppur.2010.01.008. [DOI] [Google Scholar]
- Freire M. G.; Teles A. R. R.; Canongia Lopes J. N.; Rebelo L. P. N.; Marrucho I. M.; Coutinho J. A. P. Partition Coefficients of Alkaloids in Biphasic Ionic-Liquid-Aqueous Systems and their Dependence on the Hofmeister Series. Sep. Sci. Technol. 2012, 47, 284–291. 10.1080/01496395.2011.620585. [DOI] [Google Scholar]
- Freire M. G.; Neves C. M. S. S.; Marrucho I. M.; Canongia Lopes J. N.; Rebelo L. P. N.; Coutinho J. A. P. High-performance Extraction of Alkaloids using Aqueous Two-phase Systems with Ionic Liquids. Green Chem. 2010, 12, 1715–1718. 10.1039/c0gc00179a. [DOI] [Google Scholar]
- Santos P. L.; Santos L. N. S.; Ventura S. P. M.; de Souza R. L.; Coutinho J. A. P.; Soares C. M. F.; Lima A. S. Recovery of Capsaicin from Capsicum frutescens by Applying Aqueous Two-phase Systems based on Acetonitrile and Cholinium-based Ionic Liquids. Chem. Eng. Res. Des. 2016, 112, 103–112. 10.1016/j.cherd.2016.02.031. [DOI] [Google Scholar]
- Li S.; He C.; Liu H.; Li K.; Liu F. Ionic Liquid-based Aqueous two-phase System, A Sample Pretreatment Procedure prior to High-performance Liquid Chromatography of Opium Alkaloids. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2005, 826, 58–62. 10.1016/j.jchromb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Santos J. H.; e Silva F. A.; Ventura S. P. M.; Coutinho J. A. P.; de Souza R. L.; Soares C. M. F.; Lima A. S. Ionic Liquid-based Aqueous Biphasic Systems as a Versatile Tool for the Recovery of Antioxidant Compounds. Biotechnol. Prog. 2015, 31, 70–77. 10.1002/btpr.2000. [DOI] [PubMed] [Google Scholar]
- Wang R.; Chang Y.; Tan Z.; Li F. Applications of Choline amino Acid Ionic Liquid in Extraction and Separation of Flavonoids and Pectin from Ponkan Peels. Sep. Sci. Technol. 2016, 51, 1093–1102. 10.1080/01496395.2016.1143006. [DOI] [Google Scholar]
- Cláudio A. F. M.; Ferreira A. M.; Freire C. S. R.; Silvestre A. J. D.; Freire M. G.; Coutinho J. A. P. Optimization of the Gallic Acid Extraction using Ionic-liquid-based Aqueous Two-phase Systems. Sep. Purif. Technol. 2012, 97, 142–149. 10.1016/j.seppur.2012.02.036. [DOI] [Google Scholar]
- Claudio A. F. M.; Marques C. F. C.; Boal-Palheiros I.; Freire M. G.; Coutinho J. A. P. Development of Back-extraction and Recyclability Routes for Ionic-liquid-based Aqueous Two-phase Systems. Green Chem. 2014, 16, 259–268. 10.1039/C3GC41999A. [DOI] [Google Scholar]
- Almeida M. R.; Passos H.; Pereira M. M.; Lima Á. S.; Coutinho J. A. P.; Freire M. G. Ionic Liquids as Additives to Enhance the Extraction of Antioxidants in Aqueous Two-phase Systems. Sep. Purif. Technol. 2014, 128, 1–10. 10.1016/j.seppur.2014.03.004. [DOI] [Google Scholar]
- Keremedchieva R.; Svinyarov I.; Bogdanov M. Ionic Liquid-based Aqueous Biphasic Systems—A Facile Approach for Ionic Liquid Regeneration from Crude Plant Extracts. Processes 2015, 3, 769. 10.3390/pr3040769. [DOI] [Google Scholar]
- Smirnova S. V.; Torocheshnikova I. I.; Formanovsky A. A.; Pletnev I. V. Solvent Extraction of Amino Acids into a Room Temperature Ionic Liquid with Dicyclohexano-18-Crown-6. Anal. Bioanal. Chem. 2004, 378, 1369–1375. 10.1007/s00216-003-2398-8. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Chen M.; Shentu C.; El-Sepai F.; Wang K.; Zhu Y.; Ye M. Ionic Liquids Extraction of Para Red and Sudan Dyes from Chilli Powder, Chilli Oil and Food Additive Combined with High Performance Liquid Chromatography. Anal. Chim. Acta 2009, 650, 65–69. 10.1016/j.aca.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Das A. K.; Prasad K. Extraction of Plant Growth Regulators Present in Kappaphycus Alvarezii Sap using Imidazolium Based Ionic Liquids: Detection and Quantification by using HPLC-DAD Technique. Anal. Methods 2015, 7, 9064–9067. 10.1039/C5AY02210J. [DOI] [Google Scholar]
- Larriba M.; Omar S.; Navarro P.; Garcia J.; Rodriguez F.; Gonzalez-Miquel M. Recovery of Tyrosol from Aqueous Streams using Hydrophobic Ionic Liquids: A First Step Towards Developing Sustainable Processes for Olive Mill Wastewater (OMW) Management. RSC Adv. 2016, 6, 18751–18762. 10.1039/C5RA26510J. [DOI] [Google Scholar]
- Cláudio A. F. M.; Freire M. G.; Freire C. S. R.; Silvestre A. J. D.; Coutinho J. A. P. Extraction of Vanillin using Ionic-liquid-based Aqueous Two-phase Systems. Sep. Purif. Technol. 2010, 75, 39–47. 10.1016/j.seppur.2010.07.007. [DOI] [Google Scholar]
- Albertsson P.Partition of Cell Particles and Macromolecules: Separation and Purification of Biomolecules, Cell Organelles, Membranes, and Cells in Aqueous Polymer Two-phase Systems and Their Use in Biochemical Analysis and Biotechnology, 3rd ed.; John Wiley and Sons: Chichester, 1987. [Google Scholar]
- Gutowski K. E.; Broker G. A.; Willauer H. D.; Huddleston J. G.; Swatloski R. P.; Holbrey J. D.; Rogers R. D. Controlling the Aqueous Miscibility of Ionic Liquids: Aqueous Biphasic Systems of Water-miscible Ionic Liquids and Water-structuring Salts for Recycle, Metathesis, and Separations. J. Am. Chem. Soc. 2003, 125, 6632–6633. 10.1021/ja0351802. [DOI] [PubMed] [Google Scholar]
- Passos H.; Trindade M. P.; Vaz T. S. M.; da Costa L. P.; Freire M. G.; Coutinho J. A. P. The Impact of Self-aggregation on the Extraction of Biomolecules in Ionic-liquid-based Aqueous Two-phase Systems. Sep. Purif. Technol. 2013, 108, 174–180. 10.1016/j.seppur.2013.02.008. [DOI] [Google Scholar]
- Louros C. L. S.; Cláudio A. F. M.; Neves C. M. S. S.; Freire M. G.; Marrucho I. M.; Pauly J.; Coutinho J. A. P. Extraction of Biomolecules using Phosphonium-based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. Int. J. Mol. Sci. 2010, 11, 1777. 10.3390/ijms11041777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J.-P.; Cao J.; Zhang X.-H.; Huang J.-Z.; Kong T.; Tong S.; Tian Z.-Y.; Zhu J.-H.; Ouyang X.-K. Extraction of Puerarin using Ionic Liquid Based Aqueous Two-phase Systems. Sep. Sci. Technol. 2012, 47, 1740–1747. 10.1080/01496395.2012.659787. [DOI] [Google Scholar]
- Yang Z.; Tan Z.; Li F.; Li X. An Effective Method for the Extraction and Purification of Chlorogenic Acid from Ramie (Boehmeria nivea L.) Leaves using Acidic Ionic Liquids. Ind. Crops Prod. 2016, 89, 78–86. 10.1016/j.indcrop.2016.05.006. [DOI] [Google Scholar]
- Tan Z.; Yi Y.; Wang H.; Zhou W.; Wang C. Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves using Ionic Liquid-based Ultrasonic-assisted Extraction Coupled with an Aqueous Biphasic System. Molecules 2016, 21, 262. 10.3390/molecules21030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S. P. M.; e Silva F. A.; Gonçalves A. M. M.; Pereira J. L.; Gonçalves F.; Coutinho J. A. P. Ecotoxicity Analysis of Cholinium-based Ionic Liquids to Vibrio fischeri Marine Bacteria. Ecotoxicol. Environ. Saf. 2014, 102, 48–54. 10.1016/j.ecoenv.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Petkovic M.; Ferguson J. L.; Gunaratne H. Q. N.; Ferreira R.; Leitao M. C.; Seddon K. R.; Rebelo L. P. N.; Pereira C. S. Novel Biocompatible Cholinium-based Ionic Liquids-toxicity and Biodegradability. Green Chem. 2010, 12, 643–649. 10.1039/b922247b. [DOI] [Google Scholar]
- Taha M.; e Silva F. A.; Quental M. V.; Ventura S. P. M.; Freire M. G.; Coutinho J. A. P. Good’s Buffers as a Basis for Developing Self-buffering and Biocompatible Ionic Liquids for Biological Research. Green Chem. 2014, 16, 3149–3159. 10.1039/c4gc00328d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao D.-J.; Cheng Z.; Chen F.-F.; Li Z.-M.; Hu N.; Chen X.-S. Synthesis and Thermophysical Properties of Biocompatible Cholinium-based Amino Acid Ionic Liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. 10.1021/je301103d. [DOI] [Google Scholar]
- Ventura S. P. M.; de Barros R. L. F.; de Pinho Barbosa J. M.; Soares C. M. F.; Lima A. S.; Coutinho J. A. P. Production and Purification of an Extracellular Lipolytic Enzyme using Ionic Liquid-based Aqueous Two-phase Systems. Green Chem. 2012, 14, 734–740. 10.1039/c2gc16428k. [DOI] [Google Scholar]
- Souza R. L.; Ventura S. P. M.; Soares C. M. F.; Coutinho J. A. P.; Lima A. S. Lipase Purification using Ionic Liquids as Adjuvants in Aqueous Two-phase Systems. Green Chem. 2015, 17, 3026–3034. 10.1039/C5GC00262A. [DOI] [Google Scholar]
- Tian M.; Yan H.; Row K. H. Solid-phase Extraction of Tanshinones from Salvia Miltiorrhiza Bunge using Ionic Liquid-modified Silica Sorbents. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2009, 877, 738–742. 10.1016/j.jchromb.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Luo H.-W.; Wu B.-J.; Wu M.-Y.; Yong Z.-G.; Niwa M.; Hirata Y. Pigments from Salvia Miltiorrhiza. Phytochemistry 1985, 24, 815–817. 10.1016/S0031-9422(00)84900-6. [DOI] [Google Scholar]
- Tian M.; Row K. H. SPE of Tanshinones from Salvia miltiorrhiza Bunge by using Imprinted Functionalized Ionic Liquid-modified Silica. Chromatographia 2011, 73, 25–31. 10.1007/s10337-010-1836-y. [DOI] [Google Scholar]
- Tian M.; Bi W.; Row K. H. Solid-phase Extraction of Liquiritin and Glycyrrhizic Acid from Licorice using Ionic Liquid-based Silica Sorbent. J. Sep. Sci. 2009, 32, 4033–4039. 10.1002/jssc.200900497. [DOI] [PubMed] [Google Scholar]
- Bi W.; Zhou J.; Row K. H. Solid Phase Extraction of Three Phenolic Acids from Saliconia Herbacea L. by Different Ionic Liquid-based Silicas. J. Liq. Chromatogr. Relat. Technol. 2012, 35, 723–736. 10.1080/10826076.2011.608230. [DOI] [Google Scholar]
- Bi W.; Tian M.; Row K. H. Selective Extraction and Separation of Oxymatrine from Sophora Flavescens Ait. Extract by Silica-confined Ionic Liquid. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2012, 880, 108–113. 10.1016/j.jchromb.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Nie L.-r.; Lu J.; Zhang W.; He A.; Yao S. Ionic Liquid-modified Silica Gel as Adsorbents for Adsorption and Separation of Water-soluble Phenolic Acids from Salvia militiorrhiza Bunge. Sep. Purif. Technol. 2015, 155, 2–12. 10.1016/j.seppur.2015.01.037. [DOI] [Google Scholar]
- Bi W.; Tian M.; Row K. H. Solid-phase Extraction of Matrine and Oxymatrine from Sophora Flavescens Ait using Amino-imidazolium Polymer. J. Sep. Sci. 2010, 33, 1739–1745. 10.1002/jssc.200900835. [DOI] [PubMed] [Google Scholar]
- Tian M.; Yan H.; Row K. H. Solid-phase Extraction of Caffeine and Theophylline from Green Tea by a New Ionic Liquid-modified Functional Polymer Sorbent. Anal. Lett. 2009, 43, 110–118. 10.1080/00032710903276554. [DOI] [Google Scholar]
- Bi W.; Tian M.; Row K. H. Separation of Phenolic Acids from Natural Plant Extracts using Molecularly Imprinted Anion-exchange Polymer Confined Ionic Liquids. J. Chromatogr. A 2012, 1232, 37–42. 10.1016/j.chroma.2011.08.054. [DOI] [PubMed] [Google Scholar]
- Bi W.; Tian M.; Row K. H. Solid-phase Extraction of Liquiritin and Glycyrrhizin from Licorice using Porous Alkyl-pyridinium Polymer Sorbent. Phytochem. Anal. 2010, 21, 496–501. 10.1002/pca.1227. [DOI] [PubMed] [Google Scholar]
- Wan X.; Tian M.; Row K. H. Ionic Liquid-modified Silica as a New Stationary Phase for Chromatographic Separation. J. Anal. Chem. 2010, 65, 798–802. 10.1134/S1061934810080058. [DOI] [Google Scholar]
- Tian M.; Bi W.; Row K. H. Molecular Imprinting in Ionic Liquid-modified Porous Polymer for Recognitive Separation of Three Tanshinones from Salvia miltiorrhiza Bunge. Anal. Bioanal. Chem. 2011, 399, 2495–2502. 10.1007/s00216-010-4641-4. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Bi W.; Row K. H. Optimization and Development of a SPE-HPLC-UV Method to Determine Astaxanthin in Saccharina japonica. J. Food Sci. 2011, 76, C441–C446. 10.1111/j.1750-3841.2011.02076.x. [DOI] [PubMed] [Google Scholar]
- Bucar F.; Wube A.; Schmid M. Natural Product Isolation - How to get from Biological Material to Pure Compounds. Nat. Prod. Rep. 2013, 30, 525–545. 10.1039/c3np20106f. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Yu G.; Wang W.-J.; Ren Q.; Li B.-G.; Zhu S. Design and Synthesis of Thermoresponsive Ionic Liquid Polymer in Acetonitrile as a Reusable Extractant for Separation of Tocopherol Homologues. Macromolecules 2015, 48, 915–924. 10.1021/ma502611s. [DOI] [Google Scholar]
- Nelson D. L.; Lehninger A. L.; Cox M. M.. Lehninger Principles of Biochemistry; W. H. Freeman, 2008. [Google Scholar]
- Global Oils and Fats Market 2015–2019, http://www.researchandmarkets.com/research/kk5dw5/global_oils_and (accessed on March, 15 2016).
- Cooney M. J.; Benjamin K. In Ionic Liquids in Lipid Processing and Analysis; AOCS Press, 2016. [Google Scholar]
- Pfaltzgraff L. A.; De bruyn M.; Cooper E. C.; Budarin V.; Clark J. H. Food Waste Biomass: A Resource for High-value Chemicals. Green Chem. 2013, 15, 307–314. 10.1039/c2gc36978h. [DOI] [Google Scholar]
- Xu X.; Guo Z.; Cheong L.-Z. In Ionic Liquids in Lipid Processing and Analysis; AOCS Press, 2016. [Google Scholar]
- Li M.; Li T. Enrichment of Omega-3 Polyunsaturated Fatty Acid Methyl Esters by Ionic Liquids Containing Silver Salts. Sep. Sci. Technol. 2008, 43, 2072–2089. 10.1080/01496390802064174. [DOI] [Google Scholar]
- Li M.; Pittman C. U. Jr.; Li T. Extraction of Polyunsaturated Fatty Acid Methyl Esters by Imidazolium-based Ionic Liquids Containing Silver Tetrafluoroborate—Extraction Equilibrium Studies. Talanta 2009, 78, 1364–1370. 10.1016/j.talanta.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Li M.; Pham P. J.; Wang T.; Pittman C. U. Jr; Li T. Selective Extraction and Enrichment of Polyunsaturated Fatty Acid Methyl Esters from Fish Oil by Novel π-Complexing Sorbents. Sep. Purif. Technol. 2009, 66, 1–8. 10.1016/j.seppur.2008.12.009. [DOI] [Google Scholar]
- Li M.; Pham P. J.; Pittman C. U. Jr; Li T. SBA-15-supported Ionic Liquid Compounds Containing Silver Salts: Novel Mesoporous π-complexing Sorbents for Separating Polyunsaturated Fatty Acid Methyl Esters. Microporous Mesoporous Mater. 2009, 117, 436–443. 10.1016/j.micromeso.2008.07.017. [DOI] [Google Scholar]
- Cheong L.-Z.; Guo Z.; Yang Z.; Chua S.-C.; Xu X. Extraction and Enrichment of n-3 Polyunsaturated Fatty Acids and Ethyl Esters through Reversible π–π Complexation with Aromatic Rings Containing Ionic Liquids. J. Agric. Food Chem. 2011, 59, 8961–8967. 10.1021/jf202043w. [DOI] [PubMed] [Google Scholar]
- Lateef H.; Grimes S.; Kewcharoenwong P.; Bailey E. Ionic Liquids in the Selective Recovery of Fat from Composite Foodstuffs. J. Chem. Technol. Biotechnol. 2009, 84, 1681–1687. 10.1002/jctb.2230. [DOI] [Google Scholar]
- Bollin P. M.; Viamajala S. Reactive Extraction of Triglycerides as Fatty Acid Methyl Esters using Lewis Acidic Chloroaluminate Ionic Liquids. Energy Fuels 2012, 26, 6411–6418. 10.1021/ef301101d. [DOI] [Google Scholar]
- Olkiewicz M.; Plechkova N. V.; Fabregat A.; Stüber F.; Fortuny A.; Font J.; Bengoa C. Efficient Extraction of Lipids from Primary Sewage Sludge using Ionic Liquids for Biodiesel Production. Sep. Purif. Technol. 2015, 153, 118–125. 10.1016/j.seppur.2015.08.038. [DOI] [Google Scholar]
- Teixeira R. E. Energy-efficient Extraction of Fuel and Chemical Feedstocks from Algae. Green Chem. 2012, 14, 419–427. 10.1039/c2gc16225c. [DOI] [Google Scholar]
- Fujita K.; Kobayashi D.; Nakamura N.; Ohno H. Direct Dissolution of Wet and Saliferous Marine Microalgae by Polar Ionic Liquids without Heating. Enzyme Microb. Technol. 2013, 52, 199–202. 10.1016/j.enzmictec.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Yu X.; Yang J.; Lu H.; Tu S.-T.; Yan J. Energy-efficient Extraction of Fuel from Chlorella Vulgaris by Ionic Liquid Combined with CO2 Capture. Appl. Energy 2015, 160, 648–655. 10.1016/j.apenergy.2015.04.074. [DOI] [Google Scholar]
- Kilulya K. F.; Msagati T. A. M.; Mamba B. B. Ionic Liquid-Based Extraction of Fatty Acids from Blue-Green Algal Cells Enhanced by Direct Transesterification and Determination using GC × GC-TOFMS. Chromatographia 2014, 77, 479–486. 10.1007/s10337-014-2632-x. [DOI] [Google Scholar]
- Young G.; Nippgen F.; Titterbrandt S.; Cooney M. J. Lipid Extraction from Biomass using Co-solvent Mixtures of Ionic Liquids and Polar Covalent Molecules. Sep. Purif. Technol. 2010, 72, 118–121. 10.1016/j.seppur.2010.01.009. [DOI] [Google Scholar]
- Severa G.; Kumar G.; Cooney M. J. Corecovery of Bio-Oil and Fermentable Sugars from Oil-Bearing Biomass. Int. J. Chem. Eng. 2013, 2013, 1–10. 10.1155/2013/617274. [DOI] [Google Scholar]
- Severa G.; Kumar G.; Troung M.; Young G.; Cooney M. J. Simultaneous Extraction and Separation of Phorbol Esters and Bio-oil from Jatropha Biomass using Ionic Liquid–methanol co-solvents. Sep. Purif. Technol. 2013, 116, 265–270. 10.1016/j.seppur.2013.06.001. [DOI] [Google Scholar]
- Severa G.; Kumar G.; Cooney M. J. Corecovery of Lipids and Fermentable Sugars from Rhodosporidium Toruloides using Ionic Liquid Cosolvents: Application of Recycle to Batch Fermentation. Biotechnol. Prog. 2014, 30, 1239–1242. 10.1002/btpr.1952. [DOI] [PubMed] [Google Scholar]
- Kim Y.-H.; Choi Y.-K.; Park J.; Lee S.; Yang Y.-H.; Kim H. J.; Park T.-J.; Hwan Kim Y.; Lee S. H. Ionic Liquid-mediated Extraction of Lipids from Algal Biomass. Bioresour. Technol. 2012, 109, 312–315. 10.1016/j.biortech.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Chen X.; Hu L.; Xing R.; Liu S.; Yu H.; Qin Y.; Li K.; Li R.; Li P. Ionic Liquid-assisted Subcritical Water Promotes the Extraction of Lipids from Wet Microalgae Scenedesmus sp. Eur. J. Lipid Sci. Technol. 2015, 117, 1192–1198. 10.1002/ejlt.201400189. [DOI] [Google Scholar]
- Olkiewicz M.; Caporgno M. P.; Font J.; Legrand J.; Lepine O.; Plechkova N. V.; Pruvost J.; Seddon K. R.; Bengoa C. A Novel Recovery Process for Lipids from Microalgae for Biodiesel Production using a Hydrated Phosphonium Ionic Liquid. Green Chem. 2015, 17, 2813–2824. 10.1039/C4GC02448F. [DOI] [Google Scholar]
- Choi S.-A.; Lee J.-S.; Oh Y.-K.; Jeong M.-J.; Kim S. W.; Park J.-Y. Lipid Extraction from Chlorella vulgaris by Molten-salt/ionic-liquid Mixtures. Algal Res. 2014, 3, 44–48. 10.1016/j.algal.2013.11.013. [DOI] [Google Scholar]
- Choi S.-A.; Jung J.-Y.; Kim K.; Kwon J.-H.; Lee J.-S.; Kim S. W.; Park J.-Y.; Yang J.-W. Effects of Molten-salt/ionic-liquid Mixture on Extraction of Docosahexaenoic Acid (DHA)-rich Lipids from Aurantiochytrium sp. KRS101. Bioprocess Biosyst. Eng. 2014, 37, 2199–2204. 10.1007/s00449-014-1197-2. [DOI] [PubMed] [Google Scholar]
- Choi S.-A.; Oh Y.-K.; Jeong M.-J.; Kim S. W.; Lee J.-S.; Park J.-Y. Effects of Ionic Liquid Mixtures on Lipid Extraction from Chlorella vulgaris. Renewable Energy 2014, 65, 169–174. 10.1016/j.renene.2013.08.015. [DOI] [Google Scholar]
- Kim Y.-H.; Park S.; Kim M. H.; Choi Y.-K.; Yang Y.-H.; Kim H. J.; Kim H.; Kim H.-S.; Song K.-G.; Lee S. H. Ultrasound-assisted Extraction of Lipids from Chlorella vulgaris using [Bmim][MeSO4]. Biomass Bioenergy 2013, 56, 99–103. 10.1016/j.biombioe.2013.04.022. [DOI] [Google Scholar]
- Essential Oil Market Size To Reach $11.67 Billion By 2022: Grand View Research, Inc., http://www.prnewswire.com/news-releases/essential-oil-market-size-to-reach-1167-billion-by-2022-grand-view-research-inc-531216151.html (accessed on March 24, 2016).
- Zhai Y.; Sun S.; Wang Z.; Cheng J.; Sun Y.; Wang L.; Zhang Y.; Zhang H.; Yu A. Microwave Extraction of Essential Oils from Dried Fruits of Illicium verum Hook. f. and Cuminum Cyminum L. using Ionic Liquid as the Microwave Absorption Medium. J. Sep. Sci. 2009, 32, 3544–3549. 10.1002/jssc.200910204. [DOI] [PubMed] [Google Scholar]
- Zhai Y.; Sun S.; Song D.; Sun Y.; Zhang Y.; Liu H.; Zhang H.; Yu A. Rapid Extraction of Essential Oil from Dried Cinnamomum cassia Presl and Forsythia Suspensa (Thunb.) Vahl by Ionic Liquid Microwave Extraction. Chin. J. Chem. 2010, 28, 2513–2519. 10.1002/cjoc.201190031. [DOI] [Google Scholar]
- Liu Y.; Yang L.; Zu Y.; Zhao C.; Zhang L.; Zhang Y.; Zhang Z.; Wang W. Development of an Ionic Liquid-based Microwave-assisted Method for Simultaneous Extraction and Distillation for Determination of Proanthocyanidins and Essential Oil in Cortex Cinnamomi. Food Chem. 2012, 135, 2514–2521. 10.1016/j.foodchem.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Jiao J.; Gai Q.-Y.; Fu Y.-J.; Zu Y.-G.; Luo M.; Wang W.; Zhao C.-J. Microwave-assisted Ionic Liquids Pretreatment followed by Hydro-distillation for the Efficient Extraction of Essential Oil from Dryopteris Fragrans and Evaluation of its Antioxidant Efficacy in Sunflower Oil Storage. J. Food Eng. 2013, 117, 477–485. 10.1016/j.jfoodeng.2012.10.024. [DOI] [Google Scholar]
- Jiao J.; Gai Q.-Y.; Fu Y.-J.; Zu Y.-G.; Luo M.; Zhao C.-J.; Li C.-Y. Microwave-assisted Ionic Liquids Treatment followed by Hydro-distillation for the Efficient Isolation of Essential Oil from Fructus Forsythiae Seed. Sep. Purif. Technol. 2013, 107, 228–237. 10.1016/j.seppur.2013.01.009. [DOI] [Google Scholar]
- Bica K.; Gaertner P.; Rogers R. D. Ionic Liquids and Fragrances - Direct Isolation of Orange Essential Oil. Green Chem. 2011, 13, 1997–1999. 10.1039/c1gc15237h. [DOI] [Google Scholar]
- Li Z.-Y.; Zhang S.-S.; Jie X.; Qin X.-M. Effect of Lithium Salts Addition on the Ionic Liquid Based Extraction of Essential Oil from Farfarae Flos. J. Pharm. Biomed. Anal. 2015, 102, 509–513. 10.1016/j.jpba.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Flamini G.; Melai B.; Pistelli L.; Chiappe C. How to Make a Green Product Greener: use of Ionic Liquids as Additives During Essential Oil Hydrodistillation. RSC Adv. 2015, 5, 69894–69898. 10.1039/C5RA12649E. [DOI] [Google Scholar]
- Arce A.; Marchiaro A.; Rodríguez O.; Soto A. Essential Oil Terpenless by Extraction using Organic Solvents or Ionic Liquids. AIChE J. 2006, 52, 2089–2097. 10.1002/aic.10844. [DOI] [Google Scholar]
- Arce A.; Pobudkowska A.; Rodríguez O.; Soto A. Citrus Essential Oil Terpenless by Extraction using 1-ethyl-3-methylimidazolium Ethylsulfate Ionic Liquid: Effect of the Temperature. Chem. Eng. J. 2007, 133, 213–218. 10.1016/j.cej.2007.01.035. [DOI] [Google Scholar]
- Francisco M.; Lago S.; Soto A.; Arce A. Essential Oil Deterpenation by Solvent Extraction using 1-ethyl-3-methylimidazolium 2-(2-methoxyethoxy) Ethylsulfate Ionic Liquid. Fluid Phase Equilib. 2010, 296, 149–153. 10.1016/j.fluid.2010.03.019. [DOI] [Google Scholar]
- Lago S.; Rodríguez H.; Soto A.; Arce A. Deterpenation of Citrus Essential Oil by Liquid–Liquid Extraction with 1-Alkyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)amide Ionic Liquids. J. Chem. Eng. Data 2011, 56, 1273–1281. 10.1021/je1011339. [DOI] [Google Scholar]
- Lago S.; Rodríguez H.; Soto A.; Arce A. Alkylpyridinium Alkylsulfate Ionic Liquids as Solvents for the Deterpenation of Citrus Essential Oil. Sep. Sci. Technol. 2012, 47, 292–299. 10.1080/01496395.2011.625388. [DOI] [Google Scholar]
- Lago S.; Rodríguez H.; Arce A.; Soto A. Improved Concentration of Citrus Essential Oil by Solvent Extraction with Acetate Ionic Liquids. Fluid Phase Equilib. 2014, 361, 37–44. 10.1016/j.fluid.2013.10.036. [DOI] [Google Scholar]
- Matsuda H.; Norizuki Y.; Kawai M.; Kurihara K.; Tochigi K.; Ochi K. Liquid–Liquid Equilibria for Extraction of Citrus Essential Oil Using Ionic Liquids. J. Solution Chem. 2014, 43, 1561–1573. 10.1007/s10953-014-0184-1. [DOI] [Google Scholar]
- Martins M. A. R.; Domańska U.; Schröder B.; Coutinho J. A. P.; Pinho S. P. Selection of Ionic Liquids to be Used as Separation Agents for Terpenes and Terpenoids. ACS Sustainable Chem. Eng. 2016, 4, 548–556. 10.1021/acssuschemeng.5b01357. [DOI] [Google Scholar]
- Carotenoids Market worth $1,428.12 Million by 2019, http://www.marketsandmarkets.com/PressReleases/carotenoid.asp (accessed on March 24, 2016).
- Bi W.; Tian M.; Zhou J.; Row K. H. Task-specific Ionic Liquid-assisted Extraction and Separation of Astaxanthin from Shrimp Waste. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2010, 878, 2243–2248. 10.1016/j.jchromb.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Lee Y. J.; Lee Y. R.; Tang B.; Row K. H. Optimization of Extraction of Astaxanthin from Portunus Trituberculatus by Ionic Liquids. Korean Soc. Biotechnol. Bioeng. J. 2013, 28, 238–243. 10.7841/ksbbj.2013.28.4.238. [DOI] [Google Scholar]
- Praveenkumar R.; Lee K.; Lee J.; Oh Y.-K. Breaking Dormancy: an Energy-efficient Means of Recovering Astaxanthin from Microalgae. Green Chem. 2015, 17, 1226–1234. 10.1039/C4GC01413H. [DOI] [Google Scholar]
- Desai R. K.; Streefland M.; Wijffels R. H.; Eppink M. H. M. Novel Astaxanthin Extraction from Haematococcus Pluvialis using Cell Permeabilising Ionic Liquids. Green Chem. 2016, 18, 1261–1267. 10.1039/C5GC01301A. [DOI] [Google Scholar]
- Martins P. L.; de Rosso V. V.. Carotenoids Achieving from Tomatoes Discarded using Ionic Liquids as Extracting for Application in Food Industry; XIV Safety, Health and Environment World Congress, Cubatão, São Paulo, July 20–23, 2014; Science and Education Research Council: Great Britain, 2014; 35–38.
- Lovejoy K. S.; Davis L. E.; McClellan L. M.; Lillo A. M.; Welsh J. D.; Schmidt E. N.; Sanders C. K.; Lou A. J.; Fox D. T.; Koppisch A. T.; et al. Evaluation of Ionic Liquids on Phototrophic Microbes and their use in Biofuel Extraction and Isolation. J. Appl. Phycol. 2013, 25, 973–981. 10.1007/s10811-012-9907-0. [DOI] [Google Scholar]
- Montalvo-Hernández B.; Rito-Palomares M.; Benavides J. Recovery of Crocins from Saffron Stigmas (Crocus sativus) in Aqueous Two-phase Systems. J. Chromatogr. A 2012, 1236, 7–15. 10.1016/j.chroma.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Ribeiro B. D.; Coelho M. A. Z.; Marrucho I. M. Extraction of Saponins from Sisal (Agave sisalana) and Juá (Ziziphus joazeiro) with Cholinium-based Ionic Liquids and Deep eutectic Solvents. Eur. Food Res. Technol. 2013, 237, 965–975. 10.1007/s00217-013-2068-9. [DOI] [Google Scholar]
- Wang P.; Ma C.; Chen S.; Zhu S.; Lou Z.; Wang H. Ionic Liquid-based Ultrasonic/microwave-assisted Extraction of Steroidal Saponins from Dioscorea Zingiberensis CH Wright. Trop. J. Pharm. Res. 2014, 13, 1339–1345. 10.4314/tjpr.v13i8.20. [DOI] [Google Scholar]
- Yang Q.; Xing H.; Cao Y.; Su B.; Yang Y.; Ren Q. Selective Separation of Tocopherol Homologues by Liquid–Liquid Extraction Using Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 6417–6422. 10.1021/ie801847e. [DOI] [Google Scholar]
- Yang Q.; Xing H.; Su B.; Yu K.; Bao Z.; Yang Y.; Ren Q. Improved Separation Efficiency using Ionic Liquid–cosolvent Mixtures as the Extractant in liquid–liquid Extraction: A Multiple Adjustment and Synergistic Effect. Chem. Eng. J. 2012, 181–182, 334–342. 10.1016/j.cej.2011.11.089. [DOI] [Google Scholar]
- Yang Q.; Xing H.; Su B.; Bao Z.; Wang J.; Yang Y.; Ren Q. The Essential Role of Hydrogen-bonding Interaction in the Extractive Separation of Phenolic Compounds by Ionic Liquid. AIChE J. 2013, 59, 1657–1667. 10.1002/aic.13939. [DOI] [Google Scholar]
- Liang R.; Bao Z.; Su B.; Xing H.; Yang Q.; Yang Y.; Ren Q. Feasibility of Ionic Liquids as Extractants for Selective Separation of Vitamin D3 and Tachysterol3 by Solvent Extraction. J. Agric. Food Chem. 2013, 61, 3479–3487. 10.1021/jf305558b. [DOI] [PubMed] [Google Scholar]
- Neves C. M.; Ventura S. P.; Freire M. G.; Marrucho I. M.; Coutinho J. A. Evaluation of Cation Influence on the Formation and Extraction Capability of Ionic-liquid-based Aqueous Biphasic Systems. J. Phys. Chem. B 2009, 113, 5194–5199. 10.1021/jp900293v. [DOI] [PubMed] [Google Scholar]
- Ventura S. P. M.; Neves C. M. S. S.; Freire M. G.; Marrucho I. M.; Oliveira J.; Coutinho J. A. P. Evaluation of Anion Influence on the Formation and Extraction Capacity of Ionic-Liquid-Based Aqueous Biphasic Systems. J. Phys. Chem. B 2009, 113, 9304–9310. 10.1021/jp903286d. [DOI] [PubMed] [Google Scholar]
- Li Z.; Pei Y.; Liu L.; Wang J. (Liquid + Liquid) Equilibria for (Acetate-based Ionic Liquids + Inorganic Salts) Aqueous Two-phase Systems. J. Chem. Thermodyn. 2010, 42, 932–937. 10.1016/j.jct.2010.03.010. [DOI] [Google Scholar]
- Pei Y.; Li Z.; Liu L.; Wang J. Partitioning Behavior of Amino Acids in Aqueous Two-phase Systems formed by Imidazolium Ionic Liquid and Dipotassium Hydrogen Phosphate. J. Chromatogr. A 2012, 1231, 2–7. 10.1016/j.chroma.2012.01.087. [DOI] [PubMed] [Google Scholar]
- Louros C. L. S.; Cláudio A. F. M.; Neves C. M. S. S.; Freire M. G.; Marrucho I. M.; Pauly J.; Coutinho J. A. P. Extraction of Biomolecules using Phosphonium-Based Ionic Liquids + K3PO4 Aqueous Biphasic Systems. Int. J. Mol. Sci. 2010, 11, 1777–1791. 10.3390/ijms11041777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y.; Xing H.; Yang Q.; Bao Z.; Su B.; Ren Q. Aqueous Biphasic System Containing Long Chain Anion-Functionalized Ionic Liquids for High-Performance Extraction. ACS Sustainable Chem. Eng. 2015, 3, 3365–3372. 10.1021/acssuschemeng.5b01068. [DOI] [Google Scholar]
- Freire M. G.; Neves C. M. S. S.; Canongia Lopes J. N.; Marrucho I. M.; Coutinho J. A. P.; Rebelo L. P. N. Impact of Self-Aggregation on the Formation of Ionic-Liquid-Based Aqueous Biphasic Systems. J. Phys. Chem. B 2012, 116, 7660–7668. 10.1021/jp211132z. [DOI] [PubMed] [Google Scholar]
- Zafarani-Moattar M. T.; Hamzehzadeh S. Partitioning of Amino Acids in the Aqueous Biphasic System Containing the Water-miscible Ionic Liquid 1-butyl-3-methylimidazolium Bromide and the Water-structuring Salt Potassium Citrate. Biotechnol. Prog. 2011, 27, 986–997. 10.1002/btpr.613. [DOI] [PubMed] [Google Scholar]
- Passos H.; Ferreira A. R.; Cláudio A. F. M.; Coutinho J. A. P.; Freire M. G. Characterization of Aqueous Biphasic Systems Composed of Ionic Liquids and a Citrate-based Biodegradable Salt. Biochem. Eng. J. 2012, 67, 68–76. 10.1016/j.bej.2012.05.004. [DOI] [Google Scholar]
- Zafarani-Moattar M. T.; Hamzehzadeh S.; Nasiri S. A new Aqueous Biphasic System Containing Polypropylene Glycol and a Water-miscible Ionic Liquid. Biotechnol. Prog. 2012, 28, 146–156. 10.1002/btpr.718. [DOI] [PubMed] [Google Scholar]
- Wei X.-L.; Wang X.-H.; Ping A. L.; Du P.-P.; Sun D.-Z.; Zhang Q.-F.; Liu J. Formation and Characteristics of Aqueous Two-phase Systems formed by a Cationic Surfactant and a Series of Ionic Liquids. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2013, 939, 1–9. 10.1016/j.jchromb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Luís A.; Dinis T. B. V.; Passos H.; Taha M.; Freire M. G. Good’s Buffers as Novel Phase-forming Components of Ionic-liquid-based Aqueous Biphasic Systems. Biochem. Eng. J. 2015, 101, 142–149. 10.1016/j.bej.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira J. F. B.; Lima A. S.; Freire M. G.; Coutinho J. A. P. Ionic Liquids as Adjuvants for the Tailored Extraction of Biomolecules in Aqueous Biphasic Systems. Green Chem. 2010, 12, 1661–1669. 10.1039/c003578e. [DOI] [Google Scholar]
- Hamzehzadeh S.; Vasiresh M. Ionic Liquid 1-butyl-3-methylimidazolium Bromide as a Promoter for the Formation and Extraction Capability of Poly(ethylene glycol)-potassium citrate Aqueous Biphasic System at T = 298.15 K. Fluid Phase Equilib. 2014, 382, 80–88. 10.1016/j.fluid.2014.08.029. [DOI] [Google Scholar]
- Wu D.; Zhou Y.; Cai P.; Shen S.; Pan Y. Specific Cooperative Effect for the Enantiomeric Separation of Amino Acids using Aqueous Two-phase Systems with Task-specific Ionic Liquids. J. Chromatogr. A 2015, 1395, 65–72. 10.1016/j.chroma.2015.03.047. [DOI] [PubMed] [Google Scholar]
- Carda-Broch S.; Berthod A.; Armstrong D. W. Solvent Properties of the 1-butyl-3-methylimidazolium Hexafluorophosphate Ionic Liquid. Anal. Bioanal. Chem. 2003, 375, 191–199. 10.1007/s00216-002-1684-1. [DOI] [PubMed] [Google Scholar]
- Wang J.; Pei Y.; Zhao Y.; Hu Z. Recovery of Amino Acids by Imidazolium Based Ionic Liquids from Aqueous Media. Green Chem. 2005, 7, 196–202. 10.1039/b415842c. [DOI] [Google Scholar]
- Tomé L. I. N.; Catambas V. R.; Teles A. R. R.; Freire M. G.; Marrucho I. M.; Coutinho J. A. P. Tryptophan Extraction using Hydrophobic Ionic Liquids. Sep. Purif. Technol. 2010, 72, 167–173. 10.1016/j.seppur.2010.02.002. [DOI] [Google Scholar]
- Absalan G.; Akhond M.; Sheikhian L. Partitioning of Acidic, Basic and Neutral Amino Acids into Imidazolium-based Ionic Liquids. Amino Acids 2010, 39, 167–174. 10.1007/s00726-009-0391-z. [DOI] [PubMed] [Google Scholar]
- Seduraman A.; Wu P.; Klähn M. Extraction of Tryptophan with Ionic Liquids Studied with Molecular Dynamics Simulations. J. Phys. Chem. B 2012, 116, 296–304. 10.1021/jp206748z. [DOI] [PubMed] [Google Scholar]
- Vasantha T.; Awanish K.; Pankaj A.; Pannuru V.; Devi R. S. R. The Solubility and Stability of Amino Acids in Biocompatible Ionic Liquids. Protein Pept. Lett. 2013, 21, 15–24. 10.2174/09298665113209990071. [DOI] [PubMed] [Google Scholar]
- Huaxi L.; Zhuo L.; Jingmei Y.; Changping L.; Yansheng C.; Qingshan L.; Xiuling Z.; Urs W.-B. Liquid-liquid Extraction Process of Amino Acids by a new Amide-based Functionalized Ionic Liquid. Green Chem. 2012, 14, 1721–1727. 10.1039/c2gc16560k. [DOI] [Google Scholar]
- Tang F.; Zhang Q.; Ren D.; Nie Z.; Liu Q.; Yao S. Functional Amino Acid Ionic Liquids as Solvent and Selector in Chiral Extraction. J. Chromatogr. A 2010, 1217, 4669–4674. 10.1016/j.chroma.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Wu H.; Yao S.; Qian G.; Yao T.; Song H. A Resolution Approach of Racemic Phenylalanine with Aqueous Two-phase Systems of Chiral Tropine Ionic Liquids. J. Chromatogr. A 2015, 1418, 150–157. 10.1016/j.chroma.2015.09.058. [DOI] [PubMed] [Google Scholar]
- Marwani H. M. B.; Bakhsh E. M.; Al-Turaif H. A.; Asiri A. M.; Khan S. B. Enantioselective Separation and Detection of D-Phenylalanine Based on Newly Developed Chiral Ionic Liquid Immobilized Silica Gel Surface. Int. J. Electrochem. Sci. 2014, 9, 7948–7964. [Google Scholar]
- Yang L.; Hu X.; Guan P.; Li J.; Wu D.; Gao B. Molecularly Imprinted Polymers for the Selective Recognition of l-phenylalanine based on 1-buty-3-methylimidazolium Ionic Liquid. J. Appl. Polym. Sci. 2015, 132, 42485. 10.1002/app.42485. [DOI] [Google Scholar]
- Brunzel N. A.Fundamentals of Urine and Body Fluid Analysis; Elsevier Health Sciences, 2013. [Google Scholar]
- Hoffmann R. In Advances in Health care Technology Care Shaping the Future of Medical; Spekowius G., Wendler T., Eds.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Patel R.; Kumari M.; Khan A. B. Recent Advances in the Applications of Ionic Liquids in Protein Stability and Activity: A Review. Appl. Biochem. Biotechnol. 2014, 172, 3701–3720. 10.1007/s12010-014-0813-6. [DOI] [PubMed] [Google Scholar]
- Zhao H. Protein Stabilization and Enzyme Activation in Ionic Liquids: Specific Ion Effects. J. Chem. Technol. Biotechnol. 2016, 91, 25–50. 10.1002/jctb.4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S. P. M.; Santos L. D. F.; Saraiva J. A.; Coutinho J. A. P. Ionic Liquids Microemulsions: The Key to Candida Antarctica Lipase B Superactivity. Green Chem. 2012, 14, 1620–1625. 10.1039/c2gc35197h. [DOI] [Google Scholar]
- Kumari M.; Maurya J. K.; Singh U. K.; Khan A. B.; Ali M.; Singh P.; Patel R. Spectroscopic and Docking Studies on the Interaction between Pyrrolidinium Based Ionic Liquid and Bovine Serum Albumin. Spectrochim. Acta, Part A 2014, 124, 349–356. 10.1016/j.saa.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Taha M.; Quental M. V.; Correia I.; Freire M. G.; Coutinho J. A. P. Extraction and Stability of Bovine Serum Albumin (BSA) using Cholinium-based Good’s Buffers Ionic Liquids. Process Biochem. 2015, 50, 1158–1166. 10.1016/j.procbio.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z.; Yu Y. L.; Wang J. H. Extraction of Proteins from Biological Fluids by use of an Ionic Liquid/Aqueous Two-phase System. Chem. - Eur. J. 2007, 13, 2130–2137. 10.1002/chem.200601234. [DOI] [PubMed] [Google Scholar]
- Ruiz-Angel M. J.; Pino V.; Carda-Broch S.; Berthod A. Solvent Systems for Countercurrent Chromatography: An Aqueous Two phase Liquid System Based on a Room Temperature Ionic Liquid. J. Chromatogr. A 2007, 1151, 65–73. 10.1016/j.chroma.2006.11.072. [DOI] [PubMed] [Google Scholar]
- Dreyer S.; Salim P.; Kragl U. Driving Forces of Protein Partitioning in an Ionic Liquid-based Aqueous Two-phase System. Biochem. Eng. J. 2009, 46, 176–185. 10.1016/j.bej.2009.05.005. [DOI] [Google Scholar]
- Pei Y.; Wang J.; Wu K.; Xuan X.; Lu X. Ionic Liquid-based Aqueous Two-phase Extraction of Selected Proteins. Sep. Purif. Technol. 2009, 64, 288–295. 10.1016/j.seppur.2008.10.010. [DOI] [Google Scholar]
- Lu Y.; Lu W.; Wang W.; Guo Q.; Yang Y. Thermodynamic Studies of Partitioning Behavior of Cytochrome c in Ionic Liquid-based Aqueous Two-phase System. Talanta 2011, 85, 1621–1626. 10.1016/j.talanta.2011.06.058. [DOI] [PubMed] [Google Scholar]
- Shu Y.; Liu M.; Chen S.; Chen X.; Wang J. New Insight into Molecular Interactions of Imidazolium Ionic Liquids with Bovine Serum Albumin. J. Phys. Chem. B 2011, 115, 12306–12314. 10.1021/jp2071925. [DOI] [PubMed] [Google Scholar]
- Yan H.; Wu J.; Dai G.; Zhong A.; Chen H.; Yang J.; Han D. Interaction Mechanisms of Ionic Liquids [Cnmim]Br (n = 4, 6, 8, 10) with Bovine Serum Albumin. J. Lumin. 2012, 132, 622–628. 10.1016/j.jlumin.2011.10.026. [DOI] [Google Scholar]
- Lin X.; Wang Y.; Zeng Q.; Ding X.; Chen J. Extraction and Separation of Proteins by Ionic Liquid Aqueous Two-phase System. Analyst 2013, 138, 6445–6453. 10.1039/c3an01301d. [DOI] [PubMed] [Google Scholar]
- Kohno Y.; Ohno H. Temperature-responsive Ionic Liquid/water Interfaces: Relation between Hydrophilicity of Ions and Dynamic Phase Change. Phys. Chem. Chem. Phys. 2012, 14, 5063–5070. 10.1039/c2cp24026b. [DOI] [PubMed] [Google Scholar]
- Dreyer S.; Kragl U. Ionic Liquids for Aqueous Two-phase Extraction and Stabilization of Enzymes. Biotechnol. Bioeng. 2008, 99, 1416–1424. 10.1002/bit.21720. [DOI] [PubMed] [Google Scholar]
- Pei Y.; Li L.; Li Z.; Wu C.; Wang J. Partitioning Behavior of Wastewater Proteins in Some Ionic Liquids-Based Aqueous Two-Phase Systems. Sep. Sci. Technol. 2012, 47, 277–283. 10.1080/01496395.2011.609241. [DOI] [Google Scholar]
- Sheikhian L.; Akhond M.; Absalan G.; Goltz D. M. Dye-Affinity Partitioning of Acidic, Basic, and Neutral Proteins in Ionic Liquid-Based Aqueous Biphasic Systems. Sep. Sci. Technol. 2013, 48, 2372–2380. 10.1080/01496395.2013.804086. [DOI] [Google Scholar]
- Pei Y.; Li Z.; Liu L.; Wang J.; Wang H. Selective Separation of Protein and Saccharides by Ionic Liquids Aqueous Two-phase Systems. Sci. China Chem. 2010, 53, 1554–1560. 10.1007/s11426-010-4025-9. [DOI] [Google Scholar]
- Ding X.; Wang Y.; Zeng Q.; Chen J.; Huang Y.; Xu K. Design of Functional Guanidinium Ionic Liquid Aqueous Two-phase Systems for the Efficient Purification of Protein. Anal. Chim. Acta 2014, 815, 22–32. 10.1016/j.aca.2014.01.030. [DOI] [PubMed] [Google Scholar]
- Zeng Q.; Wang Y.; Li N.; Huang X.; Ding X.; Lin X.; Huang S.; Liu X. Extraction of Proteins with Ionic Liquid Aqueous Two-phase System based on Guanidine Ionic Liquid. Talanta 2013, 116, 409–416. 10.1016/j.talanta.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Chen J.; Wang Y.; Zeng Q.; Ding X.; Huang Y. Partition of Proteins with Extraction in Aqueous Two-phase System by Hydroxyl Ammonium-based Ionic Liquid. Anal. Methods 2014, 6, 4067–4076. 10.1039/c4ay00233d. [DOI] [Google Scholar]
- Bisht M.; Kumar A.; Venkatesu P. Analysis of the Driving Force that Rule the Stability of Lysozyme in Alkylammonium-based Ionic Liquids. Int. J. Biol. Macromol. 2015, 81, 1074–1081. 10.1016/j.ijbiomac.2015.09.036. [DOI] [PubMed] [Google Scholar]
- Wu C.; Wang J.; Li Z.; Jing J.; Wang H. Relative Hydrophobicity between the Phases and Partition of Cytochrome-c in Glycine Ionic Liquids Aqueous Two-phase Systems. J. Chromatogr. A 2013, 1305, 1–6. 10.1016/j.chroma.2013.06.066. [DOI] [PubMed] [Google Scholar]
- Li Z.; Liu X.; Pei Y.; Wang J.; He M. Design of Environmentally Friendly Ionic Liquid Aqueous Two-phase Systems for the Efficient and High Activity Extraction of Proteins. Green Chem. 2012, 14, 2941–2950. 10.1039/c2gc35890e. [DOI] [Google Scholar]
- Huang S.; Wang Y.; Zhou Y.; Li L.; Zeng Q.; Ding X. Choline-like Ionic Liquid-based Aqueous Two-phase Extraction of Selected Proteins. Anal. Methods 2013, 5, 3395–3402. 10.1039/c3ay40377g. [DOI] [Google Scholar]
- Quental M. V.; Caban M.; Pereira M. M.; Stepnowski P.; Coutinho J. A. P.; Freire M. G. Enhanced Extraction of Proteins using Cholinium-based Ionic Liquids as Phase-forming Components of Aqueous Biphasic Systems. Biotechnol. J. 2015, 10, 1457–1466. 10.1002/biot.201500003. [DOI] [PubMed] [Google Scholar]
- Song C. P.; Ramanan R. N.; Vijayaraghavan R.; MacFarlane D. R.; Chan E.-S.; Ooi C.-W. Green, Aqueous Two-Phase Systems Based on Cholinium Aminoate Ionic Liquids with Tunable Hydrophobicity and Charge Density. ACS Sustainable Chem. Eng. 2015, 3, 3291–3298. 10.1021/acssuschemeng.5b00881. [DOI] [Google Scholar]
- Greaves T. L.; Drummond C. J. Protic Ionic Liquids: Evolving Structure–Property Relationships and Expanding Applications. Chem. Rev. 2015, 115, 11379–11448. 10.1021/acs.chemrev.5b00158. [DOI] [PubMed] [Google Scholar]
- Passos H.; Luís A.; Coutinho J. A. P.; Freire M. G. Thermoreversible (Ionic-Liquid-Based) Aqueous Biphasic Systems. Sci. Rep. 2016, 6, 20276. 10.1038/srep20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha M.; Almeida M. R.; Silva F. A.; Domingues P.; Ventura S. P.; Coutinho J. A.; Freire M. G. Novel Biocompatible and Self-buffering Ionic Liquids for Biopharmaceutical Applications. Chem. - Eur. J. 2015, 21, 4781–4788. 10.1002/chem.201405693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R. K.; Streefland M.; Wijffels R. H.; Eppink M. H. M. Extraction and Stability of Selected Proteins in Ionic Liquid based Aqueous Two Phase Systems. Green Chem. 2014, 16, 2670–2679. 10.1039/C3GC42631A. [DOI] [Google Scholar]
- Tan Z.-j.; Li F.-f.; Xu X.-l.; Xing J.-m. Simultaneous Extraction and Purification of Aloe Polysaccharides and Proteins using Ionic Liquid based Aqueous Two-phase System Coupled with Dialysis Membrane. Desalination 2012, 286, 389–393. 10.1016/j.desal.2011.11.053. [DOI] [Google Scholar]
- Yan J.-K.; Ma H.-L.; Pei J.-J.; Wang Z.-B.; Wu J.-Y. Facile and Effective Separation of Polysaccharides and Proteins from Cordyceps Sinensis Mycelia by Ionic Liquid Aqueous Two-phase System. Sep. Purif. Technol. 2014, 135, 278–284. 10.1016/j.seppur.2014.03.020. [DOI] [Google Scholar]
- Pereira M. M.; Pedro S. N.; Quental M. V.; Lima Á. S.; Coutinho J. A. P.; Freire M. G. Enhanced Extraction of Bovine Serum Albumin with Aqueous Biphasic Systems of Phosphonium- and Ammonium-based Ionic Liquids. J. Biotechnol. 2015, 206, 17–25. 10.1016/j.jbiotec.2015.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deive F. J.; Rodriguez A.; Pereiro A. B.; Araujo J. M. M.; Longo M. A.; Coelho M. A. Z.; Lopes J. N. C.; Esperanca J. M. S. S.; Rebelo L. P. N.; Marrucho I. M. Ionic Liquid-Based Aqueous Biphasic System for Lipase Extraction. Green Chem. 2011, 13, 390–396. 10.1039/C0GC00075B. [DOI] [Google Scholar]
- Deive F. J.; Rodríguez A.; Rebelo L. P. N.; Marrucho I. M. Extraction of Candida Antarctica Lipase A from Aqueous Solutions using Imidazolium-based Ionic Liquids. Sep. Purif. Technol. 2012, 97, 205–210. 10.1016/j.seppur.2011.12.013. [DOI] [Google Scholar]
- Ventura S. P. M.; Sousa S. G.; Freire M. G.; Serafim L. S.; Lima Á. S.; Coutinho J. A. P. Design of Ionic Liquids for Lipase Purification. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2011, 879, 2679–2687. 10.1016/j.jchromb.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Souza R. L.; Lima R. A.; Coutinho J. A. P.; Soares C. M. F.; Lima Á. S. Aqueous Two-phase Systems Based on Cholinium Salts and Tetrahydrofuran and their use for Lipase Purification. Sep. Purif. Technol. 2015, 155, 118–126. 10.1016/j.seppur.2015.05.021. [DOI] [Google Scholar]
- Ventura S. P. M.; Coutinho J. A. P. In Ionic Liquids in Lipid Processing and Analysis; Guo Z., Cheong L.-Z., Eds.; AOCS Press, 2016. [Google Scholar]
- Lee S. Y.; Vicente F. A.; e Silva F. A.; Sintra T. E.; Taha M.; Khoiroh I.; Coutinho J. A. P.; Show P. L.; Ventura S. P. M. Evaluating Self-buffering Ionic Liquids for Biotechnological Applications. ACS Sustainable Chem. Eng. 2015, 3, 3420–3428. 10.1021/acssuschemeng.5b01155. [DOI] [Google Scholar]
- Bai Z.; Chao Y.; Zhang M.; Han C.; Zhu W.; Chang Y.; Li H.; Sun Y. Partitioning Behavior of Papain in Ionic Liquids-Based Aqueous Two-Phase Systems. J. Chem. 2013, 2013, 1–6. 10.1155/2013/938154. [DOI] [Google Scholar]
- Cao Q.; Quan L.; He C.; Li N.; Li K.; Liu F. Partition of Horseradish Peroxidase with Maintained Activity in Aqueous Biphasic System Based on Ionic Liquid. Talanta 2008, 77, 160–165. 10.1016/j.talanta.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Simental-Martinez J.; Rito-Palomares M.; Benavides J. Potential Application of Aqueous Two-phase Systems and Three-phase Partitioning for the Recovery of Superoxide Dismutase from a Clarified Homogenate of Kluyveromyces marxianus. Biotechnol. Prog. 2014, 30, 1326–1334. 10.1002/btpr.1979. [DOI] [PubMed] [Google Scholar]
- Jiang B.; Feng Z.; Liu C.; Xu Y.; Li D.; Ji G. Extraction and Purification of Wheat-esterase using Aqueous Two-phase Systems of Ionic Liquid and Salt. J. Food Sci. Technol. 2015, 52, 2878–2885. 10.1007/s13197-014-1319-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvjetko M.; Žnidaršič-Plazl P. In Ionic Liquids: Theory, Properties, New Approaches; InTech, 2011. [Google Scholar]
- Novak U.; Pohar A.; Plazl I.; Žnidaršič-Plazl P. Ionic Liquid-based Aqueous two-phase Extraction within a Microchannel System. Sep. Purif. Technol. 2012, 97, 172–178. 10.1016/j.seppur.2012.01.033. [DOI] [Google Scholar]
- Shimojo K.; Kamiya N.; Tani F.; Naganawa H.; Naruta Y.; Goto M. Extractive Solubilization, Structural Change, and Functional Conversion of Cytochrome C in Ionic Liquids via Crown Ether Complexation. Anal. Chem. 2006, 78, 7735–7742. 10.1021/ac0612877. [DOI] [PubMed] [Google Scholar]
- Tzeng Y.-P.; Shen C.-W.; Yu T. Liquid–liquid Extraction of Lysozyme using a Dye-modified Ionic Liquid. J. Chromatogr. A 2008, 1193, 1–6. 10.1016/j.chroma.2008.02.118. [DOI] [PubMed] [Google Scholar]
- Kohno Y.; Saita S.; Murata K.; Nakamura N.; Ohno H. Extraction of Proteins with Temperature Sensitive and Reversible Phase Change of Ionic Liquid/water Mixture. Polym. Chem. 2011, 2, 862–867. 10.1039/c0py00364f. [DOI] [Google Scholar]
- Ito Y.; Kohno Y.; Nakamura N.; Ohno H. Design of Phosphonium-Type Zwitterion as an Additive to Improve Saturated Water Content of Phase-Separated Ionic Liquid from Aqueous Phase toward Reversible Extraction of Proteins. Int. J. Mol. Sci. 2013, 14, 18350. 10.3390/ijms140918350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G.; Gong X.; Wang B.; Chen Y.; Huang J. Affinity Ionic Liquids for the Rapid liquid–liquid Extraction Purification of Hexahistidine Tagged Proteins. Sep. Purif. Technol. 2015, 146, 114–120. 10.1016/j.seppur.2015.03.025. [DOI] [Google Scholar]
- Xu W.; Cao H.; Ren G.; Xie H.; Huang J.; Li S. An AIL/IL-based Liquid/liquid Extraction System for the Purification of His-tagged Proteins. Appl. Microbiol. Biotechnol. 2014, 98, 5665–5675. 10.1007/s00253-014-5737-0. [DOI] [PubMed] [Google Scholar]
- Cheng D. H.; Chen X. W.; Shu Y.; Wang J. H. Selective Extraction/isolation of Hemoglobin with Ionic Liquid 1-butyl-3-trimethylsilylimidazolium Hexafluorophosphate (BtmsimPF6). Talanta 2008, 75, 1270–1278. 10.1016/j.talanta.2008.01.044. [DOI] [PubMed] [Google Scholar]
- Cheng D.-H.; Chen X.-W.; Shu Y.; Wang J.-H. Extraction of Cytochrome C by Ionic Liquid 1-Butyl-3-trimethylsilylimidazolium Hexafluorophosphate. Chin. J. Anal. Chem. 2008, 36, 1187–1190. 10.1016/S1872-2040(08)60066-3. [DOI] [Google Scholar]
- Huh Y. S.; Jeong C.-M.; Chang H. N.; Lee S. Y.; Hong W. H.; Park T. J. Rapid Separation of Bacteriorhodopsin using a Laminar-flow Extraction System in a Microfluidic Device. Biomicrofluidics 2010, 4, 014103. 10.1063/1.3298608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Guerra E.; Irabien A. Ionic Liquid-Based Three Phase Partitioning (ILTPP) for Lactoferrin Recovery. Sep. Sci. Technol. 2014, 49, 957–965. 10.1080/01496395.2013.878722. [DOI] [Google Scholar]
- Alvarez-Guerra E.; Irabien A. Ionic Liquid-based Three phase Partitioning (ILTPP) Systems for Whey Protein Recovery: Ionic Liquid Selection. J. Chem. Technol. Biotechnol. 2015, 90, 939–946. 10.1002/jctb.4401. [DOI] [Google Scholar]
- Alvarez-Guerra E.; Irabien A.; Ventura S. P. M.; Coutinho J. A. P. Ionic Liquid Recovery Alternatives in Ionic Liquid-based Three-phase Partitioning (ILTPP). AIChE J. 2014, 60, 3577–3586. 10.1002/aic.14530. [DOI] [Google Scholar]
- Alvarez-Guerra E.; Ventura S. P. M.; Coutinho J. A. P.; Irabien A. Ionic Liquid-based Three Phase Partitioning (ILTPP) Systems: Ionic Liquid Recovery and Recycling. Fluid Phase Equilib. 2014, 371, 67–74. 10.1016/j.fluid.2014.03.009. [DOI] [Google Scholar]
- Shu Y.; Cheng D.; Chen X.; Wang J. A Reverse Microemulsion of Water/AOT/1-butyl-3-methylimidazolium Hexafluorophosphate for Selective Extraction of Hemoglobin. Sep. Purif. Technol. 2008, 64, 154–159. 10.1016/j.seppur.2008.09.010. [DOI] [Google Scholar]
- Mao Q.-X.; Wang H.; Shu Y.; Chen X.-W.; Wang J.-H. A Dual-Ionic Liquid Microemulsion System for the Selective Isolation of Hemoglobin. RSC Adv. 2014, 4, 8177–8182. 10.1039/c3ra46736h. [DOI] [Google Scholar]
- Vicente F. A.; Malpiedi L. P.; e Silva F. A.; Pessoa A. Jr; Coutinho J. A. P.; Ventura S. P. M. Design of Novel Aqueous Micellar Two-phase Systems using Ionic Liquids as Co-surfactants for the Selective Extraction of (Bio)molecules. Sep. Purif. Technol. 2014, 135, 259–267. 10.1016/j.seppur.2014.06.045. [DOI] [Google Scholar]
- Vicente F. A.; Lario L. D.; Pessoa A. Jr; Ventura S. P. M. Recovery of Bromelain from Pineapple Stem Residues using Aqueous Micellar Two-phase Systems with Ionic Liquids as Co-surfactants. Process Biochem. 2016, 51, 528–534. 10.1016/j.procbio.2016.01.004. [DOI] [Google Scholar]
- Ge L.; Wang X. T.; Tan S. N.; Tsai H. H.; Yong J. W.; Hua L. A novel method of protein extraction from yeast using Ionic Liquid Solution. Talanta 2010, 81, 1861–1864. 10.1016/j.talanta.2010.02.034. [DOI] [PubMed] [Google Scholar]
- Fukaya Y.; Sugimoto A.; Ohno H. Superior Solubility of Polysaccharides in Low Viscosity, Polar and Halogen-free 1,3-dialkylimidazolium Formates. Biomacromolecules 2006, 7, 3295–3297. 10.1021/bm060327d. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhang X. Optimal Extraction and Hydrolysis of Chlorella pyrenoidosa Proteins. Bioresour. Technol. 2012, 126, 307–313. 10.1016/j.biortech.2012.09.059. [DOI] [PubMed] [Google Scholar]
- Xie H.; Li S.; Zhang S. Ionic Liquids as Novel Solvents for the Dissolution and Blending of Wool Keratin Fibers. Green Chem. 2005, 7, 606–608. 10.1039/b502547h. [DOI] [Google Scholar]
- Plowman J. E.; Clerens S.; Lee E.; Harland D. P.; Dyer J. M.; Deb-Choudhury S. Ionic Liquid-assisted Extraction of Wool Keratin Proteins as an Aid to MS Identification. Anal. Methods 2014, 6, 7305–7311. 10.1039/C4AY01251H. [DOI] [Google Scholar]
- Martins M.; Vieira F. A.; Correia I.; Ferreira R. A. S.; Abreu H.; Coutinho J. A. P.; Ventura S. P. M. Recovery of Phycobiliproteins from the Red Macroalga Gracilaria sp. using Ionic Liquid Aqueous Solutions. Green Chem. 2016, 18, 4287–4296. 10.1039/C6GC01059H. [DOI] [Google Scholar]
- Shu Y.; Chen X. W.; Wang J. H. Ionic Liquid-polyvinyl Chloride Ionomer for Highly Selective Isolation of Basic Proteins. Talanta 2010, 81, 637–642. 10.1016/j.talanta.2009.12.059. [DOI] [PubMed] [Google Scholar]
- Zhao G.; Chen S.; Chen X. W.; He R. H. Selective Isolation of Hemoglobin by use of Imidazolium-modified Polystyrene as Extractant. Anal. Bioanal. Chem. 2013, 405, 5353–5358. 10.1007/s00216-013-6889-y. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ma R.; Deng Q.; Zhang L.; Liu C.; Wang S. Preparation of Ionic Liquid Polymer Materials and their Recognition Properties for Proteins. RSC Adv. 2014, 4, 52147–52154. 10.1039/C4RA05713A. [DOI] [Google Scholar]
- Chen J.; Wang Y.; Ding X.; Huang Y.; Xu K. Magnetic Solid-phase Extraction of Proteins based on Hydroxy Functional Ionic Liquid-modified Magnetic Nanoparticles. Anal. Methods 2014, 6, 8358–8367. 10.1039/C4AY01786B. [DOI] [Google Scholar]
- Bezold F.; Goll J.; Minceva M. Study of the Applicability of Non-conventional Aqueous two-phase Systems in Counter-current and Centrifugal Partition Chromatography. J. Chromatogr. A 2015, 1388, 126–132. 10.1016/j.chroma.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Gunn P.; Walsh S.; Roux C. The Nucleic Acid Revolution Continues – will Forensic Biology become Forensic Molecular Biology?. Front. Genet. 2014, 5, 44. 10.3389/fgene.2014.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly P.; Wright J. M. The Evolving Technology of DNA Fingerprinting and its Application to Fisheries and Aquaculture. J. Fish Biol. 1995, 47, 29–55. 10.1111/j.1095-8649.1995.tb06042.x. [DOI] [Google Scholar]
- Hans R.; Marwaha N. Nucleic Acid Testing-benefits and Constraints. Asian J. Transfus. Sci. 2014, 8, 2–3. 10.4103/0973-6247.126679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth C. M.; Yarmush M. L. Nucleic Acid Biotechnology. Annu. Rev. Biomed. Eng. 1999, 1, 265–297. 10.1146/annurev.bioeng.1.1.265. [DOI] [PubMed] [Google Scholar]
- Tan S. C.; Yiap B. C.. DNA, RNA, and Protein Extraction: The Past and the Present. J. Biomed. Biotechnol. 2009, 2009, 1. 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. DNA Stability in Ionic Liquids and Deep Eutectic Solvents. J. Chem. Technol. Biotechnol. 2015, 90, 19–25. 10.1002/jctb.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Liu Y.-L.; Lai P.-Y.; Tseng M.-C.; Tseng M.-J.; Li Y.; Chu Y.-H. Ionic Liquids Promote PCR Amplification of DNA. Chem. Commun. 2012, 48, 5325–5327. 10.1039/c2cc31740k. [DOI] [PubMed] [Google Scholar]
- Ohno H.; Fukaya Y.; Masuda G.; Solvent For Dissolving Nucleic Acid, Nucleic Acid-Containing Solution And Method Of Preserving Nucleic Acid, 20070196826, 2005.
- Wang J.-H.; Cheng D.-H.; Chen X.-W.; Du Z.; Fang Z.-L. Direct Extraction of Double-Stranded DNA into Ionic Liquid 1-Butyl-3-methylimidazolium Hexafluorophosphate and its Quantification. Anal. Chem. 2007, 79, 620–625. 10.1021/ac061145c. [DOI] [PubMed] [Google Scholar]
- Fister S.; Fuchs S.; Mester P.; Kilpeläinen I.; Wagner M.; Rossmanith P. The use of Ionic Liquids for Cracking Viruses for Isolation of Nucleic Acids. Sep. Purif. Technol. 2015, 155, 38–44. 10.1016/j.seppur.2015.03.035. [DOI] [Google Scholar]
- Khimji I.; Doan K.; Bruggeman K.; Huang P.-J. J.; Vajha P.; Liu J. Extraction of DNA Staining Dyes from DNA using Hydrophobic Ionic Liquids. Chem. Commun. 2013, 49, 4537–4539. 10.1039/c3cc41364k. [DOI] [PubMed] [Google Scholar]
- Clark K. D.; Nacham O.; Yu H.; Li T.; Yamsek M. M.; Ronning D. R.; Anderson J. L. Extraction of DNA by Magnetic Ionic Liquids: Tunable Solvents for Rapid and Selective DNA Analysis. Anal. Chem. 2015, 87, 1552–1559. 10.1021/ac504260t. [DOI] [PubMed] [Google Scholar]
- Clark K. D.; Yamsek M. M.; Nacham O.; Anderson J. L. Magnetic Ionic Liquids as PCR-Compatible Solvents for DNA Extraction from Biological Samples. Chem. Commun. 2015, 51, 16771–16773. 10.1039/C5CC07253K. [DOI] [PubMed] [Google Scholar]
- Li T.; Joshi M. D.; Ronning D. R.; Anderson J. L. Ionic Liquids as Solvents for in Situ Dispersive Liquid–liquid Microextraction of DNA. J. Chromatogr. A 2013, 1272, 8–14. 10.1016/j.chroma.2012.11.055. [DOI] [PubMed] [Google Scholar]
- Huang D. J.; Huang D. C. The Research for the Extraction of Yeast’s Nucleic Acid with [BMIM] BF4-H2O-KH2PO4 Ionic Liquid Aqueous Two-Phase System. Adv. Mater. Res. 2012, 455–456, 477–482. 10.4028/www.scientific.net/AMR.455-456.477. [DOI] [Google Scholar]
- Gonzalez García E.; Ressmann A. K.; Gaertner P.; Zirbs R.; Mach R. L.; Krska R.; Bica K.; Brunner K. Direct Extraction of Genomic DNA from Maize with Aqueous Ionic Liquid Buffer Systems for Applications in Genetically Modified Organisms Analysis. Anal. Bioanal. Chem. 2014, 406, 7773–7784. 10.1007/s00216-014-8204-y. [DOI] [PubMed] [Google Scholar]
- Ressmann A. K.; Garcia E. G.; Khlan D.; Gaertner P.; Mach R. L.; Krska R.; Brunner K.; Bica K. Fast and Efficient Extraction of DNA from Meat and Meat Derived Products using Aqueous Ionic Liquid Buffer Systems. New J. Chem. 2015, 39, 4994–5002. 10.1039/C5NJ00178A. [DOI] [Google Scholar]
- Wang X.; Xing L.; Shu Y.; Chen X.; Wang J. Novel Polymeric Ionic Liquid Microspheres with High Exchange Capacity for Fast Extraction of Plasmid DNA. Anal. Chim. Acta 2014, 837, 64–69. 10.1016/j.aca.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Tateishi-Karimata H.; Sugimoto N. Structure, Stability and Behaviour of Nucleic Acids in Ionic Liquids. Nucleic Acids Res. 2014, 42, 8831. 10.1093/nar/gku499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K.; Ohno H. Stable G-quadruplex Structure in a Hydrated Ion Pair: Cholinium Cation and Dihydrogen Phosphate Anion. Chem. Commun. 2012, 48, 5751–5753. 10.1039/c2cc30554b. [DOI] [PubMed] [Google Scholar]
- Mukesh C.; Mondal D.; Sharma M.; Prasad K. Rapid Dissolution of DNA in a Novel Bio-based Ionic Liquid with Long-term Structural and Chemical Stability: Successful Recycling of the Ionic Liquid for Reuse in the Process. Chem. Commun. 2013, 49, 6849–6851. 10.1039/c3cc42829j. [DOI] [PubMed] [Google Scholar]
- Sharma M.; Mondal D.; Singh N.; Trivedi N.; Bhatt J.; Prasad K. High Concentration DNA Solubility in Bio-Ionic Liquids with Long-lasting Chemical and Structural Stability at Room Temperature. RSC Adv. 2015, 5, 40546–40551. 10.1039/C5RA03512K. [DOI] [Google Scholar]
- Alfonsi K.; Colberg J.; Dunn P. J.; Fevig T.; Jennings S.; Johnson T. A.; Kleine H. P.; Knight C.; Nagy M. A.; Perry D. A.; et al. Green Chemistry Tools to Influence a Medicinal Chemistry and Research Chemistry based Organisation. Green Chem. 2008, 10, 31–36. 10.1039/B711717E. [DOI] [Google Scholar]
- Manley J. B.In Green Chemistry Strategies for Drug Discovery; The Royal Society of Chemistry, 2015. [Google Scholar]
- Roschangar F.; Sheldon R. A.; Senanayake C. H. Overcoming Barriers to Green Chemistry in the Pharmaceutical Industry - the Green Aspiration Level Concept. Green Chem. 2015, 17, 752–768. 10.1039/C4GC01563K. [DOI] [Google Scholar]
- Kümmerer K. The Presence of Pharmaceuticals in the Environment due to Human use – Present Knowledge and Future Challenges. J. Environ. Manage. 2009, 90, 2354–2366. 10.1016/j.jenvman.2009.01.023. [DOI] [PubMed] [Google Scholar]
- e Silva F. A.; Sintra T.; Ventura S. P. M.; Coutinho J. A. P. Recovery of Paracetamol from Pharmaceutical Wastes. Sep. Purif. Technol. 2014, 122, 315–322. 10.1016/j.seppur.2013.11.018. [DOI] [Google Scholar]
- Constable D. J. C.; Jimenez-Gonzalez C.; Henderson R. K. Perspective on Solvent Use in the Pharmaceutical Industry. Org. Process Res. Dev. 2007, 11, 133–137. 10.1021/op060170h. [DOI] [Google Scholar]
- Grodowska K.; Parczewski A. Organic Solvents in the Pharmaceutical Industry. Acta Polym. Pharm. 2010, 67, 3–12. [PubMed] [Google Scholar]
- Pfizer Pharmaceuticals: Green Chemistry Innovation and Business Strategy: https://www.acs.org/content/dam/acsorg/greenchemistry/industriainnovation/Pfizer-business-case-study.pdf (accessed on Feb 8, 2016).
- Examples of Green Chemistry: http://www.acs.org/content/acs/en/greenchemistry/what-is-green-chemistry/examples.html (accessed on Feb 8, 2016).
- Carr R.; Ciccone F.; Gabel R.; Guinn M.; Johnston D.; Mastriona J.; Vandermeer T.; Groaning M. Streamlined Process for the Esterification and Ketalization of Shikimic Acid en route to the Key Precursor for Oseltamivir Phosphate (Tamiflu). Green Chem. 2008, 10, 743–745. 10.1039/b801582a. [DOI] [Google Scholar]
- Dunn P. J.; Wells A. S.; Williams M. T. In Green Chemistry in the Pharmaceutical Industry; Wiley-VCH, 2010. [Google Scholar]
- Resende de Azevedo J.; Letourneau J.-J.; Espitalier F.; Ré M. I. Solubility of a New Cardioactive Prototype Drug in Ionic Liquids. J. Chem. Eng. Data 2014, 59, 1766–1773. 10.1021/je4009624. [DOI] [Google Scholar]
- Melo C. I.; Bogel-Łukasik R.; Nunes da Ponte M.; Bogel-Łukasik E. Ammonium Ionic Liquids as Green Solvents for Drugs. Fluid Phase Equilib. 2013, 338, 209–216. 10.1016/j.fluid.2012.11.029. [DOI] [Google Scholar]
- Faria R. A.; da Ponte M. N.; Bogel-Łukasik E. Solubility Studies on the System of Trihexyl(tetradecyl)phosphonium bis[(trifluoromethyl)sulfonyl]amide) Ionic Liquid and Pharmaceutical and Bioactive Compounds. Fluid Phase Equilib. 2015, 385, 1–9. 10.1016/j.fluid.2014.10.033. [DOI] [Google Scholar]
- Faria R. A.; Bogel-Łukasik E. Solubilities of Pharmaceutical and Bioactive Compounds in Trihexyl(tetradecyl)phosphonium Chloride Ionic Liquid. Fluid Phase Equilib. 2015, 397, 18–25. 10.1016/j.fluid.2015.03.053. [DOI] [Google Scholar]
- Forte A.; Melo C. I.; Bogel-Łukasik R.; Bogel-Łukasik E. A Favourable Solubility of Isoniazid, an Antitubercular Antibiotic Drug, in Alternative Solvents. Fluid Phase Equilib. 2012, 318, 89–95. 10.1016/j.fluid.2012.01.022. [DOI] [Google Scholar]
- Manic M. S.; Najdanovic-Visak V. Solubility of Erythromycin in Ionic Liquids. J. Chem. Thermodyn. 2012, 44, 102–106. 10.1016/j.jct.2011.08.004. [DOI] [Google Scholar]
- Smith K. B.; Bridson R. H.; Leeke G. A. Solubilities of Pharmaceutical Compounds in Ionic Liquids. J. Chem. Eng. Data 2011, 56, 2039–2043. 10.1021/je101040p. [DOI] [Google Scholar]
- Mizuuchi H.; Jaitely V.; Murdan S.; Florence A. T. Room Temperature Ionic Liquids and their Mixtures: Potential Pharmaceutical Solvents. Eur. J. Pharm. Sci. 2008, 33, 326–331. 10.1016/j.ejps.2008.01.002. [DOI] [PubMed] [Google Scholar]
- McCrary P. D.; Beasley P. A.; Gurau G.; Narita A.; Barber P. S.; Cojocaru O. A.; Rogers R. D. Drug Specific, Tuning of an Ionic Liquid’s Hydrophilic-lipophilic Balance to Improve Water Solubility of Poorly Soluble Active Pharmaceutical Ingredients. New J. Chem. 2013, 37, 2196–2202. 10.1039/c3nj00454f. [DOI] [Google Scholar]
- Cull S. G.; Holbrey J. D.; Vargas-Mora V.; Seddon K. R.; Lye G. J. Room-temperature Ionic Liquids as Replacements for Organic Solvents in Multiphase Bioprocess Operations. Biotechnol. Bioeng. 2000, 69, 227–233. . [DOI] [PubMed] [Google Scholar]
- Soto A.; Arce A.; Khoshkbarchi M. K. Partitioning of Antibiotics in a Two-liquid Phase System Formed by Water and a Room Temperature Ionic Liquid. Sep. Purif. Technol. 2005, 44, 242–246. 10.1016/j.seppur.2005.01.013. [DOI] [Google Scholar]
- Manic M. S.; da Ponte M. N.; Najdanovic-Visak V. Recovery of Erythromycin from Aqueous Solutions with an Ionic Liquid and High-pressure Carbon Dioxide. Chem. Eng. J. 2011, 171, 904–911. 10.1016/j.cej.2011.04.037. [DOI] [Google Scholar]
- Domańska U.; Pobudkowska A.; Bocheńska P. Extraction of Nitrofurantoin using Ionic Liquids. J. Chem. Eng. Data 2012, 57, 1894–1898. 10.1021/je201301b. [DOI] [Google Scholar]
- Matsumoto M.; Ohtani T.; Kondo K. Comparison of Solvent Extraction and Supported Liquid Membrane Permeation using an Ionic Liquid for Concentrating Penicillin G. J. Membr. Sci. 2007, 289, 92–96. 10.1016/j.memsci.2006.11.046. [DOI] [Google Scholar]
- Liu Q.; Li Y.; Li W.; Liang X.; Zhang C.; Liu H. Efficient Recovery of Penicillin G by a Hydrophobic Ionic Liquid. ACS Sustainable Chem. Eng. 2016, 4, 609–615. 10.1021/acssuschemeng.5b00975. [DOI] [Google Scholar]
- Pei Y.; Zhang J.; Song X.; Zhao M.; Wang J. Partition Behavior of Drug Molecules in Cholinium-Based Ionic Liquids. Sep. Sci. Technol. 2015, 50, 1641–1646. 10.1080/01496395.2014.978475. [DOI] [Google Scholar]
- Vitasari C. R.; Gramblička M.; Gibcus K.; Visser T. J.; Geertman R.; Schuur B. Separating Closely Resembling Steroids with Ionic Liquids in liquid–liquid Extraction Systems. Sep. Purif. Technol. 2015, 155, 58–65. 10.1016/j.seppur.2015.04.002. [DOI] [Google Scholar]
- Yue Y.; Jiang X.-Y.; Yu J.-G.; Tang K.-W. Enantioseparation of Mandelic Acid Enantiomers in Ionic Liquid Aqueous Two-phase Extraction Systems. Chem. Pap. 2013, 68, 465–471. 10.2478/s11696-013-0467-9. [DOI] [Google Scholar]
- Zhang Z.-J.; Pan J.; Ma B.-D.; Xu J.-H. In Bioreactor Engineering Research and Industrial Applications I: Cell Factories; Ye Q., Bao J., Zhong J.-J., Eds.; Springer: Berlin, 2016. [Google Scholar]
- Wang H.; Gurau G.; Kelley S. P.; Myerson A. S.; Rogers R. D. Hydrophobic vs. Hydrophilic Ionic Liquid Separations Strategies in Support of Continuous Pharmaceutical Manufacturing. RSC Adv. 2013, 3, 10019–10026. 10.1039/c3ra41082j. [DOI] [Google Scholar]
- Harini M.; Jain S.; Adhikari J.; Noronha S. B.; Yamuna Rani K. Design of an Ionic Liquid as a Solvent for the Extraction of a Pharmaceutical Intermediate. Sep. Purif. Technol. 2015, 155, 45–57. 10.1016/j.seppur.2015.07.040. [DOI] [Google Scholar]
- Li S. H.; He C. Y.; Liu H. W.; Li K. A.; Liu F. Ionic Liquid-salt Aqueous Two-phase System, a Novel System for the Extraction of Abused Drugs. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2005, 16, 1074–1076. 10.1016/j.jchromb.2005.08.005. [DOI] [Google Scholar]
- Ma C.-h.; Wang L.; Yan Y.-s.; Che G.-b.; Yin Y.-s.; Wang R.-z.; Li D.-y. Extraction of Tetracycline via Ionic Liquid Two-phase System. Chem. Res. Chin. Univ 2009, 25, 832–835. [Google Scholar]
- Pang J.; Han C.; Chao Y.; Jing L.; Ji H.; Zhu W.; Chang Y.; Li H. Partitioning Behavior of Tetracycline in Hydrophilic Ionic Liquids Two-Phase Systems. Sep. Sci. Technol. 2015, 50, 1993–1998. 10.1080/01496395.2015.1018436. [DOI] [Google Scholar]
- Marques C. F. C.; Mourão T.; Neves C. M. S. S.; Lima Á. S.; Boal-Palheiros I.; Coutinho J. A. P.; Freire M. G. Aqueous Biphasic Systems Composed of Ionic Liquids and Sodium Carbonate as Enhanced Routes for the Extraction of Tetracycline. Biotechnol. Prog. 2013, 29, 645–654. 10.1002/btpr.1708. [DOI] [PubMed] [Google Scholar]
- Shahriari S.; Tome L. C.; Araujo J. M. M.; Rebelo L. P. N.; Coutinho J. A. P.; Marrucho I. M.; Freire M. G. Aqueous Biphasic Systems: a Benign Route using Cholinium-based Ionic Liquids. RSC Adv. 2013, 3, 1835–1843. 10.1039/C2RA22972B. [DOI] [Google Scholar]
- Pereira J. F. B.; Vicente F.; Santos-Ebinuma V. C.; Araújo J. M.; Pessoa A.; Freire M. G.; Coutinho J. A. P. Extraction of Tetracycline from Fermentation Broth using Aqueous Two-phase Systems Composed of Polyethylene Glycol and Cholinium-based Salts. Process Biochem. 2013, 48, 716–722. 10.1016/j.procbio.2013.02.025. [DOI] [Google Scholar]
- Liu Q.; Yu J.; Li W.; Hu X.; Xia H.; Liu H.; Yang P. Partitioning Behavior of Penicillin G in Aqueous Two Phase System Formed by Ionic Liquids and Phosphate. Sep. Sci. Technol. 2006, 41, 2849–2858. 10.1080/01496390600786135. [DOI] [Google Scholar]
- Jiang Y.; Xia H.; Guo C.; Mahmood I.; Liu H. Phenomena and Mechanism for Separation and Recovery of Penicillin in Ionic Liquids Aqueous Solution. Ind. Eng. Chem. Res. 2007, 46, 6303–6312. 10.1021/ie070325p. [DOI] [Google Scholar]
- Jiang Y.; Xia H.; Yu J.; Guo C.; Liu H. Hydrophobic Ionic Liquids-assisted Polymer Recovery during Penicillin Extraction in Aqueous Two-phase System. Chem. Eng. J. 2009, 147, 22–26. 10.1016/j.cej.2008.11.012. [DOI] [Google Scholar]
- Li Y. F.; Han J.; Wang Y.; Ma J. J.; Yan Y. S. Partitioning of Cephalexin in Ionic Liquid Aqueous Two-Phase System Composed of 1-Butyl-3-Methylimidazolium Tetrafluoroborate and ZnSO4. J. Chem. 2013, 2013, 1–5. 10.1155/2013/193671. [DOI] [Google Scholar]
- Han J.; Wang Y.; Chen C.; Kang W.; Liu Y.; Xu K.; Ni L. (Liquid + Liquid) Equilibria and Extraction Capacity of (Imidazolium Ionic Liquids + Potassium Tartrate) Aqueous Two-phase Systems. J. Mol. Liq. 2014, 193, 23–28. 10.1016/j.molliq.2013.12.022. [DOI] [Google Scholar]
- Zhang W.; Zhang G.; Han J.; Yan Y.; Chen B.; Sheng C.; Liu Y. Phase Equilibrium and Chloramphenicol Partitioning in Aqueous two-phase System Composed of 1-hydroxylhexyl-3-methylimidazolium Chloride–salt. J. Mol. Liq. 2014, 193, 226–231. 10.1016/j.molliq.2013.12.047. [DOI] [Google Scholar]
- Chen L.-L.; Li F.-F.; Tan Z.-J. Chiral Separation of α-cyclohexylmandelic Acid Enantiomers using Ionic Liquid/salt Aqueous Two-phase System. Chem. Pap. 2015, 69, 1465–1472. 10.1515/chempap-2015-0162. [DOI] [Google Scholar]
- Zawadzki M.; e Silva F. A.; Domańska U.; Coutinho J. A. P.; Ventura S. P. M. Recovery of an Antidepressant from Pharmaceutical Wastes using Ionic Liquids-based Aqueous Biphasic Systems. Green Chem. 2016, 18, 3527–3536. 10.1039/C5GC03052H. [DOI] [Google Scholar]
- e Silva F. A.; Caban M.; Stepnowski P.; Coutinho J. A. P.; Ventura S. P. M. Recovery of Ibuprofen from Pharmaceutical Wastes using Ionic Liquids. Green Chem. 2016, 18, 3749–3757. 10.1039/C6GC00261G. [DOI] [Google Scholar]
- Kroon M. C.; van Spronsen J.; Peters C. J.; Sheldon R. A.; Witkamp G.-J. Recovery of Pure Products from Ionic Liquids using Supercritical Carbon Dioxide as a co-solvent in Extractions or as an Anti-solvent in Precipitations. Green Chem. 2006, 8, 246–249. 10.1039/B512303H. [DOI] [Google Scholar]
- Kroon M. C.; Toussaint V. A.; Shariati A.; Florusse L. J.; van Spronsen J.; Witkamp G.-J.; Peters C. J. Crystallization of an Organic Compound from an Ionic Liquid using Carbon Dioxide as Anti-solvent. Green Chem. 2008, 10, 333–336. 10.1039/b712848g. [DOI] [Google Scholar]
- Kühne E.; Peters C. J.; van Spronsen J.; Witkamp G.-J. Solubility of Carbon Dioxide in Systems with [bmim][BF4] and some Selected Organic Compounds of interest for the Pharmaceutical Industry. Green Chem. 2006, 8, 287–291. 10.1039/b513132d. [DOI] [Google Scholar]
- Kühne E.; Santarossa S.; Witkamp G.-J.; Peters C. J. Phase Equilibria in Ternary Mixtures of the Ionic Liquid bmim[BF4], (S)-naproxen and CO2 to Determine Optimum Regions for Green Processing. Green Chem. 2008, 10, 762–766. 10.1039/b801068d. [DOI] [Google Scholar]
- Weber C. C.; Kunov-Kruse A. J.; Rogers R. D.; Myerson A. S. Manipulation of Ionic Liquid Anion-solute-antisolvent Interactions for the Purification of Acetaminophen. Chem. Commun. 2015, 51, 4294–4297. 10.1039/C5CC00198F. [DOI] [PubMed] [Google Scholar]
- Viçosa A.; Letourneau J.-J.; Espitalier F.; Inês Ré M. An Innovative Antisolvent Precipitation Process as a Promising Technique to Prepare Ultrafine Rifampicin Particles. J. Cryst. Growth 2012, 342, 80–87. 10.1016/j.jcrysgro.2011.09.012. [DOI] [Google Scholar]
- An J.-H.; Kim J.-M.; Chang S.-M.; Kim W.-S. Application of Ionic Liquid to Polymorphic Design of Pharmaceutical Ingredients. Cryst. Growth Des. 2010, 10, 3044–3050. 10.1021/cg1001489. [DOI] [Google Scholar]
- An J.-H.; Kim W.-S. Antisolvent Crystallization using Ionic Liquids as Solvent and Antisolvent for Polymorphic Design of Active Pharmaceutical Ingredient. Cryst. Growth Des. 2013, 13, 31–39. 10.1021/cg300730w. [DOI] [Google Scholar]
- Smith K. B.; Bridson R. H.; Leeke G. A. Crystallisation Control of Paracetamol from Ionic Liquids. CrystEngComm 2014, 16, 10797–10803. 10.1039/C4CE01796J. [DOI] [Google Scholar]
- Weber C. C.; Kulkarni S. A.; Kunov-Kruse A. J.; Rogers R. D.; Myerson A. S. The Use of Cooling Crystallization in an Ionic Liquid System for the Purification of Pharmaceuticals. Cryst. Growth Des. 2015, 15, 4946–4951. 10.1021/acs.cgd.5b00855. [DOI] [Google Scholar]
- Calisto V.; Esteves V. I. Psychiatric Pharmaceuticals in the Environment. Chemosphere 2009, 77, 1257–1274. 10.1016/j.chemosphere.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Li C.-X.; Han J.; Wang Y.; Yan Y.-S.; Xu X.-H.; Pan J.-M. Extraction and Mechanism Investigation of Trace Roxithromycin in Real Water Samples by use of Ionic Liquid–salt Aqueous Two-phase System. Anal. Chim. Acta 2009, 653, 178–183. 10.1016/j.aca.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Han J.; Xie X.-q.; Li C.-x. Extraction of Trace Acetylspiramycin in Real Aqueous Environments using Aqueous Two-phase System of Ionic Liquid 1-butyl-3-methylimidazolium Tetrafluoroborate and Phosphate. Cent. Eur. J. Chem. 2010, 8, 1185–1191. 10.2478/s11532-010-0080-5. [DOI] [Google Scholar]
- Yang X.; Zhang S.; Yu W.; Liu Z.; Lei L.; Li N.; Zhang H.; Yu Y. Ionic Liquid-anionic Surfactant based Aqueous Two-phase Extraction for Determination of Antibiotics in Honey by High-performance Liquid Chromatography. Talanta 2014, 124, 1–6. 10.1016/j.talanta.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Dinis T. B. V.; Passos H.; Lima D. L. D.; Esteves V. I.; Coutinho J. A. P.; Freire M. G. One-step Extraction and Concentration of Estrogens for an Adequate Monitoring of Wastewater using Ionic-liquid-based Aqueous Biphasic Systems. Green Chem. 2015, 17, 2570–2579. 10.1039/C5GC00077G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.; Wang Y.; Yu C.; Li C.; Yan Y.; Liu Y.; Wang L. Separation, Concentration and Determination of Chloramphenicol in Environment and Food using an Ionic Liquid/salt Aqueous Two-phase Flotation System Coupled with High-performance Liquid Chromatography. Anal. Chim. Acta 2011, 685, 138–145. 10.1016/j.aca.2010.11.033. [DOI] [PubMed] [Google Scholar]
- Han J.; Wang Y.; Yu C.-l.; Yan Y.-S.; Xie X.-Q. Extraction and Determination of Chloramphenicol in Feed Water, Milk, and Honey Samples using an Ionic Liquid/sodium Citrate Aqueous Two-phase System Coupled with High-performance Liquid Chromatography. Anal. Bioanal. Chem. 2011, 399, 1295–1304. 10.1007/s00216-010-4376-2. [DOI] [PubMed] [Google Scholar]
- Xu X.; Su R.; Zhao X.; Liu Z.; Zhang Y.; Li D.; Li X.; Zhang H.; Wang Z. Ionic Liquid-Based Microwave-assisted Dispersive Liquid–liquid Microextraction and Derivatization of Sulfonamides in River Water, Honey, Milk, and Animal Plasma. Anal. Chim. Acta 2011, 707, 92–99. 10.1016/j.aca.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Yao C.; Li T.; Twu P.; Pitner W. R.; Anderson J. L. Selective Extraction of Emerging Contaminants from Water Samples by Dispersive Liquid–liquid Microextraction using Functionalized Ionic Liquids. J. Chromatogr. A 2011, 1218, 1556–1566. 10.1016/j.chroma.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Gao S.; Jin H.; You J.; Ding Y.; Zhang N.; Wang Y.; Ren R.; Zhang R.; Zhang H. Ionic Liquid-based Homogeneous Liquid–liquid Microextraction for the Determination of Antibiotics in Milk by High-performance Liquid Chromatography. J. Chromatogr. A 2011, 1218, 7254–7263. 10.1016/j.chroma.2011.08.063. [DOI] [PubMed] [Google Scholar]
- Vázquez M. M. P.; Vázquez P. P.; Galera M. M.; García M. D. G. Determination of Eight Fluoroquinolones in Groundwater Samples with Ultrasound-assisted Ionic Liquid Dispersive Liquid–liquid Microextraction prior to High-performance Liquid Chromatography and Fluorescence Detection. Anal. Chim. Acta 2012, 748, 20–27. 10.1016/j.aca.2012.08.042. [DOI] [PubMed] [Google Scholar]
- Song J.; Zhang Z. H.; Zhang Y. Q.; Feng C.; Wang G. N.; Wang J. P. Ionic Liquid Dispersive Liquid-liquid Microextraction Combined with High Performance Liquid Chromatography for Determination of Tetracycline Drugs in Eggs. Anal. Methods 2014, 6, 6459–6466. 10.1039/C4AY01079E. [DOI] [Google Scholar]
- Toledo-Neira C.; Álvarez-Lueje A. Ionic Liquids for Improving the Extraction of NSAIDs in Water Samples using Dispersive Liquid–liquid Microextraction by High Performance Liquid Chromatography-diode Array–fluorescence Detection. Talanta 2015, 134, 619–626. 10.1016/j.talanta.2014.11.067. [DOI] [PubMed] [Google Scholar]
- Álvarez M. S.; Esperança J. M. S. S.; Deive F. J.; Sanromán M. Á.; Rodríguez A. A Biocompatible Stepping Stone for the Removal of Emerging Contaminants. Sep. Purif. Technol. 2015, 153, 91–98. 10.1016/j.seppur.2015.08.039. [DOI] [Google Scholar]
- Almeida H. F. D.; Freire M. G.; Marrucho I. M. Improved Extraction of Fluoroquinolones with Recyclable Ionic-liquid-based Aqueous biphasic Systems. Green Chem. 2016, 18, 2717–2725. 10.1039/C5GC02464A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura S. P. M.; Coutinho J. A. P. In Toward the Recovery and Reuse of the ABS Phase-Forming Components In Ionic-Liquid-Based Aqueous Biphasic Systems: Fundamentals and Applications; Freire M. G., Ed.; Springer, 2016.