Abstract
Background
Perinatal maternal stress and low mood have been linked to offspring atopic eczema.
Objectives
To examine the relation of maternal stress/mood with atopic eczema in the offspring, focusing particularly on stress/psychological distress preconception.
Methods
At recruitment in the UK Southampton Women’s Survey, preconception maternal reports of perceived stress in daily living and the effect of stress on health were recorded; in a sub-sample psychological distress was assessed (12-item General Health Questionnaire). Infants were followed up at ages 6 (n=2956) and 12 (n=2872) months and atopic eczema ascertained (based on UK Working Party Criteria for the Definition of Atopic Dermatitis). At 6 months postpartum, mothers were asked if they had experienced symptoms of low mood since childbirth and completed the Edinburgh Post-natal Depression Scale.
Results
Preconception perceived stress affecting health (OR 1.21 (95%CI 1.08-1.35), p=0.001) and stress in daily living (OR 1.16 (1.03-1.30), p=0.014) were associated with an increased risk of offspring atopic eczema at age 12 months but not at 6 months, robust to adjustment for potentially confounding variables. Findings were similar for maternal psychological distress preconception. Low maternal mood between delivery and 6 months postpartum was associated with an increased risk of infantile atopic eczema at age 12 months, but no significant association between postnatal mood and atopic eczema was seen after taking account of preconception stress.
Conclusion & Clinical Relevance
Our data provide novel evidence linking maternal stress at preconception to atopic eczema risk, supporting a developmental contribution to the aetiology of atopic eczema and pointing to potentially modifiable influences.
Keywords: Atopic eczema, maternal stress, maternal mood
Introduction
Atopic eczema is a prevalent chronic skin condition that can have a considerable impact on quality of life and a significant financial burden. A rise in prevalence has been observed globally [1], with an estimated annual prevalence of 9.5% in children under 4 years of age in the UK [2]. There is increasing evidence that atopic eczema partly originates in utero, where genetic susceptibility and environmental exposures can result in immune dysregulation [3], influencing the risk of developing the condition. Better understanding of such early life environmental exposures could help identification of preventative strategies.
Pathways by which maternal stress can cause fetal immune dysregulation leading to a propensity to develop atopic eczema and other atopic disorders have been proposed. In mice, transplacental passage of maternal stress hormones affects fetal hypothalamic-pituitary-adrenal (HPA) axis development, with prenatally stressed offspring demonstrating airway inflammation and hyper-responsiveness [4]. Maternal stress also alters placental corticotrophin releasing hormone (CRH) regulation, with the potential to influence the fetal HPA axis [5]. In addition, glucocorticoids and catecholamines released as a result of stress can modulate differentiation of type 1 and 2 T-helper (Th1 and Th2) cells, favouring a shift towards a Th2 humoral cell type reaction with overproduction of interleukins 4 and 10 and suppression of interleukin 12 [6]. This inflammatory reaction is seen in atopic eczema and other atopic conditions.
Experimental studies in rodents and sheep have shown that maternal glucocorticoid exposure during the period around conception can have important effects on a range of organs, including the HPA axis, with implications for immune disorders such as atopic eczema [7, 8]. In humans the consequences of stress around conception remain underexplored. In a recent systematic review, Andersson et al [9] identified a number of studies linking prenatal maternal stress to an increased risk of offspring atopic eczema [10–14]. Sausenthaler et al. [13] observed a link between maternal stress during pregnancy and an increased risk of atopic eczema in the first 2 years of life but not beyond that age as a result of stress during pregnancy, suggesting that the impact of prenatal stress weakens in the presence of other influencing factors. When examining other atopic disorders in relation to prenatal maternal stress, De Marco et al. [12] reported comparable odds ratios for asthma, eczema and allergic rhinitis, supporting an immunomodulatory effect of stress and stress hormones on the development of atopy in humans. Furthermore, postpartum depression during the first 6 months of life has been linked with childhood atopic eczema at age 3 years, independently of prenatal maternal stress [15]. Early life persistent and increasing maternal postnatal depressive symptoms [16, 17], greater caregiver perceived stress [18], and prenatal and postnatal negative life events [19] have also been associated with an increased risk of offspring asthma.
It is possible that mothers experiencing stress may make poor health practice choices, including smoking, or differ in terms of their age, educational attainment, parity and history of eczema, which put their offspring at an increased risk of developing atopic eczema [20], and thus previous studies may not have controlled for all potential confounding variables. Moreover, no previous studies have explored the link between maternal preconception stress/ low mood and offspring risk of developing atopic eczema.
The objective of this study was to determine whether the risk of developing infantile atopic eczema at ages 6 and 12 months was influenced by maternal stress and low mood, controlling for a range of potential confounding factors and with particular focus on the effects of stress prior to conception.
Methods
Southampton Women’s Survey
In the UK Southampton Women’s Survey (SWS), between 1998 and 2002, 12,583 women aged 20-34 years who were not pregnant were recruited from the general population through general practitioners in Southampton, UK, and the surrounding area. Information on maternal diet, lifestyle, socioeconomic status, stress and psychological distress was collected [21]. Women who became pregnant were followed up through their pregnancies; 3158 live born infants were delivered. The findings reported in this manuscript are based on the 3008 mother-offspring dyads assessed for atopic eczema at 6 and/or 12 months. All phases of the Southampton Women’s Survey were approved by the Southampton and South West Hampshire Local Research Ethics Committee and parents gave written informed consent.
Stress and mood assessments
At recruitment (preconception) women were asked to report perceived stress using two questions (‘To what extent do you feel that the stress or pressure you have experienced in your life has affected your health?’ (n=3002), and ‘In general, how much stress or pressure have you experienced in your daily living in the last 4 weeks?’(n=3007)), grading the impact of stress into one of five groups (none, slightly/just a bit, moderately/a good bit, quite a lot, extremely/a great deal). These were adapted from the Short Form (36) Health Survey (SF-36) [22]. A subgroup of participants also completed the 12 item General Health Questionnaire (GHQ-12) (n= 1729) assessing mental wellbeing, in which a score of ≥3 is considered indicative of psychological distress [23].
At 6 months postpartum, mothers completed the Edinburgh Post-natal Depression Scale (EPDS), where higher scores indicate lower mood and a score of 13 or more is considered indicative of probable major depression [24]. A subgroup of mothers (n=1660-1713) were also asked if they had experienced symptoms of low mood between delivery and infant age 6 months (episodes of feeling sad, depressed or gloomy for most of day, unable to find pleasure in things normally enjoy, lost interest in things normally enjoy, feeling tired or worn out and episodes of having less energy than usual), these questions were adapted from the Patient Health Questionnaire- 9 (PHQ-9) [25].
Outcome assessment
Case definition of atopic eczema was based on the UK Working Party diagnostic criteria for the definition on atopic eczema [26]; as assessed by trained research nurses who administered a standardised questionnaire and ascertained other information required for the diagnostic criteria (a combination of history of itchy skin condition and two of the following: history of involvement of the skin creases such as folds of elbows, behind the knees, fronts of ankles, cheeks or around the neck, a history of a general dry skin in the last year and visible flexural eczema or eczema involving the cheeks/forehead and outer limbs in). All infants were assessed for eczema before the age of 2 years, thus this criterion was met by all infants in the study cohort. However, as the infants were not old enough to have developed clearly defined atopic disorders, a personal history of atopy was omitted as a criterion.
Statistical analysis
Confounding variables were determined prior to the analysis using a directed acyclic graph (DAGs) (Supplementary figure 1) [27]. Maternal factors that were included as potential confounding variables in our analyses were age at child’s birth, education, smoking, parity and eczema in the past 12 months; infant confounding variables were infant sex, gestational age, season of birth and breastfeeding. P values < 0.05 were considered statistically significant. Data on maternal and paternal eczema in childhood were available for most of the cohort, and additional analyses controlling for these were undertaken and are shown in supplementary tables 1 and 2. Logistic regression analyses were performed (Stata version 14.1, Statacorp LP, TX) to relate maternal stress/mood to infant atopic eczema at ages 6 and 12 months. The five-category variables describing stress preconception were analysed as continuous variables.
Further logistic regression models were used to analyse the relations between preconception stress (as determined by stress affecting health and stress in daily living) and postnatal low mood (as determined by EPDS score) and infant atopic eczema at ages 6 and 12 months. GHQ-12 scores and postnatal symptoms of low mood were not included in this analysis as these were only assessed in subsets of the study population.
Results
Cohort characteristics
Maternal and infant characteristics are summarised in Table 1. Among the study group, the mothers’ average age at their children’s birth was 30.7 years (standard deviation (SD) = 3.8); 51.4% were primiparous, 15.7% smoked during pregnancy and 7.0% of the mothers had eczema in the past 12 months. The mean duration between the initial questionnaire and conception was 2.2 years. 51.9% of infants were male; mean birthweight of infants was 3.44 kg (SD 0.55) and gestational age 40.1 weeks (IQR 39.0-41.0). 2956 infants were assessed for eczema at age 6 months, 8.9% of which had atopic eczema. At age 12 months 2872 infants were assessed and 9.4% had atopic eczema.
Table 1.
Characteristics of the study population
| Total n | n (%), Median (IQR) or Mean (SD) | |
|---|---|---|
| Maternal | ||
| Age at child’s birth (y) | 3008 | 30.7 (3.8) |
| % A level or higher | 2999 | 59.1% |
| % Smoking in pregnancy | 2870 | 15.7% |
| % Primiparous | 3005 | 51.4% |
| % Eczema in last 12 months | 2815 | 7.0% |
| Infant | ||
| % Male | 3008 | 51.9% |
| Gestational age (weeks) | 3008 | 40.1 (39.1-41.0) |
| Birthweight (kg) | 2981 | 3.44 (0.55) |
| Born in winter | 673 | 22.4% |
| Born in spring | 726 | 24.1% |
| Born in summer | 807 | 26.8% |
| Born in autumn | 802 | 26.7% |
| Breast feeding (completed months) | ||
| Never breast fed | 527 | 18.4% |
| <1 | 581 | 20.2% |
| 1 to 3 | 615 | 21.4% |
| 4 to 6 | 484 | 16.9% |
| 7 to 11 | 422 | 14.7% |
| 12 or more | 241 | 8.4% |
| 6 month assessment | ||
| Age (wks) | 2956 | 27.4 (26.1-33.7) |
| % Atopic eczema as per UK working party criteria | 2956 | 262 (8.9%) |
| 12 month assessment | ||
| Age (wks) | 2872 | 53.7 (52.6-55.0) |
| % Atopic eczema as per UK working party criteria | 2872 | 270 (9.4%) |
Table 2 provides descriptive data on maternal mood. Preconception, 28.2% had a GHQ-12 score of ≥3, suggestive of psychological distress; 17.7% reported that stress affected their health ‘quite a lot’ or ‘extremely’ preconception and 24.6% reported ‘quite a lot’ or ‘a great deal’ of stress experienced in daily living. At 6 months after delivery, 46.8% reported episodes of feeling sad, depressed or gloomy for most of the day; the median EPDS score was 10 (IQR 6-15) and 36.7% had a score of 13 or more.
Table 2.
Descriptive data of maternal mood
| Total n | n (%) or Median (IQR) | |
|---|---|---|
|
PRECONCEPTION | ||
|
Maternal stress preconception | ||
| Stress in life affecting health | 3007 | |
| None | 723 (24.0%) | |
| Slightly | 1203 (40.0%) | |
| Moderately | 548 (18.2%) | |
| Quite a lot | 448 (14.9%) | |
| Extremely | 85 (2.8%) | |
| Stress in daily living in last 4 weeks | 3002 | |
| None | 248 (8.3%) | |
| Just a bit | 1373 (45.7%) | |
| A good bit | 644 (21.5%) | |
| Quite a lot | 572 (19.1%) | |
| A great deal | 165 (5.5%) | |
|
Maternal mental wellbeing preconception | ||
| Psychological distress ascertained by General Health | 1729 | |
| Questionnaire | ||
| No (score <3) | 1241 (71.8%) | |
| Yes (score ≥3) | 488 (28.2%) | |
|
POSTNATAL | ||
|
Maternal mood in worst 2 week period between birth and infant age 6 months | ||
| Edinburgh Postnatal Depression Score | 2853 | 10 (6-15) |
| Edinburgh Postnatal Depression Score ≥13 | 1046 (36.7%) | |
|
Postnatal maternal mood between delivery and infant age 6 months | ||
| Between delivery and infant age 6 months experienced episodes of: | ||
| Feeling sad, depressed or gloomy for most of day | 1713 | 802 (46.8%) |
| Unable to find pleasure in things normally enjoy | 1660 | 502 (30.2%) |
| Lost interest in things normally enjoy | 1660 | 490 (29.5%) |
| Feeling tired of worn out | 1660 | 1319 (79.7%) |
| Less energy than usual | 1660 | 1294 (78.0%) |
Association between maternal stress and mood and infant atopic eczema at age 6 months
Table 3 shows univariate (unadjusted) and multivariate (adjusted) analyses of maternal and infant characteristics in relation to infant atopic eczema at age 6 months. Stress in daily living preconception was associated with increased risk of offspring atopic eczema (OR 1.13, 95% CI 1.01-1.28, p = 0.039); this association weakened slightly after adjusting for potential confounders (adjusted OR 1.12, 95% CI 0.99-1.28, p=0.072). Episodes of feeling sad, depressed or gloomy for most of the day experienced between delivery and infant age 6 months were associated with an increased risk of atopic eczema after taking confounding variables into account (OR 1.40, 95%CI 1.00-1.96, p= 0.048). Other measures of maternal postnatal mood and postnatal EPDS score were not associated with offspring atopic eczema at age 6 months. Additionally controlling for maternal and paternal eczema in childhood had little effect on the 6 month eczema analyses shown in Table 3 (Supplementary Table 1).
Table 3.
Maternal stress/mood preconception and postnatally in relation to infant atopic eczema at age 6 months.
| Unadjusted | Adjusted * | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | OR | 95% CI | P value | n | OR | 95% CI | P value | n |
| Preconception maternal mood | ||||||||
| Stress in life affected health (5 levels) | 1.10 | 0.98-1.24 | 0.093 | 2956 | 1.08 | 0.96-1.23 | 0.22 | 2548 |
| Stress in daily living in the past 4 weeks (5 levels) | 1.13 | 1.01-1.28 | 0.039 | 2951 | 1.12 | 0.99-1.28 | 0.072 | 2546 |
| Psychological distress ascertained by GHQ-12 (no/yes) | 1.21 | 0.85-1.73 | 0.30 | 1694 | 1.24 | 0.84-1.83 | 0.28 | 1419 |
| Postnatal maternal mood between delivery and age 6 months | ||||||||
| Edinburgh Postnatal Depression Score | 1.01 | 0.99-1.03 | 0.28 | 2816 | 1.01 | 0.99-1.04 | 0.26 | 2431 |
| Edinburgh Postnatal Depression Score ≥13 | 1.13 | 0.87-1.48 | 0.35 | 2816 | 1.12 | 0.84-1.49 | 0.44 | 2431 |
| Between delivery and infant age 6 months experienced episodes of: | ||||||||
| Feeling sad, depressed or gloomy for most of day (yes/no) | 1.33 | 0.97-1.83 | 0.08 | 1713 | 1.40 | 1.00-1.96 | 0.048 | 1646 |
| Unable to find pleasure in things normally enjoy (yes/no) | 1.34 | 0.95-1.87 | 0.09 | 1660 | 1.26 | 0.88-1.80 | 0.20 | 1596 |
| Lost interest in things normally enjoy (yes/no) | 1.27 | 0.90-1.79 | 0.17 | 1660 | 1.26 | 0.88-1.79 | 0.22 | 1596 |
| Feeling tired or worn out (yes/no) | 1.07 | 0.72-1.61 | 0.73 | 1660 | 1.01 | 0.67-1.54 | 0.95 | 1596 |
| Less energy than usual (yes/no) | 1.35 | 0.89-2.06 | 0.16 | 1660 | 1.26 | 0.81-1.94 | 0.30 | 1596 |
Adjusted for maternal age at birth, education, smoking in pregnancy, parity and eczema, and infant sex, gestational age at birth, season of birth and breastfeeding duration
Association between maternal stress and mood and infant atopic eczema at age 12 months
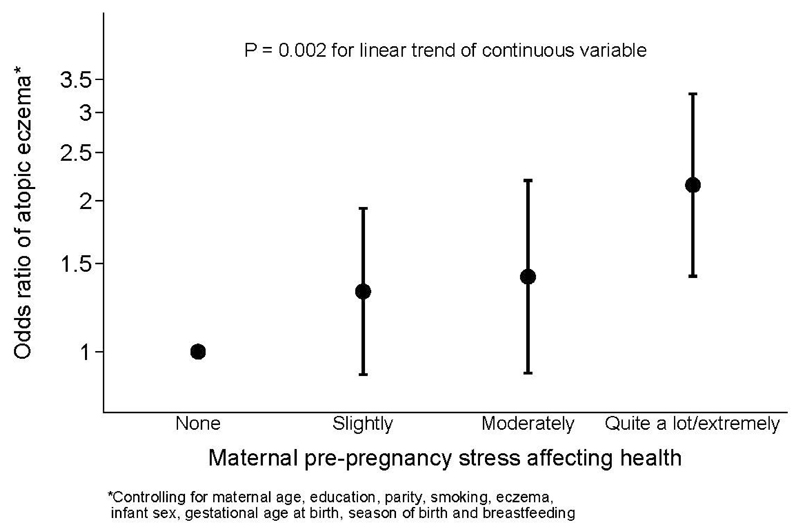
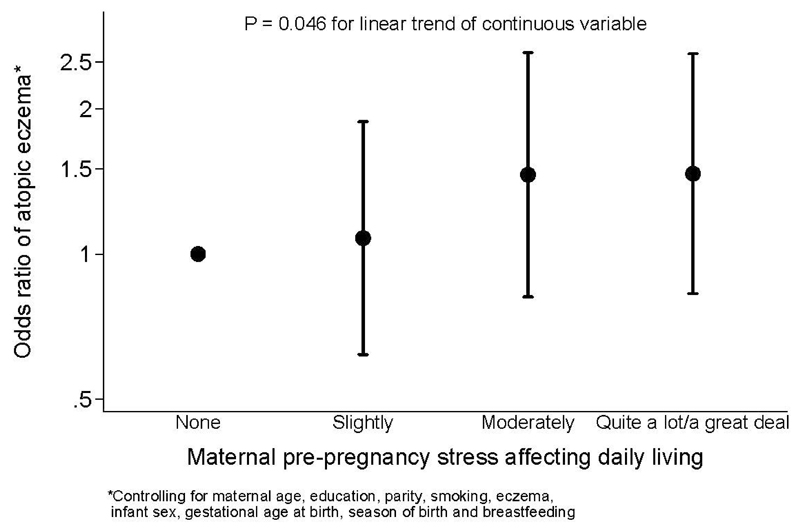
Table 4 shows similar analyses in relation to infant atopic eczema at age 12 months. Psychological distress ascertained by the GHQ-12 preconception was associated with increased risk of atopic eczema (OR 1.43, 95% CI 1.00-2.04, p = 0.044), but the association weakened slightly after adjusting for confounding variables (OR 1.37, 95% CI 0.93-2.00, p = 0.11). Increased stress in life affecting health (OR 1.21, 95% CI 1.08-1.35, p = 0.001, Figure 1) and increased stress in daily living (OR 1.16, 95%CI 1.03-1.30, p = 0.014, Figure 2) preconception were associated with an increased risk of offspring atopic eczema at age 12 months, these associations remaining significant after adjusting for confounding variables (p = 0.014 and p = 0.046, respectively). Examining postnatal maternal mood, all five measures (experiencing episodes of feeling sad, depressed or gloomy for most of the day unable to find pleasure in things normally enjoyed, loss of interest in things normally enjoyed, having less energy than usual) ascertained 6 months after delivery showed associations or trends towards lower maternal mood being associated with a higher odds of offspring atopic eczema at age 12 months in both unadjusted and adjusted analyses (p = 0.08 to p = 0.013); adjusting for potential confounders generally increased the odds ratios of atopic eczema. A higher EPDS score examined as a continuous variable was also significantly associated with increased risk of atopic eczema at 12 months (OR 1.02, 95%CI 1.00-1.04, p = 0.045), but there was no association with an EPDS score of ≥13. Additionally controlling for maternal and paternal eczema in childhood somewhat attenuated the association with maternal preconception stress in daily living in the past 4 weeks (adjusted OR 1.10, 95%CI 0.97-1.25, p = 0.14), with little effect on the associations with preconception stress in life affecting health or postnatal stress variables shown in Table 4 (Supplementary Table 2).
Table 4.
Maternal stress/mood preconception and postnatally in relation to infant atopic eczema at age 12 months.
| Unadjusted | Adjusted * | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | OR | 95% CI | P value | n | OR | 95% CI | P value | n |
| Preconception maternal mood | ||||||||
| Stress in life affected health (5 levels) | 1.21 | 1.08-1.35 | 0.001 | 2871 | 1.21 | 1.07-1.37 | 0.002 | 2448 |
| Stress in daily living in the past 4 weeks (5 levels) | 1.16 | 1.03-1.30 | 0.014 | 2867 | 1.14 | 1.00-1.29 | 0.046 | 2447 |
| Psychological distress ascertained by GHQ-12 (no/yes) | 1.43 | 1.00-2.04 | 0.044 | 1629 | 1.37 | 0.93-2.01 | 0.11 | 1340 |
| Postnatal maternal mood between delivery and age 6 months | ||||||||
| Edinburgh Postnatal Depression Score | 1.02 | 1.00-1.04 | 0.045 | 2721 | 1.02 | 1.00-1.05 | 0.041 | 2330 |
| Edinburgh Postnatal Depression Score >13 | 1.09 | 0.84-1.42 | 0.52 | 2721 | 1.08 | 0.82-1.44 | 0.58 | 2330 |
| Between delivery and infant age 6 months experienced episodes of: | ||||||||
| Feeling sad, depressed or gloomy for most of day (yes/no) | 1.47 | 1.06-2.04 | 0.022 | 1621 | 1.52 | 1.08-2.13 | 0.016 | 1574 |
| Unable to find pleasure in things normally enjoy (yes/no) | 1.54 | 1.09-2.18 | 0.013 | 1569 | 1.55 | 1.08-2.20 | 0.016 | 1524 |
| Lost interest in things normally enjoy (yes/no) | 1.56 | 1.11-2.21 | 0.011 | 1569 | 1.57 | 1.10-2.24 | 0.013 | 1524 |
| Feeling tired or worn out (yes/no) | 1.51 | 0.95-2.40 | 0.08 | 1569 | 1.52 | 0.95-2.42 | 0.08 | 1524 |
| Less energy than usual (yes/no) | 1.75 | 1.09-2.79 | 0.020 | 1569 | 1.68 | 1.05-2.70 | 0.032 | 1524 |
Adjusted for maternal age at birth, education, smoking in pregnancy, parity and eczema, and infant sex, gestational age at birth, season of birth and breastfeeding duration
Figure 1.
Figure 2.
Sensitivity analysis indicated that infants of women who reported greater perceived stress affecting health and stress in daily living and were conceived less than 1 year after the mother completed the initial preconception assessment had higher odds of developing atopic eczema at age 12 months compared with the offspring whose mothers did not report stress; this effect was greater than that seen among women in the whole study cohort (ORs of 1.25 vs 1.21 for stress affecting health, 1.22 vs 1.14 for stress in daily living for adjusted analyses) (Supplementary Table 3).
We undertook further multivariate analyses to examine the relation of offspring atopic eczema with preconception stress as determined by perceived stress affecting health and stress in daily living, and postnatal low mood as determined by EPDS (Table 5). Examining atopic eczema at age 6 months; we found no association with stress/low mood either preconception or postnatally. However, examining atopic eczema at age 12 months; stress affecting health at preconception (p= 0.015) was associated with an increased risk of atopic eczema but no significant association was seen between postnatal mood and eczema after taking account of preconception stress/mood variables.
Table 5.
Maternal preconception stress and postnatal mood in relation to infant atopic eczema at ages 6 and 12 months
| OR | 95% CI | P value | n | |
|---|---|---|---|---|
| Infant atopic eczema age 6 months | 2429 | |||
| Preconception | ||||
| Stress in life affected health (5 levels) | 1.04 | 0.91-1.20 | 0.57 | |
| Stress in daily living in the past 4 weeks (5 levels) | 1.09 | 0.94-1.25 | 0.24 | |
| Postnatal | ||||
| EPDS | 1.01 | 0.98-1.03 | 0.48 | |
| Infant atopic eczema age 12 months | 2329 | |||
| Preconception | ||||
| Stress in life affected health (5 levels) | 1.80 | 1.03-1.35 | 0.015 | |
| Stress in daily living in the past 4 weeks (5 levels) | 1.07 | 0.93-1.22 | 0.34 | |
| Postnatal | ||||
| EPDS | 1.01 | 0.99-1.04 | 0.24 | |
Adjusted for maternal age at birth, education, smoking in pregnancy, parity and eczema, and infant sex, gestational age at birth, season of birth and breastfeeding duration
A sensitivity analysis omitting 52 infants who had questionnaire data but missing data on visible eczema that could potentially have contributed to the case definition of atopic eczema (52 infants at age 6 months, 5 infants at age 12 months) showed little change in relation to the findings for either 6 month eczema and perceived stress in life affecting health (OR 1.11, 95% CI 0.99-1.25), perceived stress affecting daily living in the past 4 weeks (OR 1.13, 95% CI 1.01-1.28) and EPDS (OR 1.01, 95% CI 0.99-1.03) or 12 month eczema and the same variables OR 1.21 (95% CI 1.08-1.36), 1.16 (95% CI 1.03-1.30) and 1.02 (95% CI 1.00-1.04), respectively.
Discussion
We found that maternal preconception stress was associated with an increased risk of offspring atopic eczema at age 12 months but not at age 6 months. These associations were robust to adjustment for potentially confounding variables including maternal history of eczema in the past 12 months, maternal smoking during pregnancy, infant gestational age, sex and breastfeeding duration. Findings were similar for maternal psychological distress preconception. Low maternal mood between delivery and 6 months postpartum assessed using the EPDS as a continuous variable (but not as a dichotomous variable) was also associated with an increased risk of infantile atopic eczema at age 12 months, but no significant association between postnatal mood and eczema was seen after taking account of preconception stress/mood variables. There are many research papers utilizing EPDS as a continuous scale in relation to different outcomes. [28, 29] Using the EPDS as a continuous outcome better captures the multiple dimensions ascertained by the questionnaire, including anxiety, low mood and a sense of helplessness [30], which may not be captured by dichotomising the score (as used in clinical practice to screen for depression).
Maternal stress in the prenatal [9] and postnatal [15] periods have previously been shown to be linked to offspring atopic eczema. Our data, however, are the first to show a link between maternal stress preconception and the risk of this skin disorder.
Previous research has examined potential mechanisms for maternal prenatal stress resulting in effects in the fetus (which might lead to atopy in the offspring). For example, stress is associated with raised cortisol levels and although the majority of the maternal cortisol is metabolised as it crosses the placenta, 10-20% can pass through the placenta unmetabolised; this amount can double the much lower fetal cortisol concentration, and a linear relationship between fetal cortisol concentration and maternal cortisol concentration has been observed [31]. Placental CRH (pCRH) which is also released as a result of maternal stress, creates a positive feedback loop and a subsequent increase in pCRH, ACTH and cortisol. pCRH affects both mother and fetus and has been shown to impact fetal programming and influencing health outcomes [32]. Furthermore, mothers experiencing psychosocial stress or nervousness during pregnancy have been found to have raised maternal serum pro-inflammatory cytokines [33] and higher umbilical cord IgE concentrations [34], respectively. Despite maternal neuroendocrine changes occurring as part of pregnancy, maternal psychosocial status has also been linked with circulating plasma levels of neuroendocrine parameters including ACTH, B-endorphin and cortisol [35]. Chang et al proposed a further potential mechanism linking maternal stress with increased offspring susceptibility to atopic eczema where the placenta fails to maintain its detoxifying ability when reactive oxygen species are high during stress, exposing the developing foetus to oxidative stress [36].
The stress model is complex and has evolved in recent years with some studies exploring the link between atopic eczema and certain aspects of this model including exposure to stressors, such as significant life events, perceived stress and stress related disorders including anxiety and depression prenatally. Wen et al reported that self-reported psychological distress in the third trimester, cord blood IgE and lymphotoxin (LT-α) and high-affinity receptor for IgE (FcεRI-β) genotypes were associated with offspring atopic eczema at 2 years of age [10]. Children of mothers who experienced stressful life events (divorce, mourning, loss of job) during pregnancy were found to have a moderately increased risk of atopic eczema, wheezing, asthma and allergic rhinitis [12]. Hartwig et al [11] observed similar associations where a higher likelihood of atopic asthma and eczema in 14 year olds was seen in those whose mothers experienced negative life events in the second half of pregnancy.
Physiological responses to acute and chronic stress differ. The release of stress hormones in adaptation to acute stress in order to maintain homeostasis is referred to as allostasis [37]. Increased allostatic load develops as a result of recurring exposure to stress, protracted response to stress and incapability to adapt [38]. Although the nature, severity, significance and persistence of stress are important [39], personality traits, coping ability and physical stress are also influential in the way humans respond to stress and this makes quantifying stress difficult. As a result, detailed understanding of the exact amount of stress experienced personally by each individual, and the effects of that stress on biological events, is limited.
Exposure to stress during sensitive stages of development in early life can affect the programming of the HPA axis through epigenetic changes and may result in a Th2 dominant immune response [40]. Also, methylation of the gene encoding the glucocorticoid receptor (NR3C1) has been shown to be associated with exposure to prenatal psychological distress [41]. The stages that are most sensitive to immunomodulatory effects of maternal stress have not been identified. Evidence from animal studies support the notion that maternal exposure to stress such as undernutrition around conception [7, 8, 42, and 43] and stress such as restraint and noise and light stress in early pregnancy can affect the development of a number of systems, including the immune system and the HPA axis [44, 45]. However, evidence is scarce on the impact of periconceptional stress on HPA development in humans. Furthermore, it is unclear whether neuroendocrine changes related to maternal stress occurring around the time of conception influence the risk of atopic eczema in the same mechanistic way as if they occurred later in pregnancy.
We found that maternal stress was associated with infant eczema at age 12 months but not at age 6 months; this could reflect heterogeneity in the aetiology and pathogenesis of atopic eczema in early childhood [46]. Our data are consistent with these observations, where atopic eczema occurring in late infancy was associated with environmental factors and atopic eczema with an early onset in the first six months was mainly associated with familial factors [46]. We showed that infants conceived within 1 year of their mothers reporting increased stress affecting health and stress in daily living had higher odds of developing atopic eczema when compared with the remainder of the study cohort, suggesting that stress/low mood experienced closer to the time of conception may have a greater impact on the risk offspring atopic eczema.
Our study highlights a link between maternal stress at preconception and an increased risk of atopic eczema in the offspring at 12 months. Strengths of the study are its large sample size, its prospective nature, collection of true preconception data, the standardised assessment of eczema by trained staff and control for confounding factors. The questions on perceived stress in daily living and stress affecting health preconception were directly adapted from the Short Form (36) Health Survey, which is an established research tool for ascertaining perceived stress [22]. The General Health Questionnaire (GHQ-12) is likewise, a widely utilized standard questionnaire assessing mental wellbeing, which has been validated as measuring psychological distress [23]. Nevertheless, limitations were the lack of evaluation of stress during pregnancy and the use of questionnaire-based assessments of stress where there is potential for reporting bias. A further limitation is that 52 infants at age 6 months and 5 infants at age 12 months had missing information on visible eczema that could potentially have contributed to the case definition of atopic eczema; however, a sensitivity analysis omitting these infants with a missing skin examination information showed little change in the findings. Bioindicators relating to stress were not collected; however, indicators such as salivary cortisol may be difficult to interpret as concentrations fluctuate, particularly during pregnancy.
In summary, our study demonstrates a novel link between preconception maternal stress / low mood and the risk of atopic eczema in the offspring. The findings point to potentially modifiable maternal influences on this complex, multifactorial skin condition and add to the evidence that atopic eczema partly originates during development before birth.
Funding
This work was supported by grants from the Medical Research Council, British Heart Foundation, Food Standards Agency, Arthritis Research UK, National Osteoporosis Society, International Osteoporosis Foundation, Cohen Trust, European Union's Seventh Framework (FP7/2007-2013), projects EarlyNutrition and ODIN under grant agreement numbers 289346 and 613977, NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, NIHR Musculoskeletal Biomedical Research Unit, University of Oxford and British Lung Foundation.
Abbreviations
- GHQ
(General Health Questionnaire)
- EPDS
(Edinburgh Postnatal Depression Scale)
- OR
(Odds Ratio)
- HPA
(Hypothalamic-Pituitary-Adrenal)
- Th1 and 2
(T-helper)
- IL
(Interleukin)
- SWS
(Southampton Women’s Survey)
- SF-36
(Short Form (36) Health Questionnaire)
- PHQ9
(Patient Health Questionnaire)
- DAG
(Directed Acyclic Graph)
- SD
(Standard Deviation)
- CRH
(Corticotropin Releasing Hormone)
Footnotes
Author contributions
The authors’ responsibilities were as follows—KMG, HMI, SMR, JB, NCH, and CC: were responsible for the design and conduct of the Southampton Women's Survey; with input from SRC and EH, SEH and KMG planned the analyses and drafted the manuscript; SEH: conducted the statistical analyses with support from SRC; and all authors: read and approved the final version of the manuscript.
Conflict of interest
KMG has received reimbursement for speaking at conferences sponsored by companies selling nutritional products and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. No other author reports any potential conflicts of interest.
References
- 1.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–43. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Simpson CR, Anderson WJA, Helms PJ, Taylor MW, Watson L, Prescott GJ, et al. Coincidence of immune-mediated diseases driven by Th1 and Th2 subsets suggests a common aetiology. A population-based study using computerized general practice data. Clin Exp Allergy. 2002;32:37–42. doi: 10.1046/j.0022-0477.2001.01250.x. [DOI] [PubMed] [Google Scholar]
- 3.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–30. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pincus-Knackstedt MK, Joachim RA, Blois SM, Douglas AJ, Orsal AS, Klapp BF, et al. Prenatal Stress Enhances Susceptibility of Murine Adult Offspring toward Airway Inflammation. J Immunol. 2006;177:8484–92. doi: 10.4049/jimmunol.177.12.8484. [DOI] [PubMed] [Google Scholar]
- 5.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999;10:359–68. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 7.Langley-Evans SC, Gardner DS, Jackson AA. Maternal protein restriction influences the programming of the rat hypothalamic-pituitary-adrenal axis. J Nutr. 1996 Jun;126:1578–85. doi: 10.1093/jn/126.6.1578. [DOI] [PubMed] [Google Scholar]
- 8.Bloomfield FH, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, et al. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology. 2004;145:4278–4285. doi: 10.1210/en.2004-0424. [DOI] [PubMed] [Google Scholar]
- 9.Andersson NW, Hansen MV, Larsen AD, Hougaard KS, Kolstad HA, Schlünssen V. Prenatal maternal stress and atopic diseases in the child: a systematic review of observational human studies. Allergy. 2016;71:15–26. doi: 10.1111/all.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen H-J, Wang Y-J, Lin Y-C, Chang C-C, Shieh C-C, Lung F-W, et al. Prediction of atopic dermatitis in 2-yr-old children by cord blood IgE, genetic polymorphisms in cytokine genes, and maternal mentality during pregnancy. Pediatr Allergy Immunol. 2011;22:695–703. doi: 10.1111/j.1399-3038.2011.01177.x. [DOI] [PubMed] [Google Scholar]
- 11.Hartwig IR, Sly PD, Schmidt LA, van Lieshout RJ, Bienenstock J, Holt PG, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol. 2014;134:160–9. doi: 10.1016/j.jaci.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 12.De Marco R, Pesce G, Girardi P, Marchetti P, Rava M, Ricci P, et al. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatr Allergy Immunol. 2012;23:724–9. doi: 10.1111/j.1399-3038.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- 13.Sausenthaler S, Rzehak P, Chen CM, Arck P, Bockelbrink A, Schafer T, et al. Stress-related maternal factors during pregnancy in relation to childhood eczema: results from the LISA Study. J Investig Allergol Clin Immunol. 2009;19:481–7. [PubMed] [Google Scholar]
- 14.Larsen AD, Schlunssen V, Christensen BH, Bonde JP, Obel C, Thulstrup AM, et al. Exposure to psychosocial job strain during pregnancy and odds of asthma and atopic dermatitis among 7-year old children – a prospective cohort study. Scand J Work Environ Health. 2014;40:639–48. doi: 10.5271/sjweh.3452. [DOI] [PubMed] [Google Scholar]
- 15.Wang IJ, Wen HJ, Chiang TL, Lin SJ, Guo YL. Maternal psychologic problems increased the risk of childhood atopic dermatitis. Pediatr Allergy Immunol. 2016 Mar;27:169–76. doi: 10.1111/pai.12518. [DOI] [PubMed] [Google Scholar]
- 16.Giallo R, Bahreinian S, Brown S, Cooklin A, Kingston D, Kozyrskyj A. Maternal depressive symptoms across early childhood and asthma in school children: findings from a Longitudinal Australian Population Based Study. PLoS One. 2015;10:e0121459. doi: 10.1371/journal.pone.0121459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozyrskyj AL, Mai XM, McGrath P, Hayglass KT, Becker AB, Macneil B. Continued exposure to maternal distress in early life is associated with an increased risk of childhood asthma. Am J Respir Crit Care Med. 2008;177:142–7. doi: 10.1164/rccm.200703-381OC. [DOI] [PubMed] [Google Scholar]
- 18.Wright RJ, Cohen S, Carey V, Weiss ST, Gold DR. Parental stress as a predictor of wheezing in infancy: a prospective birth-cohort study. Am J Respir Crit Care Med. 2002;165:358–65. doi: 10.1164/ajrccm.165.3.2102016. [DOI] [PubMed] [Google Scholar]
- 19.Chiu YH, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. 2012;186:147–54. doi: 10.1164/rccm.201201-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman S, Hatch MC. Stress, social support and pregnancy outcome: a reassessment based on recent research. Paediatr Perinatal Epidemiol. 1996;10:380–405. doi: 10.1111/j.1365-3016.1996.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 21.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C, SWS Study Group Cohort profile: The Southampton Women's Survey. Int J Epidemiol. 2006;35:42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 23.Goldberg DP, Williams P. The User’s Guide to the General Health Questionnaire. NFER-Nelson; Windsor: 1988. [Google Scholar]
- 24.Matthey S, Henshaw C, Elliott S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale – implications for clinical and research practice. Arch Womens Ment Health. 2006;9:309–15. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party's Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406–16. doi: 10.1111/j.1365-2133.1994.tb08532.x. [DOI] [PubMed] [Google Scholar]
- 27.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- 28.Venkatesh KK, Zlotnick C, Triche EW, Ware C, Phipps MG. Accuracy of Brief Screening Tools for Identifying Postpartum Depression Among Adolescent Mothers. Pediatrics. 2014;133(1):e45–e53. doi: 10.1542/peds.2013-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudron LH, Szilagyi PG, Tang W, Anson E, Talbot NL, Wadkins HIM, et al. Accuracy of Depression Screening Tools for Identifying Postpartum Depression Among Urban Mothers. Pediatrics. 2010;125(3):e609–e617. doi: 10.1542/peds.2008-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reichenheim ME, Moraes CL, Oliveira AS, Lobato G. Revisiting the dimensional structure of the Edinburgh Postnatal Depression Scale (EPDS): empirical evidence for a general factor. BMC Medical Research Methodology. 2011;11:93. doi: 10.1186/1471-2288-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–8. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- 32.Sandman CA. Fetal exposure to placental corticotropin-releasing hormone (pCRH) programs developmental trajectories. Peptides. 2015;72:145–53. doi: 10.1016/j.peptides.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Lin YC, Wen HJ, Lee YL, Guo YL. Are maternal psychosocial factors associated with cord immunoglobulin E in addition to family atopic history and mother immunoglobulin E? Clin Exp Allergy. 2004;34:548–54. doi: 10.1111/j.1365-2222.2004.1928.x. [DOI] [PubMed] [Google Scholar]
- 35.Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996;58:432–46. doi: 10.1097/00006842-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Chang HY, Suh DI, Yang SI, Kang MJ, Lee SY, Lee E, et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. 2016;138:468–75. doi: 10.1016/j.jaci.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason J, editors. Handbook of Life Stress, Cognition and Health. New York: John Wiley & Sons; 1988. pp. 629–649. [Google Scholar]
- 38.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–24. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 39.von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol. 2002;109:923–8. doi: 10.1067/mai.2002.124776. [DOI] [PubMed] [Google Scholar]
- 40.Cheng TS, Chen H, Lee T, Teoh OH, Shek LP, Lee BW, et al. An independent association of prenatal depression with wheezing and anxiety with rhinitis in infancy. Pediatr Allergy Immunol. 2015;26(8):765–71. doi: 10.1111/pai.12453. [DOI] [PubMed] [Google Scholar]
- 41.Braithwaite EC, Kundakovic M, Ramchandani PG, Murphy SE, Champagne FA. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics. 2015;10:408–17. doi: 10.1080/15592294.2015.1039221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dodic M, May CN, Wintour EM, Coghlan JP. An early prenatal exposure to excess glucocorticoid leads to hypertensive offspring in sheep. Clin Sci (Lond) 1998;94:149–55. doi: 10.1042/cs0940149. [DOI] [PubMed] [Google Scholar]
- 43.De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab. 2007;293:E75–82. doi: 10.1152/ajpendo.00689.2006. [DOI] [PubMed] [Google Scholar]
- 44.Henry C, Kabbaj M, Simon H, LeMoal M, Maccari S. Prenatal stress increases the hypothalamo-pituitary-adrenal axis response in young and adult rats. J Neuroendocrinol. 1994;6:341–345. doi: 10.1111/j.1365-2826.1994.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 45.Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav. 1998;63:397–402. doi: 10.1016/s0031-9384(97)00456-3. [DOI] [PubMed] [Google Scholar]
- 46.Loo EX, Shek LP, Goh A, Teoh OH, Chan YH, Soh SE, et al. Atopic Dermatitis in Early Life: Evidence for at Least Three Phenotypes? Results from the GUSTO Study. Int Arch Allergy Immunol. 2015;166:273–9. doi: 10.1159/000381342. [DOI] [PubMed] [Google Scholar]