Abstract
Cannabis-induced acute psychotic-like states (CIAPS) represent a growing health issue, but their underlying neurobiological mechanisms are poorly understood. The use of antipsychotics and benzodiazepines against CIAPS is limited by side-effects and/or by their ability to tackle only certain aspects of psychosis. Thus, safer wide-spectrum treatments are currently needed. Although the blockade of cannabinoid type-1 receptor (CB1) had been suggested as a therapeutical means against CIAPS, the use of orthosteric CB1 receptor full antagonists is strongly limited by undesired side effects and low efficacy. The neurosteroid pregnenolone has been recently shown to act as a potent endogenous allosteric signal-specific inhibitor of CB1 receptors. Thus, we tested in mice the potential therapeutic use of pregnenolone against acute psychotic-like effects of Δ9-tetrahydrocannabinol (THC), the main psychoactive component of cannabis. We found that pregnenolone blocks a wide spectrum of THC-induced endophenotypes typically associated with psychotic-like states, including impairments in cognitive functions, somatosensory gating and social interaction. In order to capture THC-induced positive psychotic-like symptoms (e.g. perceptual delusions), we adapted a behavioral paradigm based on associations between different sensory modalities and selective devaluation, allowing the measurement of mental sensory representations in mice. Acting at hippocampal CB1 receptors, THC impaired the correct processing of mental sensory representations (reality testing) in an antipsychotic- and pregnenolone-sensitive manner. Overall, this work reveals that signal-specific inhibitors mimicking pregnenolone effects can be considered as promising new therapeutic tools to treat CIAPS.
Introduction
After tobacco, alchool and caffeine, cannabis is the most widely used psychotropic drug, with an estimated 125–227 million consumers worldwide (1). A link between cannabis intoxication and the development of psychosis has long been recognized (2–6) and psychotic-like states have been documented in numerous case-reports and estimated to occur at least once in about 20-50% of individuals who use cannabis (3, 7). Cannabinoid agonists such as the main psychoactive component of the Cannabis sativa plant, Δ9-tetrahydrocannabinol (THC), have been shown to produce a full range of positive and negative psychotic-like symptoms in humans, such as hallucinations, delusions, disorganized speech, emotional withdrawal and decreased social interaction, and other endophenotypes typically associated with psychosis, such as cognitive (memory impairments) and somatosensory gating alterations (reduction of pre-pulse inhibition, PPI) (5, 6, 8–12). Due to the high prevalence of cannabis use, it is urgent to better understand cannabis-induced acute psychotic-like states (CIAPS) and to develop novel treatments.
Antipsychotic drugs and benzodiazepines currently used against CIAPS have serious limitations and side effects (13). Typical antipsychotics are linked to extrapyramidal side effects such as tremors, spasticity, and tardive dyskinesia (14–15), whereas atypical antipsychotics can produce sedation and weight gain (16–17) and benzodiazepines induce sedation and potentially addiction (18). Moreover, these drugs can only tackle certain psychotic symptoms (e.g. positive ones), but not others (e.g. cognitive endophenotypes) (13, 19). Given these limits of currently used drugs, researchers are now investigating the role of new neurobiological substrates in the pathophysiology of psychotic-like states.
The endocannabinoid system (ECS) has been considered as an emerging target for the development of antipsychotic treatments (20–21). Some years ago, the blockade of CB1 receptors had been suggested as a therapeutical means against psychoses (22–24). However, the use of orthosteric cannabinoid antagonists is strongly limited by undesired side effects (25–26) and contradictory results have been obtained in animal models (5, 20), possibly due to the ability of these drugs to block all cellular functions of CB1 receptors, hence causing opposite effects. Indeed, the clinical use of CB1 antagonists has been stopped as an antipsychotic treatment in humans, due to lack of proven efficacy (21).
Recently, the neurosteroid pregnenolone has been shown to act as a potent endogenous signal-specific inhibitor of CB1 receptor. By binding a specific allosteric CB1 site, pregnenolone blocks THC-induced activation of extracellular-regulated kinases (ERK) and reduction of mitochondrial activity, but not other signaling pathways induced by activation of CB1 receptors (27). This is a major difference as compared to orthosteric antagonists that block all cellular effects of THC, thus explaining the lack of undesired behavioral effects of pregnenolone (27–28).
In this study, we analyzed whether pregnenolone was able to block the THC-induced endophenotypes resembling acute psychotic-like states such as cognitive impairment, alteration of somatosensory gating (i.e. decreased PPI) and reduction of social interaction in mice. Moreover, we adapted a behavioral approach recently proposed in rodents (29–31) to study alterations in mental sensory representations which are hallmarks of positive psychotic-like states. The results show that pregnenolone can block the full range of acute psychotic-like symptoms and related endophenotypes induced by THC, thereby suggesting that drugs mimicking pregnenolone activity could be used to treat CIAPS.
Material and methods
Mice
All experimental procedures were approved by the Committee on Animal Health and Care of INSERM and the French Ministry of Agriculture and Forestry (authorization number, A501350). Male C57BL/6-N mice purchased from Janvier (France) were used in this study. The age of the animals at the beginning of all the behavioral experiments was 9-10 weeks. Except for the spontaneous alternation and social interaction tasks (see below), all the behavioral approaches were performed with independent groups of mice. All experiments were performed during the first part of the light phase (9 am to 2 pm). Experimenters were always blind to treatments.
Drugs
Rimonabant, purchased from Cayman Chemical (Michigan, USA), was dissolved in a mixture of 4% ethanol, 4% Cremophor-EL and 92% of saline (NaCl 0.9%). Risperidone and lithium chloride (LiCl), obtained from Sigma-Aldrich (St. Quentin Fallavier, France), were dissolved in saline. Amphetamine, obtained from Calaire Chimie (Calais, France), was dissolved in saline. Δ9-Tetrahydrocannabinol (THC) was purchased from THC-Pharm-GmbH (Frankfurt, Germany) and dissolved in 4% ethanol, 4% Cremophor-EL and 92% saline. Pregnenolone was dissolved in Tween 80 (1 drop/3 ml) and DMSO (2.5%) diluted in saline as previously described (27). Rimonabant, risperidone, LiCl, amphetamine and THC were all administered intraperitoneally (i.p.) whereas pregnenolone was injected subcutaneously (s.c.) . All drugs were injected in a volume of 10 ml/kg. MK-801 was purchased from Tocris (Bristol, U.K.) and dissolved in saline. It was administered subchronically i.p. during 7 days in 3-weeks old mice.
Chemical compounds
The solutions used in the aversion tasks were presented in 50-ml drinking tubes in the home cage with either banana (0.05%, isoamyl acetate) or almond (0.01%, benzaldehyde) for odors, and sucrose (5%) or maltodextrin (5%) for tastes. All compounds were obtained from Sigma-Aldrich (St. Quentin Fallavier, France).
Surgery
Stereotaxical surgeries, performed as previously described (32), were aimed at implanting guide cannulas (Plastics One Inc, Virginia, USA) targeting the hippocampus with the following coordinates: AP, -1.8; ML, +/-1; DV, -1.3 (33). Drugs or vehicles were injected using a peristaltic pump (PHD 22/2000 Syringe Pump Infusion, Harvard Apparatus, Massachusetts, USA, flow rate: 0.5 μl/min). The correct placement of the hippocampal cannulas was verified post hoc by injection of 2% pontamine sky blue solution in 0.5 M sodium acetate.
Behavioral Procedures
Spontaneous alternation
Spontaneous alternation was assessed in a Y-maze (42 cm long, 8 cm wide, 120° between arms) in a room bearing a ceiling-mounted camera. Mice were placed in the maze for 8 minutes during which each arm entry was scored (with all four paws in one arm, defined by a black line in the arm entrance). A spontaneous alternation refers to a triplet of consecutive arm explorations with all 3 arms being different. Accordingly, we counted the number of correct triplets (exploration of three different consecutive arms) to calculate the percentage of alternation using the following formula:
Mice were treated with an injection of vehicle or THC (0.3, 1, 3 mg/kg) 120 minutes before the task. Pregnenolone (6 mg/kg, s.c.) was administered either 10 minutes before or 30 minutes after THC. We controlled the total locomotion by assessing that there was no difference in total number of alternations between the THC groups and the vehicle group. The dose and timing of THC administration were chosen on the basis of preliminary experiments. Whereas a dose of 3 mg/kg injected 60 minutes before the test induced a clear reduction in total alternations (Supplementary Figure 1A), the same dose administered 120 minutes before did not show effects on locomotion (Supplementary Figure 1B).
Morris Water Maze
The hippocampal-dependent delayed matching-to-place version of the Morris water maze paradigm was performed using a white circular pool as previously described (34). After a day of habituation to the pool and the platform, mice started training sessions with visual clues placed on the wall. Each training session (one per day) consisted in four trials where the mouse was initially placed in the water facing the wall until it reached the hidden platform (cut off at 90 seconds), whose location was changed every day. The interval between trials was of 30 seconds. The starting location was identical for the first and the fourth trials, but different for the other ones. The cognitive performance was assessed by the calculation of the following saving ratio formulas:
During the training phase, animals were discarded if within 3 consecutive days they did not reach the platform in any of the four trials before the cut off. 15-20 % of the animals had to be discarded due to this issue. Starting from day 5 of training, all animals received a vehicle injection 30 minutes before session. The training lasted until the average latency times and distances to reach the platform between trial 2, 3 and 4 were decreased, as compared to trial 1, for three consecutive days (generally 12 days). The average of these 3 consecutive days (with the vehicle treatment) were calculated as vehicle condition. The day after, all the animals were treated with THC (5 mg/kg, i.p.) 30 minutes before the test session. On this day, mice received also an injection of pregnenolone (6 mg/kg, s.c.) or vehicle 10 minutes before the THC injection as previously described (27). The dose and timing of THC administration were chosen in the basis of previous findings (34).
Prepulse inhibition (PPI)
PPI was measured in a startle chamber (SR-Lab San Diego Instruments, San Diego, USA). Mice were placed in the startle chamber and a 70-dB background noise was presented during a 5-minute acclimation period. The PPI session consisted of randomly presented 100 trials: a 120-dB noise trial presented alone (Startle, S), no stimulus trial, prepulse 73-dB trial, prepulse 76-dB trial, prepulse 82-dB trial, prepulse 73-dB + pulse 120-dB, prepulse 76-dB + pulse 120-dB, prepulse 82-dB + pulse 120-dB. The intervals between single trials were randomized between 10 and 30 seconds. The 100 miliseconds response after the presentation of the 120-dB pulse was analyzed by the PPI setting and we used the maximal response peak to calculate the PPI (% PPI= 100 x (S – PPiS)/S) and the startle response as a control index for the mouse reaction to the startle pulse. THC administration (1, 5, and 10 mg/kg, i.p.) was performed 60 minutes before the PPI experiment and the pretreatment with pregnenolone (6 mg/kg, s.c.) was achieved 10 minutes before THC. A dose response experiment was performed to determine the dose of THC exerting no effects on the startle response (Supplementary Figure 2A).
Social interaction
Mice were tested in an open field (35x35 cm) wide and 20 with 2 plastic containers (plastic cylinders of 8 cm diameter with holes for odor interaction) in 2 opposite corners, one of them hosting a mouse (8-10 weeks old adult male C57BL/6-N), while the other container remained empty. In each corner we defined the “social” and “non-social” zones as a 8-cm area surrounding the containers. For each experimental group, the position of the container with the mouse was counterbalanced. Mice were put in the middle of the open field whose bottom was divided into squares of 6 cm, for 5 minutes. A ceiling-mounted camera recorded animals’ movements. This behavioral test was performed immediately after the spontaneous alternation task, i.e animals had received THC (0.3, 1 and 3 mg/kg) or its vehicle 130 minutes earlier. Pregnenolone (6 mg/kg, s.c.) was injected 10 minutes before THC. A social interaction index was calculated as:
The total interaction time of the two zones and the total number of crosses were used to control locomotor exploration activity. The dose and timing of THC administration were chosen on the basis of pilot experiments revealing THC effects on social interaction but not on locomotion (Supplementary Figure 3A-C).
Locomotion
Mice were placed in a plexiglas box (wide: 35 cm; long: 45 cm) whose bottom is divided into squares (6 cm). Locomotion was assessed by the number of crossed squares, this number being manually counted during 5 minutes by the experimenter in the same room. Mice were treated with THC (0.3, 1 and 5 mg/kg) or vehicle 45 minutes before the test. Pregnenolone (6 mg/kg, i.p.) was injected 10 minutes before THC administration. Besides, amphetamine (2.5 mg/kg, i.p.) was injected 45 minutes before the test as to compare its effect with that of THC administration. The systemic pretreatment with risperidone (0.3 mg/kg, s.c.) was performed 10 minutes before amphetamine. In the MK801 mouse model, we performed the locomotion test in adult mice (9-10 weeks old).
Direct and mediated aversions
Mice were water-deprived in the room where the whole protocol occurred. The basal consumption of each odor and taste was measured in naïve mice and no preference for any stimulus was detected (Supplementary Figure 4A-B). All subjects received 60 minutes access to water during three consecutive days as habituation period. Over the following days, we performed the pre-conditioning phase with different odor-taste pairings in which animals were submitted to different schedules of pre-conditioning trainings, consisting of 1, 3, 6 or 9 odor-taste pairings, respectively (Supplementary Figure 4C-E). Each pairing consisted in two days: the first day the subjects received 60 minutes access to a flavored solution containing a new taste (either 5% sucrose or 5% maltodextrin; Taste 1, T1) and a new odorant (0.05% Banana or 0.01% Almond; Odor 1, O1) mixed with water in order to pair T1 with O1. On day two, the animals received the taste and the odor that was not provided during the previous day, i.e. Taste 2 (T2) and Odor 2 (O2). On the following 6 days, animals entered the devaluation phase where O1 (or T1) was devaluated as to become the conditioned stimulus (CS+). On days 1, 3 and 5 of this phase, the subjects have 60 minutes access to O2 (or T2) followed by an intraperitoneal (i.p.) injection of saline (CS-) whereas on days 2, 4 and 6, they received 60 minutes access to O1 or T1 immediately followed by an i.p. injection of lithium chloride (LiCl, 0.3 M, 1% b.w.) (CS+). After this conditioning, the subjects were given a recovery day during which they could only drink water during 60 minutes. On the next 2 days, mediated and direct aversions were assessed using a 60 minutes two-choice test phase. Mediated aversion was always evaluated on the first test day with a choice between the stimulus T1 (or O1) previously associated with the CS+ (called mediated CS+, mCS+) and the stimulus T2 (or O2) previously associated with the CS- (called mediated CS-, mCS-). On the second test day, we evaluate the direct aversion with a choice between the CS+ and CS- (Supplementary Figure 4D-E). Aversion was revealed by lower consumption of mCS+ over mCS- (mediated aversion) or CS+ over CS- (direct aversion) (32). Data are presented as absolute liquid intakes and as aversion indices, which were calculated using the following formula: (CS- - CS+)/(CS+ + CS-) for direct aversion or (mCS- - mCS+)/(mCS+ + mCS-) for mediated aversion, respectively.
“Reality testing” evaluation (mental representation of sensory stimuli)
One odor-taste pairing (short training) during the preconditioning phase is not sufficient to induce mediated aversion, whereas 3 pairings readily decreased the consumption of mCS+ (see results), suggesting that with intermediate training there is a similar salience between the CS+ and mCS+ (in other words, CS+ = mCS+ in terms of aversive value). However, after 6 or 9 odor-taste pairings (long training) control animals did not show mediated aversion, suggesting that they are able to separate the values of CS+ and mCS+. Considering that CS+ and mCS+ are separated in reality, this phenomenon is generally called “reality testing” in the literature (29–31), and we use this term in the present study. Thus, conditions or treatments that restore mediated aversion are considered as alteration of “reality testing”, in a way that reasonably approximate “erroneous beliefs that usually involve a misinterpretation of perception or experiences” typically observed in patients experiencing positive psychotic symptoms (35–36). Thus, using this protocol, we evaluated whether pharmacological treatments applied subchronically during adolescence (MK801, 7 days, 1 mg/kg, i.p.) or 120 minutes before the first test (2.5 mg/kg amphetamine, i.p. or 1 mg/kg THC, i.p.) impaired the “reality testing” in the 6 pairings protocol, reestablishing a specific aversion to mCS+. THC was also injected 120 minutes before the test in the 9 pairings protocol to better characterize and confirm its effects on “reality testing”. A preliminary dose-response experiment was performed to determine the dose of THC not interfering with total liquid intake. The systemic pretreatments with the antipsychotic risperidone (0.03, 0.1 and 0.3 mg/kg, i.p.), the CB1 antagonist rimonabant (1 mg/kg, i.p.) and pregnenolone (6 mg/kg, s.c.) were performed 10 minutes before THC or amphetamine (for risperidone only) administration and 120 minutes before the test of the 6 pairing protocol (Supplementary Figure 4D-E). Pregnenolone (6 mg/kg, s.c.) was also administered 30 minutes after THC administration, i.e. 90 minutes before the test. Moreover, rimonabant and pregnenolone were also perfused in the hippocampus at a dose of 3 μg/1μl per side 10 minutes before THC administration (1 mg/kg, i.p.) and 120 minutes before the test. Finally, all the systemic treatments (THC, risperidone, rimonabant and pregnenolone) were also given 120 minutes before the test after 3 pairings to control their impact on direct aversion.
Statistical analyses
Two group comparison were made by t-test. Multiple groups comparison were studied through 1-Way, 2- or 3-Way analysis of variance (ANOVA) with or without repeated values where appropriate. For ANOVA, only when significant interactions between main factors were detected, post-hoc analyses (Bonferroni’s or Fisher’s) were performed. In some cases, ANOVA is used and the p value for the main factor (e.g. mCS+ or mCS+) is showed. For detailed statistical analysis see Figure legends.
Results
1. Action of pregnenolone on the effects of THC on cognitive performance, PPI and social interaction
1.1. Pregnenolone blocks THC-induced impairment of cognitive function
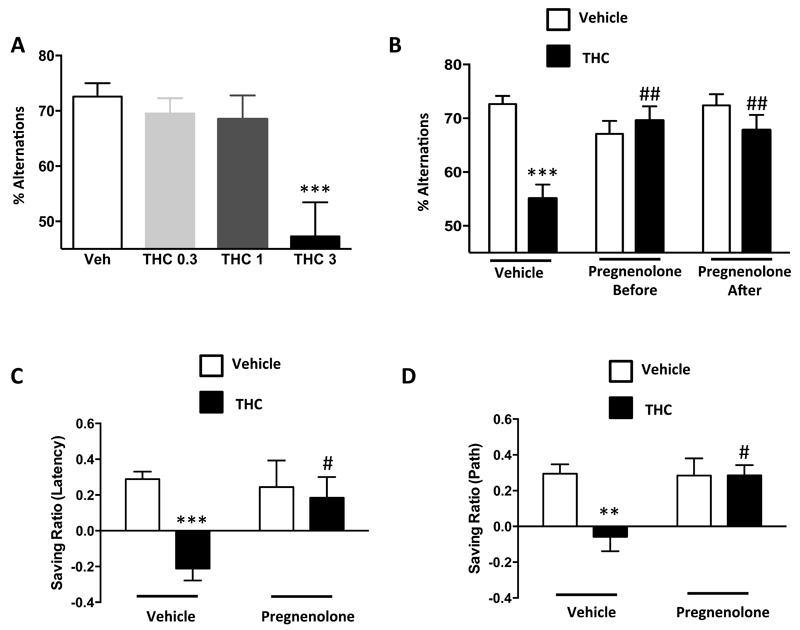
Systemic pretreatment with pregnenolone (6 mg/kg, s.c.) did not alter behavioral performance per se in the spontaneous alternation task (37) and in the hippocampal-dependent delayed matching-to-place version of the Morris water maze (38). Pregnenolone fully blocked the cannabinoid-induced cognitive impairment in the spontaneous alternation task when injected before or after THC (3 mg/kg, i.p.) (Figures 1A-B, Supplementary Figure 1A-C). Similarly, pregnenolone pretreatment abolished the effect of THC at 5 mg/kg (34) in the Morris water maze (Figures 1C-D).
Figure 1. Pregnenolone blocks the cognitive impairment induced by THC.
(A) Dose-response of THC effects in the spontaneous alternation task [1-Way ANOVA, F(3,32)=3.8, p<0.001, n=6-12]. (B) Effect of pregnenolone (6 mg/kg, s.c.) 10 min before or 30 min after THC (3 mg/kg, i.p.) administration on the impairment of spontaneous alternation [before: 2-Way ANOVA, interaction; F(1,58)=14.83, p<0.001; after: 2-Way ANOVA, interaction; F(1,62)=6.871, p<0.05, n=10-20]. (C-D) Effect of pregnenolone (6 mg/kg, s.c.) on the cognitive impairment induced by THC (5 mg/kg, i.p.) in the delayed matching-to-place version of the Morris water maze. (C) Saving ratio for the latency [2-Way ANOVA, interaction, F(1,64)=6.48, p<0.05, n=15-20]. (D) Saving ratio for the path [2-Way ANOVA, interaction, F(1,64)=5.09, p<0.05, n=15-20]. **, p<0.01; ***, p<0.001 for the effect of THC. #, p<0.05; ##, p<0.01 for the effect of pregnenolone in THC injected mice.
1.2. Pregnenolone blocks THC-induced decrease of PPI
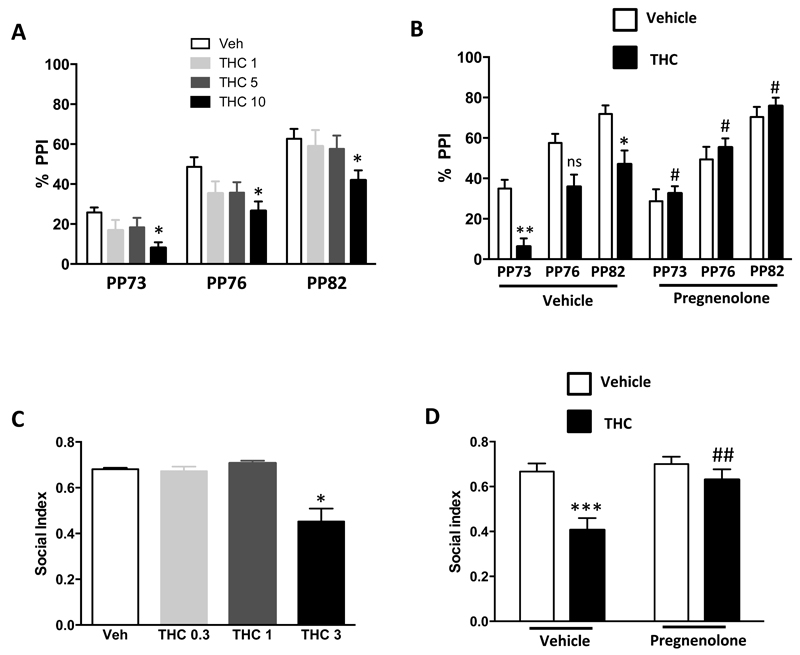
According to previous results in rats (39), dose response experiments showed that THC at 10 mg/kg impairs PPI without any significant effect on the startle response in mice (Figure 2A, Supplementary Figure 2A). Pregnenolone (6 mg/kg, s.c.) blocked this THC effect on PPI (Figure 2B) or on startle response (Supplementary Figure 2B).
Figure 2. Pregnenolone blocks the effects of THC on PPI and social interaction.
(A) Dose response of THC effects in the PPI test [1-Way ANOVA, pre-pulse 73 db (PP73), F(3,37)=0.47, p<0.01; PP76, F(3,37)=0.14, p<0.05; PP82, F(3,37)=0.11, p<0.05; n=7-13]. (B) Effect of pregnenolone (6 mg/kg, s.c.) on the reduction of PPI induced by THC (10 mg/kg, i.p.) [2-Way ANOVA, interaction; PP73 F(1,35)=11.93, p<0.01; PP76 F(1,35)=6.31, p<0.05; PP82 F(1,35)=7.63, p<0.01; n=8-12]. (C) Dose response analysis of THC effects in the social interaction task [1-Way ANOVA, F(3,33)=11.23, p<0.01, n=10-12]. (D) Effect of pregnenolone on the reduction of social interaction induced by THC (3 mg/kg, i.p.) [2-Way ANOVA, interaction, F(1,47)=4.39, p<0.05, n=10-15]. *, p<0.05; ** p<0.01; ***, p<0.001 for the effect of THC. #, p<0.05, ##, p<0.01 for the effect of pregnenolone in THC injected mice.
1.3. Pregnenolone blocks the impairment of social interaction induced by THC
The dose of 3 mg/kg of THC significantly decreased social interaction in mice (Figure 2C), without affecting possible confounding behaviors, such as locomotion (Supplementary Figure 3A-C). This effect was fully blocked by pregnenolone (6 mg/kg, s.c.), which have no effect when administered alone (Figure 2D).
2. Action of pregnenolone on the THC-induced acute positive psychotic-like states
2.1. Pregnenolone blocks the hyperlocomotor effects induced by THC
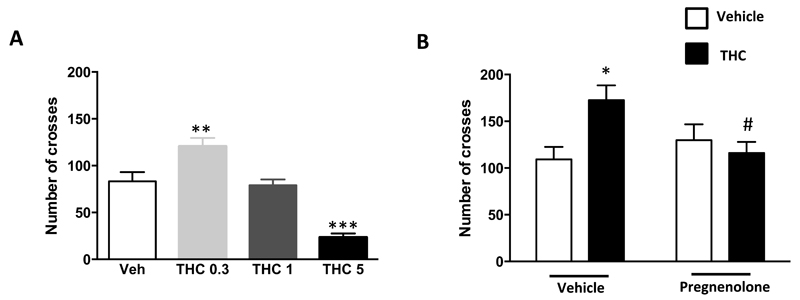
The presence of positive symptoms is a key feature of psychotic-like states (35–36, 40) and represents a major challenge to model in rodents (41–43). Rodent hyperlocomotion induced by human psychotogenic drugs has been long considered an acceptable laboratory approximation of positive symptoms of drug-induced psychotic-like states (41–43). Cannabinoids are known to exert biphasic effects on locomotion in rats, with high doses inducing sedation and low doses increasing locomotor activity (44). Whereas the 5 mg/kg (i.p.) dose of THC strongly decreased locomotion, that of 0.3 mg/kg induced hyperlocomotion in mice (Figure 3A; see ref. 45 for different results using other mouse strains and experimental settings). Administration of pregnenolone (6 mg/kg, s.c.) did not alter locomotion in control mice, but it fully blocked the hyperlocomotor effect of 0.3 mg/kg THC (Figure 3B), suggesting that the neurosteroid can block also acute “positive” psychotic-like effects of cannabinoids.
Figure 3. Pregnenolone blocks the hyperlocomotor effect induced by THC.
(A) Dose response analysis of the effects of THC on spontaneous locomotion [1-Way ANOVA, F(3,28)=0.7, p<0.001, n=8]. (B) Effect of pregnenolone (6 mg/kg, s.c.) on the hyperlocomotion induced by 0.3 mg/kg (i.p.) [2-Way ANOVA, interaction, F(1,33)=6.8, p<0.05, n=10-12]. *, p<0.05; **, p<0.01; ***, p<0.001 as compared to vehicle control. #, p<0.05 as compared to THC alone group.
2.2. Validation of a paradigm to study alterations of mental sensory representation in mice (“reality testing”)
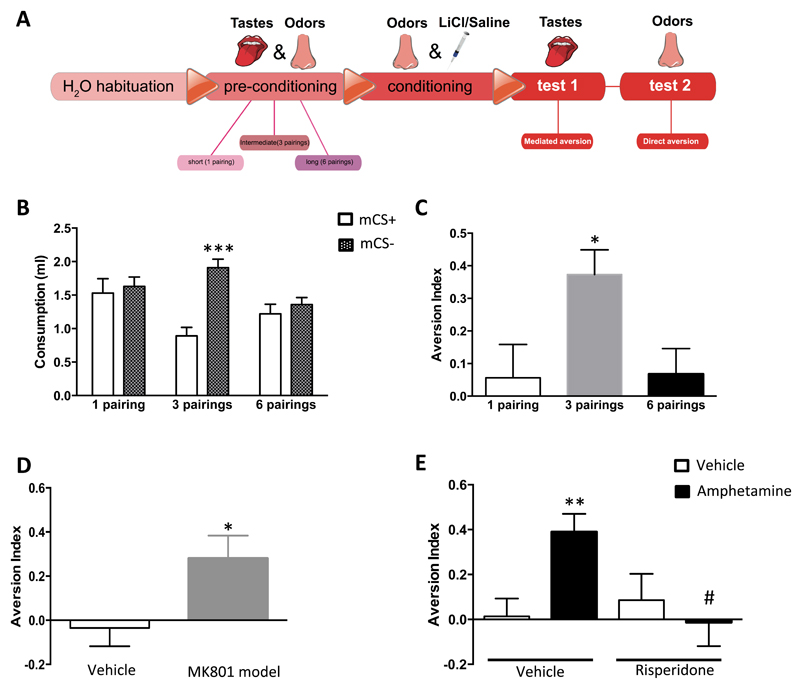
Alterations in the mental representation of stimuli leading to mismatches between perception and reality are key features of positive symptoms of psychotic-like states (35–36, 46). For instance, delusions are defined as erroneous beliefs involving a misinterpretation of perception or experiences (36). Obviously, these alterations cannot be caught by analyzing locomotor activity (41–43), but recent studies have used procedures to measure “reality testing”, defined as the accuracy of mental representation of reality (29–31, 47). Alterations of “reality testing” as an impairment of perception might therefore approximate deficits that can lead to positive psychotic-like states such as perceptual delusions or hallucinations (29–31, 47). Thus, we adapted these previous protocols (29–31, 47) to design a behavioral paradigm to measure “reality testing” in mice (see methods, Figure 4A; Supplementary Figure 4D-E).
Figure 4. Validation of the mediated aversion “reality testing” paradigm in mice.
(A) Schematic representation of the behavioral protocol. For further details see Methods and Supplementary Figure 4. (B-C) Effect of the number of odor-taste pairings during preconditioning phase on odor-mediated taste aversion: (B) Consumption of tastes (i.e. mediated CS) [2-Way ANOVA with repeated measures, interaction, F(2,27)=3.98, p<0.05; n=10]. Solid bars indicate consumption of taste paired with devaluated odor (mCS+), and dashed bars indicate consumption of taste paired with undevaluated odor (mCS-, see methods). (C) Aversive index [1-Way ANOVA, F(2,27)=0.3, p<0.05; n=10-12]. (D-E) Effects of psychotogenic treatments on mediated aversion “reality testing” paradigm (mice receiving 6 odor/taste pairings during pre-conditioning): (D) Aversion index in adult animals subchronically treated with MK801 during adolescence (post-natal days 21-28, 1 mg/kg, i.p.) (t-test, p<0.05, n=10-11). (E) Effect of acute pre-test administration of amphetamine (2.5 mg/kg, i.p.) [2-Way ANOVA, interaction, F(1,56)=5.9, p<0.05; n=10-20]. *, p<0.05; **, p<0.01 as compared to vehicle control, ***, p<0.001 as compared to mCS+; @, p<0.05 as compared to 1- and 6-pairings groups; #, p<0.05 as compared to amphetamine group.
Whereas pre-conditioning with 1 odor-taste pairing followed by a selective conditioned devaluation of one of the two stimuli did not induce mediated aversion, 3 pairings induced a reliable mediated aversion (Figure 4B-C), suggesting that the mice formed a unique mental representation of the two previously associated stimuli (odor and taste). However, 6 pairings before selective conditioned devaluation suppressed this mediated aversion (Figure 4B-C), indicating that the extended training during pre-conditioning induced a mental representation of the stimuli as two separated entities. Considering that the stimuli are separated in reality, one can argue that extended training allows animals acquiring additional information that enables the “reality testing” of the independent salience of the two stimuli (29–31), implying a correct mental representation of the values of the stimuli. Importantly, (i) total liquid consumption was similar among the groups (Supplementary Figure 5A), (ii) direct aversion was present under all pre-conditioning odor-taste pairing conditions (Supplementary Figure 5B-D) and (iii) the loss of mediated aversion was present irrespectively of whether odor or taste were devaluated during conditioning (Supplementary Figure 6A-F). This indicates that the behavioral procedure does not alter motivation to drink or direct aversive memory, and that the effects of differerent preconditioning schedules on mediated aversion are independent of the sensory modalities used.
Interestingly, previous studies showed that rodent models of psychotic-like behaviors present a “reality testing” impairment (29–31). Thus, we tested two psychotogenic pharmacological mouse models in our paradigm. Both subchronic MK801 treatment during adolescence and acute treatment with amphetamine (41), which induced hyperlocomotion in adult mice (Supplementary Figure 7A-B), impaired “reality testing” performance, hence re-establishing mediated aversion in mice pre-conditioned with 6 odor-taste pairings (Figure 4D-E; Supplementary Figures 8A-E and 9A-F). The fact that amphetamine was acutely administered before the tests excludes possible confounding factors during the pre-conditioning or conditioning associative learning phases. Moreover, both the effects of amphetamine on “reality testing” and locomotion were blocked by the acute pre-administration of the antipsychotic drug risperidone (0.3 mg/kg, i.p.) (Figure 4E; Supplementary Figures 7B and 9B-F). These results provide good face validity to this paradigm as a mouse behavioral tool to reflect perceptual alterations, typically associated with positive psychotic-like states in humans.
2.3. Pregnenolone blocks the impairment of “reality testing” induced by THC
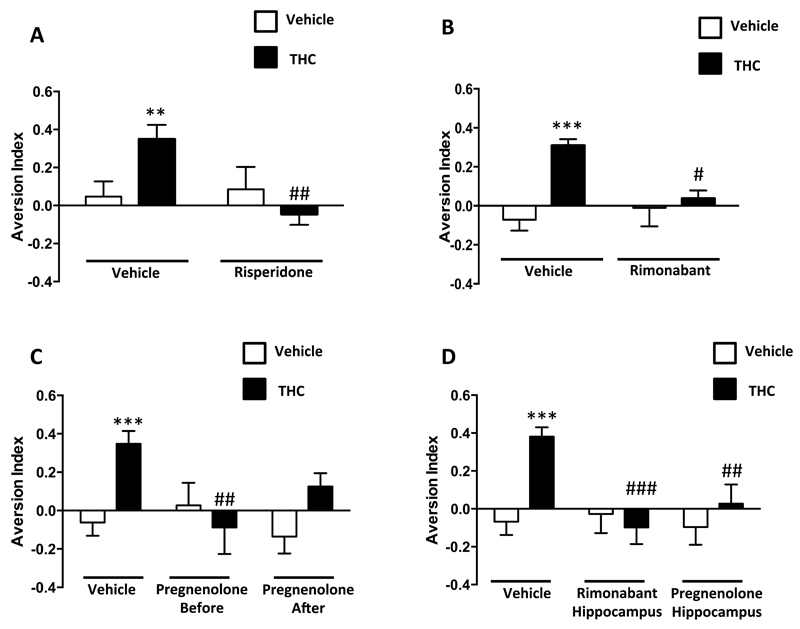
THC (1 mg/kg, i.p) did not affect liquid intake (Supplementary Figure 10A-B), but it blocked the effect of extended pre-conditioning (6 or 9 pairings before devaluation), re-establishing the mediated aversion typical of 3 pairings (Figure 5; Supplementary Figure 11A-C). Importantly, different doses of the antipsychotic risperidone acutely blocked this effect (Figure 5A, Supplementary Figure 12A-B and 13A-C), suggesting that THC impairs “reality testing” in mice, most likely triggering a psychotic-like state. The orthosteric CB1 receptor antagonist rimonabant, although slightly reducing the total amount of liquid intake (Supplementary Figure 14A-B), abolished mediated aversion in THC-treated mice (Figure 5B; Supplementary Figure 14A-B). This indicated that activation of CB1 receptors was required for this psychotic-like effect of THC. Importantly, pregnenolone treatment (6 mg/kg, s.c.) blocked the “reality testing” impairment when administered before or after THC (Figure 5C; Supplementary Figure 15A-E). Besides, none of these treatments altered direct aversion (Supplementary Figures 12C-E, 13D-F, 14C-E and 15C-E) or mediated aversion induced by the 3-pairings pre-conditioning protocol (Supplementary Figure 16A-D). Notably, hippocampal injections of rimonabant or pregnenolone fully blocked the THC-induced “reality testing” impairment (Figure 5D; Supplementary Figure 17A-B and 18A-E). Thus, activation of hippocampal CB1 receptors mediated the aforementioned pregnenolone-sensitive psychotic-like effect of THC.
Figure 5. Pregnenolone blocks the THC effects on “reality testing” in mice.
(A-C) Effect of pre-test THC administration (1 mg/kg, i.p.) on mediated aversion in mice trained with 6 odor-taste pairings. (A) Risperidone (0.3 mg/kg, i.p.) effect on the THC induced “reality testing” impairment [2-Way ANOVA, interaction, F(1,53)=5.9, p<0.05; n=11-19]. (B) Effect of the orthosteric CB1 receptor antagonist rimonabant (1 mg/kg, i.p.) [2-Way ANOVA, interaction, F(1,39)=7.1, p<0.05; n=10-11]. (C) Effect of the signal-specific allosteric inhibitor of CB1 receptors, pregnenolone (6 mg/kg, s.c.) before [2-Way ANOVA, interaction, F(1,46)=9.172, p<0.01; n=14-18] and after THC administration. (D) Effect of hippocampal injections of rimonabant [2-Way ANOVA, interaction, F(1,58)=12.04, p<0.001; n=11-21] and pregnenolone [2-Way ANOVA, interaction, F(1,55)=4.252, p<0.05; n=14-18] (3 μg/μl) before THC administration.*, p<0.05, **, p<0.01, ***, p<0.001 for the effect of THC; #, p<0.05, ##, p<0.01, ###, p<0.01 for the effect of pregnenolone in THC injected mice.
Discussion
Finding a potential drug to tackle the full psychotic-like symptomatology induced by acute cannabis consumption is a challenging issue, mostly because of the difficulty to model these symptoms in laboratory animals. In this study, we show that the signaling-specific CB1 receptor inhibitor pregnenolone reverts a wide range of acute consequences of THC that can resemble acute cannabis intoxication. This included psychotic-like negative states or endophenotypes, such as decreased social interaction, cognitive impairment and inhibition of PPI. The presence of positive symptoms is a key feature to define a psychotic-like state (35–36). Indeed, our data show that pregnenolone blocks THC-induced acute hyperlocomotion, which is often used as a surrogate for positive psychotic-like phenotypes (41–43). Considering that hyperlocomotion is an indirect means to assess positive psychotic states, thus we set a behavioral task allowing to investigate the accuracy of internal representation of stimuli (“reality testing”). We found that acute administration of THC, like other psychotogenic treatments, alters “reality testing” in mice. Importantly, pregnenolone fully reverts this effect, indicating that also THC-induced acute positive psychotic-like symptoms are also counteracted by this neurosteroid. These results are clinically relevant as these perceptual alterations can later lead to persistent delusions and other positive psychotic-like states (9,10,13). From the pharmacological point of view, it is interesting to note that the different psychotic-like effects of THC are observed in mice at different doses and at different times after administration, resembling the dose-dependence and the time course of cannabis effects in humans (48–50). Pregnenolone was able to block all effects of THC independently from dose or timing, suggesting that the whole spectrum of psychotic-like symptoms induced by acute cannabis intoxication is a target for this neurosteroid. Our data also show that pregnenolone is able to block at least some psychotic-like effects of THC (e.g. spontaneous alternation or “reality testing”) in a “real-life” scenario, in which the neurosteroid is administered after THC intoxication. Considering that chronic cannabis intake during vulnerable periods (e.g. adolescence) is often associated to the development of persistent psychoses in adult humans (51), these results might provide a proof of concept for future studies in order to extend the impact of similar treatment regimens in rodents and the potential therapeutic impact of pregnenolone.
The blockade of CB1 receptors has been previously suggested as a therapeutical means against psychoses (22–24) but its use is strongly limited by undesired side effects (25–26). Moreover, clinical studies had to be stopped due to lack of efficacy (21). Thus, the discovery of more specific, safer and efficient approaches is required for the treatment of CIAPS. Accordingly, it is worth noting that excessive activation of CB1 receptors induces large increases in pregnenolone levels in rodent brain (up to 3-4,000%) (27). Pregnenolone, in turn, acts as an endogenous signaling-specific inhibitor of excessive CB1 receptor signaling, which might explain the absence of side effects as compared to classical orthosteric antagonists (27–28). Indeed, pregnenolone binds to an allosteric site of the CB1 receptor and inhibits only several of the signaling pathways that are triggered by cannabinoids. Thus, pregnenolone has no apparent effect on CB1 receptor-dependent regulation of cAMP cellular levels, but it antagonizes the activation of ERK (27) and the CB1 receptor-dependent reduction of mitochondrial activity recently described in the brain (27, 52). Interestingly, decreased blood levels of endogenous pregnenolone are present in schizophrenic patients and exogenous pregnenolone administration slightly improves the symptomatology in psychotic women (53–55). Moreover, recent evidence from human post-mortem studies suggests that ERK signaling could contribute to the pathogenic events that occur in psychosis (56–57). The ability of antipsychotics to affect the ERK pathway has been also demonstrated in vitro and in vivo (58). In parallel, the impact of energy metabolism and mitochondria in the brain is emerging as a promising novel research field in psychopathology (59–61). For instance, brain-specific alterations of the metabolic profile in the cerebrospinal fluid of first-onset schizophrenic patients have been reported (62).
Whereas cannabinoid-induced effects on cognition, PPI and sociability have been studied in laboratory animals, psychotic-like positive symptoms are often neglected due to the lack of suitable animal models (6, 41, 63). To overcome this methodological issue, we adapted a representation-mediated learning protocol (29–31) in mice as a paradigm to evaluate the mental representation of stimuli and its potential disturbance under psychotic-like states. In this paradigm, extended pre-conditioning training allows animals becoming able to interpret the real situation in the external world and respond consequently. Thus, the term “reality testing” has been used in rodents to describe this mental process (29–31). Interestingly, commonly used animal “models” of psychotic-like states (e.g. ventral hippocampal lesion) (30, 64) and known psychotogenic pharmacological treatments (e.g. amphetamine, subchronic MK801, present results) alter “reality testing”. Moreover, this psychotogenic-like effect was also observed after acute injection of THC both in the 6- and 9-pairings pre-conditioning protocol. Human hallucinations and delusions can be viewed as mismatches between external reality and internal mental representations. For instance, the Fifth Edition of the “Diagnostic and Statistical Manual of Mental Disorders” (DSM V) defines delusions as “erroneous beliefs that usually involve a misinterpretation of perception or experiences” (36), which implies an erroneous mental representation of stimuli. For this reason, the “reality testing” impairment produced by the acute administration of THC and psychotogenic drugs before the test of the 6-pairings protocol, together with the lack of effect of THC in the 3-pairings protocol, seems to reflect a cognitive alteration linked to the perception of the stimuli during retrieval rather than their encoding, resembling human perceptual delusions, which are hallmarks and starting points of positive psychotic-like symptoms.
Importantly for the aims of the present study, the “reality testing” impairment induced by acute THC or amphetamine treatments is reversed by antipsychotic pre-treatment, supporting the psychotic-like nature of this effect. Moreover, the THC effect is also blocked by the systemic injection of the CB1 antagonist rimonabant, indicating that excessive CB1 receptor activity alters the relationship between sensory “percept” and mental “concept”. This denotes a cannabinoid-dependent top-down control of sensory representations in the brain, as we recently proposed in the olfactory system (65). Notably, this acute impairment of “reality testing” is blocked by pregnenolone, confirming that pregnenolone-like compounds represent a novel potential therapeutic tool against a wide range of acute psychotic-like states resembling the effects of acute cannabis intoxication in humans. Unfortunately, pregnenolone has a very short half-life, poor bioavailability and low efficacy in clinical studies (53–55) and it is the precursor of many other steroids, making it virtually impossible to use as a human drug (66). To obviate these limitations, we recently developed a new class of pregnenolone-derivative drugs (Synthetic Signaling Specific inhibitors of the CB1, sCB1-SSi, Ref. 28), one of which will soon enter clinical trials against cannabis use disorders and addiction. Interestingly, more than 40% of psychotic patients regularly consume cannabis, generally leading to worse prognosis of the disease (4, 9). Thus, the present results clearly suggest an additional possible application for sCB1-SSi in psychotic patients consuming cannabis. However, as psychotic symptomatology in humans mostly appear when people consume cannabis chronically during vulnerable periods (51), future studies in animals and humans will investigate the efficacy of this class of drugs in the treatment of psychotic-like states induced by acute or chronic cannabinoid intoxication.
Finally, hippocampal CB1 receptor blockade by rimonabant and pregnenolone abrogated the THC impairment of “reality testing”, corroborating recent results indeed implicated this brain region in representation-mediated learning in rats (47). Accordingly, hippocampal alterations have been demonstrated in different psychotic disorders (67–68). In particular, imaging studies showed changes in hippocampal activity and altered plasticity mechanisms of the hippocampus in schizophrenic patients (69–70). Thus, our data support the idea that cannabinoids induce psychotic-like states by altering hippocampal functions. Nevertheless, the potential involvement of other brain regions (e.g. prefrontal cortex) and the role played by different brain cell populations in these effects will require further studies to be assessed.
In summary, we show that pregnenolone fully blocks the wide range of psychotic-like effects and related endophenotypes induced by acute administration of different doses of THC in mice. These results represent a proof of concept indicating a suitable therapeutic profile of signal-specific inhibitors of excessive CB1 receptor signaling. Thus, this work identifies pregnenolone-like drugs as powerful and promising therapeutic means and provides an adapted approach to study altered mental sensory representations that can be used to model the positive symptomatology of a complex human disorder like CIAPS.
Supplementary Material
Acknowledgments
We thank Delphine Gonzales, Nathalie Aubailly, and all the personnel of the Animal Facility of the NeuroCentre Magendie for mouse care. We also thank all the members of Marsicano’s lab for useful discussions and Virginie Morales for the unvaluable help. This work was supported by INSERM (to G.M.), EU–FP7 (PAINCAGE, HEALTH-603191 to G.M. and FP7-PEOPLE-2013-IEF-623638 to A.B.-G.), European Research Council (Endofood, ERC–2010–StG–260515; CannaPreg, ERC-2014-PoC-640923, to G.M.), Fondation pour la Recherche Medicale (DRM20101220445 and DPP20151033974, to G.M.), Human Frontiers Science Program (to G.M.), Region Aquitaine (to G.M.), French State/Agence Nationale de la Recherche/LabEx BRAIN (ANR-10-LABX-0043 to G.M.), Fyssen Foundation (to E.S.-G.), CONACyT (to E.S.-G.), French State/Agence Nationale de la Recherche/IdEx (ANR-10-IDEX-03-02 to A.B.-G.), French State/Agence Nationale de la Recherche/Blanc (NeuroNutriSens ANR-13-BSV4-0006-02 to G.M.).
Footnotes
Author Contributions
A.B.-G., G.F., P.-V.P. and G.M. designed research; A.B.-G., E.S.-G., B.R, Y.M. and F.C. performed research; A.B.-G., G.F., P.-V.P. and G.M. supervised research; A.B.-G., E.S.-G., G.F. and G.M. analyzed data; A.B.-G., G.F. and G.M. wrote the manuscript. All authors edited the manuscript.
Author information
The authors declare no conflict of interests.
Competing financial interests
P. V. P. and G.M., are founders, stakeholders and consultants for the start-up company Aelis Farma.
References
- 1.United Nations Office on Drugs and Crime. World Drug Report. 2014 [Google Scholar]
- 2.Wilkinson ST, Radhakrishnan R, D'Souza DC. Impact of Cannabis Use on the Development of Psychotic Disorders. Curr Addict Rep. 2014;1:115–128. doi: 10.1007/s40429-014-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radhakrishnan R, Wilkinson ST, D'Souza DC. Gone to Pot - A Review of the Association between Cannabis and Psychosis. Front Psychiatry. 2014;22:5–54. doi: 10.3389/fpsyt.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- 5.Fakhoury M. Role of the Endocannabinoid System in the Pathophysiology of Schizophrenia. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9697-5. (In press) [DOI] [PubMed] [Google Scholar]
- 6.Rubino T, Parolaro D. The Impact of Exposure to Cannabinoids in Adolescence: Insights from Animal Models. Biol Psychiatry. 2015;3223:00643–5. doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–60. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- 8.D'Souza DC, Sewell RA, Ranganathan M. Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci. 2009;259:413–31. doi: 10.1007/s00406-009-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addict Biol. 2008;13:264–75. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–72. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- 11.Morrison PD, Zois V, McKeown DA, Lee TD, Holt DW, Powell JF, et al. The acute effects of synthetic intravenous Delta9-tetrahydrocannabinol on psychosis, mood and cognitive functioning. Psychol Med. 2009;39:1607–16. doi: 10.1017/S0033291709005522. [DOI] [PubMed] [Google Scholar]
- 12.Spaderna M, Addy PH, D'Souza DC. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl) 2013;228:525–40. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leweke FM, Gerth CW, Klosterkötter J. Cannabis-associated psychosis: current status of research. CNS Drugs. 2004;18:895–910. doi: 10.2165/00023210-200418130-00005. [DOI] [PubMed] [Google Scholar]
- 14.Dold M, Samara MT, Li C, Tardy M, Leucht S. Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst Rev. 2015;1 doi: 10.1002/14651858.CD009831.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginovart N, Kapur S. Role of dopamine D(2) receptors for antipsychotic activity. Handb Exp Pharmacol. 2012;212:27–52. doi: 10.1007/978-3-642-25761-2_2. [DOI] [PubMed] [Google Scholar]
- 16.Crossley NA, Constante M, McGuire P, Power P. Efficacy of atypical v. typical antipsychotics in the treatment of early psychosis: meta-analysis. Br J Psychiatry. 2010;196:434–439. doi: 10.1192/bjp.bp.109.066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leo RJ, Regno PD. Atypical antipsychotic use in the treatment of psychosis in primary care. Prim Care Companion. J Clin Psychiatry. 2000;2:194–204. doi: 10.4088/pcc.v02n0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77:295–301. doi: 10.1111/j.1365-2125.2012.04418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–24. doi: 10.4088/JCP.10r06264yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wyrofsky R, McGonigle P, Van Bockstaele EJ. Drug discovery strategies that focus on the endocannabinoid signaling system in psychiatric disease. Expert Opin Drug Discov. 2015;10:17–36. doi: 10.1517/17460441.2014.966680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leweke FM, Mueller JK, Lange B, Rohleder C. Therapeutic potential of Cannabinoids in Psychosis. Biol Psychiatry. 2016;79:604–12. doi: 10.1016/j.biopsych.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Roser P, Vollenweider FX, Kawohl W. Potential antipsychotic properties of central cannabinoid (CB1) receptor antagonists. World J Biol Psychiatry. 2010;11:208–219. doi: 10.3109/15622970801908047. [DOI] [PubMed] [Google Scholar]
- 23.Scatton B, Sanger DJ. Pharmacological and molecular targets in the search for novel antipsychotics. Behav Pharmacol. 2000;11:243–56. doi: 10.1097/00008877-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Zamberletti E, Rubino T, Parolaro D. The endocannabinoid system and schizophrenia: integration of evidence. Current pharmaceutical design. 2012;18:4980–4990. doi: 10.2174/138161212802884744. [DOI] [PubMed] [Google Scholar]
- 25.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 26.Rumsfeld JS, Nallamothu BK. The hope and fear of rimonabant. JAMA. 2008;99:1601–2. doi: 10.1001/jama.299.13.1601. [DOI] [PubMed] [Google Scholar]
- 27.Vallee M, Vitiello S, Bellocchio L, Hebert-Chatelain E, Monlezun S, Martin-Garcia E, et al. Pregnenolone can protect the brain from cannabis intoxication. Science. 2014;343:94–98. doi: 10.1126/science.1243985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piazza PV, Vallée M, Marsicano G, Felpin FX, Bellocchio L, Cota D, Revest JM, Vitiello S, Spampinato U, Maldonado R. Antagonists of CB1 receptor. Patent. 2012 Publication number: WO2012/160006. [Google Scholar]
- 29.McDannald M, Schoenbaum G. Toward a model of impaired reality testing in rats. Schizophr Bull. 2009;35:664–667. doi: 10.1093/schbul/sbp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDannald MA, Whitt JP, Calhoon GG, Piantadosi PT, Karlsson RM, O'Donnell P, et al. Impaired reality testing in an animal model of schizophrenia. Biol Psychiatry. 2011;70:1122–1126. doi: 10.1016/j.biopsych.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, Koh HY. Impaired Reality Testing in Mice Lacking Phospholipase Cβ1: Observed by Persistent Representation-Mediated Taste Aversion. PLoS One. 2016;11:e0146376. doi: 10.1371/journal.pone.0146376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soria-Gómez E, Busquets-Garcia A, Hu F, Mehidi A, Cannich A, Roux L, et al. Habenular CB1 Receptors Control the Expression of Aversive Memories. Neuron. 2015;88:306–13. doi: 10.1016/j.neuron.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Franklin KBJ. Academic Press; San Diego: 2001. [Google Scholar]
- 34.Han J, Kesner P, Metna-Laurent M, Duan T, Xu L, Georges F, et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell. 2012;148:1039–50. doi: 10.1016/j.cell.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. DSM. 4th ed. Washington, DC: APS; 2000. [Google Scholar]
- 36.American Psychiatric Association. DSM. 5th ed. Washington, DC: APS; 2013. [Google Scholar]
- 37.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 38.Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–36. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Martin RS, Secchi RL, Sung E, Lemaire M, Bonhaus DW, Hedley LR, et al. Effects of cannabinoid receptor ligands on psychosis-relevant behavior models in rat. Psychopharmacology (Berl) 2003;165:128–35. doi: 10.1007/s00213-002-1240-x. [DOI] [PubMed] [Google Scholar]
- 40.Tandon R. Definition of psychotic disorders in the DSM-5 too radical, too conservative, or just right! Schizophr Res. 2013;150:1–2. doi: 10.1016/j.schres.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–94. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong AH, Van Tol HH. Schizophrenia: From phenomenology to neurobiology. Neurosc Biobehav Rev. 2003;27:269–30. doi: 10.1016/s0149-7634(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 43.van den Buuse M, Garner B, Gogos A, Kusljic S. Importance of animal models in schizophrenia research. Aust N Z J Psychiatry. 2005;105:550–557. doi: 10.1080/j.1440-1614.2005.01626.x. [DOI] [PubMed] [Google Scholar]
- 44.Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM. Activational role of cannabinoids on movement. Eur J Pharmacol. 2000;391:269–74. doi: 10.1016/s0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- 45.Long LE, Chesworth R, Huang XF, McGregor IS, Arnold JC, Karl T. A behavioural comparison of acute and chronic Delta9-tetrahydrocannabinol and cannabidiol inC57BL/6JArc mice. Int J Neuropsychopharmacol. 2010;13:861–76. doi: 10.1017/S1461145709990605. [DOI] [PubMed] [Google Scholar]
- 46.Gaebel W, Zielasek J. Focus on psychosis. Dialogues Clin Neurosci. 2015;17:9–18. doi: 10.31887/DCNS.2015.17.1/wgaebel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler DS, Chang SE, Holland PC. Odor-mediated taste learning requires dorsal hippocampus, but not basolateral amygdala activity. Neurobiol Learn Mem. 2013;101:1–7. doi: 10.1016/j.nlm.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nemeth-Coslett R, Henningfield JE, O’Keeffe MK, Griffiths RR. Effects of marijuana smoking on subjective ratings and tobacco smoking. Pharmacol Biochem Behav. 1986;25:659–665. doi: 10.1016/0091-3057(86)90156-5. [DOI] [PubMed] [Google Scholar]
- 49.Hunault CC, Böcker KB, Stellato RK, Kenemans JL, de Vries I, Meulenbelt J. Acute subjective effects after smoking joints containing up to 69 mg Δ9-tetrahydrocannabinol in recreational users: a randomized, crossover clinical trial. Psychopharmacology (Berl) 2014;231:4723–33. doi: 10.1007/s00213-014-3630-2. [DOI] [PubMed] [Google Scholar]
- 50.Lagerberg TV, Kvitland LR, Aminoff SR, Aas M, Ringen PA, Andreassen OA, Melle I. Indications of a dose-response relationship between cannabis use and age at onset in bipolar disorder. Psychiatry Res. 2014;215:101–4. doi: 10.1016/j.psychres.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Aston CH. Comparing cannabis with tobacco: those who start taking cannabis young have the greatest problems. BMJ. 2003;327:165. doi: 10.1136/bmj.327.7407.165-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bénard G, Massa F, Puente N, Lourenço J, Bellocchio L, Soria-Gómez E, Matias I, et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat Neurosci. 2012;15:558–64. doi: 10.1038/nn.3053. [DOI] [PubMed] [Google Scholar]
- 53.Ritsner MS. The clinical and therapeutic potentials of dehydroepiandrosterone and pregnenolone in schizophrenia. Neuroscience. 2011;191:91–100. doi: 10.1016/j.neuroscience.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 54.Ritsner MS, Gibel A, Shleifer T, Boguslavsky I, Zayed A, Maayan R, et al. Pregnenolone and dehydroepiandrosterone as an adjunctive treatment in schizophrenia and schizoaffective disorder: an 8-week, double-blind, randomized, controlled, 2-center, parallel-group trial. The Journal of clinical psychiatry. 2010;71:1351–1362. doi: 10.4088/JCP.09m05031yel. [DOI] [PubMed] [Google Scholar]
- 55.Marx CE, Lee J, Subramaniam M, Rapisarda A, Bautista DC, Chan E, et al. Proof-of-concept randomized controlled trial of pregnenolone in schizophrenia. Psychopharmacology (Berl) 2014;231:3647–62. doi: 10.1007/s00213-014-3673-4. [DOI] [PubMed] [Google Scholar]
- 56.Kyosseva SV. Differential expression of mitogen-activated protein kinases and immediate early genes fos and jun in thalamus in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:997–1006. doi: 10.1016/j.pnpbp.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 57.Kyosseva SV. The role of the extracellular signal-regulated kinase pathway in cerebellar abnormalities in schizophrenia. Cerebellum. 2004;3:94–9. doi: 10.1080/14734220410029164. [DOI] [PubMed] [Google Scholar]
- 58.Molteni R, Calabrese F, Racagni G, Fumagalli F, Riva MA. Antipsychotic drug actions on gene modulation and signaling mechanisms. Pharmacol Ther. 2009;124:74–85. doi: 10.1016/j.pharmthera.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–66. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M. Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci Biobehav Rev. 2015;48:10–21. doi: 10.1016/j.neubiorev.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Gonçalves VF, Andreazza AC, Kennedy JL. Mitochondrial dysfunction in schizophrenia: an evolutionary perspective. Hum Genet. 2015;134:13–21. doi: 10.1007/s00439-014-1491-8. [DOI] [PubMed] [Google Scholar]
- 62.Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, Gerth CW, Nolden BM, Gross S, Schreiber D, Nicholson JK, Bahn S. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3:e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rubino T, Parolaro D. Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:41–44. doi: 10.1016/j.pnpbp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 64.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav Brain Res. 2009;204:295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, et al. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17:407–15. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 66.Vallée M. Neurosteroids and potential therapeutics: Focus on pregnenolone. J Steroid Biochem Mol Biol. 2015;160:78–87. doi: 10.1016/j.jsbmb.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 67.Mathew I, Gardin TM, Tandon N, Eack S, Francis AN, Seidman LJ, et al. Medial temporal lobe structures and hippocampal subfields in psychotic disorders: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. JAMA Psychiatry. 2014;71:769–77. doi: 10.1001/jamapsychiatry.2014.453. [DOI] [PubMed] [Google Scholar]
- 68.Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. Am J Psychiatry. 2010;167:1178–93. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- 69.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophr Res. 2014 Oct 18; doi: 10.1016/j.schres.2014.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull. 2012;38:927–35. doi: 10.1093/schbul/sbs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.