Graphical abstract

Keywords: Antimicrobial compound, Burkholderia paludis, Enterococcus faecalis, Pyochelin
Abstract
The increase in prevalence of antimicrobial-resistant bacteria (ARB) is currently a serious threat, thus there is a need for new antimicrobial compounds to combat infections caused by these ARB. An antimicrobial-producing bacterium, Burkholderia paludis was recently isolated and was able to produce a type of siderophore with antimicrobial properties, later identified as pyochelin. The chelating ability of pyochelin has been well-characterized but not for its antimicrobial characteristics. It was found that pyochelin had MIC values (MBC values) of 3.13 µg/mL (6.26 µg/mL) and 6.26 µg/mL (25.00 µg/mL) against three Enterococcus strains and four Staphylococcus strains. Pyochelin was able to inhibit E. faecalis ATCC 700802 (a vancomycin-resistant strain) in a time and dose dependent manner via killing kinetics assay. It was demonstrated that pyochelin enhanced the production of intracellular reactive oxygen species (ROS) over time, which subsequently caused a significant increase in malondialdehyde (MDA) production (a marker for lipid peroxidation) and ultimately led to cell death by disrupting the integrity of the bacterial membrane (validated via BacLight assay). This study has revealed the mechanism of action of pyochelin as an antimicrobial agent for the first time and has shown that pyochelin might be able to combat infections caused by E. faecalis in the future.
Introduction
The increase in prevalence and emergence of antimicrobial resistant bacteria (ARB) is an alarming concern. This is because ARB infections often result in increased mortality rates and cause increased healthcare costs. Enteroccocus faecalis is a major example of ARB that is difficult to treat due to its intrinsic resistance and ability to acquire resistance through mutation or horizontal gene transfer [1], [2]. As vancomycin is the last line of defence to combat enterococci infections, strains that are resistant to this antibiotic are a threat. Vancomycin-resistant enterococci (VRE) account for approximately one-third of the enterococcal healthcare-associated infections in the USA and for more than 20% of such infections in some European countries [3]. Besides that, Staphylococcus aureus is another example of ARB that causes life-threatening infections. The first line therapy for S. aureus infection is usually beta-lactam antibiotics [4]. Unfortunately, the emergence of methicillin-resistant S. aureus (MRSA) strains essentially indicates that they are resistant to all currently available beta-lactam antimicrobial agents. This limits the treatment options to three non-beta lactam antimicrobial agents such as vancomycin, daptomycin and linezolid to treat MRSA infections, but however recently there is an increase in prevalence of S. aureus strains having resistance towards these last few antibiotic options [5], [6]. Due to the limited treatment options available to treat these ARB infections, new antimicrobial compounds are needed to combat this issue.
One strategy is bioprospecting, which is defined as the exploration for potentially new bioactive compounds in unique and extreme ecological niches to treat ARB infections [7]. Bacteria thriving in these environments might produce antimicrobial compounds to gain an advantage in competing for resources and colonization of new habitats. As a result, a tropical peat swamp forest in Malaysia, characterized by its acidic (pH range of 2.9–4.5), ombotrophic and waterlogged conditions was previously chosen as a bioprospecting location for antimicrobial compounds [8]. Despite being such a harsh environment, Ong et al. [9] successfully isolated a novel bacterium Burkholderia paludis which showed potent antimicrobial activity towards methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus faecalis (VRE). The antimicrobial compound was later identified to be pyochelin.
Pyochelin is a type of siderophore commonly produced by the genus Pseudomonas and Burkholderia. The biosynthetic gene clusters of pyochelin, along with its iron-solubilizing ability are well characterized. However, pyochelin has demonstrated other biological activity recently other than being only a chelating compound. This compound can particularly inhibit S. aureus in a study conducted by Adler et al. [10] and this activity was further substantiated by another study performed by Ong et al. [9]. It was demonstrated that pyochelin is not only effective in inhibiting non-antimicrobial-resistant strains of S. aureus and E. faecalis, but also the resistant strains at 6.26 µg/mL and 3.13 µg/mL, respectively. It was postulated that pyochelin can inhibit bacterial growth by enhancing the production of reactive oxygen species (ROS) in the cells, which consequently inhibit certain essential biological processes. Nevertheless the mechanism of action of pyochelin as an antimicrobial compound is not well characterized. Thus this study aims to characterize the antimicrobial property of pyochelin.
Material and methods
Culture conditions and maintenance of bacterial strains
Test microorganism strains that were used in this study include Enterococcus faecalis ATCC 700802, Enterococcus faecalis ATCC 29212, Enterococcus faecalis JH-22, Staphylococcus aureus ATCC 700699, Staphylococcus aureus ATCC 43300, Staphylococcus aureus ATCC 6538P and Staphylococcus aureus ATCC 29213. Strains were cultured on Mueller-Hinton agar (MHA) (Oxoid, UK) at 37 °C and maintained at −80 °C in MHB (Oxoid, UK) with 25% (v/v) glycerol (Merck, Germany). As for B. paludis MSh1, it was maintained on nutrient agar (NA) (Merck, Germany) at 30 °C and in 25% (v/v) glycerol in nutrient broth (NB) (Merck, Germany) at −80 °C for long term preservation.
Extraction of pyochelin from B. paludis MSh1
The extraction of pyochelin from B. paludis MSh1 was performed according to methodology described by Ong et al. [9]. Briefly, B. paludis MSh1 was grown on NA containing 5 g/L of glycerol and incubated for 5 days at 30 °C. The whole media with the bacteria was extracted using methanol (Merck, Germany) and subsequently fractionated using dichloromethane (DCM) (Merck, Germany). The DCM fraction was purified on an open C18 column, followed by further purification using preparative high performance liquid chromatography (HPLC). The purity of pyochelin was compared with a standard purchase from Santa Cruz Biotechnology, USA.
Determination of the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of pyochelin
The MIC of pyochelin was determined using a broth microdilution assay as described by the Clinical and Laboratory Standard Institute (CLSI). The MIC is defined as the lowest concentration of an antimicrobial to inhibit the visible growth of a microorganism after 16–20 h incubation [11]. Briefly, the test microorganisms were grown in MHB at 37 °C for 20 h and adjusted to 0.5 McFarland standard (OD625 0.08–0.11), corresponding to 1.5 × 108 colony forming unit (CFU)/mL. The adjusted cultures were then diluted 100 times in MHB and used as inocula. The extracts were twofold serially diluted using sterile MHB in a 96-well flat bottomed microtiter plate. One hundred µL of the adjusted test microorganisms was added to each well. Determination of MIC was performed in triplicate. The positive control for bacteria was 200 µg/mL chloramphenicol (Calbiochem, Malaysia). The negative control contained MHB with test microorganisms. The blank control consisted only of MHB. The microtiter plate was incubated at 37 °C aerobically for 16–20 h and the MIC was determined by the concentration of extract (µg/mL) where no visible growth was observed. All clear wells containing cultures with no visible growth was streaked out onto MHA to determine the minimum bactericidal concentration (MBC). MBC is defined as the lowest concentration of antimicrobial that will prevent the growth of an organism after subculture on to antibiotic-free media. The lowest concentration of pyochelin that showed absence of growth was determined as the MBC level [11].
Killing-kinetics studies
A killing kinetic study was performed to determine the effect of different concentrations of pyochelin on E. faecalis ATCC 700802 for 24 h. As the Enterococcus strains were shown to be more susceptible to pyochelin as compared to the Staphylococcus strains, further characterization on the antimicrobial activity of pyochelin was conducted on an Enterococcus strain, with particular interest of E. faecalis ATCC 700802 due to its vancomycin-resistant property. The killing kinetics assay was performed according to the method described by Pag et al. [12] and Yan et al. [13]. Different concentrations of pyochelin corresponding to 1×, 2× and 4× the MIC determined by broth microdilution were added into 100× diluted 0.5 McFarland adjusted bacteria culture (1.5 × 106 CFU/mL) in 0.85% (w/v) saline (Fisher Scientific, USA) and incubated at 37 °C. Untreated bacterial culture was served as a negative control. The viable count was monitored up to 24 h. Aliquots were taken at defined intervals (0 h, 2nd hour, 4th hour, 8th hour and 24th hour) and appropriately diluted in 0.85% (w/v) saline. One hundred microliters of each of the dilutions was plated in triplicate on MHA. The plates were incubated at 37 °C and the cell viability was assessed by enumerating the colony forming unit (CFU) per millilitre after 24 h. Killing kinetic studies of pyochelin on E. faecalis ATCC 700802 were performed under three different conditions: (1) exponential phase culture with agitation at 200 rpm (Smith, A3555, Progressive Scientific); (2) stationary phase culture with agitation at 200 rpm (Smith, A3555, Progressive Scientific); and (3) exponential phase culture at anaerobic condition. The anaerobic cultures were cultured in an anaerobic jar (Labozone, France) with AnaeroGen pack (Oxoid, UK).
Detection of reactive oxygen species (ROS)
The production of ROS by E. faecalis ATCC 700802 after treatment with pyochelin was evaluated using a peroxynitrite indicator, 2′-7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma-Aldrich, UK), which can detect a broad range of ROS including nitric oxide and hydrogen peroxide [14]. The adjusted bacterial culture (0.5 McFarland exponential phase bacteria culture) were treated with different concentrations of pyochelin corresponding to 1, 2 and 4 times MIC in presence of DCFH-DA at a final concentration of 5 µM in 0.85% saline and incubated at 37 °C aerobically at 200 rpm (Smith, A3555, Progressive Scientific) for 24 h. Untreated bacterial culture was served as a negative control. The fluorescence emission of DCFH-DA was measured at 525 nm using a Tecan microtitre plate reader with an excitation wavelength of 485 nm [15]. The background fluorescence of 0.85% saline and autoflorescence of the bacterial cells incubated without the probe was measured to calculate the net fluorescence emitted from the assay itself. Experiment was conducted in triplicate.
Determination of malondialdehyde (MDA)
Malondialdehyde (MDA) is a natural by-product of lipid peroxidation of polyunsaturated fatty acids caused by ROS, thus is commonly used as a marker for oxidative stress. The production of MDA was quantified by using the OxiSelect™ TBARS Assay kit according to manufacturer’s protocol (Cell Biolabs Inc., USA). Briefly, the adjusted bacterial culture (0.5 McFarland adjusted exponential phase bacteria culture) were treated with different concentrations of pyochelin corresponding to 1, 2 and 4 times the MIC at 37 °C aerobically whereas the control was incubated with 0.85% (w/v) saline alone for 24 h. One hundred µl of the SDS lysis solution were added to 100 µl aliquot of the treated culture and incubated for 5 min at room temperature. The mixtures were then incubated at 95 °C for 60 min in presence of thiobarbituric acid (TBA) reagent. Each of the mixture was cooled to room temperature in an ice bath for 5 min and centrifuged at 3000g for 15 min (Eppendorf, 5810R). The supernatants were then collected and the absorbances were read at 532 nm. The concentrations of MDA in each treatment were calculated based on the standard curve of absorbance against MDA concentration. This assay was performed in triplicates.
Membrane integrity assay
As the bacterial membrane is composed of phospholipilid bilayer, the production of ROS prior to pyochelin treatment might oxidize the lipid content on the cell membrane, hence affecting the bacterial membrane integrity. Therefore, the effect of pyochelin on the membrane integrity of E. faecalis ATCC 700802 was determined by using the Live/Dead BacLight Bacterial Viability Kit (Molecular Probes, Invitrogen) according to a protocol from Ong et al. [16]. The adjusted bacterial cultures were treated with different concentrations of pyochelin corresponding to 1×, 2× and 4× the MIC at 37 °C aerobically at 200 rpm (Smith, A3555) whereas the control was incubated with 0.85% (w/v) saline alone for 24 h. After incubation, the treated cultures were pelleted by centrifugation (10,000g, 15 min) at room temperature, washed twice and resuspended in 0.85% (w/v) saline. One hundred microliters of the 2× staining solution were added into 100 µl of the bacteria suspension, and incubated in the dark for 15 min. At the end of the incubation period, green fluorescence (SYTO 9) was read at 530 nm while the red fluorescence (propidium iodide) was read at 645 nm with an excitation wavelength of 485 nm. This kit utilizes a mixture of SYTO 9 green-fluorescent nucleic acid stain and the red-fluorescent nucleic acid stain, propidium iodide. The SYTO 9 stain generally labels all bacteria in a population including those with intact membranes and those with damaged membranes. In contrast, PI is impermeable to bacterial cells with an intact cell membrane due to its large molecular size [17]. Thus, bacteria with intact cell membranes will be stained fluorescent green, whereas bacteria with damaged membranes will be stained fluorescent red. The percentage of live bacteria was determined by referring to a standard curve of G/R ratio versus percentage of live E. faecalis ATCC 700802 which was pre-plotted earlier. This assay was performed in triplicates.
Statistical analysis
The significance of results for the killing kinetics studies, detection of ROS and quantification of MDA were performed using paired-sample t-test at the significance level of α = 0.05. The significance of results for membrane integrity assay was performed using Wilcoxon test at the significance level of α = 0.05 (Kolmogoroff-Smirnow test was used to analyse the normal distribution). Statistical analysis was performed using IBM SPSS Statistics 20.
Results and discussion
MIC, MBC and killing kinetics studies of pyochelin
Pyochelin is a type of siderophore commonly produced by the genus Pseudomonas [10]. It has also been reported to be produced by certain Burkholderia species such as B. arboris, B. contaminans and B. cenocepacia [18], [19], [20]. Siderophores are important to bacteria as they are able to scavenge ferric ion in the nature for essential biological functions such as DNA synthesis [21]. Pyochelin has been extensively studied from a molecular perspective, as well as its chelating abilities. However this study had shown that pyochelin possesses other biological activity.
The MIC values of pyochelin against the Enterococcus strains (E. faecalis ATCC 700802, E. faecalis ATCC 29212, E. faecalis JH-22) and Staphylococcus strains (S. aureus ATCC 700699, S. aureus ATCC 43300, S. aureus ATCC 6538P, S. aureus ATCC 29213) were 3.13 µg/mL and 6.26 µg/mL respectively; while the MBC values were 6.26 µg/mL and 25.00 µg/mL respectively (Table 1). It was shown that the Enterococcus strains are more susceptible to pyochelin when compared to the Staphylococcus strains. Nonetheless pyochelin is bactericidal against both Enterococcus and Staphylococcus strains as the MBC values were no more than 4× the MIC values [22]. The low MIC values of pyochelin against the Enterococcus faecalis and Staphylococcus aureus strains is an advantage as it is comparable or lower than the currently available antibiotics which have MIC values of 4–64 µg/mL [11].
Table 1.
MIC and MBC of pyochelin against different test microorganisms.
| Test microorganisms | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| E. faecalis ATCC 700802 | 3.13 | 6.26 |
| E. faecalis ATCC 29212 | 3.13 | 6.26 |
| E. faecalis JH-22 | 3.13 | 6.26 |
| S. aureus ATCC 700699 | 6.26 | 25.00 |
| S. aureus ATCC 43300 | 6.26 | 25.00 |
| S. aureus ATCC 6538P | 6.26 | 25.00 |
| S. aureus ATCC 29213 | 6.26 | 25.00 |
Killing kinetics was performed to evaluate the effect of different concentrations of pyochelin on E. faecalis ATCC 700802 for 24 h. Two phases of bacterial culture were used in this study: exponential phase and stationary phase. Exponential phase culture consists of actively growing cells which consume readily available oxygen and nutrients for growth. On the other hand, stationary phase culture comprises mostly of mature non-dividing cells which are metabolically inactive [23]. Different types of antibiotics work differently depending on their mechanism of action. For instance, lipopeptides (membrane disruptors) inhibits bacterial growth (both exponential phase and stationary phase culture) instantly by puncturing their cell wall [24]; while beta lactams (cell wall biosynthesis inhibitor) only inhibit actively growing bacterial cells in a time-dependent manner, but they are effective at both aerobic and anaerobic conditions [25].
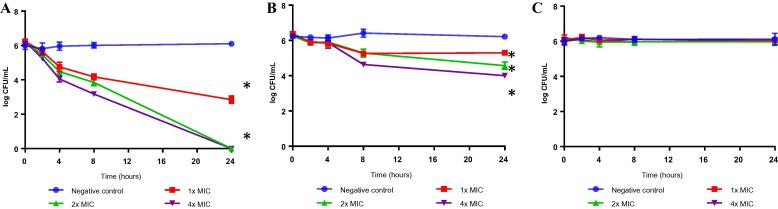
Pyochelin inhibits growth of exponential phase E. faecalis ATCC 700802 in a dose and time dependent manner (Fig. 1A). E. faecalis ATCC 700802 culture treated with 3.13 µg/mL (1× MIC) of pyochelin achieved 3 log reduction after 24 h; while bacterial culture treated with 6.26 µg/mL (2× MIC) and 12.52 µg/mL (4× MIC) of pyochelin achieved 6 log reduction after 24 h. However, a different scenario was observed when stationary phase E. faecalis ATCC 700802 was treated with pyochelin as there was only 2 log reduction after incubated for 24 h at 4× MIC aerobically (Fig. 1B). This result has revealed that pyochelin work best only on actively growing bacterial cells. Nevertheless, pyochelin is different from the beta lactams as it is ineffective against bacterial cells incubated under anaerobic condition (Fig. 1C), suggesting that oxygen might play an important role in the bactericidal effect of pyochelin on E. faecalis ATCC 700802.
Fig. 1.
Effect of different concentrations of pyochelin against (A) exponential phase E. faecalis ATCC 700802 (incubated aerobically); (B) stationary phase E. faecalis ATCC 700802 (incubated aerobically); (C) exponential phase E. faecalis ATCC 700802 (incubated anaerobically) at 37 °C for 24 h. Results are expressed as mean log CFU/mL ± SD plotted against time (n = 3). Asterisk represents significant difference (P = 0.05) between each treatment with the negative control at 24 h. As the responding data covers a range from 0 to 106, the geometric sequence of the responding data (representing bacterial growth and bacterial cell death) has been transformed into a logarithmic plot of log10 CFU/mL against time. Example: the number of bacteria (negative control) at 24 h is 1.3 × 106 CFU/mL, hence after transformation (log10 1.3 × 106), the value is 6.11.
Effect of pyochelin on the enhancement of ROS production and membrane integrity
It was hypothesized that in presence of pyochelin, the formation of ROS was enhanced in E. faecalis ATCC 700802 which can damage the iron-sulphur clusters, thereby releasing ferrous ion. This iron can react with hydrogen peroxide in the Fenton reaction, causing a chain reaction, generating hydroxyl radicals which can directly damage intracellular DNA, lipids and proteins [26]. Hence to validate the hypothesis, the intracellular ROS in E. faecalis ATCC 700802 was quantified prior to pyochelin treatment in the subsequent experiments.
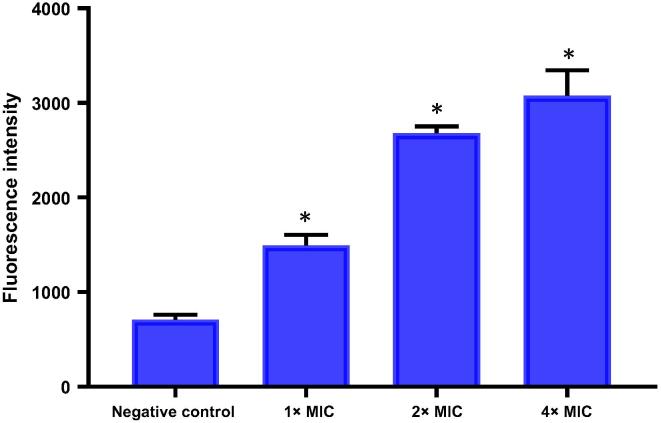
The production of ROS in healthy untreated bacterial cells is a natural side effect of aerobic respiration. These ROS can damage the RNA/DNA pool and also oxidizes lipid contents. Thus to protect themselves against the detrimental effect of ROS, bacteria are capable of producing enzymes (catalase and superoxide dismutase) to detoxify the ROS and having regulatory mechanisms (SoxRS, OxyRS and SOS regulons) to counteract the damage [26], [27]. To determine the effect of pyochelin on the enhancement of ROS production, E. faecalis ATCC 700802 was treated with different concentrations of pyochelin in presence of DCFH-DA, an unspecific probe for ROS. It was shown that the ROS production in E. faecalis ATCC 700802 was enhanced in a dose dependent manner when treated with pyochelin (Fig. 2). This suggests that the enhanced production of ROS has an indirect effect on the growth of E. faecalis ATCC 700802.
Fig. 2.
Quantitation of intracellular ROS production by E. faecalis ATCC 700802 after 24 h treatment with different concentrations of pyochelin using the DCFA-DA probe. Results are expressed as mean fluorescence intensity ± SD (n = 3). Asterisk represents significant difference (P = 0.05) between each treatment with the negative control.
As one of the side effects of increased production of ROS is lipid peroxidation, an example of the by-product in this process (malondialdehyde; MDA) was quantified in this study. The concentration of MDA in the treated E. faecalis ATCC 700802 culture was increased significantly with increasing concentrations of pyochelin. This indicates that the enhanced production of ROS (Fig. 3) in E. faecalis ATCC 700802 prior to treatment with pyochelin has caused an increase in lipid peroxidation (Fig. 3).
Fig. 3.
Quantification of MDA production in E. faecalis ATCC 700802 after 24 h treatment with different concentrations of pyochelin. Results are expressed as mean ± SD (n = 3). Asterisk represents significant difference (P = 0.05) between each treatment with the negative control.
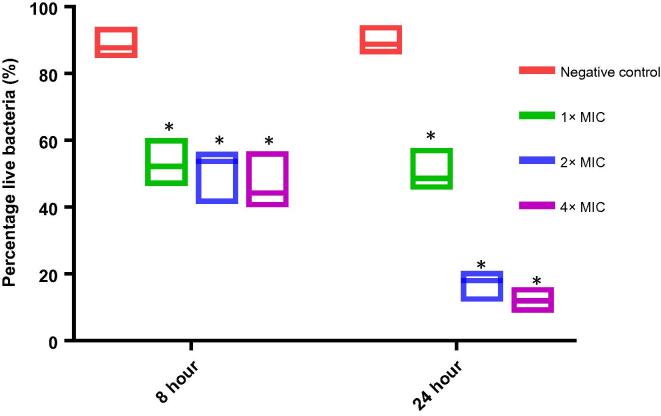
Since lipid is an essential macromolecule to the bacterial cell membrane, the membrane integrity of E. faecalis ATCC 700802 was evaluated using the Live/Dead BacLight Bacterial Viability Kits. It was found that the percentage live bacteria of E. faecalis ATCC 700802 was 52.05% at 8 h and 50.35% at 24 h when treated with 1× MIC of pyochelin (Fig. 4). This is because the enhanced generation of ROS at 1× MIC by pyochelin is not sufficient to eliminate the entire bacterial population. It was previously reported that bacterial cells are capable of lowering their metabolic activity at sub-lethal ROS concentration, hence allowing the cell’s regulatory mechanisms to repair the damaged protein or DNA clusters and concurrently producing more enzymes to detoxify the detrimental effect of ROS [28]. The results shown is consistent with the data obtained from the killing kinetics study as there was only 3 log reduction at 1× MIC of pyochelin after 24 h. Furthermore, the MDA concentration of E. faecalis ATCC 700802 treated at 1× MIC of pyochelin was not statistically significant compared to the untreated control, indicating that the ROS level generated in presence of 1× MIC of pyochelin did not trigger significant lipid peroxidation, hence the higher percentage of live bacteria. Nevertheless, the percentage live bacteria of E. faecalis ATCC 700802 decreases in a time dependent manner when treated with higher concentrations of pyochelin (2× and 4× MIC) (Fig. 4) and this is in agreement with the data obtained from the killing kinetics study.
Fig. 4.
Percentage of live E. faecalis ATCC 700802 at 8 h and 24 h after treatment with different concentrations of pyochelin using the Live/Dead BacLight Bacterial Viability Kit. Results are expressed as median with range (n = 6). Asterisk represents significant difference (P = 0.05) between each treatment with the negative control at each time-point using Wilcoxon test.
This result substantiates that pyochelin can enhance the intracellular production of ROS, which later affects the membrane integrity of E. faecalis ATCC 700802, leading to bacterial cell death. Furthermore, the lipophilicity of pyochelin might play an important role in affecting the membrane fluidity or membrane potential (proton motive force), thus allowing the initial entry of pyochelin into the bacterial cells to exert its antimicrobial effect [29]. A similar pattern can be observed from other studies conducted using aspidin BB (an alkaloid), metal oxide nanoparticles and synthesized pyrimidine derivatives, as these compounds exert their antibacterial properties by inducing the generation of ROS as well [30], [31], [32]. The killing mechanism shown in this study might potentially be useful in combating antimicrobial resistance, as it involves the bacterial cell’s redox reaction which directly influences the survival of the cells [28], [33]. However sequential passaging of the bacterial culture with sub-MIC of pyochelin should be done in the future to evaluate the development of resistance of E. faecalis ATCC 700802 towards pyochelin over generations [34]. Nevertheless, this is the first study to characterize the potential of pyochelin as an antimicrobial compound against vancomycin-resistant Enteroccocus (VRE). Further work such as in vitro cytotoxic evaluation of pyochelin using normal human cell lines and potentiation of pyochelin with existing antibiotics should be conducted. Furthermore, different strains of Enterococcus faecalis or other test microorganisms such as the Staphylococcus aureus strains should be tested to further support pyochelin as a potential therapeutic option against ARB infections.
Conclusions
Pyochelin was found to be effective in inhibiting the growth of three E. faecalis strains and four S. aureus, with MIC values (MBC values) of 3.13 µg/mL (6.26 µg/mL) and 6.26 µg/mL (25.00 µg/mL) respectively via broth microdilution. Pyochelin is able to enhance the production of intracellular ROS, subsequently causing an increase in MDA production and a decrease in membrane integrity of E. faecalis ATCC 700802 (VRE) after 24 h. This study has provided an insight that pyochelin might potentially be useful in treating infections caused by ARB, particularly VRE in the future.
Conflict of interest
The authors have declared no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Acknowledgements
The authors would like to thank Monash University Malaysia for funding this project.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Cetinkaya Y., Falk P., Mayhall C.G. Vancomycin-resistant enterococci. Clin Microbial Rev. 2000;13:686–707. doi: 10.1128/cmr.13.4.686-707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rivera A.M., Boucher H.W. Current concepts in antimicrobial therapy against select gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230–1242. doi: 10.4065/mcp.2011.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balli E.P., Venetis C.A., Miyakis S. Systemic review and meta-analysis of linezolid versus daptomycin for treatment of vancomycin-resistant enterococcal bacteremia. Antimicrob Agents Chemother. 2014;58:734–739. doi: 10.1128/AAC.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovetic S., Zhu Y., Marcone G.L., Marinelli F., Tramper J. Beta lactam and glycopeptide antibiotics: first and last line of defense? Trends Biotechnol. 2010;28:596–604. doi: 10.1016/j.tibtech.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Gorwitz R.J., Moran D.K., McAllister S.K., McQuillan G., McDougal L.K., Fosheim G.E. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J Infect Dis. 2008;197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 6.Holmes R.L., Jorgensen J.H. Inhibitory activities of 11 antimicrobial agents and bactericidal activities of vancomycin and daptomycin against invasive methicillin-resistant Staphylococcus aureus isolates obtained from 1999 through 2006. Antimicrob Agents Chemother. 2008;52:756–760. doi: 10.1128/AAC.00945-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imhoff J.F., Labes A., Wiese J. Bio-mining the microbial treasures of the ocean: new natural products. Biotechnol Adv. 2011;29:468–482. doi: 10.1016/j.biotechadv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Yule C.M. Loss of biodiversity and ecosystem functioning in Indo-Malayan peat swamp forests. Biol Conserv. 2010;19:393–409. [Google Scholar]
- 9.Ong K.S., Aw Y.K., Lee L.H., Yule C.M., Cheow Y.L., Lee S.M. Burkholderia paludis sp. nov., an antibiotic-siderophore producing novel Burkholderia cepacia complex species, isolated from Malaysian tropical peat swamp soil. Front Microbiol. 2016;7:2046. doi: 10.3389/fmicb.2016.02046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler C., Corbalan N.S., Seyedsayamdost M.R., Pomares M.F., de Creistobal R.E., Clardy J. Catecholate siderophores protect bacteria from pyochelin toxicity. PLoS ONE. 2012;7:e46754. doi: 10.1371/journal.pone.0046754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Performance standard for antimicrobial susceptibility testing; twenty-second informational supplement M100. USA: CLSI; 2014.
- 12.Pag U., Oedenkoven M., Papo N., Oren Z., Shai Y., Sahl H.-G. In vitro activity and mode of action of diastereomeric antimicrobial peptides against bacterial clinical isolates. J Antimicrob Chemother. 2004;53:230–239. doi: 10.1093/jac/dkh083. [DOI] [PubMed] [Google Scholar]
- 13.Yan J., Wang K., Dang W., Chen R., Xie J., Zhang B. Two hits are better than one: membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother. 2013;57:220–228. doi: 10.1128/AAC.01619-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakha M., Pal S., Samantarrai D., Panigrahi T.K., Mallick B.C., Pramanik K. Antimicrobial activity of iron oxide nanoparticle upon modulation of nanoparticle-bacteria interface. Sci Rep. 2015;5:1–12. doi: 10.1038/srep14813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han L., Patil S., Boehm D., Milosavljevic V., Cullen P.J., Bourke P. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl Environ Microbiol. 2016;82:450–458. doi: 10.1128/AEM.02660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong K.S., Yule C.M., Lee S.M. Antimicrobial producing bacteria isolated from tropical peat swamp soil. Malays J Microbiol. 2015;11:170–175. [Google Scholar]
- 17.Stocks S.M. Mechanism and use of the commercially available viability stain, BacLight. Cytometry Part A. 2004;61A:189–195. doi: 10.1002/cyto.a.20069. [DOI] [PubMed] [Google Scholar]
- 18.Dang L.D., Son S.W., Cheon H.M., Choi G.J., Choi Y.H., Jang K.S. Pyochelin isolated from Burkholderia arboris KRICT1 carried by pine wood nematodes exhibits phytotoxicity in pine callus. Nematology. 2011;13:521–528. [Google Scholar]
- 19.Deng P., Wang X., Baird S.M., Showmaker K.C., Smith L., Peterson D. Comparative genome-wide analysis reveals that Burkholderia contaminans MS14 possesses multiple antimicrobial biosynthesis genes but not major genetic loci required for pathogenesis. Microbiol Open. 2015;5:353–369. doi: 10.1002/mbo3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwagner S., Agnoli K., Kothe M., Feldmann F., Givskov M., Carlier A. Identification of Burkholderia cenocepacia strain H111 virulence factors using nonmammalian infections hosts. Infect Immun. 2012;81:143–153. doi: 10.1128/IAI.00768-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C. Essential functions of iron-requiring proteins in DNA replication, repair and cell cycle control. Protein Cell. 2014;5:650–760. doi: 10.1007/s13238-014-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pankey G.A., Sabath L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–870. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 23.Roostalu J., Joers A., Luidalepp H., Kaldalu N., Tenson T. Cell devision in Escherichia coli cultures monitored at single cell resolution. BMC Microb. 2008;8:1–14. doi: 10.1186/1471-2180-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinbuch K.B., Fridman M. Mechanisms of resistance to membrane-disrupting antibiotics in gram-positive and Gram-negative bacteria. Med Chem Commun. 2016;7:86–102. [Google Scholar]
- 25.Holten K.B., Onusko E.M. Appropriate prescribing of oral beta-lactam antibiotics. Am Fam Phys. 2000;62:611–621. [PubMed] [Google Scholar]
- 26.Acker H.V., Gielis J., Acke M., Cools F., Cos P., Coenye T. The role of reactive oxygen species in antibiotic-induced cell death in Burkholderia cepacia complex bacteria. PLoS ONE. 2016;11:e0159837. doi: 10.1371/journal.pone.0159837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasser V., Baco E., Cunrath O., August P.S., Perraud Q., Zill N. Catechol siderophores repress the pyochelin pathway and activate the enterobactin pathway in Pseudomonas aeruginosa: an opportunity for siderophore–antibiotic conjugates development. Environ Microbiol. 2016;18:819–832. doi: 10.1111/1462-2920.13199. [DOI] [PubMed] [Google Scholar]
- 28.Keren I., Wu Y., Inocencio J., Mulcahy L.R., Lewis K. Killing by antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 29.Mingeot-Leclercq M.-P., Decout J.-L. Bacterial lipid membranes as promising targets to fight antimicrobial resistance, molecular foundations and illustration through the renewal of aminoglycoside antibiotics and emergence of amphiphilic aminoglycosides. Med Chem Commun. 2016;7:586–611. [Google Scholar]
- 30.Dizaj S.M., Lotfipour F., Barzegar-Jalali M., Zarrintan M.H., Adibkia K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284. doi: 10.1016/j.msec.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Li N., Gao C., Peng X., Wang W., Luo M., Fu Y. Aspidin BB, a phloroglucinol derivative, exerts its antibacterial activity against Staphylococcus aureus by inducing the generation of reactive oxygen species. Res Microbiol. 2014;165:263–272. doi: 10.1016/j.resmic.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Suresh L., Kumar P.S.V., Poornachandra Y., Kumar C.G., Chandramouli G.V.P. An efficient one-pot synthesis of thiochromeno[3,4-d]pyrimidines derivatives: inducing ROS dependent antibacterial and anti-biofilm activities. Bioorg Chem. 2016;68:159–165. doi: 10.1016/j.bioorg.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Paiva C.N., Bozza M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Sign. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling L.L., Schneider T., Peoples A.J., Spoering A.L., Engels I., Conlon B.P. A new antibiotic kills pathogens without detectable resistance. Nature. 2015;517:455–459. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]