Abstract
We sought to determine the association of Life's Simple Seven (LSS) with peripheral artery disease (PAD) in African Americans. We performed a cross-sectional analysis of baseline data (2000–2004) from subjects participating in the Jackson Heart Study. African American men and women (N = 4403) age 35–84 years participated in the study. PAD was defined by an ankle-brachial index (ABI) of < 0.9. We assessed frequency of LSS (body mass index [BMI], blood pressure, total cholesterol, glucose, dietary habits, physical activity, and smoking) among participants with and without PAD. LSS variables were categorized as ideal, intermediate, or poor to indicate a participant's health status. Data were analyzed using logistic regression to assess the association of PAD with LSS. PAD was diagnosed in 113 participants (2.6%). The percentage of the cohort meeting criteria for ideal health for each of the seven LSS factors was: 14.2% for BMI, 17.1% for blood pressure, 38.0% for total cholesterol, 72.9% for glucose, 1.0% for dietary habits, 19.2% for physical activity, and 84.6% for smoking. Having ≥ 3 LSS variables within the category of poor health was associated with elevated odds for PAD (odds ratio (OR) 1.34, 95% CI 1.11–1.63) after adjusting for age. Among African American adults, LSS variables are associated with PAD. Further studies are needed to determine the association of LSS with PAD among other racial/ethnic groups.
Highlights
-
•
PAD is a debilitating disease that is associated with adverse outcomes.
-
•
Life's Simple Seven (LSS) are associated with peripheral artery disease.
-
•
Interventions that target LSS may reduce the development and/or progression of PAD.
1. Background
Peripheral artery disease (PAD), defined as atherosclerosis of the abdominal aorta and arteries of the lower extremities (McDermott and Greenland, 1998), is a common disorder that affects 20–30% of adults ≥ 50 years of age, and up to 12 million Americans overall (Fowkes et al., 1991, Hirsch et al., 2001, McDermott et al., 1999). PAD affects individuals of all races and levels of socioeconomic status (Newman et al., 1993). Epidemiologic evidence suggests an association between optimal health behaviors and a low incidence of CVD events (Warren et al., 2010). However, limited information exists on the relationship between such behaviors and prevalence of PAD among African Americans.
An American Heart Association (AHA) Strategic Planning Task Force set a goal to improve the cardiovascular health of all Americans by 20% and to concurrently reduce deaths from CVD by 20%. The task force developed definitions of ideal, intermediate, and poor cardiovascular health for adults and children based on seven CVD risk factors or health behaviors. African Americans have a high prevalence of cardiovascular risk factors and poor health behaviors as well as peripheral artery disease (PAD) (Newman et al., 1993, Collins et al., 2003). To date, no studies have assessed the association of overall cardiovascular health with PAD among African Americans. We sought to determine the association of the seven CVD risk factors or health behaviors (Life's Simple 7) with PAD among community dwelling African American men and women in Jackson, Mississippi.
2. Methods
2.1. Data source/population
Data were obtained from the Jackson Heart Study (JHS), a cohort study of risk factors of cardiovascular diseases among 5301 African American men and women residents of Jackson, MS, aged 35–84, who enrolled in the study from 2000 to 2004. In-person interviews conducted among study participants at baseline provided information on socio-demographic and clinical characteristics. Details of the study design and data collection are documented in prior publications (Carpenter et al., 2004, Wyatt et al., 2003). The JHS had IRB approval by Jackson State University, Tougaloo College, and the University of Mississippi Medical Center. Study participants signed informed consents.
2.2. Inclusions
We included all participants who completed baseline measurements for the ankle-brachial index (ABI), a sensitive and reliable noninvasive test to diagnose PAD (Tullos et al., 2013).
2.3. Outcome variable
The outcome variable was PAD, which was defined as an ABI < 0.9. The ABI was calculated by the JHS protocol (Tullos et al., 2013). Specifically, to determine the ABI, ankle and brachial systolic BP were measured by trained technicians using a sphygmomanometer along with an Ultrasonic Doppler Flow Detector (Model 811-B, Parks Medical Electronic-Inc., Aloha, Oregon USA). Technicians followed a standard protocol, using a contour wrapping technique with the midpoint of the bladder over the posterior tibial artery; the lower end of the bladder was approximately 3 cm above the medial malleolus. Systolic BP measurements were taken in each leg with ultrasound measurement of the posterior tibial artery while the participant was supine; the dorsalis pedis artery was used for measurement if the posterior tibial pulse could not be found by palpation or by Doppler. Measurements of the brachial systolic BP, usually in the right brachial, were taken twice. An ABI was calculated for each leg based on the average of the two ankle systolic BP measurements divided by the average of the two brachial readings.
2.4. Life's Simple 7 variables
Life's Simple 7 (LSS) variables were derived from variables within the JHS dataset. The definition of poor, intermediate, and ideal health for each LSS variable was based on the AHA guidelines (Djousse et al., 2015). Table 1 provides a description of the categories for each LSS variable.
Table 1.
Categories for each Life's Simple 7 variable.
| Variables | Ideal | Intermediate | Poor |
|---|---|---|---|
| AHA BMI | < 25 kg/m2 | 25 to 29.9 kg/m2 | ≥ 30 kg/m2 |
| AHA blood pressure | < 120/80 mm Hg without medication | Systolic 120 to 139 or diastolic 80 to 89 mm Hg with or without medication or medication use and < 120/80 mm Hg | Systolic ≥ 140 or diastolic ≥ 90 |
| AHA total cholesterol | < 200 mg/dL without medication | 200 to 239 mg/dL with or without medication or medication use and < 200 mg/dL | > 240 mg/dL |
| AHA glucose | < 100 mg/dL without medication | 100 to 125 mg/dL with or without medication or use of hypoglycemic medication and < 100 mg/dL | ≥ 126 mg/dL |
| AHA Healthy Diet Scorea | 4–5 points | 2–3 points | ≤ 1 |
| AHA physical activity | ≥ 150 min/week of moderate activity or ≥ 75 min/week of vigorous activity or ≥ 150 min/week of moderate + vigorous activity | 1–149 min/week of moderate activity or 1–74 min/week of vigorous activity or 1–149 min/week of moderate + vigorous activity | 0 min/week of physical activity |
| AHA smoking | Never smoker or former smoker who had quit ≥ 12 months ago | Former smoker who had quit within the past year | Current smokers |
Healthy Diet Score – scores were calculated based on one point for each of five dietary goals: at least 4.5 cups/day of fruit and vegetables; at least two 3.5-oz servings/week of fish; at least three 1-oz servings/day of fiber rich whole grains; no > 36 fluid oz/week of sugar-sweetened beverages; < 1500 mg of sodium each day.
2.5. Smoking
Participants were asked about smoking status. Based on their responses, participants were classified as a current, former, or never smoker. Former smokers were divided into those who quit smoking < 12 months or ≥ 12 months prior to the interview.
2.6. Body mass index (BMI)
Based on height, as captured to the nearest centimeter, and weight, obtained from a scale, BMI was calculated by dividing weight (kg) by height squared (m2).
2.7. Physical activity
Participants were queried about their physical activity. The interviewer-administered physical activity survey was adapted from the Baeke physical activity and Atherosclerosis Risk in Communities (ARIC) surveys (Ainsworth et al., 2000, Dubbert et al., 2005). Physical activity was categorized into four groups: sports and exercise; active living; occupational activity; and home, family, yard and garden activity. Similar to the approach of Djousse et al. (2015), only activity compiled by the sport and exercise component of the instrument was used in the current analysis. Sport and exercise was reported per a given activity as the average amount of time per week spent at that activity. Metabolic equivalent (MET) levels for each named activity were obtained from the national Compendium of Physical Activity (Ainsworth et al., 2011). Activities identified as either vigorous (N6 METs) or moderate (3–6 METS) (Omura et al., 2015) contributed to the participant's physical activity score. For each participant, the average time per week spent engaged in all activities at either a vigorous or moderate level was calculated. Based on this calculation, each participant was labeled by one of three AHA recommended levels of physical activity.
2.8. Dietary assessment
Dietary habits were assessed using a 158-food item, semi-quantitative food frequency questionnaire (FFQ) tailored to the study population (Tucker et al., 2005). The FFQ has been validated in a subset of the JHS cohort (Carithers et al., 2009, Talegawkar et al., 2008). FFQ information was available at baseline for 5065 (95.5%) of the 5301 JHS participants. We excluded 304 participants with extreme energy intakes (defined as ≤ 600 kcal/d or ≥ 4800 kcal/d). Thus, based on AHA guidelines, derived dietary data for LSS were available for 4761 participants. Individuals were given one point for each of five dietary goals. These data were obtained from the FFQ using the Nutrition Data System for Research, NDS-R, version 4.04, 2001 (Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
Assessment for total cholesterol, blood pressure, and fasting glucose were ascertained per the JHS protocol (Carpenter et al., 2004, Hickson et al., 2011). Medications were defined as antihypertensive, hypoglycemic, or statin, based on the Therapeutic Classification System (Djousse et al., 2015).
Additional variables considered as possible predictors for PAD included age, gender, alcohol use and educational attainment (< high school, some college, college graduate or more high school).
2.9. Descriptive analysis
Participants' characteristics were summarized as means and standard deviations for continuous variables and counts and percentages (%) for categorical variables. The statistical significance of differences in mean values of continuous variables between persons with PAD versus without PAD was assessed using Student's t-test. The significance of analogous differences in categorical percentages was assessed using either the χ2 test or Fisher's exact test when appropriate. Each specific statistical (null) hypothesis was tested against two-sided alternatives. A finding that resulted in a p-value ≤ 0.05 was considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute, Cary NC).
2.10. Missing values
Missing values on education, income, health insurance, alcohol use and LSS variables were imputed using multiple imputations (MI). The MIANALYZE procedure was used to combine the results based on the 10 datasets produced by the MI procedure (Rubin, 1987).
2.11. Logistic regression models
In order to have an indication of poor health, we summed the number of poor health indicators out of the seven items of the LSS. Logistic regression with splines was used to model the logarithm of the odds of PAD as a function of the number of LSS variables with poor health scores. The purpose of this process was to search for an optimum model to determine the number and location of cut points for assessing changes in odds of PAD associated with different categories of the summed number of poor LSS variables. Numerous spline models were investigated using the Akaike Information Criterion (SAS Institute Inc, 2008) to search for the best model, but none demonstrated significant improvement over the simple binary categorization for predicting elevated odds for PAD. Thus, using sensitivity and specificity (Hosmer et al., 2013), we compared different binary cut points for the number of poor health indicators to find the best predictive variable for PAD vs no PAD. The minimum Euclidean distance criterion was used. Parameters of logistic regression models were estimated to assess the association of the sum of poor health indicators (dichotomized as < 3; ≥ 3) with the prevalence of PAD. Four model types were considered, each having prevalence of PAD as the dependent variable. Model A employed a single predictor – number of poor health indicators expressed as a binary variable (< 3; ≥ 3); Model B added age to Model A; Model C added gender to Model B; and Model D added education and alcohol consumption. Results are presented as odds ratios (OR) with 95% confidence intervals.
3. Results
Data on ABI testing were available for 4403 JHS participants age 35–84 (mean age 56.3 (SD 11.3) years, male 36.7%). The prevalence of PAD in this cohort was 2.6% (N = 113). The age-adjusted prevalence of PAD was 2.1%. By age, the prevalence of PAD was as follows: 35–50, 0.5%; 50–59, 1.6%; 60–69, 3.3%; and 70–84, 8.3%. PAD was more prevalent among adults age 70 or older, and individuals who did not graduate from high school (Table 2). There was no statistically significant difference of prevalence of PAD between genders. Poor health for blood pressure was almost twice as prevalent for participants with PAD than participants without PAD (38.9% vs 20.4%, respectively). For total cholesterol, 23.9% of participants with PAD compared to 13.7% without PAD were defined as having poor health. Poor health for serum glucose was present among 18.6% of participants with PAD compared to 10.9% of participants without PAD.
Table 2.
Baseline characteristics among Jackson Heart Study Participants without and with peripheral artery disease (PAD).
| No PAD (n = 4290) | PAD (n = 113) | Total (n = 4403) | p-Valuea | |
|---|---|---|---|---|
| Age, mean (SD), years | 56.0 (11.2) | 66.6 (10.0) | 56.3 (11.3) | < 0.001 |
| Age groups, no. (%), years | < 0.001 | |||
| 35–49 | 1440 (33.6) | 7 (6.2) | 1447 (32.9) | |
| 50–59 | 1172 (27.3) | 19 (16.8) | 1191 (27.1) | |
| 60–69 | 1151 (26.8) | 39 (34.5) | 1190 (27.0) | |
| 70–84 | 527 (12.3) | 48 (42.5) | 575 (13.1) | |
| Gender, no. (%) | 0.27 | |||
| Female | 2723 (63.5) | 66 (58.4) | 2789 (63.3) | |
| Male | 1567 (36.5) | 47 (41.6) | 1614 (36.7) | |
| Education, no. (%) | < 0.001 | |||
| Less than high school (HS) | 795 (18.5) | 51 (45.1) | 846 (19.2) | |
| HS or some college | 792 (18.5) | 23 (20.4) | 815 (18.5) | |
| College or associate degree or higher | 2686 (62.6) | 38 (33.6) | 2724 (61.9) | |
| Not available | 17 (0.4) | 1 (0.9) | 18 (0.4) | |
| Alcohol use in the last year, no. (%) | 0.03 | |||
| No | 2327 (54.2) | 71 (62.8) | 2398 (54.5) | |
| Yes | 1951 (45.5) | 39 (34.5) | 1990 (45.2) | |
| Not available | 12 (0.3) | 3 (2.7) | 15 (0.3) | |
| AHA BMI categorization, no. (%) | < 0.001 | |||
| Poor | 2278 (53.1) | 40 (35.4) | 2318 (52.6) | |
| Intermediate | 1417 (33.0) | 41 (36.3) | 1458 (33.1) | |
| Ideal | 593 (13.8) | 32 (28.3) | 625 (14.2) | |
| Not available | 2 (0.1) | 0 (0) | 2 (0.1) | |
| AHA blood pressure categorization, no. (%) | < 0.001 | |||
| Poor | 875 (20.4) | 44 (38.9) | 919 (20.9) | |
| Intermediate | 2559 (59.7) | 65 (57.5) | 2624 (59.6) | |
| Ideal | 751 (17.5) | 3 (2.7) | 754 (17.1) | |
| Not available | 105 (2.4) | 1 (0.9) | 106 (2.4) | |
| AHA total cholesterol categorization, no. (%) | < 0.001 | |||
| Poor | 587 (13.7) | 27 (23.9) | 614 (13.9) | |
| Intermediate | 1543 (36.0) | 41 (36.3) | 1584 (36.0) | |
| Ideal | 1652 (38.5) | 21 (18.6) | 1673 (38.0) | |
| Not available | 508 (11.8) | 24 (21.2) | 532 (12.1) | |
| AHA serum glucose categorization, no. (%) | 0.01 | |||
| Poor | 467 (10.9) | 21 (18.6) | 488 (11.1) | |
| Intermediate | 648 (15.1) | 22 (19.5) | 670 (15.2) | |
| Ideal | 3138 (73.1) | 70 (62.0) | 3208 (72.9) | |
| Not available | 37 (0.9) | 0 (0) | 37 (0.8) | |
| AHA Healthy Diet Score, no. (%) | 0.65b | |||
| Poor | 2545 (59.3) | 63 (55.8) | 2608 (59.2) | |
| Intermediate | 1702 (39.7) | 49 (43.4) | 1751 (39.8) | |
| Ideal | 43 (1.0) | 1 (0.9) | 44 (1.0) | |
| AHA physical activity categorization, no. (%) | < 0.001 | |||
| Poor | 2090 (48.7) | 76 (67.3) | 2166 (49.2) | |
| Intermediate | 1361 (31.7) | 26 (23.0) | 1387 (31.5) | |
| Ideal | 835 (19.5) | 11 (9.7) | 846 (19.2) | |
| Not available | 4 (0.1) | 0 (0) | 4 (0.1) | |
| AHA smoking categorization, no. (%) | < 0.001b | |||
| Poor | 527 (12.3) | 33 (29.2) | 560 (12.7) | |
| Intermediate | 44 (1.0) | 4 (3.5) | 48 (1.1) | |
| Ideal | 3652 (85.1) | 73 (64.6) | 3725 (84.6) | |
| Not available | 67 (1.6) | 3 (2.7) | 70 (1.6) |
Chi-square test unless otherwise specified (Student's t-test for age as a continuous variable).
Fisher exact test.
We determined the distribution of poor health indicators (poor health for one or more indicators) to assess the frequency by disease status (Table 3). Of the total cohort, 59.8% had 2 or fewer poor health indicators on average imputed data. Among participants with PAD, 43.6% had 2 or fewer poor health indicators compared to 60.3% of participants without PAD. Among women with PAD, 42.3% had 2 or fewer poor health indicators. Among men with PAD, 45.5% had 2 or fewer poor health indicators.
Table 3.
Distribution of poor health indicators based on LSS.
| Raw data, no. (%) |
Average imputed data, (%) |
|||||
|---|---|---|---|---|---|---|
| Sum of poor health indicators | Total sample size (n = 4403) | No PAD (n = 4290) | PAD (n = 113) | Total sample size (n = 4403) | No PAD (n = 4290) | PAD (n = 113) |
| 0 | 257 (6.8) | 253 (6.9) | 4 (4.6) | 6.6 | 6.7 | 5.0 |
| 1 | 866 (23.0) | 856 (23.3) | 10 (11.4) | 22.2 | 22.5 | 11.2 |
| 2 | 1179 (31.4) | 1154 (31.4) | 25 (28.4) | 31.0 | 31.1 | 27.4 |
| 3 | 946 (25.2) | 923 (25.1) | 23 (26.1) | 25.5 | 25.5 | 24.5 |
| 4 | 423 (11.3) | 400 (10.9) | 23 (26.1) | 11.8 | 11.5 | 25.9 |
| 5 | 80 (2.1) | 77 (2.1) | 3 (3.4) | 2.5 | 2.4 | 4.6 |
| 6 | 7 (0.2) | 7 (0.2) | 0 (0) | 0.3 | 0.3 | 0.9 |
| 7 | 1 (0.0) | 1 (0.0) | 0 (0) | 0.1 | 0.0 | 0.4 |
| Missing at least one indicator | 644 | 619 | 25 | |||
Based on the minimum Euclidean distance, 3 poor health indicators or more vs < 3 is the best cut point to maximize both sensitivity and specificity with PAD using imputed data (sensitivity of 56.4% and specificity of 60.2%).
Poor health indicators that were associated with an increased odds for PAD were blood pressure, cholesterol, and physical activity (Table 4). Specifically, the adjusted ORs were as follows: blood pressure OR 1.85 (95% CI 1.18, 2.90), cholesterol OR 1.50 (95% CI 1.09, 2.06) and physical activity OR 1.35 (95% CI 1.00, 1.82). Poor status of BMI was associated with reduced odds for PAD with an adjusted OR 0.66 (95% CI 0.50, 0.88).
Table 4.
Odds ratio (95% confidence interval) from logistic regression models between each LSS and PAD adjusted for age.
| LSS variable | Distinct models | Multivariate model | |
|---|---|---|---|
| BMI | Poor | 0.65 (0.50,0.85) | 0.66 (0.50,0.88) |
| Intermediate | 0.90 (0.69,1.17) | 0.97 (0.74,1.27) | |
| Ideal | Reference | Reference | |
| Blood pressure | Poor | 1.96 (1.25,3.06) | 1.85 (1.18,2.90) |
| Intermediate | 1.30 (0.85,2.00) | 1.34 (0.87,2.07) | |
| Ideal | Reference | Reference | |
| Cholesterol | Poor | 1.52 (1.11,2.08) | 1.50 (1.09,2.06) |
| Intermediate | 0.92 (0.70,1.20) | 0.96 (0.73,1.27) | |
| Ideal | Reference | Reference | |
| Fasting glucose | Poor | 1.27 (0.90,1.78) | 1.26 (0.88,1.79) |
| Intermediate | 0.94 (0.67,1.31) | 1.01 (0.72,1.42) | |
| Ideal | Reference | Reference | |
| Dietary | Poor | 1.25 (0.63,2.51) | 1.03 (0.51,2.09) |
| Intermediate | 1.13 (0.56,2.26) | 1.16 (0.57,2.36) | |
| Ideal | Reference | Reference | |
| Physical activity | Poor | 1.44 (1.08,1.92) | 1.35 (1.00,1.82) |
| Intermediate | 0.99 (0.70,1.38) | 0.98 (0.69,1.38) | |
| Ideal | Reference | Reference | |
| Smoking | Poor | 1.49 (0.95,2.34) | 1.27 (0.80,2.03) |
| Intermediate | 2.25 (1.06,4.74) | 2.51 (1.17,5.37) | |
| Ideal | Reference | Reference |
Based on the dichotomous variable of two or fewer poor health indicators versus three or more poor health indicators, a univariate analysis showed having three or more poor health indicators was associated with having PAD with an OR 1.40 (95% CI 1.16, 1.69) (Table 5). After adjusting for age, the OR was 1.34 (95% CI 1.11, 1.63) for the association of three or more poor health indicators with having PAD. After adjusting for age, gender, education and alcohol use the association of three or more poor health indicators with PAD was OR 1.30 (95% CI 1.07, 1.58). No significant interaction effect was found between three or more poor health indicators and age or gender. As a sensitivity analysis, the models were also adjusted with complete data only. When controlling for age, three or more poor health indicator has an OR of 1.39 (95% CI 1.10, 1.78). Fig. 1 provides a graph of the association of the number of poor health indicators with the age-adjusted prevalence of PAD.
Table 5.
Odds ratio (95% confidence interval) from logistic regression models between poor health indicator and PAD with and without covariates using multiple imputed data.
| Model A | Model B | Model C | Model D | |
|---|---|---|---|---|
| Poor health indicator | ||||
| Number of poor health indicator < 3 | Reference | Reference | Reference | Reference |
| Number of poor health indicator ≥ 3 | 1.40 (1.16,1.69) | 1.34 (1.11,1.63) | 1.35 (1.11,1.64) | 1.30 (1.07,1.58) |
| Age (continuous) | 1.10 (1.07,1.12) | 1.10 (1.07,1.12) | 1.09 (1.06,1.11) | |
| Gender | ||||
| Female | 0.85 (0.70,1.03) | 0.87 (0.71,1.06) | ||
| Male | Reference | Reference | ||
| Education | ||||
| Less than high school | 1.35 (1.02,1.78) | |||
| HS/GED or some college | 1.03 (0.75,1.41) | |||
| College/associate degree or higher | Reference | |||
| Alcohol | ||||
| No alcohol during the past year | 0.94 (0.76,1.17) | |||
| During the past year | Reference |
Fig. 1.
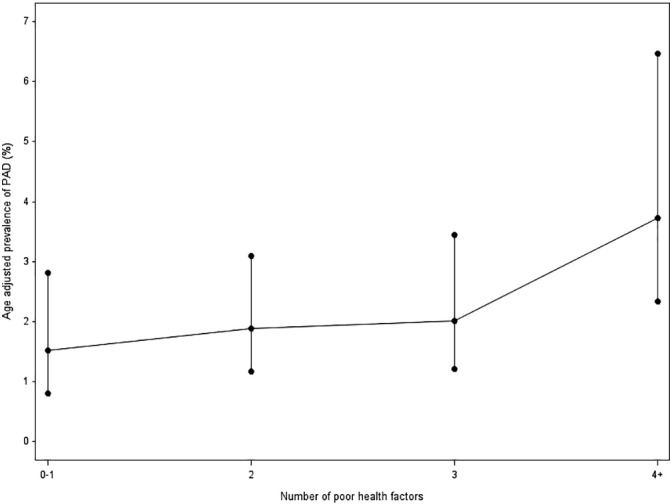
Age adjusted prevalence of PAD by number of poor health factors.
4. Discussion
In a cohort of 4403 African Americans in the JHS, the prevalence of PAD increased per the frequency of poor health indicators. The presence of three or more poor health indicators was associated with a statistically significant increased odds for PAD. The poor health indicators associated with an increased odds for PAD were blood pressure, cholesterol, and physical activity.
The prevalence of PAD in the JHS likely result from the lower mean age of its participants relative to the mean age of those in other studies (Newman et al., 1993, Collins et al., 2003, Criqui et al., 2005). For example, in the Systolic Hypertension in the Elderly Program (Newman et al., 1993) – a randomized trial of 4736 persons age 60 or older with hypertension – the prevalence of PAD was 23% for non-Hispanic white women, 25% for non-Hispanic white men, 38% for African American men, and 41% for African American women. Similarly, in work by Collins et al. and among 403 patients age 50 years or older receiving primary care in Houston, TX, 13.2% of non-Hispanic whites, 13.7% of Hispanics, and 22.8% of African Americans screened positive for PAD (Collins et al., 2003). Reasons for the lower prevalence of PAD in the JHS cohort likely result from the lower mean age of the cohort relative to the mean age of participants in other studies. The mean age for the JHS cohort was nearly one decade younger than the mean age of persons with PAD in prior work (Hirsch et al., 2006). Additionally, a community-based population like the JHS should be expected to have fewer atherosclerotic risk factors as compared to persons from clinical settings. There was also a low prevalence of smoking with < 13% of the overall cohort defined as current smokers. However, the higher prevalence of PAD in prior work has not been explained by higher levels of modifiable atherosclerotic risk factors (Criqui et al., 2005, Norgren et al., 2007). In the JHS protocol, only systolic blood pressures at the posterior tibialis were obtained for each leg; two readings were obtained and averaged. If the posterior tibialis could not be located, the dorsalis pedis was used. Two brachial systolic blood pressures were obtained from the right arm only. This protocol is not the protocol recommended by the American Heart Association and not used in most clinical trials for patients with PAD. By missing higher brachial readings from the left arm or at least obtaining readings from both arms as well as the focus on obtaining ankle systolic blood pressure from the posterior tibialis, the prevalence of PAD may have been inaccurate. In work by Allison et al. (2010), use of the lowest ankle pressure resulted in better sensitivity and use of the highest ankle pressure provided the best specificity. This conclusion is based on having calculations from both brachial arteries and both the dorsalis pedis and posterior tibialis. Given the protocol used in the JHS, it is difficult to assess whether PAD was over or underestimated. However, if the diagnosis was underestimated, this could have led to a lower reported prevalence of PAD in the cohort.
To our knowledge, this is the first study to assess the prevalence of LSS variables with PAD among African Americans. One prior study evaluated the prevalence of ideal cardiovascular health metrics, using the LSS variables, in African Americans (Djousse et al., 2015). This work revealed a low prevalence of ideal LSS variables (particularly diet, physical activity, and BMI) within the JHS cohort. Our findings add to this body of work by highlighting the significant association of the frequency of LSS variables with prevalence of PAD among African Americans.
In our bivariate analysis, we also identified that insufficient physical activity was more prevalent among subjects with PAD versus those without. The association of physical inactivity with a prevalence of PAD is not well defined (Rucker-Whitaker et al., 2004). While physical activity, notably walking therapy, is an effective treatment for PAD, less is known about the role of physical activity to reduce the risk for PAD. More work is needed to understand the implications of sufficient levels of physical activity to reduce the risk for PAD and disease progression.
Scoring in the poor category for three or more of the health indicators increased the odds for PAD. Furthermore, this increased risk remained after adjusting for multiple covariates. Modifiable risk factors for PAD are smoking, hypertension, and dyslipidemia (Fowkes et al., 1992, Fowkes, 1997, Hiatt, 2001). The presence of multiple risk factors has a synergistic effect on the risk for PAD (Dormandy and Rutherford, 2000, Kannel and McGee, 1985). Similarly, a poor score for three or more of the health indicators increases the risk for PAD. This finding warrants further study to determine if a cut point of three or more poor health indicators is an indication for diagnostic testing for PAD among African Americans, independent of reported leg symptoms.
A limitation of this study was not including arterial stiffness in our modeling. Arterial stiffness is defined as an ABI > 1.4 which limits the ability to diagnose PAD based on ABI testing. Persons with an ABI > 1.4 may have PAD but additional testing is needed to confirm. We evaluated persons with an ABI > 1.4 but the correlates were quite distinct from what we identified among persons with an ABI < 0.9.
In conclusion, we present findings on the association of LSS variables with PAD. We add to the body of PAD literature by targeting African Americans who were a part of a prospective, observational, community-based health study cohort in the southeastern United States.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Disclosures
None.
Acknowledgements
The Jackson Heart Study is supported by contracts HHSN268201300046C, HSN268201300047C, HHSN268201300048C, HHSN268201300049C, HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. The authors thank the Jackson Heart Study team (University of Mississippi Medical Center Jackson State University, and Tougaloo College) and participants for their long-term commitment and significant contributions to the study.
This research was supported in part (Johnson, Larrivee) by 1 U54 GM104940 from the National Institute of General Medical Sciences of the NIH, which funds the Louisiana Clinical and Translational Science Center. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
William D. Johnson and Sandra Larrivee, Pennington Biomedical Research Center, are responsible for data analysis.
References
- Ainsworth B.E. Comparison of three methods for measuring the time spent in physical activity. Med. Sci. Sports Exerc. 2000;32(9 Suppl):S457–S464. doi: 10.1097/00005768-200009001-00004. [DOI] [PubMed] [Google Scholar]
- Ainsworth B.E. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Allison M.A. The relevance of different methods of calculating the ankle-brachial index: the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2010;171(3):368–376. doi: 10.1093/aje/kwp382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carithers T.C. Validity and calibration of food frequency questionnaires used with African-American adults in the Jackson Heart Study. J. Am. Diet. Assoc. 2009;109(7):1184–1193. doi: 10.1016/j.jada.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M.A. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am. J. Med. Sci. 2004;328(3):131–144. doi: 10.1097/00000441-200409000-00001. [DOI] [PubMed] [Google Scholar]
- Collins T. The prevalence of peripheral arterial disease in a racially diverse population. Arch. Intern. Med. 2003;163:1469–1474. doi: 10.1001/archinte.163.12.1469. [DOI] [PubMed] [Google Scholar]
- Criqui M.H. Ethnicity and peripheral arterial disease: the San Diego population study. Circulation. 2005;112(17):2703–2707. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- Djousse L. Prevalence and changes over time of ideal cardiovascular health metrics among African-Americans: the Jackson Heart Study. Prev. Med. 2015;74:111–116. doi: 10.1016/j.ypmed.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormandy J.A., Rutherford R.B. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Concensus (TASC) J. Vasc. Surg. 2000;31(1 Pt 2):S1–S296. [PubMed] [Google Scholar]
- Dubbert P.M. Physical activity assessment methods in the Jackson Heart Study. Ethn. Dis. 2005;15(4 Suppl. 6) (p. S6-56-61) [PubMed] [Google Scholar]
- Fowkes F. Epidemiology of peripheral vascular disease. Atherosclerosis. 1997;131(Suppl):S29–S31. doi: 10.1016/s0021-9150(97)06122-4. [DOI] [PubMed] [Google Scholar]
- Fowkes F. Edinburgh Artery study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int. J. Epidemiol. 1991;20(2):384–392. doi: 10.1093/ije/20.2.384. [DOI] [PubMed] [Google Scholar]
- Fowkes F. Smoking, lipids, glucose intolerance, and blood pressure as risk factors for peripheral atherosclerosis compared with ischemic heart disease in the Edinburgh Artery study. Am. J. Epidemiol. 1992;135(4):331–340. doi: 10.1093/oxfordjournals.aje.a116294. [DOI] [PubMed] [Google Scholar]
- Hiatt W. Medical treatment of peripheral arterial disease and claudication. NEJM. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- Hickson D.A. Socioeconomic position is positively associated with blood pressure dipping among African-American adults: the Jackson Heart Study. Am. J. Hypertens. 2011;24(9):1015–1021. doi: 10.1038/ajh.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- Hirsch A.T. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- Hosmer D., Lemeshow S., Sturdivant R.X. John Wiley and Sons; 2013. Applied Logistic Regression. [Google Scholar]
- Kannel W., McGee D. Update on some epidemiologic features of intermittent claudication: the Framingham study. J. Am. Geriatr. Soc. 1985;33(1):13–18. doi: 10.1111/j.1532-5415.1985.tb02853.x. [DOI] [PubMed] [Google Scholar]
- McDermott M.M., Greenland P. Clinical significance and functional implications of peripheral arterial disease. In: Olin J.W., editor. Clinical Evaluation and Office-based Detection of Peripheral Arterial Disease. An Office-based Approach to the Diagnosis and Treatment of Peripheral Arterial Disease. Parts 1 through VIII. Society for Vascular Medicine and Biology; Manchester: 1998. pp. 20–26. [Google Scholar]
- McDermott M., Mehta S., Greenland P. Leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch. Intern. Med. 1999;159:387–392. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- Newman A., Sutton-Tyrrell K., Kuller L. Lower-extremity arterial disease in older hypertensive adults. Arterioscler. Thromb. 1993;13(4):555–562. doi: 10.1161/01.atv.13.4.555. [DOI] [PubMed] [Google Scholar]
- Norgren L. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J. Vasc. Surg. 2007;45(Suppl. S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Omura J.D. Adults Eligible for Cardiovascular Disease Prevention Counseling and Participation in Aerobic Physical Activity — United States, 2013. Weekly. 2015;64(37):1047–1051. doi: 10.15585/mmwr.mm6437a4. [DOI] [PubMed] [Google Scholar]
- Rubin D.B. The calculation of posterior distributions by data augmentation: commeent: a noniterative sampling/importance resampling alternative to the data augmentation algorithm for creating a few imputations when fractions of missing information are modest: the SIR algorithm. J. Am. Stat. Assoc. 1987;82(398):543–546. [Google Scholar]
- Rucker-Whitaker C. Peripheral arterial disease in African Americans: clinical characteristics, leg symptoms, and lower extremity functioning. J. Am. Geriatr. Soc. 2004;52(6):922–930. doi: 10.1111/j.1532-5415.2004.52259.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc . 2008. SAS/STAT(r) 9.2 User's Guide: The LOGISTIC Procedure. [Google Scholar]
- Talegawkar S.A. Serum carotenoid and tocopherol concentrations vary by dietary pattern among African Americans. J. Am. Diet. Assoc. 2008;108(12):2013–2020. doi: 10.1016/j.jada.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker K.L. A regional food-frequency questionnaire for the US Mississippi Delta. Public Health Nutr. 2005;8(1):87–96. [PubMed] [Google Scholar]
- Tullos B.W. Ankle-brachial index (ABI), abdominal aortic calcification (AAC), and coronary artery calcification (CAC): the Jackson Heart Study. Int. J. Cardiovasc. Imaging. 2013;29(4):891–897. doi: 10.1007/s10554-012-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.Y. Sedentary behaviors increase risk of cardiovascular disease mortality in men. Med. Sci. Sports Exerc. 2010;42(5):879–885. doi: 10.1249/MSS.0b013e3181c3aa7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt S.B. A community-driven model of research participation: the Jackson Heart Study Participant Recruitment and Retention Study. Ethn. Dis. 2003;13(4):438–455. [PubMed] [Google Scholar]


