Abstract
Cell therapy could be useful for the treatment of myopathies. A problem observed in mice, with different results and interpretations, is a significant death among the transplanted cells. We analyzed this problem in non-human primates, the animal model more similar to humans. Autologous or allogeneic myoblasts (with or without a reporter gene) were proliferated in vitro, labeled with [14C]thymidine, and intramuscularly injected in macaques. Some monkeys were immunosuppressed for long-term follow-up. Cell-grafted regions were biopsied at different intervals and analyzed by radiolabel quantification and histology. Most radiolabel was lost during the first week after injection, regardless of whether the cells were allogeneic or autologous, the culture conditions, and the use or not of immunosuppression. There was no significant difference between 1 hr and 1 day post-transplantation, a significant decrease between days 1 and 3 (45% to 83%), a significant decrease between days 3 and 7 (80% to 92%), and no significant differences between 7 days and 3 weeks. Our results confirmed in non-human primates a progressive and significant death of the grafted myoblasts during the first week after administration, relatively similar to some observations in mice but with different kinetics.
Keywords: cell therapy, skeletal muscle, cell death, myoblast, myopathy, satellite cell
Introduction
Cell therapy (CT) in the form of intramuscular (IM) administration of satellite cell-derived myoblasts (SCDMs) is a potential treatment for muscle pathologies. Its first indication was Duchenne muscular dystrophy,1 but later clinical applications sought treatment of selectively affected muscles, such as the cricopharyngeal muscle in oculopharyngeal muscular dystrophy,2 the tibialis anterior in patients with fascioscapular muscular dystrophy,3 the urinary sphincter in urinary incontinence,4 and the external anal sphincter in anal incontinence.5 Note that satellite cell derived is specified to make a difference between myoblasts involved in postnatal muscle regeneration (i.e., SCDMs) and somite-derived myoblasts involved in muscle embryogenesis.
The possible therapeutic effect of this CT depends on three outcomes.1 (1) When injected SCDMs fuse with the myofibers of the host tissue, graft-derived myonuclei allow gene complementation, which would be useful for the treatment of genetic myopathies. (2) To the extent that the transplanted SCDMs fuse with each other, they could generate new myofibers, which would be useful to recover muscles that lost myofibers due to pathological causes. (3) Since some transplanted SCDMs become satellite cells,6, 7, 8, 9, 10, 11, 12 this CT could repopulate the reserve of muscle-specific stem cells in the recipient. In animals, SCDM transplantation accomplished the three outcomes mentioned above.1 In clinical CT, SCDMs definitely produced the first13, 14, 15, 16 and second outcomes,14, 17 and there is potential evidence of the third one.6, 14, 18
An important issue in CT is the early post-transplantation cell survival. The fact that there is a significant death of the grafted cells was in fact reported in several types of CTs, including transplantation of neurons,19 hepatocytes,20 cardiac cells,21 and islets.22 With regard to SCDM transplantation, it was reported using different methods of detection in mice11, 23, 24, 25, 26, 27, 28, 29, 30 and pigs31 that the grafted SCDMs experience considerable mortality within the first days after their IM administration. However, the observations in mice showed significant differences between different research teams, especially in relation to the dynamics and intensity of the cell death.28 However, this problem was never studied in non-human primates (NHPs), which are the optimal model for human extrapolation in preclinical transplantation research because of the narrow phylogenetic relationship and biological similitudes.32 Therefore, we analyzed the post-CT death of SCDMs in macaques (Table 1), both to obtain information more extrapolable to the human and to help clarify an issue in which there are conflicting interpretations. For this, we proliferated autologous or allogeneic SCDMs in vitro, which were radiolabeled with [14C]thymidine before IM administration to quantify the grafted-cell death by measuring the radioactivity in the DNA isolated from the cell-grafted muscles.33
Table 1.
Monkeys Included in the Study, Indicating Details of the Protocol and Quantification of Acute Rejection
| Monkey | Species | IS | Type of Graft | Origin of Cellsa | Reporter Protein | Amount of Cells per Site | Cell Death Pre-CT (%)b | Follow-up | Dead Cellsc | CT Ex Vivod | CD8+ Cells at Post-CT Day 7e |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | rhesus | – | allo | monkey 2 | – | 2 × 106 | 2.4 | 7 days | X | X | – |
| auto | monkey 1 | – | 1.8 × 106 | 3.3 | 7 days | – | X | – | |||
| 2 | rhesus | – | allo | monkey 1 | – | 2.9 × 106 | 4.5 | 7 days | X | X | + |
| auto | monkey 2 | – | 2.8 × 106 | 2.3 | 7 days | – | X | + | |||
| 3 | rhesus | – | allo | monkey 2 | – | 3.5 × 106 | 12.6 | 7 days | X | X | – |
| auto | monkey 3 | – | 2.9 × 106 | 11.4 | 7 days | – | X | – | |||
| 4 | rhesus | – | allo | monkey 2 | – | 3.3 × 106 | 4.8 | 7 days | – | X | – |
| auto | monkey 4 | – | 3.5 × 106 | 8 | 7 days | – | X | – | |||
| 5 | rhesus | – | allo | monkey 2 | – | 3.4 × 106 | 8.5 | 7 days | X | X | + |
| auto | monkey 5 | – | 2.9 × 106 | 6.9 | 7 days | – | X | – | |||
| 6 | cyno | – | allo | NS19 | β-gal | 1.1 × 106 | 17 | 7 days | – | – | ++ |
| 7 | cyno | – | allo | NS19 | β-gal | 1.1 × 106 | 17 | 7 days | – | – | ++ |
| 8 | cyno ♀ | – | allo | NS19 | β-gal | 1 × 106 | 4 | 7 days | – | – | +++ |
| 9 | cyno | – | allo | NS19 | β-gal | 1 × 106 | 10 | 7 days | – | – | +++ |
| 10 | cyno | – | allo | NS19 | β-gal | 1.5 × 106 | 7 | 7 days | – | – | +++ |
| 11 | rhesus | yes | allo | monkey 2 | β-gal | 1.7 × 106 | 13 | 3 weeks | – | – | – |
| 12 | cyno | yes | allo | NS19 | β-gal | 3 × 106 | 5 | 3 weeks | – | – | – |
| 13 | cyno | yes | allo | NS19 | β-gal | 2.7 × 106 | 7.9 | 3 weeks | – | – | – |
β-gal, β-galactosidase; allo, allogeneic; auto, autologous; CT, cell transplantation; cyno, cynomolgus; IS, immunosuppression; ♀, the macaque female included in the study.
NS19: identification of the cynomolgus donor of the cells.
Estimated by a trypan blue exclusion test.
Injection of cells killed by freezing and thawing.
Cells injected into pieces of muscle taken at euthanasia.
Number of CD8+ cells per mm2 of muscle section as follows: –, < 3; +, 3–10; ++, 10–30; +++, >30.
Results
Cell Injection Ex Vivo versus In Vivo
We tested whether there were differences in the radiolabel detection when the cells were injected ex vivo on a muscle fragment (where we could ensure that all cells were inside the muscle sample before freezing) compared to a cell-grafted site performed in vivo and biopsied 1 hr later (we waited 1 hr to allow the saline of the cell suspension to be absorbed), which was our first choice as a reference for the radiolabel loss.
For this, we performed ten sites in five monkeys (Table 1). The radioactivity in the muscles injected with the [14C]thymidine-labeled cells was measured in the total DNA isolated from the muscle samples in a liquid scintillation counter as counts per minute (CPM) and normalized per million of injected cells. The radioactivity measured in the muscle fragments injected ex vivo was 3,763 ± 1,076 CPM/106 cells and in the biopsies sampled 1 hr post-CT in vivo was 3,324 ± 1,139/106 cells, with no significant difference (Figure 1A).
Figure 1.
Quantification of [14C]thymidine in Validation Tests
(A) Comparing the radiolabel measured in the scintillation counter in CPM per 106 of grafted cells, there was no significant difference between muscle fragments injected with cells ex vivo and biopsies of the regions injected with cells in vivo and sampled 1 hr later. (B and C) Since the presence or absence of radioactivity in the biopsies is extrapolated as survival or death of the grafted cells, we wanted to evaluate the effectiveness of the monkey organism in the present context to remove the radioactivity present in dead cells after 1 day of their administration. This was evaluated by transplanting cells killed by freezing and thawing in a site that was sampled 1 day later. Comparing the results in CPM per 106 cells (B), there was again no significant difference between muscle fragments injected with cells ex vivo and biopsies 1 hr post-CT in vivo. There was a significant decrease of radioactivity detected at day 1 in the site injected with the dead cells. (C) Taking as 100% either muscle fragments injected ex vivo or biopsies 1 hr post-CT, the loss of [14C]thymidine detected in the site grafted with dead cells was very similar, respectively, at 86.6% ± 7.4% and 86.2% ± 6.4% (indicated by arrows). The bars correspond to the mean with the SD except for those used for the 100% reference, which were fixed to that value.
Accuracy of the Method to Detect Donor Cell Death
To verify the accuracy of [14C] thymidine quantification to reveal the grafted-cell death, a cell pellet from those prepared for transplantation was submitted to three cycles of freezing and thawing to kill the cells in four monkeys (Table 1), and it was injected similarly as the live cells at one site that was sampled 1 day later. The results in CPM/106 cells (Figure 1B) showed again no significant differences between muscle fragments injected ex vivo (4,111 ± 803 CPM/106 cells) and biopsies 1 hr post-CT in vivo (3,745 ± 456 CPM/106 cells). Although substantially reduced, there was some radioactivity in the biopsies of the sites injected with dead cells (516 ± 233 CPM/106 cells). In Figure 1C we compared the radiolabel remaining in the site injected with dead cells, taking as reference both the muscle fragments injected ex vivo and the biopsies 1 hr post-CT. The remaining radiolabel was, respectively, 13.4% ± 7.4% and 13.8% ± 6.4%. Not only was there no significant difference but also the values were quite similar.
Therefore, we chose the biopsies at 1 hr post-CT as a reference to analyze the loss of radiolabel, since the cell injection and muscle-sampling conditions were the same as the other biopsies in the post-CT follow-up.
Dynamics of Grafted-Cell Death
There were four different immuno-transplantation conditions in the study (Table 1), which we consider separately. Note that (1) and (2) were done in the same monkeys, which received autologous SCDMs on one side of the muscle and allogeneic SCDMs on the other side.
-
(1)
Allogeneic CT into non-immunosuppressed rhesus monkeys (Figure 2A). The radioactivity at post-CT day 1 was 92.3% ± 13.7% of that detected 1 hr post-CT (no significant difference), at day 3 was 17.4% ± 8.3% (significant difference with day 1), and at day 7 was 3.1% ± 1.6% (significant difference with day 3).
-
(2)
Autologous CT into non-immunosuppressed rhesus monkeys (Figures 2B and 2C). The radioactivity at post-CT day 1 was 102.9% ± 15.6% of that detected 1 hr post-CT (no significant difference), at day 3 was 40.3% ± 29.4% (significant difference with day 1), and at day 7 was 14.3% ± 20% (significant difference with day 3) (Figure 2B). The SD was wide at post-CT days 3 and 7 because in monkey 5 the radioactivity at those periods was much higher than in the others monkeys: 91% at post-CT day 3 and 49.9% at post-CT 7. This was not due to a problem with the reference at 1 hr post-CT, given that in the muscle fragment injected ex vivo the radiolabel was 105.3% of 1 hr post-CT, that is, quite similar. Excluding monkey 5, the mean and SD of the other four monkeys are closer to those of the sites of allo-transplantation (Figure 2C): the radioactivity at day 1 was 98.9% ± 14.7% of 1 hr post-CT (no significant difference), at day 3 was 27.7% ± 9.1% (significant difference with day 1), and at day 7 was 5.4% ± 2.2% (significant difference with day 3). Given this striking difference of monkey 5, Figure 2C shows the mean of monkeys 1–4, overlapping monkey 5 as gray bars.
-
(3)
Allogeneic CT (LacZ labeled) into non-immunosuppressed cynomolgus monkeys (Figure 2D). We tested SCDMs labeled with lacZ because this is the standard in our CT studies in macaques.34 The radioactivity at day 1 was 87.9% ± 19.6% of that detected 1 hr post-CT (no significant difference), at day 3 was 52.1% ± 18.1% (significant difference with day 1), and at day 7 was 4.4% ± 2.5% (significant difference with day 3).
-
(4)
Allogeneic CT (LacZ labeled) into immunosuppressed cynomolgus and rhesus monkeys (Figure 2E). The radioactivity at day 3 was 55.3% ± 11.4% of that detected 1 hr post-CT (significant difference), at day 7 was 8.4% ± 6.5% (significant difference with day 3), and at 3 weeks was 11.5% ± 7.9% (no significant difference with day 7).
Figure 2.
Quantification of [14C]thymidine in the Four Immuno-transplantation Conditions of the Study
The arrows indicate the percentage of radiolabel loss between the means in the bars indicated by the fractional lines. Note that (A)–(C) correspond to the same monkeys, which received autografts in one side of the muscles and allografts in the other side. (A) Allogeneic transplantation of non-β-gal-labeled cells into non-immunosuppressed recipients (monkeys 1–5). (B and C) Autologous transplantation of non-β-gal-labeled cells into non-immunosuppressed recipients (monkeys 1–5). In (B) we represent the five monkeys used for this condition, while in (C) we represent in black bars the mean and SD of monkeys 1–4, overlapping the results of monkey 5 in gray bars, to show the striking difference of this monkey with the others. (D) Allogeneic transplantation of β-gal-labeled cells into non-immunosuppressed recipients (monkeys 6–10). (E) Allogeneic transplantation of β-gal-labeled cells into immunosuppressed recipients (monkeys 11–13). (F) Comparison of the values of each immuno-transplantation condition within each period post-CT. When not indicated, there were no significant differences within each post-CT period. There are significant differences only between some values at post-CT day 3, as indicated by brackets (*p < 0.05, **p < 0.01, and ***p < 0.001; n/s, not significant). The bars correspond to the mean with the SD except for those used for the 100% reference, which were fixed to that value.
Comparing the values obtained for each immuno-transplantation condition in each post-CT period, there were significant differences between some values at post-CT day 3, but not between values on day 1 or day 7 (Figure 2F).
Histology
In addition to the sites injected with radiolabeled cells to quantify the cell death, a nearby site of CT was performed for histological analysis to detect potential acute rejection. Clear evidence of ongoing acute rejection,35 in the form of dense accumulations of lymphocytes with an important constituent of CD8+ cells (31.4 ± 17.8 CD8+ cells/mm2), was observed at day 7 in monkeys transplanted with LacZ-labeled SCDMs without immunosuppression. There was no histological evidence of acute rejection in the other groups, even in monkeys that were not immunosuppressed and received the allogeneic SCDMs without a reporter gene. In this case, a few CD8+ lymphocytes were observed in the tissues at day 7 (2.7 ± 1.8 CD8+ cells/mm2), scattered in the muscle and with a few small focal accumulations, generally perivascular. This was not significantly different from the muscles that received autologous SCDMs (1.9 ± 2.3 CD8+ cells/mm2). The density of CD8+ cells in all monkeys is indicated in Table 1.
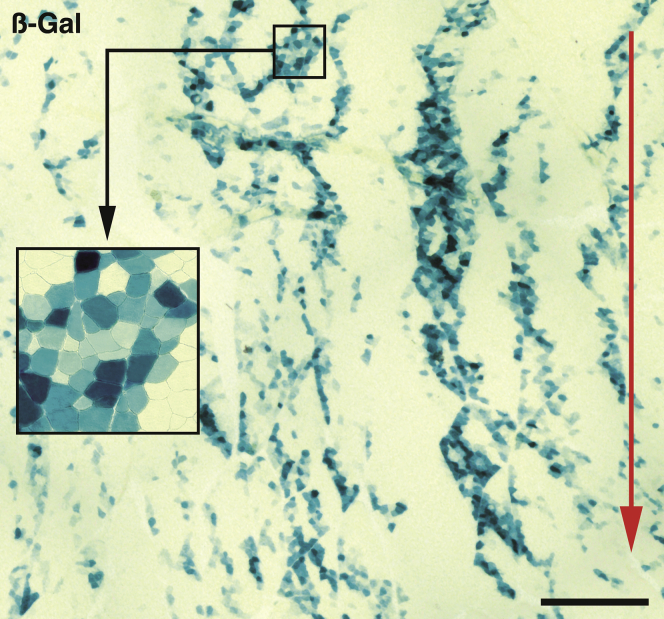
We observed many β-galactosidase (β-gal)+ myofibers at 3 weeks post-CT in monkeys 11–13, with the pattern of bands of engraftment previously described in NHPs36 (Figure 3). This confirmed the consistency of the long-term results in this study in the context of previous studies of CT in NHPs.
Figure 3.
Histological Graft Result at 3 Weeks
Cross-section of a biopsy taken 3 weeks post-transplantation in a muscle region grafted with LacZ-labeled SCDMs in a monkey of the immunosuppressed group, stained for β-gal demonstration (blue-greenish stain). Many β-gal+ myofibers are observed, following the pattern of bands of engraftment, which correspond to the original trajectories of the cell injections (indicated by the red arrow). This was the area of the biopsy in which more β-gal+ myofibers were observed: in the complete injected region, about 14% of the surface that was β-gal+. The inset is a higher magnification of the indicated region to more clearly show the myofiber profiles. Scale bar, 1 mm.
Discussion
In vitro radiolabeling of DNA is the most widely used method to evaluate the post-CT death of IM-transplanted cells.27, 28, 31, 33, 37, 38 With this method, the post-CT loss of radioactivity is extrapolated as cell death. Since radiolabeled DNA divides between daughter cells during mitosis, its quantification evidences the dynamics of cell death regardless of whether some surviving cells proliferate.27, 33 This method does not immediately detect cell death because the radiolabeled DNA can remain in the tissue as long as the DNA of the dead cells is not eliminated, essentially by macrophages.28 Therefore, the radiolabel loss reflects the magnitude and kinetics of grafted-cell death, but there could be a delay between the actual cell death and its demonstration by radiolabel loss. For this reason, we indicate “remaining radiolabel” instead of “cell survival” in the graphs. In this study, 1 day was sufficient to eliminate 86.2% ± 6.4% of the [14C]thymidine present in cells killed by freezing and thawing just before transplantation. This contrasts with that observed in mice, where 1 day was sufficient to essentially eliminate the entire radiolabel present in grafted cells killed by the same method.28 This difference could be explained by the faster elimination of necrotic tissues by macrophages in small rodents.39
The comparison of radioactivity in muscle fragments injected ex vivo and in biopsies taken 1 hr after in vivo CT coincides with our previous findings in mice, where there were no significant differences between muscles injected ex vivo and muscles sampled even 6 hr after in vivo CT.28 In fact, 6 hr post-CT in mice was not sufficient to detect cell death by this method, even when cells were killed before CT,28 most likely because 6 hr is too early for macrophage infiltration.28, 40
Dynamics of Grafted-Cell Death
Overall, most of the radiolabel present in the grafted cells was eliminated from muscle biopsies during the first 7 days after CT, both in allogeneic and autologous conditions (with the exception of autologous CT in monkey 5, in which only approximately half was eliminated). The loss of the radiolabel was also independent of the following: (1) the use of cells after a few in vitro passages with no genetic manipulation or cells more proliferated in vitro after introduction of the lacZ gene, (2) the use of immunosuppression or not, (3) the cell injection method, and (4) the presence or absence of acute rejection evidence at day 7. In all cases, there was no difference between the radiolabel detected 1 hr and 1 day post-CT, there was a significant decrease between day 1 and day 3 (with variations, sometimes significant, between the different experimental groups, range: ∼45%–83%), and another significant decrease (range: ∼80%–92%, no significant differences between the different experimental groups) detected between day 3 and day 7. In the subsequent period, although the sample was not very large (n = 3) and showed a wide variation, there was no significant difference between the radiolabel present at day 7 and 3 weeks post-CT.
This relative homogeneity in post-CT cell death in NHPs is noteworthy because in mice some differences in cell treatment greatly affected cell death, which was much faster and more intense using cloned SCDMs immortalized with T antigen than using primary SCDMs barely proliferated in vitro.28, 40 SCDMs immortalized with T antigen were used in several studies of post-CT cell death in mice,25, 26, 27, 33, 40, 41 which may be considered as one small animal model of CT among others. The extrapolation of these results in a clinical situation is questionable, due to the numerous differences in nature between models and their size, cell lines, and immunological contexts.
The fact that there were no significant differences between the radiolabel detected at 7 days and 3 weeks post-CT can be explained by the fact that the vast majority of the grafted SCDMs had already fused at post-CT day 7 and were no longer mononuclear cells. Since they became myonuclei, they were not exposed to the same threats to which grafted cells are exposed in the early post-CT period. Indeed, long-term CT studies in monkeys (up to a year and a half) revealed that the only threat to graft-derived myonuclei is acute rejection under allogeneic conditions.35
Although the scope of this study was not to investigate immunological aspects (which will be studied more deeply later), it is interesting to observe that there was histological evidence of acute rejection35 at post-CT day 7 in non-immunosuppressed monkeys grafted with allogeneic cells labeled with LacZ, but there was no histological evidence of acute rejection at the same period in non-immunosuppressed monkeys that received allogeneic SCDMs without a reporter gene. Indeed, this can indicate that the expression of transgenic β-gal provokes a stronger specific acquired immune reaction leading probably to a faster rejection. On the other hand, our observations confirm in NHPs the observations made in mice25 that the acquired immune response is not responsible for the early death of the grafted SCDMs.
Final Considerations
Increasing the amount of SCDMs transplanted in macaques increases the CT outcome until an optimal graft is achieved.36 Therefore, the post-CT cell death does not prevent an optimal graft if a sufficient amount of cells is transplanted, as long as the cells are distributed to avoid the formation of large accumulations in which ischemia will develop.42 In mice, in fact, a part of the grafted cells always survives and partially or totally compensates the cell death.27, 28, 43 Thus, a method to significantly reduce this post-CT cell death would have the advantage of grafting smaller amounts of cells, thereby reducing the resources and costs of cell culture (which has practical importance), but it is not a sine qua non condition for the success of CT.
Of the various hypotheses on the cause of this cell death, so far there is no conclusive evidence of any. Initial studies in mice blamed acute inflammation,25, 41 but specific depletion of macrophages, neutrophils, and natural killer cells did not prevent this post-CT cell death.29 Hypoxia37 and anoikis38 were supposed to induce apoptosis in the grafted SCDMs, but experiments to control each produced only minimal survival improvements. An unresolved enigma is that, whatever the factor that causes the cell death, it never eliminates all grafted SCDMs. It was postulated that there is a specific subpopulation of SCDMs capable of evading the post-CT cell death,27, 43 although without elucidating the causes of the cell death. Only one mechanism of cell death was unequivocally confirmed following SCDM transplantation in NHPs: ischemic necrosis of the internal region of the cell accumulations, due to the limited diffusion of oxygen from the surrounding tissue.42 Ischemic necrosis depends on the size of the cell collections, and this was the reason why in the present study we delivered small amounts of cells per injection. Apart from this mechanism, the overall post-CT cell death is still not well understood, and further studies are needed to solve this problem.
Finally, it is important to remark that the present observations apply to CT in a muscle whose structure is preserved. It could be interesting to analyze what occurs in muscles that have partially or totally lost myofibers and were replaced by fibrous and/or adipose tissue, as is the case in several degenerative myopathies.
Materials and Methods
Animals
The study included seven cynomolgus (Macaca fascicularis, six males and one female, age range 3–6 years, weight 3.9–7.9 kg) and six rhesus (Macaca mulatta, males, age range 4–5 years 9 months, weight 8–10 kg) macaques (Table 1). For transplantations and biopsies, monkeys were kept under general anesthesia using isofluorane (1.5%–2% in oxygen) after induction with IM ketamine (10 mg/kg) and glycopyrrolate (0.05 mg/kg). Buprenorphine (0.01 mg/kg twice daily [b.i.d.] for 3 days) was given IM for post-operative analgesia. Due to the several muscle samples to be taken, euthanasia was done at the end of the experiments by intravenous pentobarbital overdose (120 mg/kg) after anesthesia with IM ketamine (15 mg/kg). The Laval University Animal Care Committee authorized these procedures.
Cell Culture
Cynomolgus monkeys were transplanted with SCDMs from a primary cell line obtained previously from another cynomolgus monkey and labeled with a LacZ reporter gene. Most rhesus monkeys were transplanted with SCDMs of primary cell lines without genetic manipulation, produced from muscle biopsies taken in the same monkeys of the study. The exception was monkey 11 (Table 1), for which the cells of another rhesus monkey were labeled with LacZ.
In all cases, muscle samples were minced with fine scissors into fragments of less than 1 mm3 and then dissociated with 0.2% collagenase (Sigma) in Hank’s balanced salt solution (HBSS, Gibco) for 1 hr, followed by another dissociation in 0.125% trypsin (Gibco) in HBSS for 45 min. The cells were sub-cultured in MCDB-12044 with 15% fetal bovine serum (FBS, HyClone), 10 ng/mL basic fibroblast growth factor (Feldan), 0.5 mg/mL BSA (Sigma), 1.0 μM dexamethasone (Sigma), and 5 μg/mL human insulin (Sigma). LacZ+ cells were produced by in vitro infection with a replication-defective retroviral vector LNPoZC7 (gift of Dr. Constance Cepko, Harvard University) encoding a LacZ reporter gene and a neomycin resistance gene, selected with 600 μg/mL Geneticin (Invitrogen), proliferated until confluence, and frozen for storage in liquid nitrogen. Rhesus SCDMs without reporter genes were frozen for storage in liquid nitrogen after the first passage. In preparation for CT, the frozen cells were thawed and proliferated during one or two passages in culture. Cell radiolabeling was performed by adding 0.25 μCi/mL [methyl-14C] thymidine (Amersham Biosciences) to the growth medium 24 hr prior to CT.
Cell Transplantation
For transplantation, the cells were detached from the flasks using 0.1% trypsin in HBSS, washed three times with HBSS, and resuspended in HBSS for injection. The cell viability (Table 1) was verified with a trypan blue exclusion test, adding 0.1 mL 0.4% trypan blue (Sigma) solution to 1 mL cell suspension to be examined in a hemocytometer. To verify the accuracy of the [14C] thymidine quantification to reveal the grafted-cell death, cell pellets were submitted to three cycles of freezing and thawing to kill the cells,28 and they were injected similarly as the living cells in one site of four monkeys (Table 1).
The muscles used for CT were the biceps brachii, the quadriceps femoris, and, less frequently, the gastrocnemius. One muscle per period was used in each monkey, varying the muscle throughout the study. For radiolabeled cells, CT was performed by exposing the muscle after a skin incision, visually controlling the cell injection, and confirming that there were no leaks. In monkeys 1–10, we delivered the cells in small superficial pockets to visually control that they went into the region to be sampled later. We used 50-μL precision syringes (Hamilton) and 27-gauge 1/2” length disposable needles, delicately injecting 20 or 30 μL cell suspension at each site. In monkeys 11–13 (long-term follow-up under immunosuppression), we delivered the cells by matrices of parallel equidistant injections,36 which is our standard for long-term studies of CT in monkeys and humans. Matrices were smaller than in our routine CT in monkeys (0.25 cm2, ∼5 mm depth) because we wanted to sample the entire grafted region in biopsies that did not exceed 1 cm3. We used 100-μL precision syringes (Hamilton) attached to PB600-1 repeating dispensers (Hamilton) and 27-gauge 1/2” length disposable needles. The inter-injection distance was 1 mm, delivering 2 μL cell suspension per IM needle path.
In addition, a nearby site of CT was performed for histological analysis to evidence potential acute rejection. The cells injected into these sites were not radiolabeled.
To identify cell-grafted sites during biopsies, stitches of inert non-absorbable polypropylene 4.0 suture (Prolene, Ethicon) were placed at ∼5 mm on the sides of each site.
Immunosuppression
Monkeys 11–13 were immunosuppressed with an IM formulation of tacrolimus (a generous gift from Astellas Pharma), using our standard protocol for CT studies in NHPs.34 Immunosuppression started 5–7 days before CT and was maintained until the end of the experiment. Tacrolimus was injected IM once a day, beginning at 0.5 mg/kg/day and adjusting the dosage to target blood levels of >50 μg/L, as quantified in blood samples with an IMx tacrolimus II kit for micro-particle enzyme immunoassay (Abbott).
Sampling
Muscle biopsies were performed in the CT sites 1 hr, 1 day, 3 days, and 7 days post-CT in non-immunosuppressed monkeys (1–10) and 1 hr, 3 days, 7 days, and 3 weeks post-CT in immunosuppressed monkeys (11–13). Sites grafted with dead cells were sampled 1 day post-CT. Biopsies to quantify the radiolabel were placed in microtubes, frozen in liquid nitrogen, and stored at −80°C until DNA extraction. Biopsies for histology were mounted in embedding medium, snap-frozen in liquid nitrogen, and stored at −80°C until performing serial sections of 10–15 μm in a cryostat at −25°C.
Cell Injection Ex Vivo
To compare the radiolabel detection after cell injection performed ex vivo on a muscle fragment with in vivo CT biopsied 1 hr later, we froze a cell pellet similar to those used for in vivo CT in some monkeys. After euthanasia in these monkeys, we dissected a fragment of ungrafted muscle (similar to biopsies of cell-grafted sites), thawed the frozen cells, and injected them into the muscle fragment, which was frozen in liquid nitrogen and stored at −80°C until DNA extraction.
Radiolabel Detection
The amount of radiolabel in the muscle injected with the [14C]thymidine-labeled cells was measured in the total DNA isolated from the muscle samples as in previous mouse studies.28 Similar aliquots of the DNA solution were mixed with 5 mL liquid scintillation counting cocktail (Sigma), and the radioactivity was measured in a Wallac 1409 liquid scintillation counter (PerkinElmer) as CPM.
Histological Analysis
Sections were stained with H&E for standard analysis. For β-gal histochemical detection in muscles transplanted with LacZ+ cells, sections were fixed 3 min in 0.25% glutaraldehyde, rinsed with PBS, and incubated 24 hr at room temperature in 0.4 mg/mL X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) (Boehringer Mannheim) with 1 mM MgCl2, 3 mM Ke3F(CN)6,·3 mM Ke4F(CN)6, and·3H20 in PBS. To depict acute rejection,35 we immunodetected CD8+ lymphocytes using a mouse anti-human/macaque CD8 monoclonal antibody (BD Biosciences), followed by a 30-min incubation with a biotinylated anti-mouse antibody (1/150, Dako) and either a 30-min incubation with streptavidin-Cy3 (1/700, Sigma) or a 30-min incubation in streptavidin-peroxidase (1/200, Dako). Peroxidase activity was revealed with 3,3′ diaminobenzidine (0.25 mg/mL) and 0.03% hydrogen peroxide. Reagents for immunodetection were diluted in PBS with 1% FBS. Stained sections were analyzed using an Axiophot microscope with epifluorescence and bright-field optics (Zeiss). To estimate the density of CD8+ cells, we counted the number of CD8+ cells per muscle section and the area of the section was measured using ImageJ software.
Statistical Analysis
For the bar graphs, we estimated the mean value of n = 3–10 monkeys ± 1 SD. A paired t test was used to assess the probability of significant differences between successive periods in the follow-up of the radiolabel loss (i.e., comparing each period with the precedent, as indicated in Figures 1 and 2A–2E), given that the number of values was similar for each period and each sequence of values corresponded to a same monkey. When comparing values from different experimental groups, we used an unpaired t test (Figure 2F). Statistical significance was defined as p < 0.05.
Author Contributions
Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Visualization, Project Administration, and Writing – Original Draft, D.S.; Writing – Review & Editing, Funding Acquisition, and Resources, D.S. and J.P.T.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by a grant of the Jesse’s Journey Foundation for Gene and Cell Therapy of Canada to D.S. and a grant of the Canadian Institutes of Health Research to J.P.T. The authors wish to express their gratitude to Ms. Marlyne Goulet and Mr. Martin Paradis for their excellent technical work and to Steve Brochu for his technical assistance during monkey procedures.
References
- 1.Skuk D., Tremblay J.P. Cell therapy in muscular dystrophies: many promises in mice and dogs, few facts in patients. Expert Opin. Biol. Ther. 2015;15:1307–1319. doi: 10.1517/14712598.2015.1057564. [DOI] [PubMed] [Google Scholar]
- 2.Périé S., Trollet C., Mouly V., Vanneaux V., Mamchaoui K., Bouazza B., Marolleau J.P., Laforêt P., Chapon F., Eymard B. Autologous myoblast transplantation for oculopharyngeal muscular dystrophy: a phase I/IIa clinical study. Mol. Ther. 2014;22:219–225. doi: 10.1038/mt.2013.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vilquin J.T., Marolleau J.P., Sacconi S., Garcin I., Lacassagne M.N., Robert I., Ternaux B., Bouazza B., Larghero J., Desnuelle C. Normal growth and regenerating ability of myoblasts from unaffected muscles of facioscapulohumeral muscular dystrophy patients. Gene Ther. 2005;12:1651–1662. doi: 10.1038/sj.gt.3302565. [DOI] [PubMed] [Google Scholar]
- 4.Peters K.M., Dmochowski R.R., Carr L.K., Robert M., Kaufman M.R., Sirls L.T., Herschorn S., Birch C., Kultgen P.L., Chancellor M.B. Autologous muscle derived cells for treatment of stress urinary incontinence in women. J. Urol. 2014;192:469–476. doi: 10.1016/j.juro.2014.02.047. [DOI] [PubMed] [Google Scholar]
- 5.Frudinger A., Kölle D., Schwaiger W., Pfeifer J., Paede J., Halligan S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010;59:55–61. doi: 10.1136/gut.2009.181347. [DOI] [PubMed] [Google Scholar]
- 6.Skuk D., Paradis M., Goulet M., Chapdelaine P., Rothstein D.M., Tremblay J.P. Intramuscular transplantation of human postnatal myoblasts generates functional donor-derived satellite cells. Mol. Ther. 2010;18:1689–1697. doi: 10.1038/mt.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghostine S., Carrion C., Souza L.C., Richard P., Bruneval P., Vilquin J.T., Pouzet B., Schwartz K., Menasché P., Hagège A.A. Long-term efficacy of myoblast transplantation on regional structure and function after myocardial infarction. Circulation. 2002;106(12, Suppl 1):I131–I136. [PubMed] [Google Scholar]
- 8.Irintchev A., Langer M., Zweyer M., Theisen R., Wernig A. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. J. Physiol. 1997;500:775–785. doi: 10.1113/jphysiol.1997.sp022057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irintchev A., Rosenblatt J.D., Cullen M.J., Zweyer M., Wernig A. Ectopic skeletal muscles derived from myoblasts implanted under the skin. J. Cell Sci. 1998;111:3287–3297. doi: 10.1242/jcs.111.22.3287. [DOI] [PubMed] [Google Scholar]
- 10.Heslop L., Beauchamp J.R., Tajbakhsh S., Buckingham M.E., Partridge T.A., Zammit P.S. Transplanted primary neonatal myoblasts can give rise to functional satellite cells as identified using the Myf5nlacZl+ mouse. Gene Ther. 2001;8:778–783. doi: 10.1038/sj.gt.3301463. [DOI] [PubMed] [Google Scholar]
- 11.Xu X., Yang Z., Liu Q., Wang Y. In vivo fluorescence imaging of muscle cell regeneration by transplanted EGFP-labeled myoblasts. Mol. Ther. 2010;18:835–842. doi: 10.1038/mt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brimah K., Ehrhardt J., Mouly V., Butler-Browne G.S., Partridge T.A., Morgan J.E. Human muscle precursor cell regeneration in the mouse host is enhanced by growth factors. Hum. Gene Ther. 2004;15:1109–1124. doi: 10.1089/hum.2004.15.1109. [DOI] [PubMed] [Google Scholar]
- 13.Mendell J.R., Kissel J.T., Amato A.A., King W., Signore L., Prior T.W., Sahenk Z., Benson S., McAndrew P.E., Rice R. Myoblast transfer in the treatment of Duchenne’s muscular dystrophy. N. Engl. J. Med. 1995;333:832–838. doi: 10.1056/NEJM199509283331303. [DOI] [PubMed] [Google Scholar]
- 14.Skuk D., Goulet M., Roy B., Chapdelaine P., Bouchard J.P., Roy R., Dugré F.J., Sylvain M., Lachance J.G., Deschênes L. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol. 2006;65:371–386. doi: 10.1097/01.jnen.0000218443.45782.81. [DOI] [PubMed] [Google Scholar]
- 15.Skuk D., Goulet M., Roy B., Piette V., Côté C.H., Chapdelaine P., Hogrel J.Y., Paradis M., Bouchard J.P., Sylvain M. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul. Disord. 2007;17:38–46. doi: 10.1016/j.nmd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Skuk D., Tremblay J.P. Confirmation of donor-derived dystrophin in a duchenne muscular dystrophy patient allotransplanted with normal myoblasts. Muscle Nerve. 2016;54:979–981. doi: 10.1002/mus.25129. [DOI] [PubMed] [Google Scholar]
- 17.Hagège A.A., Carrion C., Menasché P., Vilquin J.T., Duboc D., Marolleau J.P., Desnos M., Bruneval P. Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet. 2003;361:491–492. doi: 10.1016/S0140-6736(03)12458-0. [DOI] [PubMed] [Google Scholar]
- 18.Gussoni E., Blau H.M., Kunkel L.M. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat. Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 19.Boonman Z., Isacson O. Apoptosis in neuronal development and transplantation: role of caspases and trophic factors. Exp. Neurol. 1999;156:1–15. doi: 10.1006/exnr.1999.7056. [DOI] [PubMed] [Google Scholar]
- 20.Weber A., Groyer-Picard M.T., Franco D., Dagher I. Hepatocyte transplantation in animal models. Liver Transpl. 2009;15:7–14. doi: 10.1002/lt.21670. [DOI] [PubMed] [Google Scholar]
- 21.Robey T.E., Saiget M.K., Reinecke H., Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J. Mol. Cell. Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall M., Shapiro A.M. Update on islet transplantation. Cold Spring Harb. Perspect. Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beauchamp J.R., Morgan J.E., Pagel C.N., Partridge T.A. Quantitative studies of the efficacy of myoblast transplantation. Muscle Nerve. 1994;17:S261. [Google Scholar]
- 24.Huard J., Acsadi G., Jani A., Massie B., Karpati G. Gene transfer into skeletal muscles by isogenic myoblasts. Hum. Gene Ther. 1994;5:949–958. doi: 10.1089/hum.1994.5.8-949. [DOI] [PubMed] [Google Scholar]
- 25.Guérette B., Skuk D., Célestin F., Huard C., Tardif F., Asselin I., Roy B., Goulet M., Roy R., Entman M., Tremblay J.P. Prevention by anti-LFA-1 of acute myoblast death following transplantation. J. Immunol. 1997;159:2522–2531. [PubMed] [Google Scholar]
- 26.Qu Z., Balkir L., van Deutekom J.C., Robbins P.D., Pruchnic R., Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J. Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beauchamp J.R., Morgan J.E., Pagel C.N., Partridge T.A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J. Cell Biol. 1999;144:1113–1122. doi: 10.1083/jcb.144.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skuk D., Caron N.J., Goulet M., Roy B., Tremblay J.P. Resetting the problem of cell death following muscle-derived cell transplantation: detection, dynamics and mechanisms. J. Neuropathol. Exp. Neurol. 2003;62:951–967. doi: 10.1093/jnen/62.9.951. [DOI] [PubMed] [Google Scholar]
- 29.Sammels L.M., Bosio E., Fragall C.T., Grounds M.D., van Rooijen N., Beilharz M.W. Innate inflammatory cells are not responsible for early death of donor myoblasts after myoblast transfer therapy. Transplantation. 2004;77:1790–1797. doi: 10.1097/01.tp.0000131150.76841.75. [DOI] [PubMed] [Google Scholar]
- 30.Fan Y., Maley M., Beilharz M., Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;19:853–860. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 31.Holzer N., Hogendoorn S., Zürcher L., Garavaglia G., Yang S., König S., Laumonier T., Menetrey J. Autologous transplantation of porcine myogenic precursor cells in skeletal muscle. Neuromuscul. Disord. 2005;15:237–244. doi: 10.1016/j.nmd.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Anderson D.J., Kirk A.D. Primate models in organ transplantation. Cold Spring Harb. Perspect. Med. 2013;3:a015503. doi: 10.1101/cshperspect.a015503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beauchamp J.R., Pagel C.N., Partridge T.A. A dual-marker system for quantitative studies of myoblast transplantation in the mouse. Transplantation. 1997;63:1794–1797. doi: 10.1097/00007890-199706270-00015. [DOI] [PubMed] [Google Scholar]
- 34.Skuk D., Goulet M., Paradis M., Tremblay J.P. Myoblast transplantation: techniques in nonhuman primates as a bridge to clinical trials. In: Soto-Gutierrez A., Navarro-Alvarez N., Fox I.J., editors. Methods in bioengineering: cell transplantation. Artech House; Boston: 2011. pp. 219–236. [Google Scholar]
- 35.Skuk D. Acute rejection of myofibers in nonhuman primates: key histopathologic features. J. Neuropathol. Exp. Neurol. 2012;71:398–412. doi: 10.1097/NEN.0b013e31825243ae. [DOI] [PubMed] [Google Scholar]
- 36.Skuk D., Goulet M., Tremblay J.P. Intramuscular transplantation of myogenic cells in primates: importance of needle size, cell number, and injection volume. Cell Transplant. 2014;23:13–25. doi: 10.3727/096368912X661337. [DOI] [PubMed] [Google Scholar]
- 37.Bouchentouf M., Benabdallah B.F., Bigey P., Yau T.M., Scherman D., Tremblay J.P. Vascular endothelial growth factor reduced hypoxia-induced death of human myoblasts and improved their engraftment in mouse muscles. Gene Ther. 2008;15:404–414. doi: 10.1038/sj.gt.3303059. [DOI] [PubMed] [Google Scholar]
- 38.Bouchentouf M., Benabdallah B.F., Rousseau J., Schwartz L.M., Tremblay J.P. Induction of Anoikis following myoblast transplantation into SCID mouse muscles requires the Bit1 and FADD pathways. Am. J. Transplant. 2007;7:1491–1505. doi: 10.1111/j.1600-6143.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 39.Borisov A.B. Regeneration of skeletal and cardiac muscle in mammals: do nonprimate models resemble human pathology? Wound Repair Regen. 1999;7:26–35. doi: 10.1046/j.1524-475x.1999.00026.x. [DOI] [PubMed] [Google Scholar]
- 40.Skuk D., Caron N., Goulet M., Roy B., Espinosa F., Tremblay J.P. Dynamics of the early immune cellular reactions after myogenic cell transplantation. Cell Transplant. 2002;11:671–681. doi: 10.3727/000000002783985378. [DOI] [PubMed] [Google Scholar]
- 41.Guérette B., Asselin I., Skuk D., Entman M., Tremblay J.P. Control of inflammatory damage by anti-LFA-1: increase success of myoblast transplantation. Cell Transplant. 1997;6:101–107. doi: 10.1177/096368979700600203. [DOI] [PubMed] [Google Scholar]
- 42.Skuk D., Paradis M., Goulet M., Tremblay J.P. Ischemic central necrosis in pockets of transplanted myoblasts in nonhuman primates: implications for cell-transplantation strategies. Transplantation. 2007;84:1307–1315. doi: 10.1097/01.tp.0000288322.94252.22. [DOI] [PubMed] [Google Scholar]
- 43.Cousins J.C., Woodward K.J., Gross J.G., Partridge T.A., Morgan J.E. Regeneration of skeletal muscle from transplanted immortalised myoblasts is oligoclonal. J. Cell Sci. 2004;117:3259–3269. doi: 10.1242/jcs.01161. [DOI] [PubMed] [Google Scholar]
- 44.Ham R.G., St Clair J.A., Webster C., Blau H.M. Improved media for normal human muscle satellite cells: serum-free clonal growth and enhanced growth with low serum. In Vitro Cell. Dev. Biol. 1988;24:833–844. doi: 10.1007/BF02623656. [DOI] [PubMed] [Google Scholar]