Abstract
The extensive existing knowledge on bi-directional communication between astrocytes and neurons led us to hypothesize that not only ischemia-preconditioned (IP) astrocytes can protect neurons but also IP neurons protect astrocytes from lethal ischemic injury. Here, we demonstrated for the first time that neurons have a significant role in protecting astrocytes from ischemic injury. The cultured medium from IP neurons (IPcNCM) induced a remarkable reduction in LDH and an increase in cell viability in ischemic astrocytes in vitro. Selective neuronal loss by kainic acid injection induced a significant increase in apoptotic astrocyte numbers in the brain of ischemic rats in vivo. Furthermore, TUNEL analysis, DNA ladder assay, and the measurements of ROS, GSH, pro- and anti-apoptotic factors, anti-oxidant enzymes and signal molecules in vitro and/or in vivo demonstrated that IP neurons protect astrocytes by an EPO-mediated inhibition of pro-apoptotic signals, activation of anti-apoptotic proteins via the P13K/ERK/STAT5 pathways and activation of anti-oxidant proteins via up-regulation of anti-oxidant enzymes. We demonstrated the existence of astro-protection by IP neurons under ischemia and proposed that the bi-directionally protective communications between cells might be a common activity in the brain or peripheral organs under most if not all pathological conditions.
Abbreviations: CNS, central nervous system; EPO, erythropoietin; ERK, extracellular signal-regulated kinase; GFAP, glial fibrillary acadic protein; HIF-1alpha, hypoxia-inducible factor-1 alpha; IP, ischmia-preconditionning; IPcNCM, ischemia-preconditioned neuron culture medium; JAK-2, Janus kinase-2; KA, kainic acid; MAP2, microtubule-associated protein 2; MTT, 3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolinum bromide; OGD, oxygen glucose deprivation; PI3K, phosphatidylinositol 3-kinase; rhEPO, recombinant human EPO; STAT5, signal transducer and activator of transcription 5
Keywords: Bi-directional communication, Astro-protection, Anti-apoptosis and anti-oxidant
1. Introduction
The brain is very sensitive to ischemia, which can be caused by cerebrovascular diseases such as stroke. In addition to neurons, astrocytes, the main supporting cells in the brain, can also be irreversibly injured [1]. The damage of these cells lead to lethal consequences or permanent neurological deficits. For this reason, extensive research has been aimed at finding effective strategies and drugs to ameliorate or prevent brain ischemic injury, although few have achieved a satisfactory effect. One strategy that has been shown to provide effective and powerful protection against such harmful stress is ischemic/hypoxic preconditioning [2], [3], which was first described in a dog model of myocardial injury in which sublethal ischemia enabled cells to better tolerate subsequent, usually lethal ischemia [4]. A number of studies have demonstrated that preconditioning induced by ischemia or hypoxia can produce a significant effect for protection of neurons or brain tissues in experimental animals and humans [5]. The discovery of preconditioning has opened a window for utilizing the endogenous protection mechanisms of the body for treating patients of stroke and other central nervous system (CNS) disorders [6].
Although the molecular mechanisms underlying preconditioning have not been completely elucidated, it has been well confirmed that ischemic preconditioned (IP) astrocytes play a significant role in the protection of neurons or brain tissues against ischemia/hypoxia or oxygen glucose deprivation (OGD)-induced injury [7], [8], [9]. During past years, a number of studies have also been conducted to investigate the mechanisms involved in neuro-protection by IP astrocytes. By influencing glutamate excitotoxicity, oxidative stress and acidosis, which are primary mediators of neuronal death during ischemia and reperfusion [10], IP astrocytes could effectively protect neurons from ischemia and reperfusion-induced injury.
However, it is unknown whether preconditioned neurons have a role in protecting astrocytes from lethal ischemia/hypoxia injury. In the last decade, knowledge on the bi-directional communication between astrocytes and neurons in the brain has been dramatically expanded. It has been demonstrated that there is not only chemical transmitter-mediated astrocyte-to-neuron modulation but also neurotransmitter-mediated neuron-to-astrocyte signaling in the brain under physiological conditions [11], [12], [13], [14]. It has also been shown that IP neurons, like astrocytes, can release protective factor(s) and hence protect un-preconditioned neurons against lethal ischemia/hypoxia injury [7], [9]. These published data made us speculate that there may also be bi-directional protective communication between neurons and astrocytes under pathological (ischemic) conditions. We hypothesized that not only preconditioned astrocytes can protect neurons, but also preconditioned neurons can protect astrocytes from ischemia/hypoxia injury. In the present study, we tested this hypothesis and demonstrated for the first time that ischemia-preconditioned medium from neurons (IPcNCM) has a significant role in protecting astrocytes from ischemia-induced injury.
2. Materials and methods
2.1. Materials
Unless otherwise stated, all chemicals were obtained from the Sigma Chemical Co., St. Louis, MO, USA. Primary polyclonal rabbit anti-Akt, phosphorylated Akt (p-Akt), extracellular signal-regulated kinase (ERK) 42/44, phosphorylated ERK 42/44 (p-ERK42/44), Bad, phosphorylated Bad Ser112 (112p-Bad), phosphorylated Bad Ser136 (136p-Bad), Bcl-2, cleaved caspase-3, caspase-3, phosphorylated signal transducer and activator of transcription 3 (p-STAT3) and 5 (p-STAT5) antibodies were purchased from Cell Signaling Technology, Beverly, MA, USA; primary monoclonal mouse anti-hypoxia-inducible factor-1 alpha (HIF-1 alpha) antibody from Novus Biologicals, Inc., Littleton, CO, USA; primary polyclonal rabbit anti-EPO antibody from Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; antibodies against neuron microtubule-associated protein 2 (MAP2) and astrocyte glial fibrillary acadic protein (GFAP) from Chemicon International Ltd, Hampshire, UK; and both mouse anti-Histone 3 monoclonal antibody and Ab175819-8 isoprostane from Abcam, Cambridge, UK. The TUNEL detection kit was purchased from Roche Applied Science, Indianapolis, IN, USA; Bradford assay kit from Bio-Rad, Hercules, CA, USA, goat anti-rabbit or anti-mouse IRDye 800 CW secondary antibody from Li-Cor, Lincoln, NE, USA; Superoxide dismutas (SOD), catalase (CAT) and glutathione peroxidase (GSH-PX) assay kit from Jiancheng Bioengineering Institute, Nanjing, JS, China; and EPO ELISA kit from BioScience, Minneapolis, MN, USA.
2.2. Animals
Rats were supplied by the Centralized Animal Facilities of The Chinese University of Hong Kong (CUHK), housed in stainless steel cages at 21±2 °C and had free access to food and water. The animal rooms were in a cycle of 12-h of light (7:00 to 19:00) and darkness (19:00 to 7:00). The Departments of Health of Hong Kong and the Shanghai Government and the Animal Research Ethics Committees of The Chinese University of Hong Kong and Fudan University approved the experimental procedures of this study.
2.3. Primary cortical neurons
Primary cortical neurons were prepared from 15 to 16 day-old rats embryos (E15-16) as described previously [15]. The purity of the neurons was assessed by staining with neuron-specific antibody against MAP2. In our case, over 98% of cells obtained were positively stained.
2.4. Primary cortical astrocytes culture
Primary cortical astrocytes were prepared from newborn SD rats at 1–3 days postnatal as described previously [16]. The purity of the astrocytes was assessed via anti-GFAP antibody (1:5000), reaching approximately 99%.
2.5. Oxygen glucose deprivation (OGD)
To mimic ischemic preconditioning, cells were exposed to OGD which was achieved by culturing cells in serum-free DMEM without glucose in a dedicated chamber (NAPCO 7101FC-1) with 1% O2, 94% N2 and 5% CO2 at 37 °C for a given period, as previously described [17].
2.6. Ischemia-preconditioned neuron culture medium (IPcNCM)
To prepare IPcNCM, neurons were exposed to OGD for 0, 0.5, 1 or 2-h and then incubated in a normoxic incubator for 24-h. Afterwards, the media were collected and referred to as IPcNCMs; IPc-0h, IPc-0.5h, IPc-1h or IPc-2h NCM respectively.
2.7. A neuronal loss model in vivo
To find out whether neurons have a protective effect on astrocytes in IP rats (270–280 g) in vivo, a neuronal loss model was established by injecting 0.5 nmol of kainic acid (KA) (1.5 µl of a 0.333 mM solution with PBS) stereotaxically into the cortical regions of rats at the following coordinates: 1.8 mm anterior to bregma, 2.0 mm lateral to the midline and 1.8 mm ventral to the dura. This dosage of KA had been reported to selectively destroy neurons but not astrocytes [18], [19]. This model was verified by immunocytochemistry staining against MAP2 and GFAP in brain slices. 24-h after injection of KA, the rats were treated with IP (forebrain ischemia for 4-min) and then subjected to forebrain ischemia for 20-min, followed by reperfusion for 24-h (I/R). The sham-operation rats underwent an identical surgery but did not have KA injection, IP and ischemic injury. Forebrain ischemia was induced by bilateral common carotid artery occlusion plus hypotension, by removal of blood until 50 mm Hg from the jugular vein into heparinized sterile tubing before carotid clamping [20].
2.8. MAP2 and GFAP double staining
Rats were deeply anesthetized and transcardially perfused with normal saline solution, followed by 4% paraformaldehyde in 0.1 M PBS 24-h after ischemia-reperfusion. The brains were removed and post-fixed in 4% paraformaldehyde for 4-h, then transferred into 30% sucrose solution, until they sank to the bottom of the container. Coronal sections (20 µm) were made using a Leica CM3050S cryostat (Leica Microsystems, Wetzlar, Germany). Sections were blocked with 3% normal goat serum (diluted in PBS containing 0.3% Triton X-100) for 1-h and incubated with primary antibodies (anti-MAP2 and anti-GFAP, 1:1000, Chemicon) overnight at 4 °C. After rinsing with PBS, sections were incubated with rhodamine-conjugated goat anti-rabbit IgG (for MAP2, Millipore) and FITC-conjugated goat anti-mouse IgG (for GFAP, Invitrogen) as secondary antibodies (1:200) for 1-h. Fluorescent images were captured by a Nikon C-1 confocal imaging system (Nikon, Japan).
2.9. MTT assay
An MTT (3-(4,5-dimethylthazol-2-yl)-2,5-diphenyltetrazolinum bromide) assay was conducted as described previously [21]. Optical density was measured at the 570 nm wavelength by the use of an ELX-800 microplate assay reader (Bio-Tek, USA).
2.10. LDH release
The quantity of LDH (unit ml−1 min−1) released into the medium was determined by the decrease in absorbance at an optical density of 340 nm (Mod-756) for NADH disappearance within 3 min [22].
2.11. Determination of dichlorofluorescein and 8-isoprostane
After diffusion into the cell, 2′,7′–dichlorofluorescin diacetate (DCFHDA) is deacetylated by cellular esterases to a non-fluorescent compound, which is later oxidized by ROS into 2′, 7′–dichlorofluorescein (DCF). It was measured in the present study using a CytoFluor 4000 fluorescence spectrophotometer with excitation at 485 nm and emission at 530 nm, as described previously [23]. Background fluorescence was corrected by the inclusion of parallel blanks. ROS production was quantified from a DCF standard curve and expressed as pmol DCF formed/mg protein/min. 8-isoprostane, a group of prostaglandin-like compounds resulting from the peroxidation process of arachidonic acid induced by ROS [24], was determined by using an ELISA Kit (ab175819). Cells were processed according to the manufacturer's instructions. Each ELISA sample was tested in duplicates according to the manual of the 8-isoprostane ELISA Kit. Absorbance readings at 450 nm were normalized to readings of Maximum Binding Control, and quantified into pg/ml using an 8-isoprostane standard curve [24].
2.12. Measurements of GSH, superoxide dismutase (SOD), CAT and GSH-PX
Astrocytes receiving different treatments were washed three times with ice-cold PBS and collected into eppendorf tubes, followed by centrifugation at 2000 rpm at 4 °C for 5-min. The pellets were then resuspended in 100 µl PBS and sonicated. After centrifugation at 4000 rpm for 10 min, the supernatants were collected and stored at −80 °C until assayed. The level of GSH and the activities of total SOD, manganese superoxide dismutase (Mn-SOD), copper & zinc superoxide dismutase (CuZn-SOD), CAT, and GSH-PX were measured spectrophotometrically by assay kits according to the manufacturers’ instructions.
2.13. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
The astrocytes grown on poly-d-lysine pre-coated glass slides were fixed, permeabilized and then treated with 30 µl of labeling solution, containing 3 µl of terminal deoxynucleotidyl transferase (TdT) for 1-h at 37 °C according to the manufacturer's instructions. Subsequently, the slides were washed and nuclei staining was performed by incubation in 1 μg/ml DAPI for 10-min. The green fluorescein-labeled DNA was visualized by using a Nikon D-Eclipse confocal microscope (Nikon, UK). The percentages of nuclei displaying green fluorescence were calculated as a percentage of total nuclei visible in the field [23].
In the in vivo experiments, the brain slices were fixed, permeabilized, blocked with 3% goat serum (Invitrogen-Life Technologies, USA) containing 0.3% Triton X-100 in PBS for 1-h, and then incubated with mouse anti-GFAP primary antibody in a humidified chamber at 4 °C overnight, followed by incubation with the goat anti-mouse secondary antibody conjugated to Alexa fluor 488 for 1-h. After washing with PBS, the sections were incubated in 50 µl of labeling solution containing 5 µl of TdT at 37 °C for 1-h. Sham sections were subjected to DNase treatment as a positive control, and labeling solution without enzymes was equivalently used as a negative control. Fluorescent images were captured by using a Nikon D-Eclipse confocal microscope (Nikon, UK).
2.14. DNA laddering assay
Astrocytes were washed and lysed in 500 µl lysis buffer followed by centrifugation at 7000 rpm for 15-min (4 °C). The supernatants were removed and the pellet was mixed with equal volumes of phenol (pH 8.0) followed by centrifugation at 13,000 rpm for 10-min (4 °C). The upper aqueous layer was transferred into a fresh vial and mixed with 100 µl TE buffer followed by centrifugation at 13,000 rpm for 10-min (4 °C). DNA in the upper layer was extracted by a solution of phenol: chloroform:isoamylalcohol (at 25:24:1) and then precipitated by 40 µl of 3 M NaOAc, pH 5.2 and 1 ml of ice-cold 100% ethanol. After centrifugation at 14,000 rpm for 15-min (4 °C), the DNA pellet was washed with 70% ethanol, resuspended in 15 µl TE buffer and incubated with 1 µl RNAase (10 mg/ml) at room temperature for 1-h. Finally, DNA samples were run in 2% TAE (Tris-acetate-EDTA) agarose gel, containing 0.5 µg/ml ethidium bromide.
2.15. Enzyme-linked immunosorbent assay (ELISA)
The concentrations of EPO in IPcNCM (in vitro) and the contents of EPO in KA or PBS injected-areas (in vivo) were measured by ELISA kits according to the manufacturer's instructions. Optical density was read at a wavelength of 450 nm by an ELX-800 microplate assay reader (Bio-tek, USA).
2.16. Western blot analysis
Astrocytes were washed, homogenized with lysis buffer and subjected to sonication using a Soniprep 150 (MSE Scientific Instruments, London, UK) followed by centrifugation at 12,000g for 10-min at 4 °C [25]. The supernatants were then collected and stored at −80 °C for future use. The protein concentrations were determined using the Bradford protein assay kit. The primary antibodies used were mouse anti-HIF-1 alpha (1:500), rabbit anti-Bad (1:500), anti-112p-Bad, anti-136p-Bad, rabbit anti-Akt, anti-p-Akt, anti-Bcl-2, anti-Bax, anti-Bcl-xL, anti-caspase-3, anti-cleaved caspase-3, anti-p-STAT3, anti-p-STAT5 (1:1000), anti-ERK42/44 and anti-p-ERK42/44 (1:3000), and the secondary antibody was either goat anti-rabbit or anti-mouse IRDye 800 CW IgG (1:10000, Li-Cor). The intensities of the specific bands were detected and analyzed by the Odyssey infrared imaging system at a resolution of 169 µm (Li-Cor). Anti-β-actin (1:20000) and anti-Histone 3 monoclonal antibodies (1:10000) were used as internal protein controls.
2.17. Statistical analysis
Statistical analyses were performed using Graphpad Prism. Data were presented as mean ±SEM. The differences between the means were all determined by two-way analysis of variance (ANOVA). A probability value of p < 0.05 was taken to be statistically significant.
3. Results
3.1. Effects of different durations of ischemia on cell viability and hypoxia-inducible factor-1 alpha expression in neurons
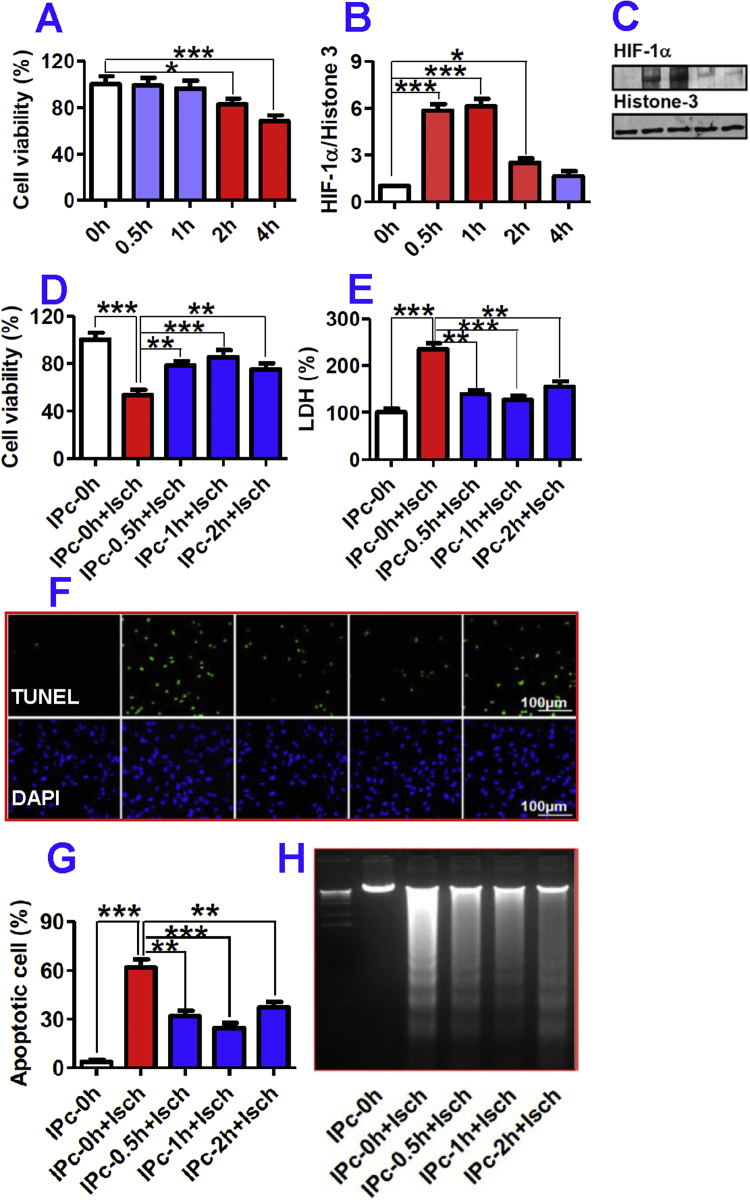
In order to find out an optimal and effective IP treatment for neurons, we first investigated the effects of the different durations (0, 0.5, 1, 2 or 4-h) of ischemia on cell viability and also HIF-1 alpha expression in neurons. The MTT assay showed that 0.5 or 1-h ischemia did not lead to any significant reduction in neuronal viability as compared with the control (0-h ischemia) (Fig. 1A). A significant reduction in neuronal viability began to appear after 2-h ischemia, which amounted to an 18% decrease in comparison with the control. More serious injury was observed when the neurons were exposed to 4-h ischemia, reflected by a decrease of cell viability to about 68% of the control. The levels of HIF-1 alpha were dramatically increased to 5.8, 6.1 and 2.5-fold of the control in neurons after 0.5, 1 and 2-h ischemia respectively, but only 1.6-fold after ischemia for 4-h (Fig. 1B and C). Thus, 0.5–2-h ischemia were used to precondition the neurons, as this represents the period in which hypoxia-induced HIF production takes place while the neurons remain intact.
Fig. 1.
A–C: Effects of different durations of ischemia on cell viability and expression of hypoxia-inducible factor-1 alpha in neurons. Primary neurons were subjected to combined conditions of 1% O2 and serum-free DMEM without glucose for 0, 0.5, 1, 2, 3 or 4-h, and then back to normoxia in DMEM containing 5% FBS for 24-h. The cell viability (A, n = 12) of neurons was then measured by MTT assay and HIF-1 alpha level in neuronal nuclei (B&C, n = 5) detected by western blot. Data were Mean ± SD. *p < 0.05, ***p < 0.001 versus the control (0-h). D-H: Ischemia-preconditioned neurons protected astrocytes against ischemic injury by inhibition of apoptosis. Astrocytes were pre-incubated with IPc-0 h, 0.5 h, 1 h or 2 h NCM for 48-h before undergoing ischemia (12-h). Cell viability (D) and LDH release (E) were measured, and the degree of apoptosis analyzed by TUNEL and DAPI staining. F. TUNEL (upper) and DAPI (lower) staining; G, apoptotic cells (% of total cells); H, DNA laddering analysis (M = marker). Data were Mean ± SD (n = 10). **P < 0.01, ***P < 0.001 versus ‘IPc-0h’ or ‘IPc-0h + Isch’ (Isch = Ischemia).
3.2. Ischemia-preconditioned neurons protect astrocytes against ischemic injury
To explore whether IP neurons could protect astrocytes against OGD injury, astrocytes were pre-incubated with IPc-0h, IPc-0.5h, IPc-1h or IPc-2h NCM for 48-h before being subjected to 12-h OGD. OGD (IPc-0h + Isch) induced a marked increase in LDH (2.3-fold of the control) and a significant reduction in astrocyte viability (53% of the control) (Fig. 1D&E). However, in astrocytes treated with 12-h OGD pre-treatment with IPcNCMs for 48-h induced a significant increase in cell viability (Fig. 1D), as well as a decrease in LDH (Fig. 1E) as compared with the cells treated without IPcNCM (IPc-0h + Isch). Astrocytes were significantly preserved by pre-treatment with IPcNCMs as reflected by the increased cell viability, to 79% (IPc-0.5h + Isch), 85% (IPc-1h + Isch) and 75% (IPc-2h + Isch) of the control (IPc-0h + Isch) respectively (Fig. 1D). LDH leakage from astrocytes pretreated with IPc-0.5h, IPc-1h or IPc-2h NCM were 1.3, 1.2 and 1.5-fold respectively of the control (Fig. 1E), significantly less than those of the OGD astrocytes pretreated with IPc-0h NCM.
3.3. Inhibition of apoptosis confers the protective effect of ischemia-preconditioned neurons on astrocytes
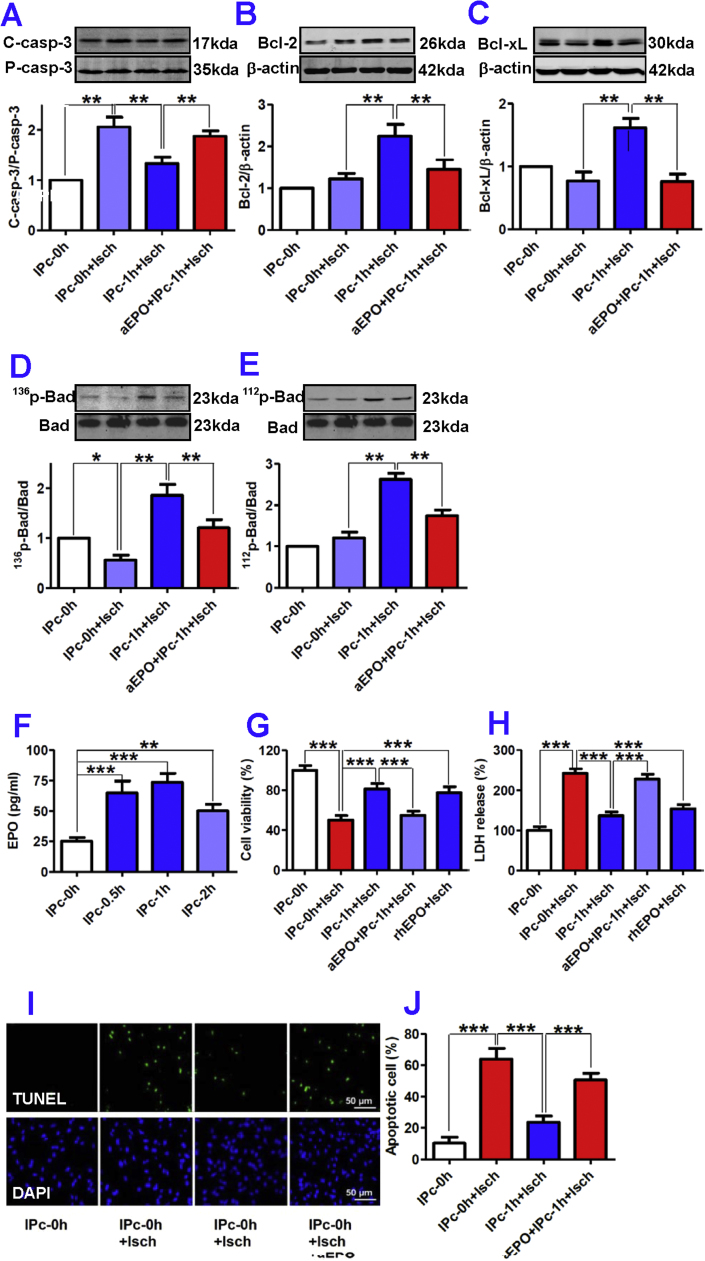
In order to understand the mechanisms by which IP neurons protect astrocytes against OGD injury in vitro, we examined the effects of pre-treatment with IPcNCMs on OGD-induced apoptosis in astrocytes. As shown in Fig. 1F and G, OGD dramatically increased the number of TUNEL positive astrocytes to 62% of the total cell population (3.7% in the control). However, pre-treatment with IPc-0.5h, IPc-1h or IPc-2h NCM for 48-h induced a significant decrease in the number of TUNEL positive astrocytes. This finding was also verified by DNA ladder assay in which the IPcNCM displayed a similar anti-apoptotic profile (Fig. 1H). We then examined the effects of the IPc-1h NCM on pro-apoptotic factor caspase-3 and anti-apoptotic factors Bcl-2, Bad and Bcl-xL in the astrocytes treated with 12-h OGD. The IPc-1h NCM was used because 1-h of IP achieved the maximal protective (Fig. 1D and E) and anti-apoptotic effect (Fig. 1F–H). It was found that OGD induced a significant activation of caspase-3 (2.2-fold of the control), while IPc-1h NCM markedly suppressed this increase by almost 75% (Fig. 2A). On the other hand, treatment with IPc-1h NCM caused a 2-fold increase in expression of anti-apoptotic factors Bcl-2 (Fig. 2B) and Bcl-xL (Fig. 2C), which was paralleled by greatly enhanced phosphorylation of pro-apoptotic protein Bad on serine 136 (136p-Bad) (Fig. 2D) and 112 sites (112p-Bad) (Fig. 2E) in OGD astrocytes.
Fig. 2.
Effects of EPO secreted by ischemia-preconditioned neurons on apoptosis of astrocytes in ischemia. Astrocytes were incubated with IPc-0 h or IPc-1 h NCM in the presence or absence of EPO antibody (aEPO) or recombinant human EPO (rhEPO, 75 pg/ml) followed by treatment with ischemia. The contents of cleaved/active caspase-3 (A), Bcl-2 (B), Bcl-xL (C), 136p-Bad (D), 112p-Bad (E), cell viability (G), LDH release (H), TUNEL-positive cells (I, Representative photographs of TUNEL (upper) and DAPI (lower) staining; J, The percentage of apoptotic cells in total cells) were measured as described in ‘Methods’. F, EPO contents in IPcNCMs measured by ELISA. Data were Mean ± SD (A-E: n = 4; F: n = 6). *p < 0.05, **P < 0.01, ***P < 0.001 versus ‘IPc-0h’, ‘IPc-0h + Isch’ or ‘IPc-1h + Isch’ (Isch = Ischemia).
3.4. Anti-apoptosis induced by IP neurons is an EPO-mediated process
To elucidate why IP neurons can affect expression of apoptotic factors, we then investigated the role of EPO because EPO has been reported to protect neurons by preventing apoptosis [7], [9], [26], [27] and we speculated that similar mechanisms might also operate in astrocytes. First, the EPO levels in IPc-0.5h, IPc-1h and IPc-2h NCMs were found to be significantly higher than those in IPc-0h NCM (Fig. 2F). The maximum effect was achieved by 1-h IP, with a nearly 3-fold increase in EPO content. This showed that IP could induce a significant increase in EPO release from neurons. Second, astrocyte viability was significantly lower (Fig. 2G) and LDH significantly higher (Fig. 2H) in 12-h OGD astrocytes treated with IPc-1h NCM plus anti-EPO (aEPO + IPc-1h + Isch), than in those treated with IPc-1h NCM only (IPc-1h + Isch), implying that the astro-protective effect of IPc-1h NCM could be largely blocked by anti-EPO. Third, rhEPO, at a concentration comparable to that of EPO in the IPc-1h NCM has a similar astro-protective effect as revealed by MTT (Fig. 2G) and LDH assays (Fig. 2H). Finally, the number of TUNEL positive astrocytes (Fig. 2I and J) and caspase-3 (Fig. 2A) was significantly higher and the expression of Bcl-2 (Fig. 2B), Bcl-xL (Fig. 2C), 136p-Bad (Fig. 2D) and 112p-Bad (Fig. 2E) remarkably lower in 12-h OGD astrocytes treated with IPc-1h NCM and anti-EPO (aEPO + IPc-1h + Isch) than in those treated with IPc-1h NCM only (IPc-1h + Isch).
3.5. Activation of ERK, P13K and STAT5 pathways are involved in EPO-mediated protection of ischemic astrocytes by IP neurons
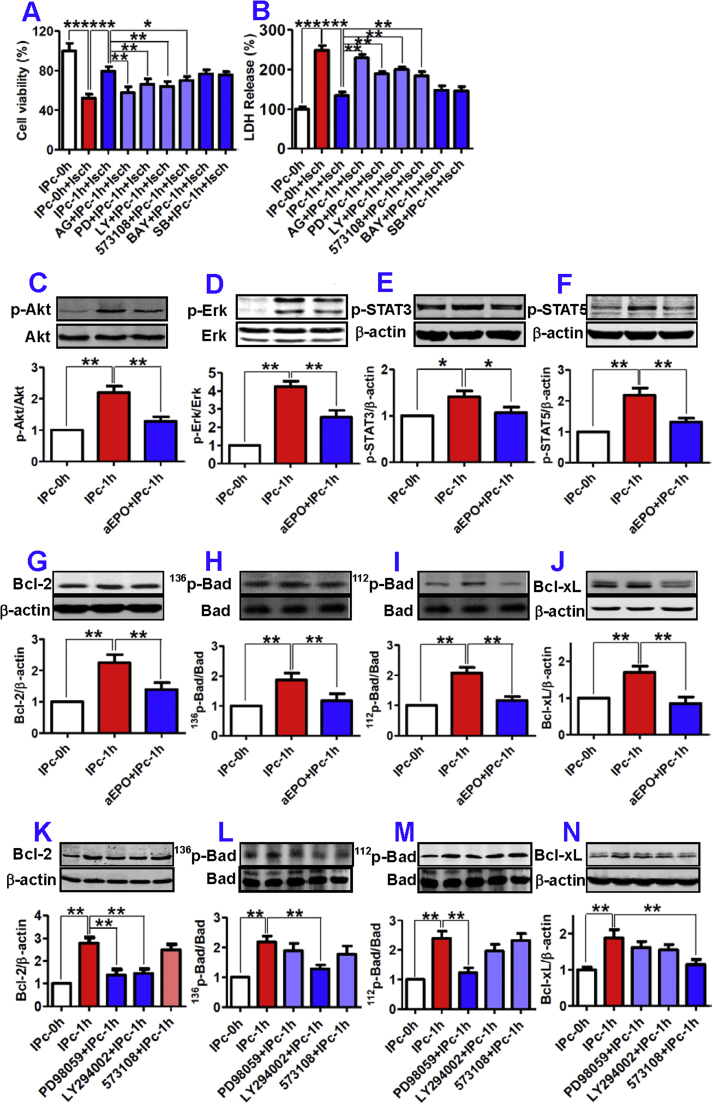
Next, we explored the signaling pathways involved in EPO-mediated anti-apoptosis. We first determined whether Jak2 dependent signal transduction pathways are involved in the protective effect of IP neurons, as Jak2 and its downstream effectors have been demonstrated to play a central role in EPO-mediated neuroprotection against ischemic injury in the brain [28]. As shown in Fig. 3A, the improved viability of 12-h OGD astrocytes induced by IPc-1h NCM was significantly attenuated by pre-treatment of the cells with Jak2 inhibitor AG490 (25 µM), Jak2 downstream PI3K inhibitor LY294002 (10 µM), ERK inhibitor PD98059 (25 µM) or STAT5 inhibitor 573108 (50 µM) for 20-min. However, no change was detected when NF-κB inhibitor BAY11-7082 (2 µM) and p38 MAPK inhibitor SB203580 (50 µM) were applied. These results were corroborated by the increase in the measurements of LDH release (Fig. 3B).
Fig. 3.
Involvement of ERK, PI3K and STAT5 in EPO-mediated anti-apoptosis in astrocytes. In A (n = 10) and B (n = 6), astrocytes were incubated with IPc-0 h NCM (IPc-0h) with or without ischemia (Isch), or with IPc-1h in the presence of AG490 (AG), PD98059 (PD), LY294001 (LY), 573108 (STAT5 inhibitor), BAY11-7082 (BAY) and SB203850 (SB) for 1-h, followed with IPc-1h NCM for 48-h and exposure to ischemia for 12-h. Viabilities were then assayed. In C to F, astrocytes were incubated in IPc-1h NCM (IPc-1h) with or without anti-EPO antibody (aEPO) for 20 min, and p-Akt (C), p-Erk (D), p-STAT3 (E) and p-STAT5 (F) were then measured by Western blot (n = 3). G–J: Astrocytes were incubated in IPc-1h NCM with or without aEPO for 48-h, and Bcl-2 (G), 136p-Bad (H), 112p-Bad (I) and Bcl-xL (J) were then measured by Western blot (n = 3). K-N: Astrocytes were pre-treated with or without PD98059, LY294002 or 573108 for 1-h before being incubated in IPc-1h NCM for 48-h, and Bcl-2 (K), 136p-Bad (L), 112p-Bad (M) and Bcl-xL (N) were then measured by Western blot (n = 3). Data were Mean ±SD. *p < 0.05, **p < 0.01,***p < 0.001. versus the corresponding control (Isch = Ischemia).
We then examined the effects of anti-EPO on the phosphorylation of Akt, ERK, STAT3 and STAT5 in astrocytes pre-treated with IPcNCM. Western blot analysis showed that IPc-1h NCM induced a significant increase in phosphorylation of Akt, ERK, STAT3 and STAT5 in astrocytes, all of which were however markedly inhibited by anti-EPO (Fig. 3C–F). Also, treatment with IPc-1h NCM significantly increased Bcl-2 expression and Bad and Bcl-xL phosphorylation in astrocytes, while these effects were also blocked by anti-EPO (Fig. 3G–J).
Also, we investigated the effects of the pathway-specific inhibitors on expression of pro- and anti-apoptotic factors. It was found that pre-treatment with LY294002 inhibited the IPc-1h NCM-induced increase in Bcl-2 and 136p-Bad, but not 112p-Bad and Bcl-xL levels in astrocytes (Fig. 3K–N). Pre-incubation with PD98059 blocked the effects of IPc-1h NCM on Bcl-2 and 112p-Bad but not 136p-Bad and Bcl-xL contents (Fig. 3K–N). The inhibitor 573108 could significantly inhibit the IPc-1h NCM-induced increase in Bcl-xL expression but had no effects on the other anti-apoptotic factors (Fig. 3K–N).
3.6. Ischemia-preconditioned neurons protect astrocytes against ischemia-induced apoptosis via up-regulation of EPO in the rat cortex in vivo
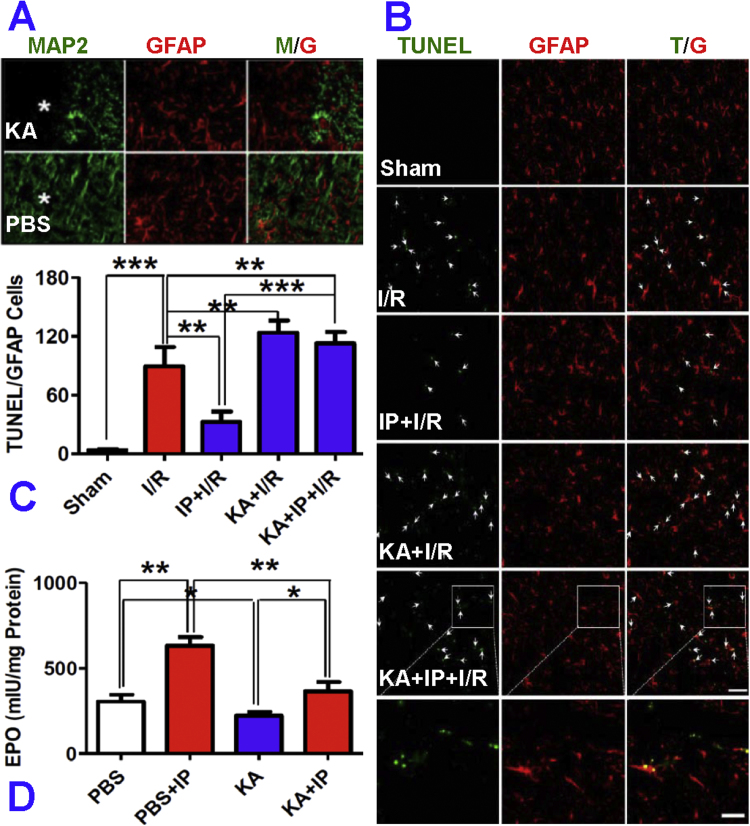
To find out whether IP neurons can protect astrocytes against I/R-induced apoptosis in vivo, a neuronal loss model was established by injecting kainic acid (KA) stereotaxically into the cortex. After 24-h of injection, the rats were treated with IP and then subjected to I/R. TUNEL-positive cells were counted by using rectangular grids placed randomly on the investigated areas. Data from 2 sections (40 µm of interval) of each investigated area from both sides were averaged for each animal (n = 6 rats in each group). It was found that the number of apoptotic astrocytes was increased to 89.28 ± 19.55 cells/0.1 mm2 in the rats treated with 20-min forebrain ischemia (I) followed by reperfusion (R) for 24 h, in comparison with 3.76 ± 0.97 cells/0.1 mm2 in the sham rats (Fig. 4A–C). However, the number of apoptotic astrocytes was significantly reduced, to 32.56 ± 9.59 cells in the I/R rats pre-treated with 4-min forebrain ischemia (IP + I/R). These indicated that 4-min of forebrain ischemia as IP successfully protected astrocytes against subsequent severe injury, induced by I/R via an anti-apoptotic effect. It was also found that the number of apoptotic astrocytes in the KA + IP + I/R group (112.55 ± 11.66 cells/0.1 mm2, the absence of neurons) was significantly higher than that of the IP + I/R rats (32.56 ± 9.59 cells/0.1 mm2, the presence of neurons). There were no significant differences in the numbers of apoptotic astrocytes between KA + IP + I/R and KA + I/R (123.48 ± 12.57 cells/0.1 mm2) groups (Fig. 4C). These data indicate that IP neurons were able to protect astrocytes against ischemia-induced apoptosis in vivo as well.
Fig. 4.
Effects of ischemia-preconditioned neurons on apoptosis of astrocytes in the cortex of rats after ischemia-reperfusion in vivo. Rats received 0.5 nmol kainic acid (KA, in 1.5 µl PBS) injections in the left cerebral cortex. After 24-h, the animals were treated with 4 min forebrain ischemia (IP) and then subjected to 20 min forebrain ischemia followed by reperfusion (I/R) for 24-h. A, Neurons and astrocytes in the peri-injected area (asterisk) were identified by immunostaining for MAP2 (green) and GFAP (red) respectively. In this area, selective loss of neurons but not astrocytes was observed. B, Astrocytic apoptosis under different conditions was analyzed by TUNEL (green) and GFAP (red) double staining. C, the number of TUNEL positive cells co-localized with GFAP staining was counted in the area 0.5–1.0 mm from the injection site (n = 12). D, Rats were injected with KA or PBS into the left or right cerebral cortex. After 24-h, the animals were treated with 4 min forebrain ischemia followed by a 24-h interval. The tissues around the injection site were cut out and used to measure the content of EPO (mIU/mg protein) by ELISA (n = 11). Data were presented as mean ± SD. * p < 0.05; **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.).
In some experiments, KA and PBS were injected into the left and right cerebral cortices of rats at corresponding coordinates before treatment with IP, and the tissues of the injection area were then harvested for measurement of EPO by ELISA. It was found that EPO content in PBS-injected areas was highly elevated in the IP rats (572.5 mIU/mg protein) compared to that of the non-IP rats (385.8 mIU/mg protein) (Fig. 4D). On the other hand, EPO content in the KA-injected areas was significantly lower (234.5 mIU/mg protein) than in the PBS-injected areas. There was no significant difference in EPO content in the KA-injected areas between KA (234.5 mIU/mg protein) and IP + KA (253.8 mIU/mg protein) rats (Fig. 4D). These results indicate that the reduced EPO content in the cerebral cortex of KA and KA + IP rats was due to KA-induced neuronal loss, and implied that neurons are an important source of EPO in IP, and that astro-protection by IP neurons in vivo is also EPO-associated.
3.7. Ischemia-preconditioned neurons inhibited oxidative stress induced by ischemia/reperfusion in astrocytes
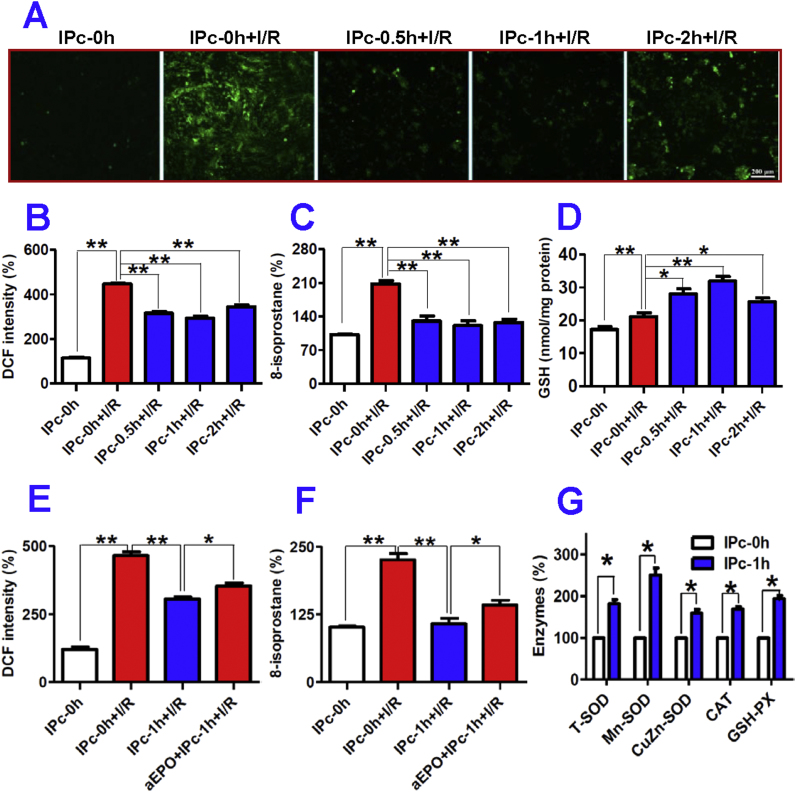
We also examined the effects of IP neurons on ROS and GSH levels in astrocytes treated with I/R by pre-treatment of astrocytes with IPcNCMs for 48-h before being exposed to ischemia for 12-h and reperfusion for 24-h. The ROS levels were measured by a fluorometric assay with 2′,7′-dichlorofluorescin diacetate (DCFH-DA). The confocal microscopy pictures obtained (Fig. 5A) showed that ROS levels dramatically increased in astrocytes treated by I/R (IPc-0h + I/R). However, when astrocytes were pre-incubated with IPc-0.5h, IPc-1h or IPc-2h NCM for 48-h before being exposed to I/R, ROS levels significantly decreased. In comparison with the control (IPc-0h), DCF fluorescence intensity increased about 4-fold in astrocytes treated with I/R only (IPc-0h + I/R) and decreased to 70.6% (IPc-0.5h + I/R), 65.7% (IPc-1h + I/R) and 77.1% (IPc-2h + I/R) in astrocytes pre-treated with IPcNCMs (Fig. 5B). Very similar changes were also found in 8-isoprostane content (Fig. 5C). In addition, I/R induced a significant increase (IPc-0h + I/R), and pre-treatment with IPc-0.5h, IPc-1h or IPc-2h NCM for 48-h led to a further increase in GSH levels in I/R astrocytes (Fig. 5D). Furthermore, DCF fluorescence intensity and 8-isoprostane content (ROS level) in OGD astrocytes treated with IPc-1h NCM and anti-EPO (aEPO + IPc-1h + Isch) were significantly higher than in those treated with IPc-1h NCM only (IPc-1h + Isch), but still significantly lower than in IPc-0h + I/R cells (Fig. 5E and F).
Fig. 5.
Ischemia-preconditioned neurons protect astrocytes against I/R injury by inhibiting oxidative stress. A–D: Astrocytes were pre-incubated with IPc-0h, IPc-0.5h, IPc-1h or IPc-2h NCM for 48-h before being exposed to ischemia (I) for 12-h followed by reperfusion (R) for 24-h. The ROS and GSH contents were determined, and fluorescence intensities were observed under confocal microscopy and quantified by using a fluorescent spectrophotometer as described in Methods. A, Representative confocal images indicating ROS levels in astrocyte which were measured by a fluorometric assay with 2′, 7′-dichlorofluorescin diacetate (DCFH-DA); B, DCF intensity (% IPc-0h, n = 10); C, 8-isoprostane contents (% IPc-0h, n = 10); D, GSH contents (n = 6). E and F: Astrocytes were incubated with IPc-0h or IPc-1h NCM in the presence or absence of EPO antibody (aEPO) followed by treatment with ischemia, and DCF intensity (% IPc-0h, n = 10) (E) and 8-isoprostane contents (%I Pc-0h, n = 10) (F) were then determined as described in Methods. G: Effects of ischemia-preconditioned neurons on expression of anti-oxidant enzymes in astrocytes. Astrocytes were incubated with IPc-0h or IPc-1h NCM for 48-h, and the contents of T-SOD, Mn-SOD, CuZn-SOD, CAT (catalase) and GSH-PX were then measured (n = 6). Data were Mean ± SD. *P < 0.05, **P < 0.01 vs. IPc-0h or IPc-0h + I/R.
3.8. Ischemia-preconditioned neurons increased activities of superoxide dismutase, catalase and glutathione peroxidase in astrocytes
To understand how IP neurons suppressed ROS levels, we investigated the effects of IPc-1h NCM on the activities of anti-oxidant enzymes T-SOD, Mn-SOD, CuZn-SOD, CAT and GSH-PX in astrocytes by treating the cells with IPc-0h or IPc-1h NCM for 48-h and then determining the activities of these enzymes. It was found that the activities of all enzymes we measured in astrocytes treated with IPc-1h NCM were significantly higher than those in the cells treated with IPc-0h NCM (Fig. 5G). The activities of T-SOD, Mn-SOD, CuZn-SOD, CAT and GSH-PX in astrocytes treated with IPc-0h NCM were 181.67, 250.73, 159.36, 169.01 and 193.86 of the respective corresponding values in astrocytes treated with IPc-1h NCM.
3.9. Ischemia-preconditioned astrocytes protected neurons from ischemia-induced injury
See 'Supplementary information: Supplementary Figs. 1–5'.
4. Discussion
For a long time, neurons have traditionally been considered as targets that need to be protected by IP astrocytes under ischemia/hypoxia. It is completely unknown whether IP neurons can protect astrocytes from lethal ischemia/hypoxia-induced injury, and one of the major objectives of this study was to investigate this issue. Our surprising findings demonstrate for the first time that pre-treatment with the culture medium from ischemia-preconditioned neurons (IPcNCM) can induce a significant reduction in LDH leakage and a remarkable increase in cell viability in ischemic astrocytes in vitro. We also showed that neurons play an essential and key role in significantly reducing the number of apoptotic astrocytes induced by IP in the rats subjected to I/R in vivo. Furthermore, we showed that IP astrocytes can protect neurons against ischemia-induced injury (Supplementary Figs. 1–5). Our findings provide direct evidence for our hypothesis, that not only preconditioned astrocytes protect neurons but also that preconditioned neurons protect astrocytes from ischemia/hypoxia injury. The results also imply that there is bi-directional communication between neurons and astrocytes, not only under physiological conditions but also under pathological (ischemic) circumstances. In addition, the current study supports the notion that the astro-protective effect of IP neurons should be recognized as a part of the molecular mechanisms underlying the beneficial roles of ischemic preconditioning.
Our findings showed that the astro-protective effects are at least partly associated with the anti-apoptotic ability of IP neurons. We demonstrated that pre-treatment of astrocytes with IPcNCM can significantly inhibit the increase in the number of TUNEL positive astrocytes induced by OGD in vitro. Similar results were also found in IP rats treated with I/R in vivo. In addition, pre-treatment with IPcNCM markedly suppressed the OGD-induced increase in pro-apoptotic factor caspase-3, and, at the same time caused a significant increase in the expression of anti-apoptotic factors Bcl-2 and Bcl-xL, as well as the phosphorylation of pro-apoptotic protein Bad on serine 136 (136p-Bad) and 112 sites (112p-Bad) in OGD astrocytes. Activation of caspase-3 is a key event in the execution of apoptotic cascade in CNS diseases, including the extrinsic (initiated by caspase-8) and the intrinsic (initiated by caspase-9) pathways [29], [30]. Thus, the ability of IPcNCM to suppress caspase-3 enables it to inhibite the extrinsic and the intrinsic pathways of apoptotic cascade in ischemic astrocytes. The generation of anti-apoptotic proteins Bcl-2 and BclxL and the phosphorylation of 136p-Bad and 112p-Bad [31], [32] also provide evidence for the involvement of an anti-apoptotic role in the astro-protective effects of IP neurons.
Existing evidence has shown that EPO protects neurons by preventing apoptosis [7], [9], [26], [27]. We therefore speculated that similar mechanisms might also operate in astrocytes. This hypothesis was strongly supported by the following findings; first, IP (1-h) led to a dramatic increase in HIF-1 alpha content in neurons as well as in EPO in the culture medium, evidencing that IP could lead to a remarkable increase in EPO release from neurons via increased HIF-1 alpha. Second, rhEPO, at a concentration comparable to that of EPO in the IPc-1h NCM, has a similar astro-protective effect as IPc-1h NCM. Third, the astro-protective effect of IPcNCM could be largely blocked by anti-EPO. Fourth, the anti-apoptotic properties of IPc-1h NCM, including reduction of the number of TUNEL positive astrocytes and caspase-3 level, and the increase in expression of Bcl-2 and BclxL and phosphorylation of 136p-Bad and 112p-Bad, could also be largely inhibited by anti-EPO. Finally, pre-injection with KA to destroy neurons induced a significant reduction in EPO content, as well as an increase in the number of apoptotic astrocytes in the brain of IP rats treated with I/R in vivo. Taken together, these results strongly imply that the anti-apoptotic effect on ischemic astrocytes is mediated by the up-regulation of EPO in IP neurons.
JAK-2 is a key kinase in the signal transduction pathways activated by EPO [7], [33]. The significant inhibition by Jak2 inhibitor AG490 of the IPcNCM-induced astro-protection strongly suggests that JAK-2 also plays a central role in EPO-mediated protection by IP neurons of astrocytes against ischemic injury, under our experimental conditions. Downstream from JAK-2, at least three different signaling pathways have been implicated in EPO-mediated anti-apoptosis: PI3K, MAPK and STAT. Bad is one major target of Akt, linking the PI3K pathway directly to the apoptotic machinery [34], and 136p-Bad has been shown to be a substrate of Akt kinase and thereby is sufficient to promote cell survival [26]. We showed that the inhibition of PI3K by LY294002 and the blockade of EPO by anti-EPO both could significantly diminish the IPcNCM-induced increase in phosphorylation of the serine-threonine protein kinase Akt, Bcl-2, 136p-Bad and 112p-Bad contents. The data indicate that the PI3K/Akt/136p-Bad and Bcl-2 pathway is involved in the EPO-mediated anti-apoptotic effect of IP neurons on astrocytes.
We also found that ERK inhibitor PD98059 significantly attenuated the improvement in astrocyte viability, Bcl-2 expression and 112p-Bad content induced by IPcNCM. Also, anti-EPO markedly blocked the significantly increased ERK phosphorylation, Bcl-2 expression and Bad and Bcl-xL phosphorylation in OGD astrocytes pre-treated with IPcNCM. These findings imply that the ERK/112p-Bad/Bcl-2 pathway is also associated with the EPO-mediated astro-protection induced by IP neurons. So far, only STAT5 has been demonstrated in the anti-apoptotic signaling of EPO [35], [36], and only STAT3 is expressed constitutively throughout the brain in glial cells as well as in neurons [37]. Here, we demonstrated that anti-EPO could markedly block the activation of STAT3 and STAT5 induced by IPcNCM. Also, STAT5 inhibitor 573108 could significantly inhibit the IPcNCM-induced increase in Bcl-xL expression. These suggest that the STAT5/Bcl-xL signal pathway might also be involved in the EPO-mediated anti-apoptotic effect of IP neurons on astrocytes.
It is well known that the irreversible damage to both neurons and astrocytes induced by reperfusion can be caused by oxidative stress [38], [39]. A large amount of ROS was generated during the reperfusion period through various pathways [40]. ROS can directly oxidize lipids, proteins, and nucleic acids or indirectly cause cellular damage through signaling pathways [41]. Because of the detrimental role of ROS, successful suppression of ROS levels can rescue neurons and astrocytes from oxidative damage induced by I/R. We therefore examined the effects of IP neurons on ROS levels in astrocytes treated with I/R and demonstrated that IPcNCM markedly suppressed increased ROS levels and oxidative stress in astrocytes exposed to I/R. We also investigated the effects of IPcNCM on GSH content in astrocytes treated with I/R, because GSH directly reacts with ROS to form oxidized glutathione disulfide (GSSG) [42] and is an indicator of ROS, such that lower GSH content indicate higher ROS levels [39]. It was found that treatment with IPcNCM induced a further increase in GSH levels in I/R astrocytes. These results imply that IP neuron-induced astro-protection against I/R-induced injury is achieved by an ability to inhibit I/R induced-oxidative stress. This inhibition by IP neurons is partially mediated by EPO released from the IP neurons, as the addition of anti-EPO antibody was found to partially block the increased anti-oxidant effects induced by the IP neurons in astrocytes.
It is well established that IP can up-regulate the activities of anti-oxidant enzymes in the brain [2], [3], [43], [44]. To understand the mechanisms involved in the inhibition by IP neurons of I/R induced-oxidative stress, we investigated the effects of IPcNCM on the activities of total superoxide dismutase (T-SOD), including mitochondrial manganese superoxide dismutase (Mn-SOD) and cytosolic copper, zinc superoxide dismutase (CuZn-SOD), catalase (CAT) and glutathione peroxidase (GSH-PX) in astrocytes, and demonstrated that treatment with IPc-1 h NCM could significantly increase all of these anti-oxidant enzymes as measured. These findings evidence that IP neurons have the ability to enhance the activities of anti-oxidant enzymes in astrocytes. A number of studies have demonstrated that superoxide, hydrogen peroxide and other peroxides are the major reactive oxygen species over-produced during reperfusion, and are responsible for oxidative damage [2], [44], [45]. Evidence has also shown that CAT and GSH-PX can specially scavenge hydrogen peroxides and other peroxides, while SOD mainly detoxifies superoxide anions [40]. Therefore, it is reasonable to believe that the up-regulation of these anti-oxidant enzymes is associated with the ability of IP neurons to inhibit I/R induced-oxidative stress or reduce ROS levels in astrocytes when exposed to I/R.
It has been demonstrated that EPO expression can be induced by hypoxia in astrocytes as well as in neurons [46], and that EPO receptor (EpoR) can also be detected in astrocytes as well as neurons [47], [48], [49]. These results are in agreement with our finding of the significant increase in EPO in the cultured medium of IP neurons. Ruscher et al. [7] compared the absolute levels of EPO expression in astrocytes and neurons, and reported that only astrocytes, but not neurons, express and release sufficient amounts of EPO for paracrine neuroprotection. In the present study, our findings all provide evidence that IP neurons have the ability to express and release sufficient amounts of EPO for paracrine astro-protection. A number of differences in experimental conditions, including IP and the purity of the neurons used, might be partly associated with the discrepancy between these two studies. A further study to find the relevant reasons is necessary.
5. Summary
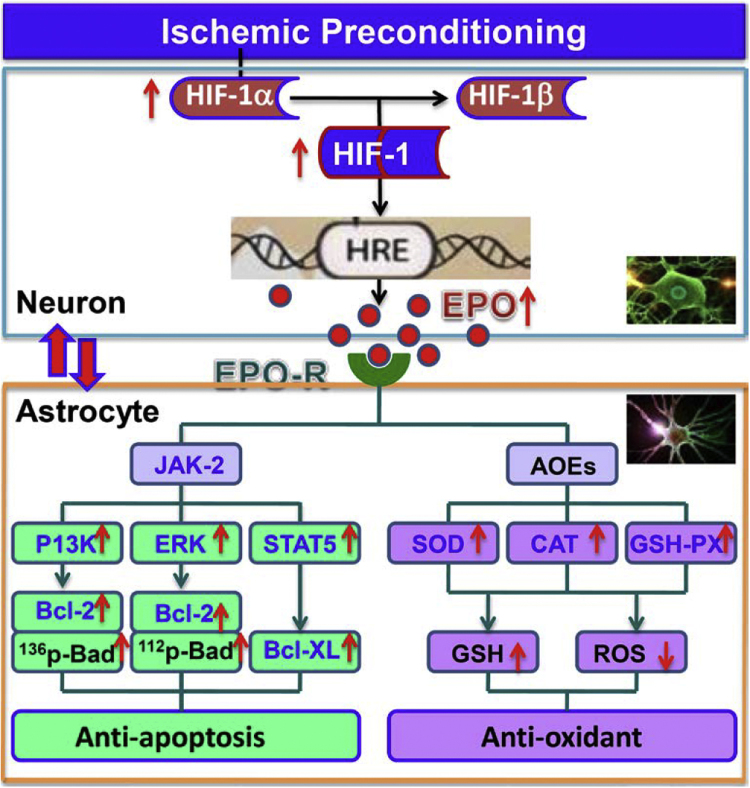
We demonstrated for the first time that IP neurons have a significant role in protecting astrocytes from ischemia-induced injury by an EPO-mediated anti-apoptosis and anti-oxidant effect, via inhibition of pro-apoptotic signals, activation of antiapoptotic proteins and up-regulation of anti-oxidant enzymes (Fig. 6). We also provide strong evidence to support the growing belief that bi-directional communication between neurons and astrocytes are important not only under normal physiological conditions, but also pathological conditions. This study may have a broad significance for a better understanding of cell biology, especially neurobiology, under physiological as well as pathological conditions. Based on the findings in the present study and the accumulated literature, we propose that the bi-directionlly protective communications between cells may be a common activity in the brain or peripherial organs under most if not all pathological conditions. Different types of cells in a particular organ may all have a response to pathophysiological stimulation and help each other by generating and releasing signals or chemicals, especially at the earlier stages of disease. A holistic approach that aims at multiple cellular targets will likely achieve a higher therapeutic efficacy through the synergistic interactions among the different cell types in the brain or other organs. Further studies on these possibilities are worth conducting.
Fig. 6.
A hypothetical scheme for the mechanisms involved in the astro-protection by ischemia-preconditioned neurons. IP neurons have the ability to express and release sufficient amounts of EPO for paracrine astro-protection via the increased HIF-1 alpha induced by IP. The increased EPO binds to EPOR on the membrane of astrocytes, triggering dimerization of EPOR and inducing Jak2 activation, which in turn activates three different downstream-signaling pathways to prevent apoptosis: PI3K/Akt, ERK and STAT5. Activated PI3K/Akt can phosphorylate Bad at ser-136 and up-regulate Bcl-2 expression, and in turn preventing apoptosis. ERK signaling pathway-mediated anti-apoptosis is via enhanced Bcl-2 levels and phosphorylation of Bad at ser-112, while STAT5 suppresses astrocyte apotosis via the up-regulated expression of Bcl-xL. The anti-apoptotic effects of EPO from IP neurons on astrocytes require the combined activation of these three pathways. In addition, IP neurons were able to inhibit I/R induced-oxidative stress by up-regulation of anti-oxidant enzymes (SOD, CAT, GSH-PX) partly via EPO. Therefore. IP neurons protect astrocytes from ischemia-induced injury by an EPO-mediated anti-apoptosis and anti-oxidant effect.
Conflict of interests
The authors declare no conflict of interest.
Funding sources
The studies in our laboratories were funded by the Competitive Earmarked Grants of The Hong Kong Research Grants Council (GRF466713, GRF14106914 and GRF14111815 - KY), National Natural Science Foundation of China (31371092 - KY, 31330035, 31271132 and 31571195 - ZMQ) and National 973 Programs (2014CB541604 - ZMQ).
Author contributions
Y. K. and Z.M.Q. conceived, organized and supervised the study; X.M.W., C.Q., Y.F.Z., Y.Y.C., Q.Q.L., F.L.Z. and L.R.J. performed the experiments; W.H.Y. and X.M.W. contributed to the analysis and interpretation of data. Y.K., Q.C. and Z.M.Q. prepared, wrote and revised the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.05.010.
Appendix A. Supplementary material
Supplementary material
References
- 1.Martin L.J., Brambrink A.M., Lehmann C., Portera-Cailliau C., Koehler R., Rothstein J., Traystman R.J. Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann. Neurol. 1997;42:335–348. doi: 10.1002/ana.410420310. [DOI] [PubMed] [Google Scholar]
- 2.Gidday J.M. Cerebral preconditioning and ischaemic tolerance. Nat. Rev. Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 3.Dirnagl U., Becker K., Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murray C.E., Jennings R.B., Reimer K.A. Preconditioning with ischaemia: a delay of lethal cell injury in ischaemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 6.Narayanan S.V., Dave K.R., Perez-Pinzon M.A. Ischemic preconditioning and clinical scenarios. Curr. Opin. Neurol. 2013;26:1–7. doi: 10.1097/WCO.0b013e32835bf200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruscher K., Freyer D., Karsch M., Isaev N., Megow D., Sawitzki B., Priller J., Dirnagl U., Meisel A. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J. Neurosci. 2002;22:10291–10301. doi: 10.1523/JNEUROSCI.22-23-10291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trendelenburg G., Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50:307–320. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 9.Chavez J.C., Baranova O., Lin J., Pichiule P. The transcriptional activator hypoxia inducible factor 2 (HIF-2/EPAS-1) regulates the oxygen-dependent expression of erythropoietin in cortical astrocytes. J. Neurosci. 2006;26:9471–9481. doi: 10.1523/JNEUROSCI.2838-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson R.A., Ying W., Kauppinen T.M. Astrocyte influences on ischemic neuronal death. Curr. Mol. Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 11.Araque A., Carmignoto G., Haydon P.G. Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 12.Nedergaard M., Ransom B., Goldman S.A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Haydon P.G., Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 14.Verkhratsky A., Rodríguez J.J., Parpura V. Neurotransmitters and integration in neuronal-astroglial networks. Neurochem. Res. 2012;37:2326–2338. doi: 10.1007/s11064-012-0765-6. [DOI] [PubMed] [Google Scholar]
- 15.Ho K.P., Li L., Zhao L., Qian Z.M. Genistein protects primary cortical neurons from iron-induced lipid peroxidation. Mol. Cell. Biochem. 2003;247:219–222. doi: 10.1023/a:1024142004575. [DOI] [PubMed] [Google Scholar]
- 16.Qian Z.M., To Y., Tang P.L., Feng Y.M. Transferrin receptors on the plasma membrane of cultured rat astrocytes. Exp. Brain Res. 1999;129:473–476. doi: 10.1007/s002210050916. [DOI] [PubMed] [Google Scholar]
- 17.Wu X.M., Qian Z.M., Ke Y., Du F., Zhu L. Ginkgolide B preconditioning protects neurons against ischaemia-induced apoptosis. J. Cell. Mol. Med. 2009;13:4474–4483. doi: 10.1111/j.1582-4934.2008.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz G., Manozzo L., Gottardo S., Achaval M., Salbego C., Rodnight R. Temporal profiles of the in vitro phosphorylation rate and immunocontent of glial fibrillary acidic protein (GFAP) after kainic acid-induced lesions in area CA1 of the rat hippocampus: demonstration of a novel phosphoprotein associated with gliosis. Brain Res. 1997;764:188–196. doi: 10.1016/s0006-8993(97)00456-3. [DOI] [PubMed] [Google Scholar]
- 19.Vezzani A., Moneta D., Richichi C., Aliprandi M., Burrows S.J., Ravizza T., Perego C., De Simoni M.G. Functional role of inflammatory cytokines and antiinflammatory molecules in seizures and epileptogenesis. Epilepsia. 2002;43(Suppl 5):S30–S35. doi: 10.1046/j.1528-1157.43.s.5.14.x. [DOI] [PubMed] [Google Scholar]
- 20.Miyawaki T., Mashiko T., Ofengei D., Flannery R.J., Noh K.M., Fujisawa S., Bonanni L., Bennett M.V., Zukin R.S., Jonas E.A. Ischemic preconditioning blocks BA D translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W., Qian Z.M., Zhu L., Christopher Q., Du F., Yung W.H., Ke Y. Ginkgolides mimic the effects of hypoxic preconditioning to protect C6 cells against ischemic injury by up-regulation of hypoxia-inducible factor-1 alpha and erythropoietin. Int. J. Biochem. Cell. Biol. 2008;40:651–662. doi: 10.1016/j.biocel.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L., Wu X.M., Yang L., Du F., Qian Z.M. Up-regulation of HIF-1alpha expression induced by ginkgolides in hypoxic neurons. Brain Res. 2007;1166:1–8. doi: 10.1016/j.brainres.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L., Qian Z.M., Zhang C., Wing H.Y., Du F., Ya K. Amyloid beta-peptide 31-35-induced neuronal apoptosis is mediated by caspase-dependent pathways via cAMP-dependent protein kinase A activation. Aging Cell. 2008;7:47–57. doi: 10.1111/j.1474-9726.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 24.Gong J., Du F., Qian Z.M., Luo Q.Q., Sheng Y., Yung W.H., Xu Y.X., Ke Y. Pre-treatment of rats with ad-hepcidin prevents iron-ind uced oxidative stress in the brain. Free Radic. Biol. Med. 2016;90:126–132. doi: 10.1016/j.freeradbiomed.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Zhu L., Ya K., Xiao-Mei W., Lei Y., Yang L., Ming Q.Z. Ginkgolides protect PC12 cells against hypoxia-induced injury by p42/p44 MAPK pathway-dependent upregulation of HIF-1alpha expression and HIF-1DNA-binding activity. J. Cell. Biochem. 2008;103:564–575. doi: 10.1002/jcb.21427. [DOI] [PubMed] [Google Scholar]
- 26.Digicaylioglu M., Lipton S.A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 27.Obrenovitch T.P. Molecular physiology of preconditioning-induced brain tolerance to ischemia. Physiol. Rev. 2008;88:211–247. doi: 10.1152/physrev.00039.2006. [DOI] [PubMed] [Google Scholar]
- 28.Kilic E., Kilic U., Soliz J., Bassetti C.L., Gassmann M., Hermann D.M. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J. 2005;19:2026–2028. doi: 10.1096/fj.05-3941fje. [DOI] [PubMed] [Google Scholar]
- 29.Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 30.Man S.M., Kanneganti T.D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Delft M.F., Huang D.C. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–213. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 32.Wu X.M., Qian Z.M., Zhu L., Du F., Yung W.H., Gong Q., Ke Y. Neuroprotective effect of ligustilide against ischaemia-reperfusion injury via up-regulation of erythropoietin and down-regulation of RTP801. Br. J. Pharmacol. 2011;164:332–343. doi: 10.1111/j.1476-5381.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broxmeyer H.E. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013;210:205–208. doi: 10.1084/jem.20122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datta S.R., Brunet A., Greenberg M.E. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 35.Socolovsky M., Fallon A.E., Wang S., Brugnara C., Lodish H.F. Fetal anemia and apoptosis of red cell progenitors in STAT5a_/_/5b_/_ mice: a direct role for STAT5 in Bcl-XL induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 36.Constantinescu S.N., Huang L.J., Nam H., Lodish H.F. The erythropoietin receptor cytosolic juxtamembrane domain contains an essential, precisely oriented, hydrophobic motif. Mol. Cell. 2001;7:377–385. doi: 10.1016/s1097-2765(01)00185-x. [DOI] [PubMed] [Google Scholar]
- 37.Murata S., Usuda N., Okano A., Kobayashi S., Suzuki T. Occurrence of a transcription factor, signal transducer and activators of transcription 3 (STAT3) in the postsynaptic density of the rat brain. Mol. Brain Res. 2000;78:80–90. doi: 10.1016/s0169-328x(00)00077-2. [DOI] [PubMed] [Google Scholar]
- 38.Danilov C.A., Chandrasekaran K., Racz J., Soane L., Zielke C., Fiskum G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57:645–656. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bragin D.E., Zhou B., Ramamoorthy P., Muller W.S., Connor J.A., Shi H. Differential changes of glutathione levels in astrocytes and neurons in ischemic brains by two-photon imaging. J. Cereb. Blood Flow. Metab. 2010;30:734–738. doi: 10.1038/jcbfm.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Candelario-Jalil E., Fiebich B.L. Cyclooxygenase inhibition in ischemic brain injury. Curr. Pharm. Des. 2008;14:1401–1418. doi: 10.2174/138161208784480216. [DOI] [PubMed] [Google Scholar]
- 41.Chan P.H. Reactive Oxygen Radicals in Signaling and Damage in the Ischemic Brain. J. Cereb. Blood Flow. Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Leopold J.A., Zhang Y.Y., Scribner A.W., Stanton R.C., Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arter. Thromb. Vas. Biol. 2003;23:411–417. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 43.Blondeau N., Widmann C., Lazdunski M., Heurteaux C. Activation of the nuclear factor-kappaB is a key event in brain tolerance. J. Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Y., Swanson R.A. Astrocytes and Brain Injury. J. Cereb. Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 45.Ravati A., Ahlemeyer B., Becker A., Krieglstein J. Preconditioning-induced neuroprotection is mediated by reactive oxygen species. Brain Res. 2000;866:23–32. doi: 10.1016/s0006-8993(00)02210-1. [DOI] [PubMed] [Google Scholar]
- 46.Bernaudin M., Bellail A., Marti H.H., Yvon A., Vivien D., Duchatelle I., Mackenzie E.T., Petit E. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30:271–278. [PubMed] [Google Scholar]
- 47.Masuda S., Okano M., Yamagishi K., Nagao M., Ueda M., Sasaki R. A novel site of erythropoietin production. oxygen-dependent production in cultured rat astrocytes. J. Biol. Chem. 1994;269:19488–19493. [PubMed] [Google Scholar]
- 48.Digicaylioglu M., Bichet S., Marti H.H., Wenger R.H., Rivas L.A., Bauer C., Gassmann M. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc. Natl. Acad. Sci. USA. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergeron M., Gidday J.M., Yu A.Y., Semenza G.L., Ferriero D.M., Sharp F.R. Role of hypoxia-inducible factor-1 in hypoxia-induced ischemic tolerance in neonatal rat brain. Ann. Neurol. 2000;48:285–296. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material