Abstract
Objective:
To assess the association of the number and anatomic location of cerebral microbleeds (CMBs), visible indicators of microvascular damage on MRI, with incident cognitive disease in the general population of older people.
Methods:
In the longitudinal population-based Age, Gene/Environment Susceptibility (AGES)–Reykjavik Study, 2,602 participants 66 to 93 years of age and free of prevalent dementia underwent brain MRI and cognitive testing of verbal memory, processing speed, and executive function at baseline and a mean of 5.2 years later. Adjudicated incident dementia cases were diagnosed according to international guidelines.
Results:
In the multiple linear regression models adjusted for demographic, genetic, cardiovascular risk, and other cerebrovascular MRI markers, the presence of CMBs located in deep or mixed (deep and lobar) areas was associated with a greater decline in all 3 cognitive domains. Mixed CMBs were the strongest correlate for decline in memory and speed. Compared to those with no CMBs, participants with ≥3 CMBs had a steeper decline in a composite measure of global cognitive function, memory, and speed. Among those with ≥3 deep or mixed CMBs, associations were strongest for memory; the association with speed was strongest in those having ≥3 strictly lobar CMBs. People with ≥3 CMBs, regardless of their locations, had a higher incidence of all-cause dementia and vascular dementia.
Conclusions:
Mixed or a higher load of CMBs, with some specificity for location, is associated with accelerated cognitive decline in older people. These findings suggest a role for hypertensive vasculopathy and the combined effect of hypertensive and cerebral amyloid angiopathy in the pathogenesis of cognitive deterioration.
Cognitive impairment and dementia are increasingly recognized as a continuum of overlapping neurologic syndromes in older people with both cerebrovascular and neurodegenerative pathology.1–3 Exactly how the 2 processes interact to confer an increased risk of cognitive dysfunction is an area of intense investigation. Cerebral microbleeds (CMBs) may provide an intriguing link between them.4,5 Depending on location, CMBs commonly relate to 2 different small vessel disease (SVD) pathologies: hypertensive vasculopathy (deep regions) and cerebral amyloid angiopathy (CAA) (lobar regions).4
The cognitive consequences of CMBs in the general population remain uncertain,5,6 and previous studies, mostly of cross-sectional design,7–12 have yielded inconsistent results. While some studies7,9 found an association between the presence of CMBs and worse cognitive performance that was strongest for deep CMBs, others8,12 showed an association with a higher number of CMBs most strongly for lobar CMBs, and still others reported no region-specific associations.10,11 Longitudinal studies are scant.13,14 In particular, although mixed (deep and lobar) CMBs appear to increase the risk of developing dementia in patients with elevated vascular risk burden,13 it remains unclear whether older people ostensibly without dementia with such preexisting CMBs also experience more cognitive decline over time.
In the population-based Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, we sought to investigate whether baseline CMBs by number and location are associated with the rate of cognitive decline and incident dementia over a 5-year period. We hypothesized that people with a high load of CMBs or with mixed deep and lobar CMBs, suggesting more severe and extensive SVD, are predisposed to progressive cognitive deterioration.
METHODS
Participants.
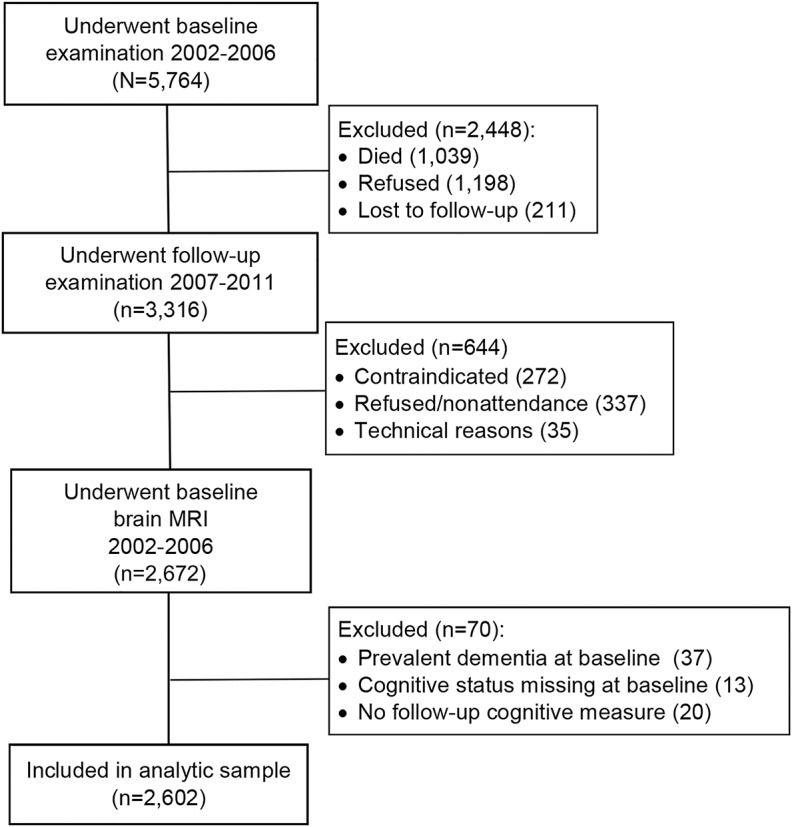
The present study was based on longitudinal data from the AGES-Reykjavik Study, which originates from the Reykjavik Study (1967–1996), as described fully elsewhere.15 From 2002 to 2006, 5,764 surviving men and women of the Reykjavik Study cohort (born 1907–1935) underwent an extensive physical, cognitive, and brain MRI examination (AGES I).15 From 2007 to 2011, there was a follow-up examination of surviving participants (AGES II). Of the 3,316 participants who attended the follow-up examination, we excluded 644 participants who had missing MRI data on CMBs at baseline and 70 participants because of a diagnosis of prevalent dementia at baseline, missing data on either baseline cognitive status or follow-up cognitive measures. The cohort at risk of dementia thus comprised 2,602 participants (figure 1 and appendix e-1 at Neurology.org).
Figure 1. Study population.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Icelandic National Bioethics Committee (VSN 00-063), and by the National Institute on Aging Intramural Institutional Review Board. All participants gave written informed consent.
Brain MRI and rating of CMB.
We acquired brain MRI scans on a single study-dedicated 1.5T General Electric Signa Twinspeed system (Waukesha, WI).16,17 For CMB detection, we used a 2-dimensional T2*-weighted gradient echo-type echo planar sequence (echo time 50 milliseconds, repetition time 3,050 milliseconds, flip angle 90°, field of view 220 mm, matrix 256 × 256, slice thickness 3 mm) and a proton density/T2-weighted fast spin echo sequence (first echo time 22 milliseconds, second echo time 90 milliseconds, repetition time 3,220 milliseconds, echo train length 8, flip angle 90°, field of view 220 mm, matrix 256 × 256, slice thickness 3 mm).16 Two trained radiographers evaluated CMBs on the MRI scan in terms of size and anatomic location with good intrarater and interrater reliabilities for CMB detection.17 CMBs were defined as a focal area of signal void within the brain parenchyma that is observable on T2*-weighted gradient echo-type echo planar sequence scans and smaller or unobservable on T2-weighted fast spin echo scans.16 We counted up to 30 CMBs in lobar regions (frontal, parietal, temporal, and occipital) and in deep (basal ganglia and thalamus, corpus callosum, and brainstem) or cerebellar regions.17 If there were >30 CMBs, they were coded as 30. People with ≥1 CMBs confined to lobar regions were regarded as having strictly lobar CMBs, and those with CMBs in a deep region, with or without coexisting lobar CMBs, were regarded as having deep or mixed CMBs.17 CMBs in the cerebellum were classified as a separate group given that there is no general agreement on their presumed underlying etiology.18,19
Assessment of cognitive function.
Participants underwent a neuropsychological test battery assessing 3 cognitive domains.20 We constructed composite scores for each cognitive domain based on a theoretical grouping of tests, as reported previously.21,22 The memory composite included a modified version of the California Verbal Learning test consisting of immediate and delayed recall subtests.20 The processing speed composite included the Digit Symbol Substitution Test, the Figure Comparison Test, and the Stroop test parts I and II (word naming and color naming).20 The executive function composite included the Digit Backwards Test and the Stroop test part III (word-color interference).20 We transformed the raw test scores into standardized z scores and then averaged them across all tests for the cognitive domain. The composite score for global cognitive function was the average of the z scores for all these domains. Higher z scores reflect a better cognitive performance. For each participant, we computed z scores for both baseline and follow-up using the mean and SD of the baseline test scores. Change in cognitive functioning was calculated by subtracting the baseline z scores for memory, processing speed, executive function, and global cognitive function from the follow-up z scores.
Diagnosis of dementia and subtypes.
Incident dementia cases were identified at follow-up with a 3-step procedure.7 All participants underwent the Mini-Mental State Examination and the Digit Symbol Substitution Test. People who were positive at screening on either test underwent additional diagnostic testing that included the Trail-Making Tests A and B and the Rey Auditory Verbal Learning tests.20 On the basis of these tests, those who were then suspected to have dementia went for a final evaluation that included a proxy interview and neurologic examination.20 The diagnosis of dementia and all subtypes was made in accordance with international criteria23–25 (appendix e-1) and assigned at a consensus conference by a panel made up of a geriatrician, neurologist, neuropsychologist, and neuroradiologist.7
Statistical analysis.
Because experimental and empiric evidence7,8 shows that a higher number of CMBs is a strong indicator of underlying pathology and a single lesion is not uncommon among individuals without pathologic evidence of SVD,26 we categorized the number of CMBs into 0, 1, 2, and ≥3 CMBs per person on the basis of the skewed distribution of CMBs counts.
We first estimated the association between the CMB count categories at baseline and subsequent cognitive decline by multiple linear regression analyses. The change scores for processing speed and global cognitive function were skewed, so we transformed them with a natural logarithmic transformation. All analyses were initially adjusted for age and sex (model 1), followed by further adjustment for coil type, the time interval between the 2 waves of neuropsychological tests, primary education level, depressive symptomology at follow-up, hypertension, total cholesterol, use of statin, brain infarcts, white matter hyperintensity volume as a percentage of total intracranial volume, and APOE ε4 carriership (model 2). We evaluated the interactions between CMBs and other covariates with respect to effects on cognitive decline by including cross-product terms of each covariate with CMBs in the fully adjusted models. Second, we investigated the association between CMB count categories and incident dementia. Given that events for dementia, Alzheimer disease (AD), and vascular dementia (VaD) are relatively rare (n < 5) within some CMB count categories, we merged 4 categories into 3 categories (0, 1 or 2, and ≥3 CMBs) and applied the Fisher exact test to examine the associations with dementia (there were not enough events to obtain reliable estimates of the odds ratios with logistic regressions). All analyses were repeated according to CMB location. To test the robustness of the results, we did several sensitivity analyses, details of which are described in appendix e-1. Analyses were performed with SAS version 9.3. Associations were considered significant at the 0.05 level. Given that multiple a priori statistical tests were performed, the likelihood of type I errors increased. Statistical adjustment would be overly stringent because cognitive domains were correlated, and we acknowledge that very small p values tended to indicate replicable associations.14,27
RESULTS
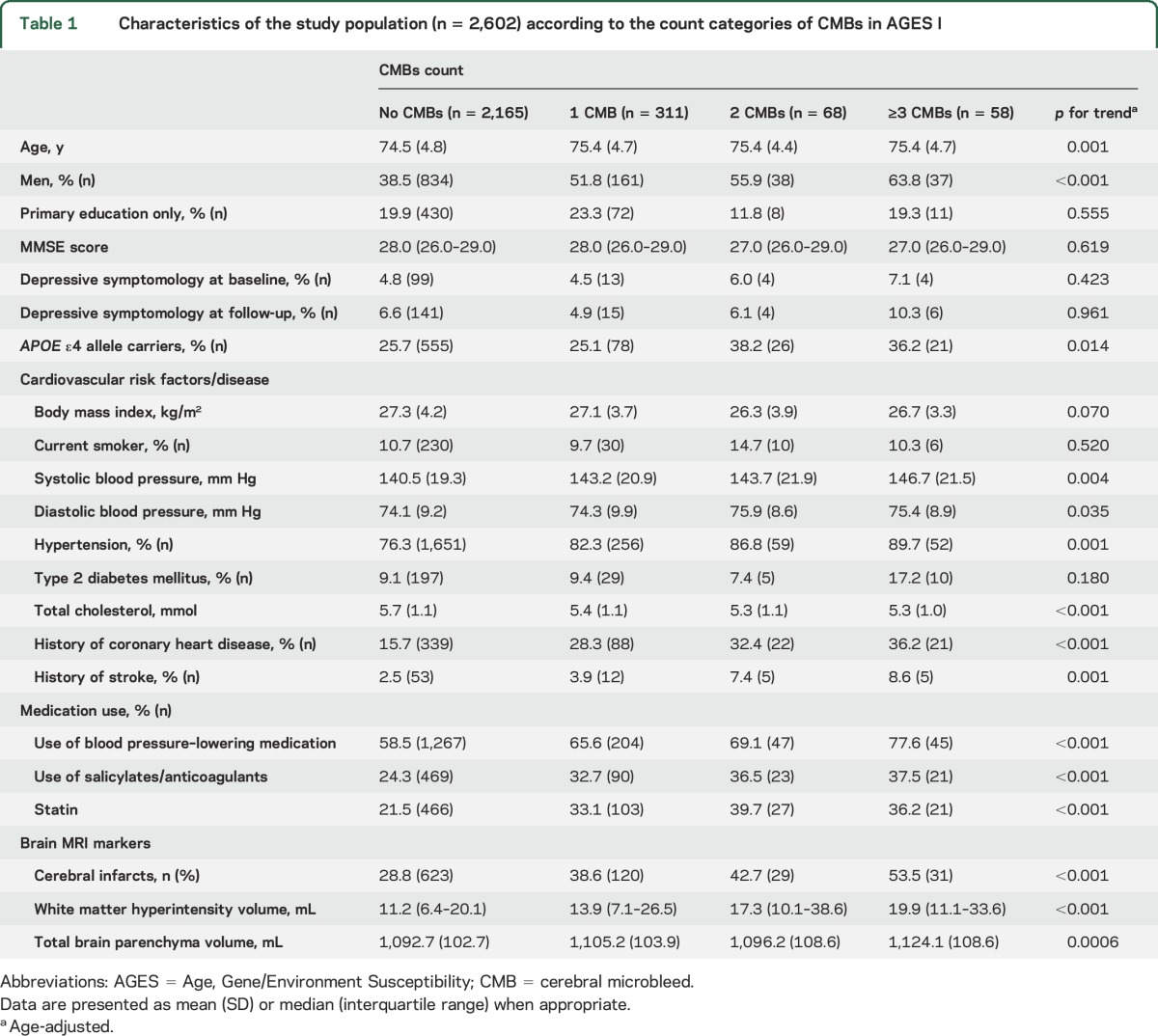
Of the 2,602 total participants, the mean age at baseline was 74.6 years (SD 4.8 years), and 41% were men. The prevalence of CMBs was 16.8% (n = 437) (median number of CMBs 1 [range 1–21]), of a single CMB was 12.0%, of 2 CMBs was 2.6%, and of ≥3 CMBs was 2.2% (table 1). Compared to participants with no CMBs, the other 3 CMB groups were older and more likely to be male, to be APOE ε4 allele carriers and hypertensive, to use statin and antithrombotic medications, to have lower average total cholesterol level and higher total brain volume, and to have cardiovascular disease and ischemic vascular lesions on brain MRI.
Table 1.
Characteristics of the study population (n = 2,602) according to the count categories of CMBs in AGES I
Among participants with CMBs (n = 437), 71.2% (n = 311) had CMBs in a strictly lobar location, 13.3% (n = 58) had CMBs in deep or mixed locations (including those with mixed CMBs in both deep and lobar locations, n = 25), and 15.5% (n = 68) had CMBs in the cerebellum. Among those with ≥3 CMBs (n = 58), 55.2% (n = 32) had strictly lobar CMBs, 27.6% (n = 16) had deep or mixed CMBs, and 17.2% (n = 10) had cerebellar CMBs.
Prevalent CMBs at baseline and cognitive decline.
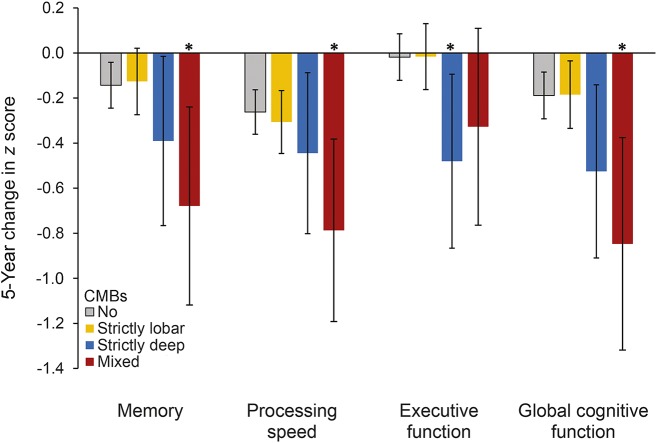
In the fully adjusted models, presence of deep or mixed CMBs was significantly associated with a steeper decline in a composite measure of global cognitive function and specifically in performance on all 3 cognitive domains: memory, information processing speed, and executive function (table 2). Mixed CMBs were most strongly associated with a decline in memory and speed (figure 2 and table e-1). Presence of strictly lobar CMBs was not associated with cognitive decline. Compared to participants with no CMBs, those with ≥3 CMBs had a greater decline in, separately, global cognition, memory, and speed. Associations in those with ≥3 CMBs in deep or mixed locations were strongest for memory. Participants with ≥3 CMBs in a strictly lobar location had significantly greater decline in processing speed. No association was observed for cerebellar CMBs with respect to CMB count categories or location (table e-2).
Table 2.
Association (β regression coefficient [95% confidence interval]) of prevalent CMBs with 5-year cognitive changea (decline in z scores) between baseline and follow-up in those who were free of prevalent dementia at baseline (n = 2,561)b
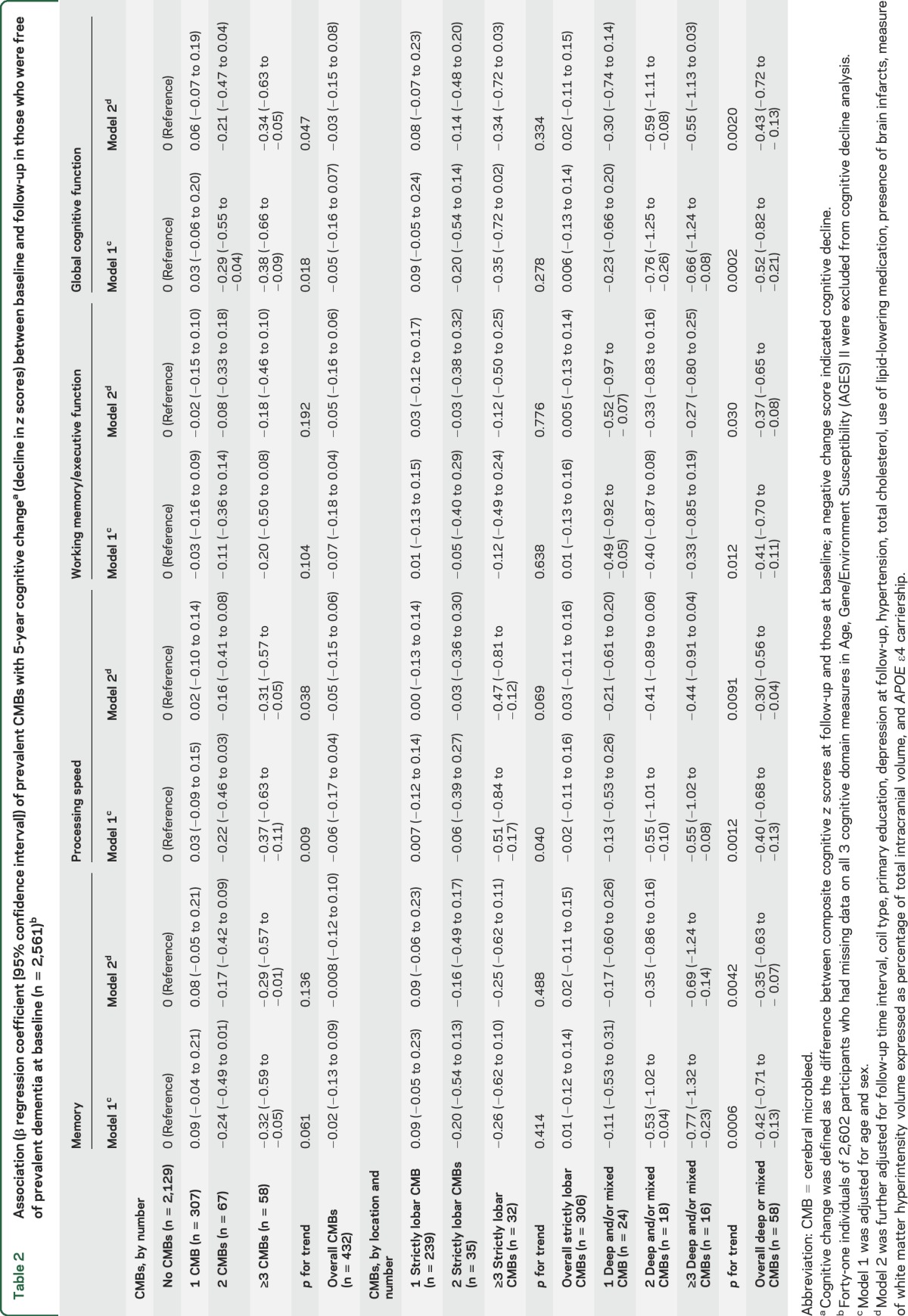
Figure 2. Multivariable-adjusted 5-year change in cognitive domains for CMB locations.
Error bars represent 95% confidence intervals. Adjusted for age, sex, follow-up interval, coil type, primary education, depression at follow-up, hypertension, total cholesterol, use of lipid-lowering medication, presence of brain infarcts, measure of white matter hyperintensity volume expressed as percentage of total intracranial volume, and APOE ε4 carriership. *p < 0.05.
Prevalent CMBs at baseline and incident dementia.
Over a mean follow-up of 5.2 years (SD 0.2 years), 4.5% of participants (n = 119) developed all-cause dementia, of whom 68.9% (n = 82) had AD, 14.3% (n = 17) had VaD, and 3.4% (n = 4) had both possible AD and possible VaD. The remaining 16 cases were attributed to other subtypes such as dementia in Parkinson disease and Lewy body dementia. The cumulative incidence of dementia and its subtypes according to CMBs count and location is shown in table 3.
Table 3.
Association of prevalent CMBs with incident dementia (n = 2,601)a
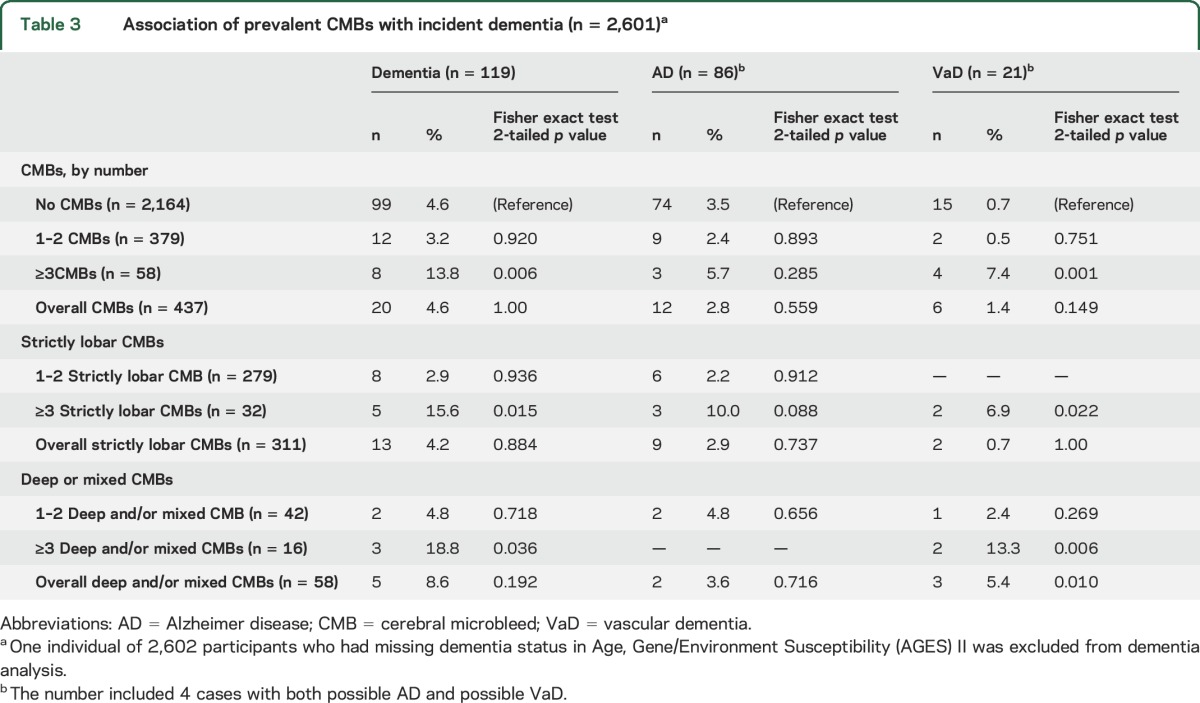
The presence of deep or mixed CMBs was associated with a higher incidence of VaD. Compared to those with no CMBs, participants with ≥3 CMBs had a higher incidence of all-cause dementia and VaD. Similar association patterns were observed for those with ≥3 strictly lobar CMBs or with ≥3 deep or mixed CMBs. Cerebellar CMBs revealed no significant associations (table e-2).
Sensitivity analyses.
The association between mixed CMBs and cognitive decline persisted for memory and global cognitive function, while the association with processing speed lost statistical significance after further controlling for CMBs count (table e-3). Additional adjustment for the use of anticoagulants/salicylates, brain atrophy, or prevalent stroke or analyses with imputed covariate values generated similar results with respect to the associations between CMBs and cognitive decline. When including participants with baseline prevalent dementia (n = 37) in the analysis, we observed similar slopes on cognitive decline (table e-4).
DISCUSSION
In a community-based sample of older people free of prevalent dementia at baseline, we found that mixed CMBs or ≥3 CMBs, with some specificity for location, are associated with accelerated cognitive decline. These results were independent of education level, depression, APOE ε4 carriership, cardiovascular risk factors, and other MRI markers of cerebrovascular disease, including brain atrophy. Furthermore, there is suggestive evidence of a higher rate of incident dementia and VaD in participants with ≥3 CMBs.
Previously, 2 small longitudinal studies13,28 among selected individuals or clinic-based patients have reported an association between multiple or mixed CMBs and cognitive decline or dementia. Recently, another large population-based study showed an association of a high number of lobar CMBs with cognitive decline in executive function and processing speed.14 Those investigators did not find an association with deep or mixed CMBs. However, compared to the AGES-Reykjavik study, the cohort had a lower mean age and fewer cardiovascular risk factors, including hypertension and duration of hypertension, which may moderate the associations we found. The current results thus add significantly to our understanding of the cognitive consequences of CMBs in a large, well-described community-based sample of older adults free of dementia. Our findings are consistent with the hypothesis that CMBs, especially mixed or a high load of CMBs, exert deleterious effects on cognitive decline, eventually leading to full-blown dementia.
Although the precise underlying mechanisms of the observed associations between, on the one hand, mixed or a higher number (≥3) of CMBs and, on the other, accelerated cognitive decline have not been established, there are several possible explanations. CMBs may reflect focal damage of brain tissue and concomitant microstructural damage of the surrounding tissue (e.g., microinfarcts or gliosis).29 As a result, they may disrupt connections of functionally important cortical and subcortical tracts that are critical for cognitive processes, ultimately leading to damage of neural networks and interfering with cognition.29 Alternatively, CMBs are more likely to imply more generalized microvascular damage that disrupts the blood-brain barrier or causes hypoxia.13,29 In this scenario, CMBs are only the tip of the iceberg of SVD, and mixed or a higher number of CMBs may thus indicate more extensive and severe subclinical microvascular damage.
We found that the associations with cognitive decline differed according to the spatial location of CMBs and thus possibly differed with underlying vasculopathy. Our results suggest that ≥3 CMBs in strictly lobar regions, presumably resulting from CAA, were related to a faster decline in processing speed. On the other hand, participants with ≥3 strictly lobar CMBs had the highest prevalence of cerebral infarcts and largest volume of white matter hyperintensities at baseline compared to those with no, 1, or 2 strictly lobar CMBs in the present study. Thus, this finding of impaired speed associated with a relatively high load of lobar CMBs, shown previously,8,12 suggests that the vascular damage and ischemia30 caused by (or predisposing to) CAA31 can reflect overall vascular damage.
Presence of CMBs in deep or mixed regions, resulting primarily from hypertensive arteriopathy, was associated with more rapid decline in all cognitive domains, and in particular, our results suggest that ≥3 CMBs in deep or mixed regions were associated with the fastest decline in verbal memory. The associations with speed and executive function are consistent with the hypothesis that cerebral microvascular damage preferentially affects white matter and subcortical gray matter, with disruption of integrity of frontal-subcortical circuits.22,32 Our finding of deep or mixed CMBs in association with verbal memory, independently of brain atrophy, might suggest disruption of thalamic nuclei, which are involved in storage and short-term memory,32 and is consistent with memory impairment, the hallmark of AD, also being present in vascular-related cognitive impairment.31
Of note, mixed CMBs were most strongly associated with a decline in memory and global cognitive function compared to CMBs either in strictly lobar or in strictly deep locations. These findings may also underscore the role of interplay between hypertensive vasculopathy and CAA in the pathogenesis of cognitive decline. It is possible that vascular β-amyloid deposition impairs reactivity of cerebral microvasculature and causes functional loss with ischemic and hemorrhagic lesions.6 In parallel, hypertensive damage to small vessels impairs arterial pulsation and results in failure of perivascular drainage, which reduces the clearance of β-amyloid and leads to further deposition of β-amyloid in vessel walls.6,33
Apart from cognitive decline, our findings pointed in the direction of adverse effects of ≥3 CMBs on dementia incidence, particularly for VaD. However, the relatively small number of subtyped dementia events limited our ability to conduct in-depth, adjusted statistical analysis. Further meta-analysis of individual population-based studies with adequate statistical power is warranted.
Major strengths of the present study include the longitudinal design, the large population-based sample of old people without dementia at baseline who were followed up for 5 years on average, the use of a comprehensive cognitive battery and standard MRI, reliable assessment of baseline CMBs, and the extensive characterization of participants that enabled us to control for a series of potential confounders, including other MRI markers of cerebrovascular disease.
There are several issues, however, that affect the interpretation of these data. Although we administered a wide range of neuropsychological tests to assess each particular cognitive domain, there is still a chance to underestimate the complex executive functions, which is the leading presentation of cognitive decline in vascular disease. Moreover, pure VaD is relatively rare, and the current diagnostic criteria for VaD have low sensitivity.25,34 We cannot fully rule out the possibility that the presence of CMBs may have provided information that weighed the diagnosis toward VaD. The categorization of CMBs by location is presumably suggestive of specific underlying small vessel pathology. Although in line with current research and clinical practice, the categorization does not accurately reflect the multifactorial nature of CMBs35 and thus may be an oversimplification of underlying cognition-related pathology of location-specific CMBs. Furthermore, the relatively lower field strength and spatial resolution of the MRI scanner we used may have resulted in underestimation of the number of CMBs and affected the assessment of other MRI lesions. Finally, people who were included in the analysis were younger, were more educated, and had better vascular health and cognitive profiles at baseline than those who were excluded. If those excluded were affected by CMBs similarly to those included in the analysis, our results may be underestimated.
Our findings support the hypothesis that CMBs are important indicators of a microvascular contribution to cognitive impairment in older people and highlight the role for hypertensive vasculopathy and the combined effect of hypertensive angiography and CAA in cognitive deterioration. Multimodal neuroimaging assessments of brain metabolism, fiber tract integrity, and amyloid burden may help explain the underlying pathophysiology and integration of vascular and neurodegenerative lesions of CMBs and associated cognitive impairment.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- AGES
Age, Gene/Environment Susceptibility
- CAA
cerebral amyloid angiopathy
- CMB
cerebral microbleed
- SVD
small vessel disease
- VaD
vascular dementia
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
J.D., M.A.v.B., V.G., and L.J.L. designed the study. S.S., G.E., P.V.J., O.K., O.L.L., M.A.v.B., V.G., and L.J.L. directed implementation of the study and collected data. J.D. and O.M. carried out statistical analyses. J.D. and L.J.L. drafted the manuscript. All authors were involved in the critical revision of the intellectual content of this manuscript.
STUDY FUNDING
The AGES-Reykjavik Study was funded by the NIH (contract N01-AG-12100), Intramural Research Program of the National Institute on Aging, Icelandic Heart Association, and Icelandic Parliament. None of the funding organizations or sponsors were involved in study design; in collection, analysis, or interpretation of data; in writing of the report; or in the decision to submit the paper for publication.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol 2010;120:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fotuhi M, Hachinski V, Whitehouse PJ. Changing perspectives regarding late-life dementia. Nat Rev Neurol 2009;5:649–658. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer's disease: innocent observation or key player? Brain 2011;134(pt 2):335–344. [DOI] [PubMed] [Google Scholar]
- 6.Charidimou A, Werring DJ. Cerebral microbleeds and cognition in cerebrovascular disease: an update. J Neurol Sci 2012;322:50–55. [DOI] [PubMed] [Google Scholar]
- 7.Qiu C, Cotch MF, Sigurdsson S, et al. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology 2010;75:2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poels MM, Ikram MA, van der Lugt A, et al. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology 2012;78:326–333. [DOI] [PubMed] [Google Scholar]
- 9.Yakushiji Y, Noguchi T, Hara M, et al. Distributional impact of brain microbleeds on global cognitive function in adults without neurological disorder. Stroke 2012;43:1800–1805. [DOI] [PubMed] [Google Scholar]
- 10.Hilal S, Saini M, Tan CS, et al. Cerebral microbleeds and cognition: the Epidemiology of Dementia in Singapore Study. Alzheimer Dis Assoc Disord 2014;28:106–112. [DOI] [PubMed] [Google Scholar]
- 11.Takashima Y, Mori T, Hashimoto M, et al. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. J Stroke Cerebrovasc Dis 2011;20:105–110. [DOI] [PubMed] [Google Scholar]
- 12.Meier IB, Gu Y, Guzaman VA, et al. Lobar microbleeds are associated with a decline in executive functioning in older adults. Cerebrovasc Dis 2014;38:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miwa K, Tanaka M, Okazaki S, et al. Multiple or mixed cerebral microbleeds and dementia in patients with vascular risk factors. Neurology 2014;83:646–653. [DOI] [PubMed] [Google Scholar]
- 14.Akoudad S, Wolters FJ, Viswanathan A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol 2016;73:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol 2007;165:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sveinbjornsdottir S, Sigurdsson S, Aspelund T, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J Neurol Neurosurg Psychiatry 2008;79:1002–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Sigurdsson S, Garcia M, et al. Risk factors associated with incident cerebral microbleeds according to location in older people: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study. JAMA Neurol 2015;72:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh Y, Yamada M, Hayakawa M, Otomo E, Miyatake T. Cerebral amyloid angiopathy: a significant cause of cerebellar as well as lobar cerebral hemorrhage in the elderly. J Neurol Sci 1993;116:135–141. [DOI] [PubMed] [Google Scholar]
- 19.De Reuck JL, Deramecourt V, Auger F, et al. The significance of cortical cerebellar microbleeds and microinfarcts in neurodegenerative and cerebrovascular diseases: a post-mortem 7.0-tesla magnetic resonance study with neuropathological correlates. Cerebrovasc Dis 2015;39:138–143. [DOI] [PubMed] [Google Scholar]
- 20.Saczynski JS, Sigurdsson S, Jonsdottir MK, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke 2009;40:677–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 2005;128(pt 9):2034–2041. [DOI] [PubMed] [Google Scholar]
- 22.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA 2002;287:742–748. [DOI] [PubMed] [Google Scholar]
- 23.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 24.Chui HC, Victoroff JI, Margolin D, Jagust W, Shankle R, Katzman R. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer's disease diagnostic and treatment centers. Neurology 1992;42(pt 1):473–480. [DOI] [PubMed] [Google Scholar]
- 25.Lopez OL, Kuller LH, Becker JT, et al. Classification of vascular dementia in the Cardiovascular Health Study Cognition Study. Neurology 2005;64:1539–1547. [DOI] [PubMed] [Google Scholar]
- 26.De Reuck J, Deramecourt V, Cordonnier C, Leys D, Maurage CA, Pasquier F. The impact of cerebral amyloid angiopathy on the occurrence of cerebrovascular lesions in demented patients with Alzheimer features: a neuropathological study. Eur J Neurol 2011;18:913–918. [DOI] [PubMed] [Google Scholar]
- 27.Ding J, Strachan MW, Fowkes FG, et al. Association of retinal arteriolar dilatation with lower verbal memory: the Edinburgh Type 2 Diabetes Study. Diabetologia 2011;54:1653–1662. [DOI] [PubMed] [Google Scholar]
- 28.Chiang GC, Cruz Hernandez JC, Kantarci K, Jack CR Jr, Weiner MW; Alzheimer's Disease Neuroimaging Initiative. Cerebral microbleeds, CSF p-tau, and cognitive decline: significance of anatomic distribution. AJNR Am J Neuroradiol 2015;36:1635–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Werring DJ, Gregoire SM, Cipolotti L. Cerebral microbleeds and vascular cognitive impairment. J Neurol Sci 2010;299:131–135. [DOI] [PubMed] [Google Scholar]
- 30.Park JH, Seo SW, Kim C, et al. Pathogenesis of cerebral microbleeds: in vivo imaging of amyloid and subcortical ischemic small vessel disease in 226 individuals with cognitive impairment. Ann Neurol 2013;73:584–593. [DOI] [PubMed] [Google Scholar]
- 31.Resnick SM, Sojkova J, Zhou Y, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology 2010;74:807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222. [DOI] [PubMed] [Google Scholar]
- 33.Tarasoff-Conway JM, Carare RO, Osorio RS, et al. Clearance systems in the brain: implications for Alzheimer disease. Nat Rev Neurol 2015;11:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1143–1153. [DOI] [PubMed] [Google Scholar]
- 35.Akoudad S, Portegies ML, Koudstaal PJ, et al. Cerebral microbleeds are associated with an increased risk of stroke: the Rotterdam study. Circulation 2015;132:509–516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.