Abstract
Background
We assessed the cancer risks of four different Finnish asbestos-exposed cohorts. We also explored if the cohorts with varying profiles of asbestos exposure exhibited varying relative risks of cancer.
Methods
The incident cancer cases for the asbestos-exposed worker cohorts were updated to the end of 2012 using the files of the Finnish Cancer Registry. The previously formed cohorts consisted of asbestos mine workers, asbestosis patients, asbestos sprayers, and workers who had taken part in a screening study based on asbestos exposure at work.
Results
The standardized incidence ratio (SIR) for mesothelioma varied from about threefold to > 100-fold in the different cohorts. In the screening cohort the SIR for mesothelioma was highest in 2003–2007, In other cohorts it was more constant in 5-year period inspection. The SIR for lung cancer was about twofold to tenfold in all except the screening cohort. Asbestos sprayers were at the highest risk of mesothelioma and lung cancer.
Conclusion
The SIR for mesothelioma is high in all of the cohorts that represent different kinds of asbestos exposure. The smaller SIR for mesothelioma in the screening cohort with lowest level of asbestos exposure might suggest dose-responsiveness between asbestos exposure and mesothelioma. It does seem that the highest risk of lung cancer in these cohorts except in the youngest of the cohorts, the screening cohort, is over. The highest SIR for lung cancer of the asbestosis patient and sprayers cohort is explained by their heavy asbestos exposure.
Keywords: asbestos exposure, cohort study, follow-up, lung cancer, mesothelioma
1. Introduction
Asbestos is the name given to a group of naturally occurring fibrous minerals. The two main groups of these minerals are serpentine, which includes chrysotile; and amphiboles, which includes crocidolite, anthophyllite, and amosite. The adverse health effects of asbestos have been known since the first half of the 20th century. When handled, asbestos fibers may emanate into the air and be easily inhaled. After inhalation, fibers end up in the smallest bronchial tubes and the alveoli. They can also be transferred via the lymphatic veins to different parts of the body. Asbestos exposure may cause, for example, pleural plaques, pleural effusion, pulmonary fibrosis (asbestosis), lung cancer, and mesothelioma of the pleura or peritoneum. The Helsinki Criteria for the diagnosis and attribution of asbestos were updated in 2014. Laryngeal and ovarian cancers were considered asbestos-caused diseases [1], [2]; the International Agency for Research of Cancer (IARC) found sufficient evidence of asbestos causation of these cancers in humans [3], [4].
The latency period of asbestos diseases is from 10 years to 40 years or even longer. All types of asbestos fibers are known to cause health hazards, crocidolite being the most potent. After the widespread use of asbestos in the 20th century, hundreds of thousands of workers in industrialized countries have contracted an asbestos-related disease. According to the World Health Organization (WHO) estimates, > 107,000 people die each year from asbestos-related lung cancer, mesothelioma, and asbestosis resulting from occupational exposures [5].
In Finland, unlike in other countries, anthophyllite asbestos has been widely used because of its domestic production in the Paakkila and Maljasalmi mines in 1918‒1975. Approximately 40% of all asbestos used in Finland was anthophyllite. Finland also imported asbestos in the forms of chrysotile, amosite, and crocidolite. Asbestos was widely used in construction materials, especially during the 1960s and 1970s. Although all uses of asbestos were banned by 1994 in Finland, it is estimated that half of the asbestos that was previously used in construction is still in place. Due to earlier asbestos exposure, new asbestos-related diseases are still diagnosed in Finland. The numbers of work-related asbestos-induced lung cancer and mesothelioma cases show an almost flat trend, with ∼50 new cases of lung cancer and 50 new cases of mesothelioma emerging each year in Finland [6]. It is estimated that the incidence of mesothelioma and lung cancer will begin to diminish by the end of the 2010s. The number of registered asbestosis cases has already fallen by ∼50% from the highest values of the mid-1990s.
In this study we assessed the cancer risks of four different Finnish asbestos-exposed cohorts. Our main aim was to determine whether the cessation of the use of asbestos some 20 years ago has affected the risks of asbestos-related cancers. We also explored whether the cohorts with somewhat varying profiles of asbestos exposure exhibited varying relative risks of cancer and whether an excess of cancers of the larynx or ovaries could be identified in these cohorts.
2. Materials and methods
Table 1 shows the number of workers in the different cohorts and subcohorts, followup period, person-years, and the proportion of men and tobacco smokers among the workers.
Table 1.
The number of workers (N), followup period, and person years at followup, and percentages of men and current and ex-smokers in the different cohorts and subcohorts
| Cohort | n | Follow up period | Person y by age |
Men % | Smokers %∗ | |
|---|---|---|---|---|---|---|
| < 60 y | ≥ 60 y | |||||
| Asbestos mine workers | 734 | 1967–2012 | 11,049 | 8,333 | 80 | 67 |
| Moderate exposure | 257 | 4,316 | 3,199 | 76 | 55 | |
| Heavy exposure | 477 | 6,733 | 5,134 | 82 | 74 | |
| Asbestosis patients | 128 | 1978–2012 | 684 | 1,211 | 92 | 82 |
| Asbestos sprayers | 133 | 1967–2012 | 3,468 | 504 | 97 | 83 |
| Screening cohort | 24,214 | 1988–2012 | 187,024 | 254,849 | 96 | 69 |
| Construction | 17,236 | 130,343 | 187,724 | 97 | 70 | |
| Shipyard | 117 | 283 | 1,658 | 91 | 67 | |
| Asbestos industry | 496 | 4,120 | 4,619 | 80 | 61 | |
| Spontaneous† | 672 | 7,965 | 5,085 | 94 | 68 | |
| Questionnaire‡ | 5,693 | 44,313 | 55,763 | 96 | NA | |
NA, not applicable.
Percentage out of persons with known smoking status.
Those who spontaneously contacted the researchers willing to participate.
Those who only answered the preliminary questionnaire.
“Asbestos mine workers” is the oldest cohort, and comprises workers of the former Paakkila and Maljasalmi anthophyllite asbestos mines in Finland. The Paakkila mine began operating in 1918; Maljasalmi in 1943. A total of 734 workers were followed up: they had been employed in these mines for at least 3 months between January 1953 and July 1967. Altogether 28% of the workers were exposed for over 5 years. Workers of the mine and the refinery were considered to have heavy asbestos exposure while the rest of the personnel had moderate asbestos exposure. [7]. The Maljasalmi mine was closed in 1953, and the Paakkila mine in 1975. It is possible that these workers were also exposed to asbestos afterwards in, for example, the construction industry.
The “asbestosis patient” cohort was formed in 1977‒1985, and consisted of patients who visited the Finnish Institute of Occupational Health for a periodic health examination due to previously diagnosed asbestosis. They had worked as insulation (53 workers), asbestos mine (24 cases) or asbestos cement factory (24 cases) workers, sprayers (14 cases), or in other asbestos-exposing work (13 cases). Their mean duration of asbestos exposure was 21 (range, 4‒40) years. [8]
The “asbestos sprayer” cohort consisted of 133 asbestos sprayers who were identified in 1987 from employee registers and other sources. The mean duration of the asbestos exposure of the 60 sprayers who took part in the health examinations was 3 (range, 0.2‒13) years. Asbestos spraying with crocidolite was performed in Finland between the years 1955 and 1976, after which spraying and the use of crocidolite was prohibited [8].
The largest and most recent of the four cohorts is the “asbestos screening” cohort. The asbestos-related illnesses of exposed workers were screened in Finland in 1990‒1992. A questionnaire was sent to study participants, based on the registers of trade unions and employment pension institutions. The final cohort consisted of 24,214 people. At the time of the screening project, the mean duration of work that exposed workers to asbestos was 26 years. A total of 71% of the workers of this cohort were construction workers who had a history of at least 10 years of employment in the construction industry, starting before 1980. Asbestos use in construction materials in Finland ceased at the end of the 1980s, which can be regarded as materially decreasing any potential exposure in the construction industry. Of course, the material that was already in place also contained asbestos, which may have caused some exposure in renovation work even after the end of the 1980s. In Finland, asbestos cement products were manufactured between 1923 and 1988. Asbestos cement contained 10‒15% chrysotile. In shipbuilding, crocidolite asbestos was sprayed in 1955‒1975. Boards containing amosite were used in the interior furnishing of ships until the 1970s [9]. The screening cohort also had 672 workers who participated voluntarily. Their interviewed asbestos exposure was similar to the other workers' in the cohort. There were also 5,693 workers who answered the preliminary questionnaire but did not participate in the actual screening study. Their detailed asbestos exposure remained unclear, although they had been employed in asbestos exposing industry over 10 years [10].
The participants were identified and followed up for death and emigration in 1967–1994 via the Population Register Centre, using the unique identification number given to everyone residing in Finland since January 1, 1967 as the key. Three men from the cohort of asbestos sprayers had to be excluded because of missing identification data. Follow-up for cancer was carried out automatically using the files of the Finnish Cancer Registry. The follow-up started on January 1, 1967; on January 1 of the year following the first periodic health examination since 1977 for the asbestosis patients, on January 1 of the year following the year of first employment as an asbestos sprayer, or from the date of screening. or from January 1 1991 for the screening cohort. The calculation of person-years ended at emigration or death, or on December 31, 2012, whichever occurred first. The number of incident cancer cases and the person-years at risk, were counted separately for 5-year (1st period 6 years) calendar periods (1967–1972, 1973–1977, 1978‒1982, 1983‒1987, 1988‒1992, 1993‒1997, 1998‒2002, 2003‒2007, and 2008‒2012). The expected numbers of cases for total cancer and specific cancer types were calculated by multiplying the number of person-years in each age group by the corresponding average cancer incidence in Finland during the period of observation.
As we did not know the smoking habits of the workers after the cohorts were formed, we analyzed smokers and ex-smokers together. As no smoking-specific reference data were available for cancer incidence, the smoking-specific risks of cancer were calculated using the expected numbers of the general Finnish population.
To calculate the standardized incidence ratio (SIR), the observed number of cases was divided by the expected number. Exact 95% confidence intervals (CI) were defined on the presumption that the number of observed cases followed a Poisson distribution.
3. Results
3.1. Mesothelioma
There were altogether 108 mesotheliomas during the follow-up period. The SIR varied from about threefold to > 100-fold in the different cohorts (Table 2). Table 3 shows the SIRs for mesothelioma and lung cancer in the four cohorts in the 5-year calendar periods of the follow-up. The asbestos mine workers' as well as asbestosis patients' and sprayers' SIRs for mesothelioma have been highest in the 1980s and 1990s. In the screening cohort the highest SIR for mesothelioma was in the beginning of this century.
Table 2.
The mesothelioma incidence of the cohorts 1967–2012, men and women
| Cohort | O | SIR | 95% CI |
|---|---|---|---|
| Asbestos mine workers | 8 | 13.2 | 5.70–26.0 |
| Moderate exposure | 2 | 8.81 | 1.07–31.8 |
| Heavy exposure | 6 | 15.8 | 5.81–34.4 |
| Asbestosis patients | 5 | 49.6 | 16.1–115 |
| Asbestos sprayers | 11 | 127 | 63.3–226 |
| Screening cohort | 84 | 2.95 | 2.35–3.65 |
| Construction | 56 | 2.66 | 2.01–3.44 |
| Shipyard | 0 | 0.00 | 0.00–22.0 |
| Asbestos industry | 5 | 11.0 | 3.57–25.7 |
| Spontaneous ∗ | 12 | 18.4 | 9.53–32.2 |
| Questionnaire † | 11 | 1.79 | 0.90–3.21 |
95% CI, 95% confidence interval; O, observed number; SIR, standardized incidence ratio.
Those who spontaneously contacted the researchers willing to participate.
Those who only answered the preliminary questionnaire.
Table 3.
The mesothelioma and lung cancer incidence of the cohorts in 5-year periods, men and women
| Cancer type | Period | Asbestos mine workers |
Asbestosis patients |
Asbestos sprayers |
Screening cohort |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | ||
| Mesothelioma | 1967–1972 | 0 | 0.00 | 0.00–300 | NA | 0 | 0.00 | 0.00–7,180 | NA | ||||
| 1973–1977 | 0 | 0.00 | 0.00–199 | 0 | 0.00 | 0.00–572,000 | 0 | 0.00 | 0.00–3,580 | NA | |||
| 1978–1982 | 0 | 0.00 | 0.00–84.8 | 0 | 0.00 | 0.00–533 | 0 | 0.00 | 0.00–1,870 | NA | |||
| 1983–1987 | 1 | 14.9 | 0.38–82.9 | 2 | 108 | 13.1–391 | 1 | 204 | 5.16–1,140 | NA | |||
| 1988–1992 | 2 | 25.9 | 3.13–93.6 | 1 | 53.4 | 1.35–297 | 3 | 395 | 81.4–1,150 | 1 | 0.90 | 0.02–4.99 | |
| 1993–1997 | 3 | 33.9 | 7.00–99.2 | 0 | 0.00 | 0.00–207 | 2 | 186 | 22.5–670 | 15 | 3.19 | 1.79–5.26 | |
| 1998–2002 | 0 | 0.00 | 0.00–37.0 | 1 | 58.77 | 1.49–327 | 2 | 119.95 | 14.5–433 | 15 | 2.34 | 1.31–3.85 | |
| 2003–2007 | 1 | 9.64 | 0.24–53.7 | 0 | 0.00 | 0.00–286 | 0 | 0.00 | 0.00–171 | 37 | 4.69 | 3.30–6.45 | |
| 2008–2012 | 1 | 10.5 | 0.26–58.3 | 1 | 111 | 2.81–618 | 3 | 138 | 28.5–403 | 16 | 1.91 | 1.09–3.10 | |
| Lung cancer | 1967–1972 | 14 | 4.09 | 2.24–6.86 | NA | 1 | 44.2 | 1.12–246 | NA | ||||
| 1973–1977 | 15 | 3.90 | 2.18–6.42 | 0 | 0.00 | 0.00–2,750 | 1 | 23.3 | 0.59–130 | NA | |||
| 1978–1982 | 8 | 1.86 | 0.80–3.66 | 7 | 8.94 | 3.59–18.4 | 3 | 33.1 | 6.81–96.6 | NA | |||
| 1983–1987 | 16 | 3.67 | 2.10–5.95 | 15 | 12.3 | 6.90–20.3 | 3 | 21.4 | 4.42–62.6 | NA | |||
| 1988–1992 | 5 | 1.31 | 0.42–3.04 | 9 | 8.93 | 4.08–17.0 | 1 | 5.33 | 0.13–29.7 | 65 | 1.27 | 0.98–1.62 | |
| 1993–1997 | 8 | 2.26 | 0.97–4.44 | 6 | 7.51 | 2.76–16.4 | 3 | 11.4 | 2.35–33.3 | 196 | 1.11 | 0.96–1.26 | |
| 1998–2002 | 3 | 0.93 | 0.19–2.73 | 2 | 3.52 | 0.43–12.7 | 4 | 11.70 | 3.19–30.0 | 230 | 1.21 | 1.05–1.36 | |
| 2003–2007 | 6 | 2.09 | 0.77–4.54 | 2 | 4.86 | 0.59–17.5 | 4 | 9.81 | 2.67–25.1 | 251 | 1.25 | 1.10–1.40 | |
| 2008–2012 | 3 | 1.33 | 0.27–3.89 | 0 | 0.00 | 0.00–16.9 | 2 | 4.50 | 0.54–16.3 | 252 | 1.34 | 1.18–1.51 | |
95% CI, 95% confidence interval; NA, not applicable; O, observed number; SIR, standardized incidence ratio.
3.2. Lung cancer
During the follow-up, we observed a total of 1,135 lung cancers among men and women in these four cohorts (Table 2). The SIRs for lung cancer to be seen in the 5-year period inspection seem to have diminished since late 1990s in all except the screening cohort (Table 3).
The screening cohort had 6,197 cancer cases in all and 994 lung cancer cases (SIR 1.23, 95% CI 1.16–1.30; Table 2). Table 4 presents lung cancer SIRs in the different cohorts and subcohorts. The highest SIRs for lung cancer were among smokers in these different exposure groups except for the asbestos sprayer cohort.
Table 4.
The lung cancer incidence of the different cohorts and the subcohorts of the asbestos mine workers and the screening cohorts, men and women
| Cohort | Smoking category | O | SIR | 95% CI |
|---|---|---|---|---|
| Asbestos mine workers∗ | Nonsmokers | 3 | 0.36 | 0.07–1.05 |
| Smokers | 75 | 3.21 | 2.53–4.02 | |
| All smoking categories | 78 | 2.46 | 1.95–3.07 | |
| Moderate exposure | Nonsmokers | 1 | 0.24 | 0.01–1.32 |
| Smokers | 15 | 2.29 | 1.28–3.77 | |
| All smoking categories | 16 | 1.49 | 0.85–2.41 | |
| Heavy exposure | Nonsmokers | 2 | 0.49 | 0.06–1.76 |
| Smokers | 60 | 3.57 | 2.73–4.59 | |
| All smoking categories | 62 | 2.97 | 2.28–3.80 | |
| Asbestosis patients∗ | Nonsmokers | 1 | 0.97 | 0.02–5.38 |
| Smokers | 40 | 10.97 | 7.20–13.71 | |
| All smoking categories | 41 | 8.19 | 5.88–11.1 | |
| Asbestos sprayers | Nonsmokers | 2 | 10.4 | 1.26–37.7 |
| Smokers | 10 | 10.3 | 4.93–18.9 | |
| Unknown smoking habits | 10 | 12.9 | 6.17–23.6 | |
| All smoking categories | 22 | 11.3 | 7.10–17.2 | |
| Screening cohort | Nonsmokers | 18 | 0.09 | 0.06–0.14 |
| Smokers | 699 | 1.61 | 1.49–1.72 | |
| Unknown smoking habits | 277 | 1.53 | 1.35–1.71 | |
| All smoking categories | 994 | 1.23 | 1.16–1.30 | |
| Construction | Nonsmokers | 15 | 0.08 | 0.05–0.13 |
| Smokers | 657 | 1.58 | 1.47–1.70 | |
| Unknown smoking habits | 0 | 0.00 | 0.00–18.5 | |
| All smoking categories | 672 | 1.13 | 1.05–1.21 | |
| Shipyard∗ | Nonsmokers | 0 | 0.00 | 0.00–1.89 |
| Smokers | 8 | 2.19 | 0.95–4.32 | |
| All smoking categories | 8 | 1.43 | 0.62–2.81 | |
| Asbestos industry | Nonsmokers | 2 | 0.49 | 0.06–1.76 |
| Smokers | 16 | 2.18 | 1.25–3.54 | |
| Unknown smoking habits | 0 | 0.00 | 0.00–173 | |
| All smoking categories | 18 | 1.57 | 0.93–2.48 | |
| Spontaneous∗,† | Nonsmokers | 1 | 0.20 | 0.01–1.10 |
| Smokers | 18 | 1.91 | 1.13–3.02 | |
| All smoking categories | 19 | 1.31 | 0.79–2.05 | |
| Questionnaire‡ | All with unknown smoking habits | 277 | 1.53 | 1.35–1.71 |
95% CI, 95% confidence interval; NA, not applicable; O, observed number; SIR, standardized incidence ratio.
No persons with unknown smoking status in the cohort.
Those who spontaneously contacted the researchers willing to participate.
Those who only answered the preliminary questionnaire.
3.3. Other cancers
Table 5 shows the SIRs for all sites cancer in the different cohorts according to smoking status.
Table 5.
The all sites cancer incidence of the different cohorts 1967–2012, men and women
| Cohort | Nonsmokers |
Smokers |
Smoking unknown |
||||||
|---|---|---|---|---|---|---|---|---|---|
| O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | |
| Asbestos mine workers | 59 | 0.95 | 0.72–1.22 | 161 | 1.47 | 1.25–1.70 | NA | ||
| Asbestosis patients | 9 | 1.47 | 0.67–2.79 | 64 | 3.27 | 2.52–4.18 | NA | ||
| Asbestos sprayers | 3 | 2.16 | 0.45–6.31 | 27 | 3.91 | 2.58–5.69 | 23 | 3.91 | 2.48–5.86 |
| Screening cohort | 1,242 | 0.89 | 0.84–0.93 | 3,504 | 1.16 | 1.13–1.20 | 1,451 | 1.17 | 1.11–1.22 |
95% CI, 95% confidence interval; NA, not applicable; O, observed number; SIR, standardized incidence ratio.
We observed three laryngeal cancer cases among the nonsmoking men and women of the screening cohort (SIR 0.26, 95% CI 0.05–0.77), 34 cases among the smokers (SIR 1.31, 95% CI 0.90–1.82), and 15 cases among those whose smoking status was unknown (SIR 1.40, 95% CI 0.78–2.31). In other cohorts, only one case of laryngeal cancer was observed (data not shown).
Six cases of cancer of the ovaries were observed, all of which were in the screening cohort. Of these, four were among the nonsmokers (SIR 1.14, 95% CI 0.31–2.92) and two were among the smokers (SIR 1.02, 95% CI 0.12–3.67; data not shown).
4. Discussion
The first mesothelioma cases in the asbestos mine worker cohort exposed to anthophyllite asbestos occurred in the 1980s, which reflects the long latency period of mesothelioma, as the asbestos exposure of some of the workers had begun already in 1918 and ceased by 1975. Karjalainen et al [11] reported the first four mesotheliomas in this cohort and calculated the latency period to be 39‒58 years. The asbestos sprayers were at the highest risk of mesothelioma. This can be explained by their earlier exposure to high concentrations of crocidolite asbestos, which is considered the most potential type of asbestos to cause health problems. The asbestos exposure of the screening cohort workers varied according to their profession and working years, and the cohort also included less exposed workers. Part of the asbestos used in construction was chrysotile, which is regarded to be less potent in causing mesothelioma [4]. The question still remains, however, as to why the mesothelioma risk in the screening cohort is lower than that in the other cohorts. It is unclear whether the level of asbestos exposure has affected the risk of mesothelioma, but a recent French study found a clear dose-response relationship between occupational asbestos exposure and pleural mesothelioma [12]. One earlier study [13] showed a dose–response relationship between mesothelioma risk and the asbestos fiber concentration of lung tissue. In the future we will see whether the risk increases in the screening cohort, or stays lower, which would support the theory of dose-responsiveness.
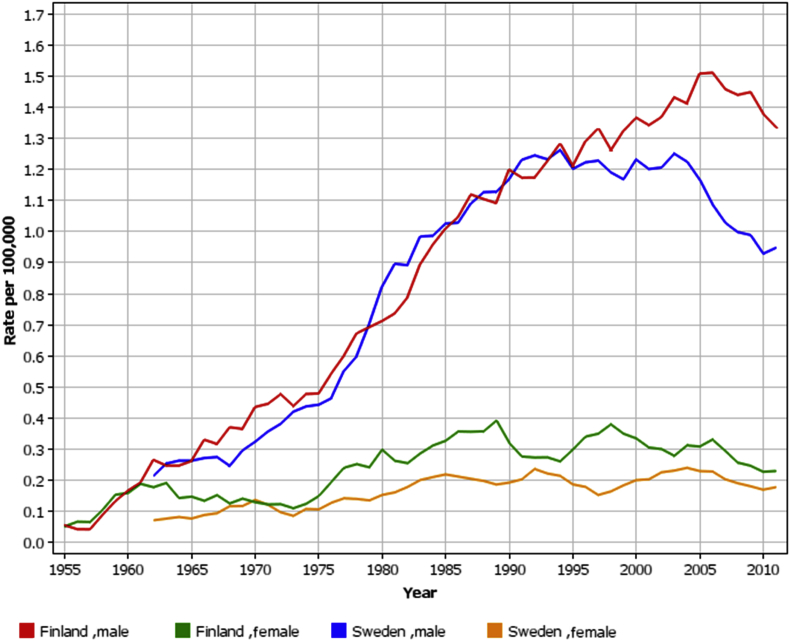
The SIRs for mesothelioma have been constant in the asbestos mine workers and sprayers cohorts (Table 3). There is no clear trend of the SIR for mesothelioma either in the asbestosis patient cohort, where the asbestos exposure ceased at the latest in 1985; because of the disease, they were not at work anymore. The SIR for mesothelioma in the screening cohort was highest in 2003–2007. The cohorts are heterogenic regarding the beginning and ending of the workers' asbestos exposure, which with the very variable latency period of mesothelioma makes it impossible to make conclusions of when the mesothelioma risk starts to diminish after the ending of asbestos exposure. The overall incidence of pleural mesothelioma has been constant in Finland in recent years, whereas in Sweden, for example, it has been decreasing since the asbestos ban in 1982, 12 years earlier than in Finland (Fig. 1) [14].
Fig. 1.
The age-standardised rate (ASR) of pleural mesothelioma in Finland 1953–2013 and Sweden 1960–2013, by sex (5-year floating averages) [15].
A previous study [16] showed that the workers of the asbestos mine and associated factory had excess overall mortality for lung cancer already in 1936‒1966, after rather a short period of asbestos exposure. In this cohort, the prevalence of smoking among men was 81%. Fourteen percent of the female workers smoked. According to Rimpelä [17], ∼60% of Finnish men smoked in the early 1960s. Thus smoking plays an important role in the excess risk of lung cancer, perhaps amplifying the effect of anthophyllite exposure. The lung cancer risk is smaller in the asbestos mine workers cohort than in the asbestosis patients and sprayers cohorts. Rather than being a sign of a smaller carcinogenicity of anthophyllite asbestos, this is likely due to different grades of asbestos exposure among workers of the mines. The measurements made in the enrichment plant in Paakkila mine showed 200 anthophyllite fibers in cm3 before 1970 [18], but of course the level of the asbestos exposure depended on the working time and the particular duty in the mine. According to an article published in 1968 [19], the number of workers in Paakkila mine was 132, 12 of whom were white collar workers. As the cohort was formed of people who had worked in Paakkila and Maljasalmi mines in 1953–1967 and Maljasalmi was closed already in 1953, there were also those who had worked for a shorter time and thus were less exposed in this cohort. That would explain the smaller SIR for lung cancer of this cohort, compared with asbestosis patients and sprayers, who are known to have a heavy asbestos exposure.
Asbestosis patients' risk of lung cancer is high. This is logical, because the development of asbestosis is known to require heavy asbestos exposure. The higher the asbestos exposure, the higher the risk of lung cancer, regardless of smoking. The highest observed risk of lung cancer was among asbestos sprayers. Sprayers were exposed to high concentrations (as measured, up to 100 fibers per cm3; [9]) of crocidolite. The SIR for lung cancer in the screening cohort, which mainly consisted of construction workers, is moderately increased. The asbestos exposure of workers in this cohort was very heterogenic, which probably explains why their overall risk was lower than that in the other cohorts. However, the SIR for lung cancer of nonsmokers is surprisingly low, although we have no nonsmoking population to compare it with. As detected earlier [10], the SIR for lung cancer was higher (Table 3) among those who only answered the preliminary questionnaire and did not participate in the actual study. Not participating may reflect the fact that they were not so interested in health issues and therefore, might have had an unhealthier lifestyle, which also elevates the overall risk of cancer. Nevertheless, in the screening cohort, the SIR for lung cancer was still at the same level as it was over 25 years ago (Table 3). Exposure to asbestos continued until at least 1994 in this cohort, so the cancer risk is expected to start to decrease in the near future.
As we do not know the smoking habits of the workers after the cohorts were formed, we analyzed the smokers and ex-smokers together. Although it is of course possible that smoking habits changed later, it is less probable that nonsmokers started smoking, because active campaigning against it has been targeted at asbestos-exposed workers [20]. In the screening cohort, the prevalence of smoking was similar to that of the Finnish general population in 1979 [8].
We found no increased risk of ovarian cancer or laryngeal cancer in these asbestos-exposed cohorts. The total number of women was small (1,091). Most of them (539 women) worked in the construction industry, where asbestos exposure in typical women's work was probably low. Why the asbestosis patient and sprayer cohorts had no cases of laryngeal cancer and why there was only one in the asbestos mine worker cohort is explained by the small sizes of these cohorts and the relative rarity of laryngeal cancer. Although the evidence of an increased risk of laryngeal cancer associated with asbestos exposure [1] is consistent, even in our largest cohort the risk remained at the same level as that of the overall population.
It has to be kept in mind that overall SIRs of different cohorts are not directly comparable even in this case when the reference incidence rates for all cohorts are derived from the same population, if the person-year distributions of the cohorts are different in terms of calendar period or age. Therefore we stratified our data according to these variables and compared SIRs in a given calendar period and age range in each of the cohorts.
As a conclusion, even if the SIRs for mesothelioma are high, the number of cases per year is small. Asbestos sprayers, who had been exposed to high amounts of crocidolite, were at the highest risk of mesothelioma and lung cancer. The smaller SIR for mesothelioma in the screening cohort would suggest dose-responsiveness of asbestos exposure. It does seem that, the highest risk of lung cancer in the older cohorts is over, but remains elevated in the most recent of the cohorts, the screening cohort. The highest SIR for lung cancer is in the asbestosis patient and sprayers cohort, which is explained by their heavy asbestos exposure. We did not find an increased risk of ovarian cancer, or laryngeal cancer in these asbestos-exposed cohorts.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Weissman D, Gustavsson P, Miller A, Rushton L, Stayner L, Pallasaho P, Wolff H. New asbestos-related disease entities in Panu Oksa, Henrik Wolff, Tapio Vehmas, Paula Pallasaho, and Heikki Frilander (Ed). Asbestos, asbestosis, and cancer. Helsinki criteria for diagnosis and attribution. [Internet]. Helsinki (Finland): Finnish Institute of Occupational Health; 2014. p. 49–109. Available from: http://www.julkari.fi/bitstream/handle/10024/116909/Asbestos_web.pdf?sequence=1.
- 2.Wolff H., Vehmas T., Oksa P., Rantanen J., Vainio H. Asbestos, asbestosis, and cancer the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health. 2015;41:5–15. doi: 10.5271/sjweh.3462. [DOI] [PubMed] [Google Scholar]
- 3.Straif K., Benbrahim-Tallaa L., Baan R., Grosse Y., Secretan B., El Ghissassi F., Bouvard V., Guha N., Freeman C., Galichet L., Cogliano V., World Health Organization (WHO) International Agency for Research on Cancer Monograph Working Group A review of human carcinogens – part C: metals, arsenic, dusts and fibers. Lancet Oncol. 2009;10:453–454. doi: 10.1016/s1470-2045(09)70134-2. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research of Cancer (IARC) International Agency for Research on Cancer; Lyon (France): 2012. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). Arsenic, metals, fibres, and dusts Volume 100C A review of human carcinogens IARC monographs on evaluation of carcinogenic risks to humans; pp. 219–309. [Google Scholar]
- 5.World Health Organization (WHO). Asbestos: elimination of asbestos-related diseases. Fact sheet no. 343 [Internet]. 2010. Available from: http://www.who.int/mediacentre/factsheets/fs343/en/index.html.
- 6.Oksa P, Palo L, Saalo A, Jolanki R, Mäkinen I, Pesonen M, Virtanen S. Occupational diseases in Finland in 2012. New cases of recognized and suspected occupational diseases [Internet]. Helsinki (Finland): Finnish Institute of Occupational Health; 2014. Available from: http://www.julkari.fi/bitstream/handle/10024/131563/Occupational_diseases_2012.pdf?sequence=1.
- 7.Meurman L.O., Pukkala E., Hakama M. Incidence of cancer among antophyllite asbestos miners in Finland. Occup Environ Med. 1994;51:421–425. doi: 10.1136/oem.51.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oksa P., Pukkala E., Karjalainen A., Ojajärvi A., Huuskonen M.S. Cancer incidence and mortality among Finnish asbestos sprayers and in asbestosis and silicosis patients. Am J Ind Med. 1997;31:693–698. doi: 10.1002/(sici)1097-0274(199706)31:6<693::aid-ajim4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 9.Riala R. Finnish Institute of Occupational Health and the Finnish Work Environment Fund; Helsinki (Finland): 1991. Exposures at work: Asbestos. [in Finnish] [Google Scholar]
- 10.Koskinen K., Pukkala E., Reijula K., Karjalainen A. Incidence of cancer among the participants of the Finnish Asbestos Screening Campaign. Scand J Work Environ Health. 2003;29:64–70. doi: 10.5271/sjweh.706. [DOI] [PubMed] [Google Scholar]
- 11.Karjalainen A., Meurman L.O., Pukkala E. Four cases of mesothelioma among Finnish anthophyllite miners. Occup Environ Med. 1994;51:212–215. doi: 10.1136/oem.51.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacourt A., Gramond C., Rolland P., Ducamp S., Audignon S., Astoul P., Chamming's S., Gilg Soit Ilg A., Rinaldo M., Raherison C., Galateau-Salle F., Imbernon E., Pairon J.C., Goldberg M., Brochard P. Occupational and nonoccupational attributable risk of asbestos exposure for malignant pleural mesothelioma. Thorax. 2014;69:532–539. doi: 10.1136/thoraxjnl-2013-203744. [DOI] [PubMed] [Google Scholar]
- 13.Rogers A.J., Leigh J., Berry G., Ferguson D.A., Mulder H.B., Ackad M. Relationship between lung asbestos fiber type and concentration and relative risk of mesothelioma. A case-control study. Cancer. 1991 Apr 1;67:1912–1920. doi: 10.1002/1097-0142(19910401)67:7<1912::aid-cncr2820670716>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Järvholm B., Englund A. The impact of asbestos exposure in Swedish construction workers. Am J Ind Med. 2014;57:49–55. doi: 10.1002/ajim.22264. [DOI] [PubMed] [Google Scholar]
- 15.Engholm G, Ferlay J, Christensen N, Kejs AMT, Johannesen TB, Khan S, Leinonen M, Milter MC, Ólafsdóttir E, Petersen T, Stenz F, Storm HH. NORDCAN: Cancer incidence, mortality, prevalence, and survival in the Nordic countries, Version 7.1. [Internet]. Association of the Nordic Cancer Registries, Danish Cancer Society. 2015 Jul 9. Available from http://www.ancr.nu.
- 16.Nurminen M. A study of the mortality of workers in an anthophyllite asbestos factory in Finland. Work Environ Health. 1972;9:112–118. [Google Scholar]
- 17.Rimpelä M. Trends in the smoking habits in Finland in 1949 and 1960–1977. J Soc Med. 1978;3:112–123. [Google Scholar]
- 18.Santonen T., Oksa P., editors. Memorandum of the working group for occupational cancer (in Finnish) Finnish Institute of Occupational Health; Helsinki (Finland): 2013. [Google Scholar]
- 19.Palomäki A., Halonen O. The anthophyllite asbestos mine of Paakkila. Vuoriteollisuus. 1968;2:92–98. [In Finnish] [Google Scholar]
- 20.Huuskonen M.S., Koskinen K., Tossavainen A., Rinne J.P., Karjalainen A., Rantanen J. Finnish Institute of Occupational Health asbestos program 1987–92. Am J Ind Med. 1995;28:123–142. doi: 10.1002/ajim.4700280111. [DOI] [PubMed] [Google Scholar]