Abstract
Background
Benzene is a known occupational and environmental pollutant. Its urinary metabolite trans, trans-muconic acid (tt-MA) has been introduced by some environmental and occupational health regulatory associations as a biological index for the assessment of benzene exposure; however, recently, doubts have been raised about the specificity of tt-MA for low-level benzene exposures. In the present study, we investigated the association between urinary levels of tt-MA and inhalational exposure to benzene in different exposure groups.
Methods
Benzene exposure was assessed by personal air sampling. Collected benzene on charcoal tube was extracted by carbon disulfide and determined by a gas chromatograph (gas chromatography with a flame ionization detector). Urinary tt-MA was extracted by a strong anion-exchange column and determined with high-performance liquid chromatography–UV.
Results
Urinary levels of tt-MA in intensive benzene exposure groups (chemical workers and police officers) were significantly higher than other groups (urban and rural residents), but its levels in the last two groups with significant different exposure levels (mean = 0.081 ppm and 0.019 ppm, respectively) showed no significant difference (mean = 388 μg/g creatinine and 282 μg/g, respectively; p < 0.05). Before work shift, urine samples of workers and police officers showed a high amount of tt-MA and its levels in rural residents’ samples were not zero.
Conclusion
Our results suggest that tt-MA may not be a reliable biomarker for monitoring low-level (below 0.5 ppm) benzene exposures.
Keywords: benzene; biological monitoring; exposure assessment; trans, trans-muconic acid
1. Introduction
Benzene is an important industrial chemical used worldwide for the production of other chemicals and solvents such as styrene, cumene, and cyclohexane. It is also used in the manufacture of some types of plastics, rubbers, lubricants, dyes, detergents, drugs, and pesticides.
Although the International Program on Chemical Safety in 1993 reported that about 60% of workers are exposed to benzene at levels lower than 0.1 ppm and only 1% of them are exposed to levels higher than 10 ppm [1], workers in different industries and workplaces are among the higher-risk groups exposed to benzene.
Benzene is emitted into the environment from industrial processes, gasoline, engine exhausts, and combustion of organic materials, causing significant exposure of the general population. It is reported that about 1% of benzene is found in gasoline in most countries [2], [3]. Many foodstuff and consumer products, along with tobacco smoke (about 87 μg/cigarette), contain considerable levels of benzene. The average background level of benzene intake for nonsmokers has been estimated to be about 0.5 mg/d [4]. Significant environmental distribution of benzene and its dangerous health consequences have raised deep concerns. It has been reported that benzene's concentrations in the environmental air range from 0.5 μg/m3 in uninhabited regions to 200–300 μg/m3 in urban environments of large cities and capitals [5], [6].
Benzene can either be absorbed through the respiratory system or have considerable dermal absorption, due to its lipophilic structure. Therefore, the individual uptake of benzene among different people can be highly variable, depending on the rate of pulmonary ventilation and some other factors [7], [8]. As a result, ambient air monitoring by itself could not reliably assess benzene exposure. Alternative ways for comprehensive exposure assessment are biological monitoring methods that determine exact entrance of pollutants into the human body. In the case of benzene, the question is whether or not some effective biological indicators exist and which one is suitable for biological monitoring of low benzene exposure.
In addition, measurement of the unmetabolized benzene excreted in urine (which represents about 1% of the absorbed quantity) has been proposed as a useful biomarker for benzene exposure [9], but a recent study has claimed that, at very low occupational or environmental exposure to benzene, urinary benzene is less valid than urinary metabolites of benzene, even if both are strongly influenced by smoking habit [10].
Traditionally, the most common method used for biological monitoring of benzene exposure is based on the measurement of different benzene metabolites in the blood and urine as well as adducts of reactive benzene metabolites with cellular component such as DNA [11], [12].
Urine metabolites that might serve as indices of occupational or environmental exposures to benzene include phenol, hydroquinone, trans, trans-Muconic acid (tt-MA), and S-phenylmercapturic acid [13]. Among them, urinary tt-MA is increasingly recognized as a reliable and relatively convenient biomarker [14], [15], [16]. The compound tt-MA is an open-ring (nonphenolic) metabolite of benzene [17]. About 2% of the total benzene uptake in humans is excreted via urine as tt-MA, and its excretion half-life is reported to be < 6 hours [18]. Despite the low metabolic conversion of benzene to tt-MA, this metabolite appears to be more suitable than phenolic metabolites, and the American Conference of Governmental Industrial Hygienists (ACGIH) has recommended it as an appropriate biomarker for the assessment of occupational exposure to benzene (above 0.25 ppm), with limit values (biological exposure indices) of 500 μg/g creatinine [19].
Several competing metabolic pathways have been proposed for benzene metabolism, and recent studies indicated that some of these pathways were saturated at specific exposure levels and some other activated in certain conditions [11]. By contrast, the specificity of tt-MA as a biomarker for low-level benzene exposure may be limited because it is also reported to be a metabolite of sorbic acid, a widely used food preservative [20], [21]. Owing to these findings, recently some doubts have been raised about the specificity of tt-MA as a reliable biomarker for low-level benzene exposure [22]. Therefore, in the present study, urinary concentrations of tt-MA in different groups with various benzene exposure levels have been measured and compared, to investigate the reliability of this index for biological monitoring of low-level benzene exposures.
2. Materials and methods
2.1. Participants and study protocol
For comprehensive evaluation of selectivity of tt-MA as a biomarker of benzene exposure, four study groups were selected: (1) 61 workers of a chemical company (producer of linear alkyl benzene); (2) 52 police officers of a large city (Ahvaz, Iran); (3) 73 urban residents of a large city (Ahvaz, Iran); and (4) 75 rural residents. All participants were 22–45-year-old males. In the case of the chemical company, all the workers participated in the study, but for other study groups, the participants were selected randomly. For all participants, inhalational exposure to benzene and urinary levels of tt-MA (in morning and evening urine samples) were determined on the same day. Necessary information (demographic characteristics, smoking, medical and occupational history, special diet, home location, work shift, etc.) was collected by a questionnaire before sampling, and reviewed by the investigator for identifying and avoiding confounding factors. Ahvaz University of Medical Sciences Ethics Review Committee, Ahvaz, Iran has approved the study proposal (ID: 2301), and all participants provided written informed consent before participation.
2.2. Benzene exposure monitoring
Inhalational benzene exposure for all study participants was monitored by active personal sampling. Benzene was collected by a sampling train consisting of a pump (SKC Inc., Eighty Four, PA., USA) and a charcoal tube [SKC number 226-01, 100 mg; SKC; National Institute of Occupational Safety and Health (NIOSH) approved]. Flow rates of the pump were calibrated prior to each run using a wet test meter. The charcoal tube was clipped on the collar and the pump was installed on the belt of all participants. At the end of the sampling time, the charcoal tubes were sealed and sent to the laboratory for analyses. The samples were kept at 4°C prior to desorption with carbon disulfide (benzene free) and analysis. The analysis method was according to the NIOSH method (method numbers: 1500 and 1501), which is based on gas chromatography with a flame ionization detector. To eliminate possible intensive dermal exposures of chemical company workers, it was necessary for them to use suitable personal protective equipment.
For urban residents, police officers, and rural residents (with regard to their low-level exposures), sampling was continued for 8 h/d (from 9 am to 5 pm), but for chemical workers with higher benzene exposure levels, multiple sampling during work shift (30-minute–4-hour samples based on environmental benzene concentrations and job condition) were used, and by this means, time-weighted average of benzene exposure for all study groups was calculated.
2.3. Measurement of urinary levels of tt-MA
Each participant provided two (morning and evening) 5 mL urine samples. The first sampling was conducted in the early morning (the 1st urination of day), and the second samples were collected at the end of the 8-hour work shift (for police officers and chemical workers) or at the end of 8-hour inhalation exposure sampling (for urban and rural residents). These samples were collected in polypropylene tubes, preserved with 100 μL of 6 mol/L hydrochloric acid, and were immediately transported to the laboratory to be stored at −20°C until the day of analysis. The levels of tt-MA were measured by high-performance liquid chromatography according to the method described by Lee et al [23] with some modifications. In this method, vanilic acid was used as the internal standard. Samples from all study groups were analyzed in the same series, without the knowledge of exposure status. Urinary concentrations of tt-MA were corrected for creatinine content. The concentration of tt-MA in the samples was determined using a calibration curve, which was prepared by spiking of different confrontations of tt-MA (10,100,1000 and 10,000 μg/L) in a blank urine sample.
Analysis was carried out by high-performance liquid chromatography (JASCO Corporation, Tokyo, Japan) with a reversed-phase C18 column [250 × 5 mm2 (i.d.) with 5-μm particle size; Merck, Darmstadt, Germany]. The flow rate was equal to 1.2 mL/min. The mobile phase consisted of (per liter) 10 mL of acetic acid, 100 mL of methanol, and 890 mL of 5 mmol/L sodium acetate (pH, 3.5). The detector (UV/visible) was set at a wavelength of 259 nm.
Creatinine levels of the urine samples were determined by Golestan Hospital Medical Diagnostic Laboratory (Ahvaz, Iran) by its commercial kit (Pars Azmon, Tehran, Iran) and spectrophotometer at a wavelength of 500 nm according to the manufacturer's instruction.
2.4. Statistical analysis
The relationship between benzene exposure and urinary levels of tt-MA was assessed by making comparisons among the different study groups and subgroups, using correlation/regression techniques. The t test and analysis of variance with a confidence level of ≤ 0.05 were applied to determine whether there was a significant difference between one or more confounding factors and different variables. The criterion for significance was set at p < 0.05. All statistical analyses were carried out by the SPSS software (version 18.0 for Windows; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Participants and study groups
As mentioned above, during the study some samples were lost. Final numbers of samples, along with other characteristics of the participants and study groups, are presented in Table 1.
Table 1.
Characteristics of participants and study groups
| Study group | Participants | Final number of samples∗ | Smoker | Consumption of preservative-containing food† |
|---|---|---|---|---|
| Chemical company workers | 61 | 57 | 31 | 8 |
| Police officers | 52 | 51 | 12 | 5 |
| Urban residents | 73 | 69 | 22 | 12 |
| Rural residents | 75 | 71 | 21 | 1 |
Both morning and evening samples were provided correctly.
Eating a meal containing sorbic acid-preserved foods within 24 hours prior to sampling.
3.2. Inhalational benzene exposure
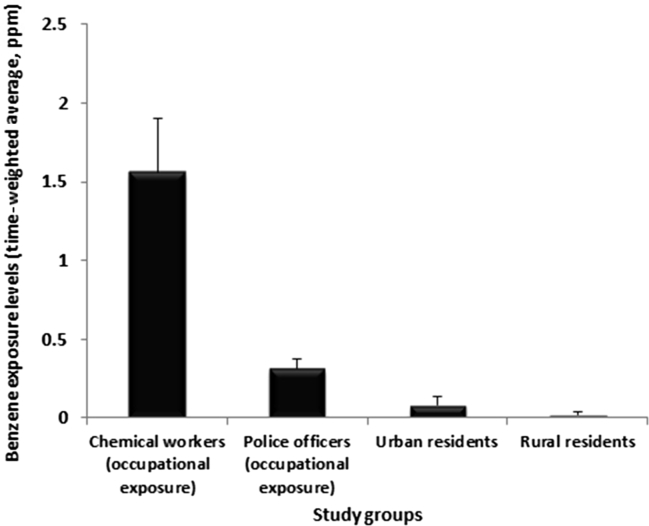
Method limits of detection were 0.01 ppm. The within-day coefficient of variation was 4–9% and between-day coefficient of variation 8–13%. Time-weighted average levels of benzene exposure [mean ± standard deviation (SD)] for different study groups are presented in Fig. 1. Exposure levels for study groups were quite different. While benzene exposure among chemical workers rises to 3.5 ppm (mean ± SD = 1.56 ± 0.34 ppm), its mean ± SD level for rural residents was 0.019 ± 0.016 ppm.
Fig. 1.
Comparing the time-weighted average of benzene exposure levels (ppm) among four different study groups. Data are presented as mean ± SD.
3.3. Urinary levels of tt-MA
The tt-MA and internal standard were detected at 5.2 minutes and 10.2 minutes, respectively. Linearity between 10 μg/L and 10,000 μg/L was very good (r2 = 0.982); limits of detection and quantification were 0.53 μg/L and 2.11 μg/L, respectively. The mean coefficients of variation for replicate analysis with spiked samples were 7.4% (repeatability) and 11.2% (between-day precision); the mean recovery of tt-MA in this method was 86.1%.
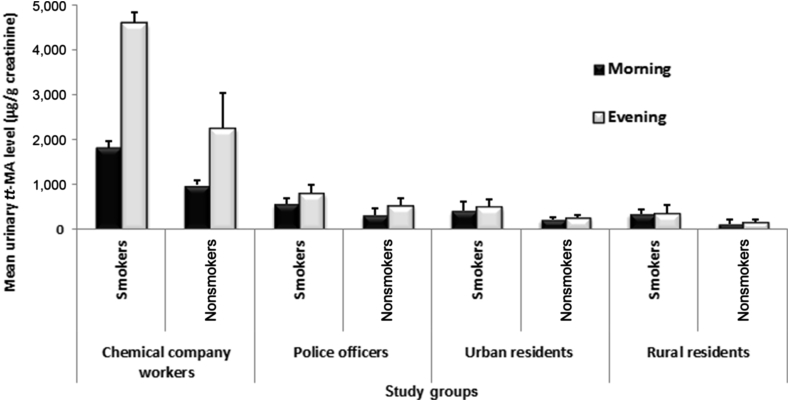
Evening urine samples of chemical company workers showed the highest concentration of tt-MA, which rose to 4,500 μg/g creatinine (for some workers who were exposed to benzene while washing tanks). In both morning and evening samples, the mean urinary levels of tt-MA in chemical company workers' urine samples were significantly higher than those of the other study groups. However, these levels in other study groups showed no significant differences. The mean urinary concentrations of tt-MA for smokers were significantly higher than those for nonsmokers in all study groups (Fig. 2 and Table 2).
Fig. 2.
Comparing mean urinary levels of tt-MA (μg/g creatinine) in different study groups based on their groups and smoking status. Data are presented as mean ± SD. tt-MA trans, trans-muconic acid.
Table 2.
Levels of tt-MA in analyzed urine samples
| Study group | Smoking status | Urine sample | tt-MA (μg/g creatinine) |
|---|---|---|---|
| Chemical company workers | Smokers | Morning | 1,824 ± 147 |
| Evening | 4,616 ± 225 | ||
| Nonsmokers | Morning | 987 ± 111 | |
| Evening | 2,271 ± 780 | ||
| Police officers | Smokers | Morning | 571 ± 120 |
| Evening | 820 ± 187 | ||
| Nonsmokers | Morning | 321 ± 145 | |
| Evening | 544 ± 160 | ||
| Urban residents | Smokers | Morning | 425 ± 198 |
| Evening | 501 ± 161 | ||
| Nonsmokers | Morning | 224 ± 55 | |
| Evening | 275 ± 57 | ||
| Rural residents | Smokers | Morning | 352 ± 102 |
| Evening | 351 ± 201 | ||
| Nonsmokers | Morning | 125 ± 87 | |
| Evening | 160 ± 52 |
Data are presented as mean ± SD from three independent experiments.
tt-MA trans, trans-muconic acid.
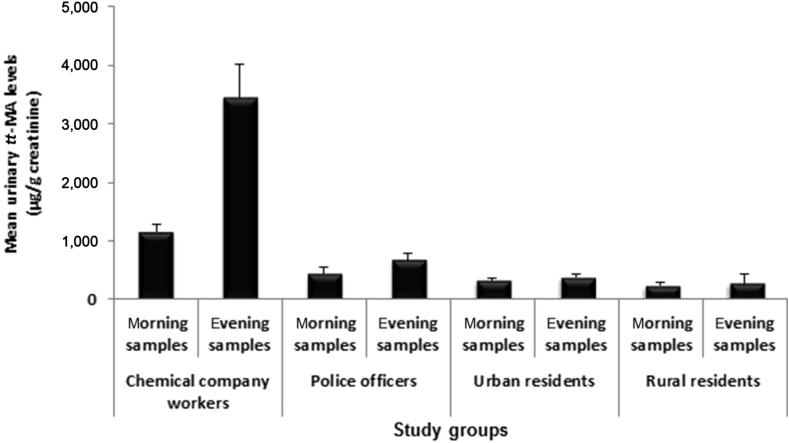
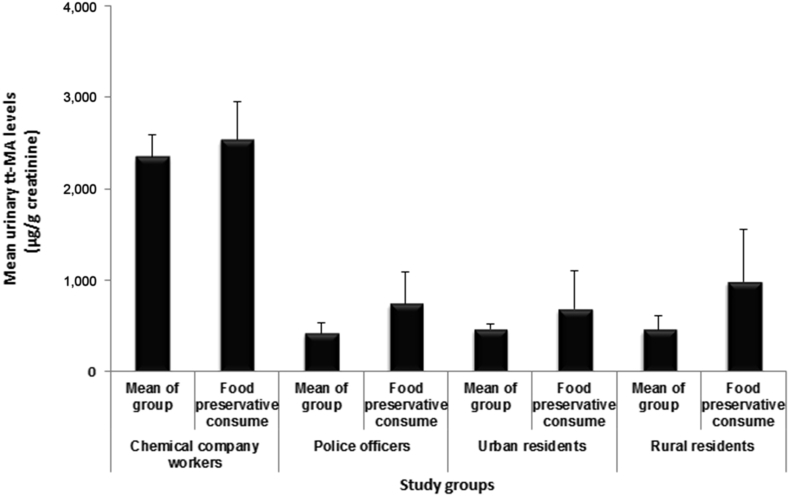
Urinary levels of tt-MA in morning and evening samples showed a significant difference only for chemical company workers and police officers, and evening levels were significantly higher than morning levels (p < 0.05; Fig. 3). Urinary levels of tt-MA in rural resident urine samples were not zero (even for nonsmokers). The urinary tt-MA levels of participants consuming preservative-containing foods (such as sausage and canned foods) were separately analyzed. Except the chemical company workers, the mean urinary levels of tt-MA for preservative-containing food-consuming participants in all study groups were significantly higher than those for other participants in their respective study groups (p < 0.05; Fig. 4).
Fig. 3.
Comparing mean urinary levels of tt-MA (μg/g creatinine) in morning and evening samples of all study groups. Data are presented as mean ± SD. tt-MA trans, trans-muconic acid.
Fig. 4.
Comparing mean urinary levels of tt-MA (μg/g creatinine) in different study groups, based on preservative-containing food-consuming status. Data are presented as mean ± SD. tt-MA trans, trans-muconic acid.
4. Discussion and conclusion
Benzene, as one of the most widely used industrial solvents and a ubiquitous environmental pollutant, has very serious effects on human health. Some of its destructive effects such as chromosomal damage, depression of the immune system [24], [25], [26], progressive degeneration of the bone marrow, and induction of aplastic anemia and leukemia [16] resulted in its classification as a human carcinogen by the International Agency for Research on Cancer [27] and, consequently, in a reduction of its acceptable occupational exposure levels. Its occupational threshold limit value has been lowered to 0.5–1 ppm by many occupational health organizations such as NIOSH and Occupational Safety and Health Administration.
Hence, monitoring of its exposure in various populations seems to be of utmost importance. Although it is more than two decades that ACGIH and some other occupational and environmental organizations introduced tt-MA as a selective and sensitive biomarker for monitoring of benzene exposure, recently some evidence has been presented about the insufficiency of this index for monitoring low-level benzene exposures. For example, a study by Weaver and colleagues [21] showed that tt-MA is a specific biomarker for benzene only for exposure levels above 0.5 ppm. However, a study on major industries with benzene exposure revealed that 60% of workers currently have exposure levels below 0.1 ppm [28].
The results of this study showed that the time-weighted average of benzene exposure among chemical company workers is about three times higher than the levels recommended by ACGIH and also the standard of Iran (0.5 ppm). Despite the fact that environmental exposure levels of other study groups (police officers, and urban and rural residents) were not very high (below 0.5 ppm and for some cases close to zero), tt-MA was found in all urine samples, confirming that benzene can be a ubiquitous environmental pollutant and there should be other sources for tt-MA in the urine. As seen in Fig. 2, the SD of benzene exposure levels in all study groups, especially for chemical company workers, is relatively high, which might be a consequence of the differences in the nature of the tasks, locations, and sampling conditions.
Following monitoring of benzene exposure in a study, it was estimated that an 8-hour exposure to 1 ppm benzene would result in tt-MA levels ranging from 500 μg/g creatinine to 1,500 μg/g creatinine, while another study evaluated these values to be about 1,900 μg/g creatinine [16]. In the present study, the mean urinary levels of tt-MA measured in the evening samples of chemical workers (3,443 μg/g creatinine) was statistically different from those of other three study groups (police officers, and urban and rural residents), but the mean tt-MA levels of these three groups showed no significant between-group differences (p < 0.05).
Urinary tt-MA in chemical company workers with exposure levels above 1 ppm showed a good linear regression with exposure levels (r2 = 0.904, data not shown), but in the other study groups (with exposure levels below 0.5 ppm) this correlation was not seen. Considering the results of exposure levels and urinary tt-MA concentration in the four study groups, it can be concluded that for exposure levels below 0.5 ppm, inhalational exposure levels and urinary tt-MA concentration do not have a linear correlation, and tt-MA cannot be used as a sensitive and reliable biomarker for low-level benzene exposure.
No significant difference in the urinary tt-MA level of morning and evening samples of urban and rural residents (p < 0.05) and the presence of a considerable amount of tt-MA in the morning samples of workers and police officers (2,352 μg/g creatinine and 419 μg/g creatinine, respectively) indicated that, following exposure to benzene and its distribution in the body, a large amount of benzene may accumulate in some body organs and tissues (such as fat tissues), and over time, it may redistribute, metabolize, and excrete from the body in the form of tt-MA. Dermal absorption (particularly for chemical workers) can be another important source of delayed appearance of tt-MA in urine.
It has been known that tt-MA is also a metabolite of sorbic acid. Weaver and colleagues [21] reported that sorbic acid-preserved foods have the potential to cause substantial interference with tt-MA as a biomarker for both occupational and environmental benzene exposure. The average peak of urinary tt-MA levels after ingestion of some sorbic acid-preserved foods (such as Hostess cupcakes and Sunny Delight) was 452 μg/g creatinine and 675.3 μg/g creatinine, respectively, which are greater than the predicted values for 0.1–0.5 ppm benzene exposure and in some cases even above the predicted values for 1 ppm benzene exposure [29]. In our study, according to the information extracted from questioners, some participants consume sorbic acid-preserved foods (such as canned foods and sausage). As mentioned above, the results of tt-MA measurements in urine samples of these participants were analyzed separately. The results showed that except the chemical company workers, the mean levels of urinary tt-MA in all groups were about two-fold higher than mean levels of their respective groups. Based on the results of previous studies, increased urinary tt-MA levels in these participants were predictable, but what we should take into consideration is that nowadays preservative-containing foods are an integral component of most community's nutrition and diet, and will have a significant effect on urinary tt-MA; hence, tt-MA cannot be a selective and sensitive biomarker for benzene exposure. The other important point is that, in chemical workers’ groups, the mean urinary tt-MA levels of preservative-containing food-consuming participants showed no significant difference with those of non-preservative-containing food-consuming individuals, which might be due to saturation of metabolic pathways.
Benzene is a component of environmental tobacco smoke, and the results of a study by Melikian and colleagues [30] showed that the levels of urinary tt-MA range from 20 μg/g creatinine to 1,300 μg/g creatinine, resulting in a mean of 290 ± 40 μg/g creatinine in smokers, and corresponding values in nonsmokers ranged from “not detectable” to 520 μg/g creatinine with an average of 90 ± 20 μg/g creatinine (the levels of tt-MA in smokers were about 3 times higher than those in nonsmokers). The results of the present study are also in agreement with these findings. Levels of urinary tt-MA for smokers in all study groups except the chemical company workers were about two times higher than those of nonsmokers. Based on information extracted from questionnaires, urinary levels of tt-MA showed a weak linear relation with the number of cigarettes smoked (data not shown). As usual, urinary levels of tt-MA in smokers and nonsmokers showed no significant difference due to high levels of occupational exposure to benzene (p < 0.05). Despite the introduction of tt-MA as a selective biomarker for occupational and environmental benzene exposure by ACGIH, NIOSH, and some other regulatory organizations, several factors such as exposure levels, smoking status, and diet may influence its urinary concentration significantly, in particular for low-level exposure (≤ 0.5 ppm), and if ACGIH would decide to reduce benzene's threshold limit value (threshold limit value–time-weighted average) to 0.3 ppm [31], tt-MA cannot be used as a reliable biomarker for biological monitoring of benzene any longer.
Conflicts of interest
The authors declare that they have no conflicts of interest.
References
- 1.Pezzagno G., Maestri L. The specificity of trans, trans-muconic acid as a biological indicator for low levels of environmental benzene. Indoor Built Environ. 1997;6:12–18. doi: 10.1002/(sici)1097-0274(199905)35:5<511::aid-ajim8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Brugnone F., Perbellini L., Romeo L., Cerpelloni M., Bianchin M., Tonello A. Benzene in blood as a biomarker of low level occupational exposure. Sci Total Environ. 1999;235:247–252. doi: 10.1016/s0048-9697(99)00197-7. [DOI] [PubMed] [Google Scholar]
- 3.Carrieri M., Bonfiglio E., Scapellato M.L., Maccà I., Tranfo G., Faranda P., Paci E., Bartolucci G.B. Comparison of exposure assessment methods in occupational exposure to benzene in gasoline filling-station attendants. Toxicol Lett. 2006;162:146–152. doi: 10.1016/j.toxlet.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Melikian A.A., Qu Q., Shore R., Li G., Li H., Jin X., Cohen B., Chen L., Li Y., Yin S., Mu R., Zhang X., Wang Y. Personal exposure to different levels of benzene and its relationships to the urinary metabolites S-phenylmercapturic acid and trans, trans-muconic acid. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:211–221. doi: 10.1016/s0378-4347(01)00454-6. [DOI] [PubMed] [Google Scholar]
- 5.Cocheo V., Sacco P., Boaretto C., De Saeger E., Ballesta P.P., Skov H., Goelen E., Gonzalez N., Caracena A.B. Urban benzene and population exposure. Nature. 2000;404:141–142. doi: 10.1038/35004651. [DOI] [PubMed] [Google Scholar]
- 6.Wester R.C., Maibach H.I., Gruenke L.D., Craig J.C. Benzene levels in ambient air and breath of smokers and nonsmokers in urban and pristine environments. J Toxicol Environ Health A. 1986;18:567–573. doi: 10.1080/15287398609530894. [DOI] [PubMed] [Google Scholar]
- 7.Dor F., Dab W., Empereur-Bissonnet P., Zmirou D. Validity of biomarkers in environmental health studies: the case of PAHs and benzene. CRC Crit Rev Toxicol. 1999;29:129–168. doi: 10.1080/10408449991349195. [DOI] [PubMed] [Google Scholar]
- 8.Kirkeleit J., Riise T., Bråtveit M., Pekari K., Mikkola J., Moen B.E. Biological monitoring of benzene exposure during maintenance work in crude oil cargo tanks. Chem Biol Interact. 2006;164:60–67. doi: 10.1016/j.cbi.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Basilicata P., Miraglia N., Pieri M., Acampora A., Soleo L., Sannolo N. Application of the standard addition approach for the quantification of urinary benzene. J Chromatogr B. 2005;818:293–299. doi: 10.1016/j.jchromb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Lovreglio P., Carrieri M., Barbieri A., Sabatini L., Fracasso M., Doria D., Iavicoli S., Drago I., D'errico M.N., Imbriani M., Violante F.S., Bartolucci G.B., Soleo L. Applicability of urinary benzene to biological monitoring of occupational and environmental exposure to very low benzene concentrations. G Ital Med Lav Ergon. 2011;33:41–46. [PubMed] [Google Scholar]
- 11.Kim S., Vermeulen R., Waidyanatha S., Johnson B.A., Lan Q., Rothman N., Smith M.T., Zhang L., Li G., Shen M., Yin S., Rappaport S.M. Using urinary biomarkers to elucidate dose-related patterns of human benzene metabolism. Carcinogenesis. 2006;27:772–781. doi: 10.1093/carcin/bgi297. [DOI] [PubMed] [Google Scholar]
- 12.Sørensen M., Skov H., Autrup H., Hertel O., Loft S. Urban benzene exposure and oxidative DNA damage: influence of genetic polymorphisms in metabolism genes. Sci Total Environ. 2003;309:69–80. doi: 10.1016/S0048-9697(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 13.Medeiros A., Bird M., Witz G. Potential biomarkers of benzene exposure. J Toxicol Environ Health A. 1997;51:519–539. doi: 10.1080/00984109708984042. [DOI] [PubMed] [Google Scholar]
- 14.Amodio-Cocchieri R., Prete U.D., Cirillo T., Agozzino E., Scarano G. Evaluation of benzene exposure in children living in Campania (Italy) by urinary trans, trans-muconic acid assay. J Toxicol Environ Health A. 2001;63:79–87. doi: 10.1080/15287390151126388. [DOI] [PubMed] [Google Scholar]
- 15.Suwansaksri J., Wiwanitkit V. Urine trans, trans-muconic acid determination for monitoring of benzene exposure in mechanics. Asian J Trop Med Public Health. 2000;31:587–589. [PubMed] [Google Scholar]
- 16.Scherer G., Renner T., Meger M. Analysis and evaluation of trans,trans-muconic acid as a biomarker for benzene exposure. J Chromatogr B Biomed Sci Appl. 1998;717:179–199. doi: 10.1016/s0378-4347(98)00065-6. [DOI] [PubMed] [Google Scholar]
- 17.Rappaport S.M., Kim S., Lan Q., Vermeulen R., Waidyanatha S., Zhang L., Li G., Yin S., Hayes R.B., Rothman N., Smith M.T. Evidence that humans metabolize benzene via two pathways. Environ Health Perspect. 2009;117:946–952. doi: 10.1289/ehp.0800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue O., Seiji K., Nakatsuka H., Watanabe T., Yin S.N., Li G.L., Cai S.X., Jin C., Ikeda M. Urinary t,t-muconic acid as an indicator of exposure to benzene. Br J Ind Med. 1989;46:122–127. doi: 10.1136/oem.46.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Conference of Governmental Industrial Hygienists (ACGIH) ACGIH; Cincinnati (OH): 2011. Threshold limit values and biological exposure indices for 2010–2011. [Google Scholar]
- 20.Marrubini G., Coccini T., Maestri L., Manzo L. Effect of sorbic acid administration on urinary trans, trans-muconic acid excretion in rats exposed to low levels of benzene. Food Chem Toxicol. 2002;40:1799–1806. doi: 10.1016/s0278-6915(02)00185-0. [DOI] [PubMed] [Google Scholar]
- 21.Weaver V.M., Buckley T., Groopman J.D. Lack of specificity of trans, trans-muconic acid as a benzene biomarker after ingestion of sorbic acid-preserved foods. Cancer Epidemiol Biomarkers Prev. 2000;9:749–755. [PubMed] [Google Scholar]
- 22.Sanguinetti G., Accorsi A., Barbieri A., Raffi G.B., Violante F.S. Failure of urinary trans, trans-muconic acid as a biomarker for indoor environmental benzene exposure at PPB levels. J Toxicol Environ Health A. 2001;63:599–604. doi: 10.1080/152873901316857770. [DOI] [PubMed] [Google Scholar]
- 23.Lee B.L., New A.L., Kok P.W., Ong H.Y., Shi C.Y., Ong C.N. Urinary trans, trans-muconic acid determined by liquid chromatography: application in biological monitoring of benzene exposure. Clin Chem. 1993;39:1788–1792. [PubMed] [Google Scholar]
- 24.Krewski D., Snyder R., Beatty P., Granville G., Meek B., Sonawane B. Assessing the health risks of benzene: a report on the benzene state-of-the-science workshop. J Toxicol Environ Health A. 2000;61:307–338. [PubMed] [Google Scholar]
- 25.Hayes R.B., Yin S., Rothman N., Dosemeci M., Li G., Travis L.T., Smith M.T., Linet M.S. Benzene and lymphohematopoietic malignancies in China. J Toxicol Environ Health A. 2000;61:419–432. doi: 10.1080/00984100050166442. [DOI] [PubMed] [Google Scholar]
- 26.Whysner J. Benzene-induced genotoxicity. J Toxicol Environ Health A. 2000;61:347–351. doi: 10.1080/00984100050166343. [DOI] [PubMed] [Google Scholar]
- 27.International Agency for Research on Cancer (IARC) International Agency for Research on Cancer; Lyon (France): 2003. IARC monographs programme on the evaluation of carcinogenic risks to humans. [Google Scholar]
- 28.Pezzagno G., Maestri L., Fiorentino M.L. Trans, Trans-muconic acid, a biological indicator to low levels of environmental benzene: some aspects of its specificity. Am J Ind Med. 1999;35:511–518. doi: 10.1002/(sici)1097-0274(199905)35:5<511::aid-ajim8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 29.Kivistö H., Pekari K., Peltonen K., Svinhufvud J., Veidebaum T., Sorsa M., Aitio A. Biological monitoring of exposure to benzene in the production of benzene and in a cokery. Sci Total Environ. 1997;199:49–63. doi: 10.1016/s0048-9697(97)05481-8. [DOI] [PubMed] [Google Scholar]
- 30.Melikian A.A., Prahalad A.K., Hoffmann D. Urinary trans, trans-muconic acid as an indicator of exposure to benzene in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 1993;2:47–51. [PubMed] [Google Scholar]
- 31.Verma D.K., des Tombe K. Measurement of benzene in the workplace and its evolution process, part I: overview, history, and past methods. Am Ind Hyg Assoc J. 1999;60:38–47. doi: 10.1080/00028899908984421. [DOI] [PubMed] [Google Scholar]