Abstract
Infectious Bursal Disease is the second important viral disease of poultry which affects the young growing pullets. The end fate appears in huge economic losses to poultry industry. Throughout the world, cheapest source of animal protein is chicken meat. It was initially reported in Europe; soon it spreads worldwide and causes drastic losses. In Pakistan, first of all this disease was reported in 1971. It is the first review to track the IBDV history in Pakistan. It provides comprehensive details of forty-six years researchers work in controlling this important disease. Different scientists worked to fill the gap areas to achieve the goal. Present review covers all the research aspects being explored in Pakistan since first report.
Keywords: Infectious bursal disease, Forty-six years, Pakistan
1. Introduction
Poultry sector is second biggest organisation in Pakistan (Anjum et al., 1993a, Anjum et al., 1993b, Siddique et al., 1987). Gumboro is economically important disease causing great losses to this emerging industry (Anjum et al., 1993a, Anjum et al., 1993b, Tsai and Lu, 1993). IBD is continuously threat for the growing pullets. In 1962 this disease was discovered in town Gumboro (Cosgrove, 1962). Qureshi that Gumboro was first time diagnosed in 1971 in Pakistan (Qureshi, 1999). Since its first report it has not been given priority by the research workers. In next 10–15 years it appeared as havoc for poultrymen (Siddique et al., 1987). The Infectious bursal disease (IBD) is the second considerable threat among infectious poultry diseases (Alexander, 1996). In early ninety’s this disease contributes in major losses to poultry farmers due to unawareness of controlling measures in Pakistan (Anjum et al., 1994). The causative agent of “Gumboro disease” is infectious bursal disease virus (IBDV) (Müller et al., 2003). It is an acute important disease of young chickens of 3–5 weeks old (Yunus et al., 2008), which is caused by a dsRNA virus belonging to Birnaviridae (Ferrero et al., 2015).
2. Incidence, prevalence and epidemiology
In a short tenure the IBD spreads throughout the world. Limited resources and insufficient measures taken by the poultry breeders to control the viral diseases have declared the farming risky business. Poor vaccination programme added losses of farmers (Farooq et al., 2000). In early eighty’s mortality in layers increased that might be due to viral infection as the mortality pattern repeated each time (Ahmad and Irfan, 1981). Increase incidence of poultry diseases in Pakistan change the scenario of poultry sector in future (Qureshi, 1981). In 1987, outbreak was quoted in Fayoumi birds in Government Poultry Farm, Peshawer during the months January–February in Pakistan. The morbidity rate was 70% while mortality rate raised up to 15% in affected ones (Khan et al., 1988b). Initially the diseased flocks were evaluated on basis of post mortem. Data analysis showed that the flock size, bird type and vaccination (strain and schedule) has significant effects in occurence of disease (Farooq et al., 2000). During the year 1997 and 1998 data from 50 poultry farms was collected from Mirpur and Kotli districts of Kashmir. Concurrent occurence of coccidiosis in a rearing flock had a significant effect (P < 0.01) on the prevalence of IBD. Average losses from IBD were 15.31 ± 1.04%. Financial losses due to IBD per year were approximately Rs. 31701.38 ± 2345.36 from single farming unit. To prevent any stress or unsanitary conditions its better to maintain two weeks flock interval to avoid existing diseases (Farooq et al., 2003). It is recommended to improve vaccination programme and other managemental tools for better production (Abbas et al., 2015). The epidemiological studies poultry anomalies during 1998 (Farooq et al., 2002). In 2000–01 losses from IBD were reduced due to effective vaccination strategy and biosecurity measures in commercial sector. The net losses due to different factors were 50.4% in brooding and 31.3% during laying as compared to growing i-e 18.3%. Mortality was negatively correlated with peak lay. Losses of IBD were given in tabulated form mapped that IBD was present 60% as compared to all prevalent diseases (Farooq et al., 2002). (Table A).
Table A.
Impact of IBD losses at different ages of commercial flocks.
| Disease | Brooding | Growing | Laying | Overall |
|---|---|---|---|---|
| Infectious Bursal disease | 5.35 ± 1.89 | 3.44 ± 1.78 | 6.12 ± 2.02 | 4.97a ± 2.91 |
Means with different subscripts are significantly different at α = 0.05.
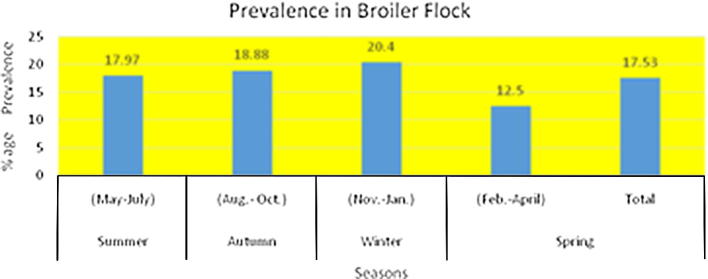
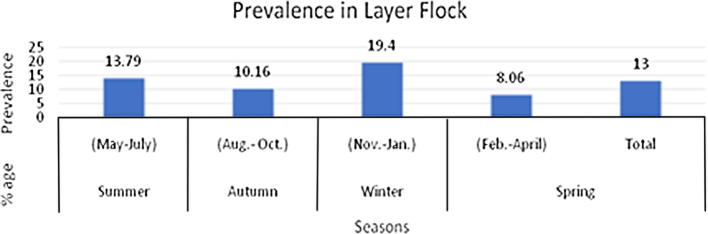
Experts documented that IBD was one of the disease which was not affected by weather (Yunus et al., 2009). In broiler and layer flocks incidence of IBD was measured with respect of age and flock size. It is noted that IBD decreased with increase in flock size. This threat is at peak during third and fourth week of age (Khan., 1991, Sultana et al., 2008, Yunus et al., 2008, Sarfraz et al., 2017). (Figure A.1, Figure A.2).
Figure A.1.
Prevalence of Infectious Bursal Disease in Broiler Flocks throughout the year.
Figure A.2.
Prevalence of Infectious Bursal Disease in Layer throughout the year.
In the year 2007 the data was evaluated on the parameters of PM and previous history from fifty sheds. The prevalence of IBD in 7.75%. The overall mortality and morbidity rates were to be 6.38% and 1.35% accordingly. There was maximum mortality in fourth week 38% (Khan et al., 2009). IBD initiated in the north Islamabad area, severe form of disease appear in Karachi. It came to notice that it is present in both vaccinated and non vaccinated flocks. It may cause 20% mortality in a flocks throughout the year in private and government poultry farms. The incidence of clinical Gumboro is higher in the commercial layers than broilers (Amin et al., 1991). Razia and co-workers investigated that 17.53% broiler sheds were affected from Gumboro whereas 13.45% of layer keepers suffered during the whole year. For future control strategies it was recommended to regulate surveillance and characterization of field strains (Sultana et al., 2008). Its interesting that occurring of disease in smaller flocks (<1000) was higher than larger flocks (>4000) but season had not major influence in disease spread (Farooq et al., 2000, Yunus et al., 2009, Zaheer et al., 2017). Anjum mentioned that if pullet vaccinated at age of 3 weeks its immunity lasts till 100 days. Booster should be given at age of 25 days. Severity of IBD was more in non-vaccinated flocks than in vaccinated flocks.
3. Transmission/spread
This disease prevails around the year however stress factors enhances its occurence in birds. In winter months the sheds were kept air tight to maintain temperature so the virus load increases inwards when the fresh air moves through shed ventilation system it becomes the vital source of aerosol infection in nearby sheds (Sultana et al., 2008).
4. Clinical and sub-clinical disease
IBD is present in clinical and subclinical forms. The recovered bird may serve as carrier. Affected ones were anorexic, dull and depressed in the initial stages. There were ruffled feathers sticked around the vent and mucoid diarrhea was found in majority of affected birds (Khan et al., 1988a). For the comparison of different IBD vaccines pathogenic lesions were evaluated in the three week old chickens on the basis of hemorrhages on the thigh and breast muscles, bursa weight to body weight ratio and virulence. The result showed that two could be classified as mild, two were moderate and two of the strains were for the suspectable host. These vaccines claimed that can be used in presence of maternal Ab which was not appreciated. High mortality was reported in broiler flocks in different regions of Pakistan. Severe immunosuppression was recorded in all the groups by analyzing the serum. Lack of antibodies to endemic pathogens was reflection of very high degree of immunosuppression in the broiler groups. The presence of Ab against IBDV showed that extreme immunosuppression might be due to IBD (Rehman, 2001). The co-relation between the mycotoxins and feed (commercial and home mix) was ensured in terms of disease occurence. Toxin levels increased due to more moisture in feed, it result in immunosuppression (Yunus et al., 2009, Jamal et al., 2017).
5. Adaptation of IBDV
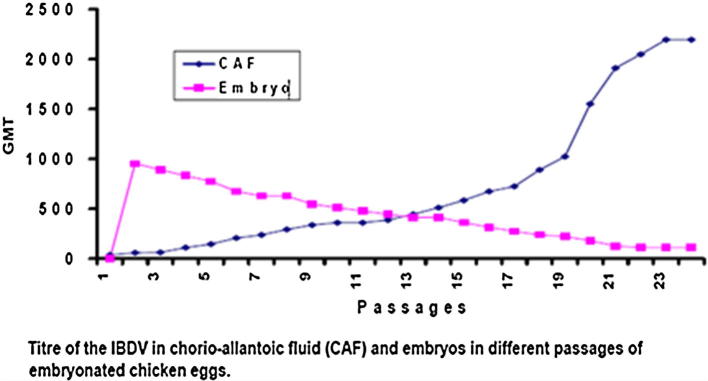
The IBDV differs in infection and growth kinetics in cell culture and it depends on virulence of strain. IBDV was confirmed through AGPT and counter current immune electrophoresis (CCIE). The concentration of virus was checked by reversepassive haemagglutination (RPHA) test and egg infective dose fifty (EID50). During first three passages through chorio-allantoic membrane (CAM) Geometric mean titre (GMT) of the virus in CAF was 37–64 but titre of virus at 24th passage raised to 2195. On the other hand virus titre in the embryos was 1024–512 in first 10 passages, while the virus titre decreased to 111 up to 24th passage. Similarly, embryos were monitored for lesions and mortality. During first seven passages severe lesions were present on the CAM while moderate to mild haemorrhagic lesions were seen from 8th to 16th passages and no lesions were observed in 17–24th passages. The result of present study revealed that local IBDV was fully adapted and attenuated in embryonated chicken eggs. In early passages the virus titre was low in CAF but boost with the passages. In initial passages the lesions were severe on CAM and embryos, but their severity decreased in later passages. The first passages of adapted and attenuated virus may be a good candidate for hot vaccine, the middle passages for the intermediate and last passages i.e., 20–24th as mild vaccines, respectively (Ahmad et al., 2005) (Fig. A.3).
Figure A.3.
Graph shows the titre of IBDV in chorio-allantoic fluid (CAF) vs embruos in different passages of embryonated chicken eggs.
6. IBDV antigenicity/immunity
There is no alternative of vaccination in the prevention of IBD or Gumboro disease. Immune reflection was variable in each selected schedule depending upon level of maternal antibodies and time of vaccination. The affected birds showed depression of immune response (Khan et al., 1988b). For the evaluation of antigenic response of IBDV, a trail was conducted against various types of IBD vaccines purchased from market in broilers. The decline pattern of maternal antibodies was observed in non-vaccinated birds in comparison to different IBD vaccine protocols. It was recommended to adopt vaccination programme in preview of prevailing maternal antibodies in day old chicks. Single shot vaccination was compared with the two shot procedure of immunization, it was found that last one was proved better than the first programme (Ahmed et al., 2003, Shareef et al., 2017). It was investigated by selecting ten breeder flocks from the field for serum and egg yolk antibody titres against IBD. Geometric mean titre (GMT) ranged from 9 to 59 in serum and 20 to 127 in the eggs. The titres were reached at peak in 4 weeks post-vaccination while it fell down at 8 weeks. Antibodies (Ab) titres were higher in eggs rather than serum. To maintain protective levels for longer interval it is suggested to re-vaccinate breeder at age of 40–45 weeks for better maternal immunity. The level of Abs titre was not affected by age of the breeder in serum and the egg yolk of flock. The similar studies might be carried on Specific Pathogen Free (SPF) flocks in a controlled environment for valuable results (Ahmad and Siddique, 1998). The study was planned to observe the antigenic response of three commercial vaccinal strains [two intermediate plus (hot) vaccines (228-E and BUR-706) and one mild (Gumboral CT)] on bursa, spleen and thymus in birds. Present findings pointed that intermediate plus strains have good protective cover against infectious bursal disease. Some degenerative changes were noted in the immune organs but they recovered soon. There should be proper replacement with rationally designed subunit and/or recombinant viral vector vaccines will be recommended (Azhar et al., 2012). The effect of three IBD vaccines were noted in the low titre of ND flock. 228-E, D-78 and Bursine 2 (IBD vaccines) were evaluated against other viral vaccine, it was noted that strain Bursine 2 live vaccine can be used in the layer flock which was already at risk. It was applied on the account of showing least immunopathological response in the immune organs (Ayyub et al., 2003). Recently, protein profile of local strains and imported vaccine (D-78) was compared and found that 60kd molecular weight protein band was common in all groups but it was absent in the vaccine D-78 strain. SDS-PAGE and Western blot techniques were used for studying structural polypeptides and protein analysis. It confirmed that antigenic heterogenicity was prevalent among the native strains and the imported vaccine strains of IBDV. Its observed that local isolates provide better protection due to more antigenic relatedness. Its the need of time to manufacture indigenous vaccines using locally availing strains of IBDV for broader protection profile (Mannan et al., 2015, Rashid et al., 2017). Three immunoboosting products (levamisole, vitamin E and bursinex) from market were compared against IBD. Vitamin E played a leading role in response to others. Levamisole role was intermediate while bursinex did not show much protection against IBDV (Mushtaq et al., 2003). Recently, the trail evaluated hematology and immune responses against infectious bursal disease on two groups of broilers. One group serves as control while other challenged with mixed Eimeria species. Both the parameters were adversely altered (Akhtar et al., 2015, Sindhu et al., 2017). The immunosuppressive effects of IBD vaccine were checked against ND vaccination. The immunosuppressant behaviour of IBDV had adversely affect immunity produced against ND. In comparison, birds that received intermediate strain of IBD were found well immunoprotected (Rehman et al., 2014). In the experimental cages 20 commercial layers were reared. To enhance titre of IgY in eggs, multiple shots of oil based IBD vaccine were used. AGPT and ELISA tests were performed to determine the Abs level. Immune egg yolk having 64 AGPT units provide 80% recovery in diseased ones. Its noted that significant antibody titre was lost during the purification process from egg yolk. Hyperimmune yolk bearing titre about 6000 gives full protection in infected chickens however semi purified Ig did not give full protection to IBDV infected birds. However semi purified IgY and immune yolk having titre more than 4000 were relatively effective in prevention. It disclosed that after killed vaccination protective antibody titre persist in yolk for two and half month (Farooq et al., 2012). Its recommended that 2–3 shots of vaccine in routine farming provides better outcome while it might be given more in breeder for maximum maternal antibodies in next progeny for early protection (Hasan et al., 1998).
7. Inactivation of IBDV
IBDV is very virulent and highly resistant to inactivation. The genetic alterations responsible for the attenuation of infectious bursal disease virus (IBDV) have been under research at the molecular level, although passage of the virus in cell culture ends in the loss of virulence. The study was conducted for the inactivation of infectious bursal disease virus (IBDV). It was isolated from the bursa of infected birds. Confirmation of IBDV was done by agar gel precipitation test. Binary Ethylenimine (BEI) was used at concentration of 0.001 and 0.002 mol/L. It showed completely inactivation of the virus after 36 h, whereas 0.1% and 0.2% formalin inactivated the virus after 24 h of incubation at 37 °C. These findings declared that BEI inactivated IBDV for further use as vaccine in chickens (Habib et al., 2006). IBDV is continuously present in poultry sheds as it is found resistant to inactivation by both heat and many disinfectants.
8. Histopathology
After postmortem samples were taken for investigations of pathological changes. The affected group showed typical changes from partial depletion of lymphocytes to cyst formation in the center of many follicles. In the medulla of follicles several small cysts were found. The bursae had post-necrotic follicular atrophy at age of 34 days (Khan et al., 1988a). In 2012, Azhar and co-workers noted histopathological changes in vaccinated birds challenged with vvIBDV. Two intermediate plus and one mild strain was used in different groups. Intermediate strains exerts adverse effects on the development of immune organs harboring B cells. In nutshell it is need of the hour to develop IBD vaccine with low virulence conferring excellent protection against the disease (Azhar et al., 2012). An experiment was designed to compare the commercial vaccine with the egg attenuated live vaccines. Different vaccine groups were given separate challenges and found that the egg attenuated vaccine having 24 passages proved best among all groups. In short its results were evaluated on histopathological lesion in different selected organs (kidney, muscles and thymus), better Abs titre and protection against IBD (Ahmad et al., 2014). Histologically, there was also proliferation of fibrous tissue in the interfolicular region (Khan et al., 1988b). In morbid tissue haemorrhages, lymphoid cell necrosis and depletion of lymphocyte were noted (Hasan et al., 1998).
9. Vaccines
IBD is one of the economically major diseases that affects growing chickens throughout world. To prevent IBD strict biosecurity programmes along with efficient vaccination strategies have been in practice. Commercial vaccines with egg attenuated live vaccines had been compared, final results declared that live vaccines provide wider protective profile (Ahmad et al., 2014). A new concept of ovo route vaccination was given. Different strains of IBDV were practiced hot strain proved significantly better than others (hatched chicks). It concludes that in ovo vaccination was effective and highly safe in protecting the growing pullets against IBD. Higher immune titre was recorded which enabled maximum safety against exposure to virus (Riaz et al., 2004). Different adjuvants were tried for best selection. Serum was raised against IBD in rabbits from field isolates. Three different preparations in rabbits were used. Adjuvanted with incomplete Freund’s adjuvant provides better immune effect Hussain et al., 2004.
10. Cell culture
For better understanding of relation between host and IBDV interaction its better to observe growth pattern on cell culture. For provision of immunoprotected candidate for local live attenuated vaccine will be provided by cultivation in embryonated eggs. Adoption was successfully achieved and no pathological lesions were found in natural hosts. Protection level was much better in limited vaccination study as compared to commercial vaccine (Anjum et al., 2010). Different beneficial micro organisms were used as pro-biotics and found that it has positive co-relation to water and feed intake which leads to weight gain and increase in antibody titres. It was found that group “G” had maximum IBD titre at age of 15, 30 and 45 days. Antibiotic growth promotors were found to develop antibiotic so these probiotic (beneficial microbes) were considered highly value (Ashraf et al., 2005). A local wild type (vvIBDV) was successfully adapted for growth on chicken embryofibroblast (CEF) cell culture after thirteenth passage with concurrent loss of pathological imprints in specific pathogen free (SPF) chicks. Adapted virus showed 1:1024 titre at fifteenth passage with complete loss of pathological lesions (Khan et al., 2007). Successful attempt was made for production of cost effective cultured base vaccine from local strains on chicken embryo fibroblast cells. Its observed that maximum level of immunogenicity was efficiently adapted to grow local IBDV in Vero-cell line within limited passages. The live vaccine prepared from this cell line produced high immunogenicity as compared to other commercially available live cell culture vaccines (Rasool and Hussain, 2006). In this study it pointed out that ISCOM-based IBD vaccine manufactured from local vvIBDV attenuated Vero cell is more friendly in terms of VN-antibody titres as compared to commercial vaccines. In commercial layers and breeders this type of vaccine will provide prolong immune response (Rasool, 2008).
11. Post mortem
Post-mortem examination showed haemorrhages in the thigh and breast muscles. The lesions in the bursa ranges from flakes of pus to haemorrhages (Khan et al., 1988b). In addition to above said kidneys were also found swollen along with deposition of urates (Khan et al., 2009). It was noted that 20.05% hemorrhages in the bursa and 18.85% were found in thigh muscles. Bursal atrophy was found in 61% where as 11% showed haemorrhages in breast muscles. 89% affected with nephritis and only 4% pictured necroric liver (Sultana et al., 2008).
12. Diagnosis
Initial diagnosis can usually be made based on flock history, clinical signs and PM examinations. However, definitive diagnosis can only be made by the specific detection and/or isolation and characterization of IBDV. The disease was diagnosed on the basis of clinical signs and postmortem findings first time in Pakistan (Khan et al., 1988a). The level of Abs were calculated. Serum antibodies against infectious bursal disease were detected by IHA (Indirect Hemagglutination) test. It was found that maximum positive sample were between 6 and 8 weeks of age and minimum Abs were present in group below 3 weeks of age. For the characterization of Pakistan isolates, a reverse transcriptase polymerase chain reaction/restriction fragment length polymorphism (RT-PCR/RFLP) technique was used. SspI restriction enzyme was used. By this study it was confirmed that vvIBDVstrains were existed in commercial set up. RT-PCR/RFLP is a very useful and rapid method for characterization and identification of existing and evolving strains of IBDV (Zahoor et al., 2011). In this study three immunodetective techniques were compared to assess the best one. For the IBDV detection reverse transcription polymerase chain reaction (RT-PCR), reverse passive haemagglutination assay (RPHA) and agar gel precipitation test (AGPT) were compared by screening suspected virus in bursa of Fabricius, liver, kidneys, spleen and thymus of chicken. Its noted that RPHA were less time consuming and sensitive ones as compared to AGPT. In field conditions RPHA has given economical results in contrast to other techniques. RT-PCR was found more sensitive than other local available techniques (Mahmood and Siddique, 2006, Ali et al., 2017). A strip was developed in Dot-ELISA for quick diagnosis of multiple pathogens in a single kit during field inspections. This diagnostic tool was successful with little false results but its highly specific, very sensitive and less time consuming for trained field workers (Alam et al., 2012). The performance of DOT-ELISA was weighed with agar gel precipitation test (AGPT). The DOT-ELISA showed better sensitivity than diagnostic AGPT. The other edge of DOT-ELISA was that it has ability to detect lBDV at early stage of infection so it was best low cost screening test (Sultana et al., 1999).
13. Therapy
The pathogenic viruses spreads infections in the targeted populations. There is no effective remedy for the viral diseases but by adopting control measures and supportive therapy, the losses can be reduced. Vaccination may not be successful 100% for raising immunity. The multivitamin therapy combined with vaccination showed boosting effects in protection. IBD strain D-78 caused low stress with good protective titre in the birds (Khan et al., 2003). The comparative immunostimulatory studies of two available market products i-e Livol (herbal supplement) and immunotone (selenium and vit E) by ELISA were noted. Livol showed more encouraging results than immunotone to minimize the adverse effect of IBDV vaccine. Different immunogenic products were tried to select the better one available in commercial markets (Arshad et al., 2005, Qayyum et al., 2012, Saleem and Mehmood, 2013, Zahid et al., 2015). It promoted immune status against IBD and result in better feed conversion ratio (FCR) (Mahmood et al., 2014). It was observed that hyperimmunized yolk controlled IBD infection in rural and hybrid birds (Malik et al., 2006). Medicinal plants extracts were used in designed experiments. Immunological and hematological profile of the birds showed that there was significant improve in antibodies titres against IBD. Findings depicts that oral administration enhances immunological profiles (Mushtaq et al., 2012, Muhammad et al., 2017) In this study high molecular mass glycoproteins (HMMGs) derived from sugar cane (Saccharum officinarum) juice were tried. It was found that it had immunostimulatory response of both cellular and humoral aspects on growth rates in commercial broiler. HMMGs were extracted from sugar cane juice by size exclusion chromatography (Awais et al., 2013, Raziq et al., 2012).
14. Conclusion
In Pakistan during last forty-three years most of work was done on incidence, prevalence and epidemiology of IBD. In initial stages disease was traced on the basis of clinical signs and post mortem lesions. During intermediate period histopathology confirms the changing culprit. The other diagnostic tools were IHA, AGPT, different kinds of ELISA, CCIE, egg infective dose fifty (EID)50, RPHA, and RT-PCR/RFLP. The second largest aspect was use of immunostimulants to combat immunosuppression. Immunoboosters may be natural or synthetic in nature. The sophisticated work of virology required huge funding so this aspect was not touched up to the required levels. Although different characters of IBDV were studied in cell culture and embryonated eggs. For preparation of local IBD vaccine trails were proved quite successful. But final products were not commercialized due to many reasons. Pakistan is still spending huge revenue for importing IBD vaccine. Imported vaccines are not providing more than 80% protection. The developed countries are working on novel aspects along with handsome knowledge of continuously circulating local strains. Production of successful IBD vaccine based upon native viruses is the only cost effective tool to save major losses from this immunosupressive disease.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas G., Khan S.H., Hassan M., Mahmood S., Naz S., Gilani S.S. Incidence of poultry diseases in different seasons in Khushab district, Pakistan. J. Adv. Veter. Anim. Res. 2015;2:141–145. [Google Scholar]
- Ahmad A., Hussain I., Siddique M., Mahmood M. Adaptation of indigenous infectious bursal disease virus (IBDV) in embryonated chicken eggs. Pakistan Veterin. J. 2005;25:71. [Google Scholar]
- Ahmad A.N., Hussain I., Akhtar M., Bibi F. Comparative study of commercially available infectious bursal disease vaccine with egg attenuated live vaccine. Pakistan J. Zool. 2014;46:959–966. [Google Scholar]
- Ahmad I., Irfan M. The causes of mortality in layers around Lahore [viral diseases, Pakistan] Pakistan Veterin. J. 1981 [Google Scholar]
- Ahmad M., Siddique M. Kinetics of antibodies in serum, egg yolk and day-old chicks against infectious bursal disease in chicken broiler breeders. Pakistan Veterin. J. 1998;18:187–191. [Google Scholar]
- Ahmed Z., Inayat S., Naeem K., Malik S. Comparative immune response pattern of commercial infectious bursal disease vaccines against field isolates in Pakistan. Int. J. Poultry Sci. 2003;2:449–453. [Google Scholar]
- Akhtar M., Awais M.M., Anwar M.I., Ehtisham-ul-Haque S., Nasir A., Saleemi M.K., Ashraf K. The effect of infection with mixed Eimeria species on hematology and immune responses following Newcastle disease and infectious bursal disease booster vaccination in broilers. Veterin. Quart. 2015;35:21–26. doi: 10.1080/01652176.2014.991048. [DOI] [PubMed] [Google Scholar]
- Alam J., Muhammad F., Siddiqui M.U., Khan S.A., Rehmani S., Ahmad A. Dot-ELISA for Newcastle disease, infectious bursal disease and Mycoplasmosis. Pakistan J. Zool. 2012;44:1301–1305. [Google Scholar]
- Alexander D. Office International des Epizooties; Paris: 1996. Newcastle Disease Manual of Standards for Diagnostic Tests and Vaccines. [Google Scholar]
- Ali W., Habib M., Sajid S., Khan R.S.A., Mazhar M.U., Khan I.U., Saliha U., Farooq M., Shah M.S.D., Muzammil H.M. A reverse transcription-polymerase chain reaction (RT-PCR) based detection of foot-and-mouth disease in District Faisalabad, Pakistan during the Year 2016. Matrix Sci. Med. 2017;1(1):27–29. [Google Scholar]
- Amin M., Rana A., Hussain T. Annual Progress Report of Poultry Development Center; Rawalpindi: 1991. Prevalence of Gumboro disease in broilers and around Multan. [Google Scholar]
- Anjum A., Hassan S., Arbi G. Infectious bursal disease in chickens in Pakistan. Pakistan Veterin. J. 1993;13:54. [Google Scholar]
- Anjum A., Sabri G., Jamshidi K. Proceedings of 1st PPAPVMA Punjab, International Poultry Conference March. 1994. Occurrence spread and control of infectious bursal disease in Pakistan; pp. 57–59. [Google Scholar]
- Anjum A.A., Hussain I., Mahmood M.S., Anwar M.I. Adaptation of infectious bursal disease virus by cultivation in embryonated chicken eggs and evaluation as potential candidate for local live attenuated vaccine. Pakistan J. Life Social Sci. 2010;8:30–34. [Google Scholar]
- Anjum A.D., Hassan S., Arbi G.S. Infectious Bursal Disease chickens in Pakistan. Pakistan Veterin. J. 1993;13:54–58. [Google Scholar]
- Arshad M., Siddique M., Ashraf M., Khan H. Effect of selenium supplementation on antibody titres against infectious bursal disease vaccine in broiler chicks. Pakistan Veterin. J. 2005;25:203. [Google Scholar]
- Ashraf M., Siddique M., Rahman S., Arshad M., Khan H. Effect of various microorganisms culture feeding against Salmonella infection in broiler chicks. J. Agric. Soc. Sci. 2005;1:2. [Google Scholar]
- Awais M.M., Akhtar M., Iqbal Z., Muhammad F. Saccharum officinarum derived mid molecular mass glycoproteins as native BRMs in chickens. Pak. J. Life Soc. Sci. 2013;11:200–207. [Google Scholar]
- Ayyub R., Aslam A., Khan S., Munir M. Comparative immunopathological and immunosuppressive effects of three different Gumboro vaccine strains against Newcastle disease vaccination in broilers. Pakistan Veterin. J. 2003;23:181–186. [Google Scholar]
- Azhar S., Akhtar S., Munir M. Post-vaccinal observation of lymphoid organs in broiler chicks inoculated with hot and mild vaccinal strains of infectious bursal disease virus. J. Vetern. Anim. Sci. 2012;2:72–78. [Google Scholar]
- Cosgrove A. An apparently new disease of chickens: avian nephrosis. Avian Dis. 1962;6:385–389. [Google Scholar]
- Farooq A., Rabbani M., Muhammad K., Akram Z., Ahad A., Fatima Z., Kamal T., Anwar Z. Passive immunization in infectious bursal disease virus infected birds using chemically purified immune yolk immunoglobulins (IgY) Afr. J. Microbiol. Res. 2012;6:2993–2998. [Google Scholar]
- Farooq M., Durrani F., Faisal S., Asghar A., Khurshid A. Incidence of infectious bursal disease among birds submitted to a diagnostic laboratory in NWFP, Pakistan. Pakistan Veterin. J. 2000;20:77–80. [Google Scholar]
- Farooq M., Durrani F., Imran N., Durrani Z., Chand N. Prevalence and economic losses due to infectious bursal disease in broilers in Mirpur and Kotli districts of Kashmir. Int. J. Poult. Sci. 2003;2:267–270. [Google Scholar]
- Farooq M.Z., Durrani F.R., Mian M.A., Chand N., Ahmed J. Prevalent diseases and overall mortality in broilers. Pakistan Veterin. J. 2002;22:111–115. [Google Scholar]
- Ferrero D., Garriga D., Navarro A., Rodríguez J.F., Verdaguer N. Infectious bursal disease virus VP3 upregulates VP1-mediated RNA-dependent RNA replication. J. Virol. 2015;89:11165–11168. doi: 10.1128/JVI.00218-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib M., Hussain I., Fang W., Rajput Z., Yang Z., Irshad H. Inactivation of infectious bursal disease virus by binary ethylenimine and formalin. J. Zhejiang Univ. Sci. B. 2006;7:320–323. doi: 10.1631/jzus.2006.B0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S., Fawad N., Siddique B. A study on the Infectious Bursal Disease around Rawalpindi/Islamabad. Pakistan J. Livestock Res. 1998;8:84–88. [Google Scholar]
- Hussain I., Rasool M., Mahmood M. Production of hyperimmune serum against infectious bursal disease virus in rabbits. Pak. Vet. J. 2004;24:179–183. [Google Scholar]
- Jamal M., Shareef M., Sajid S. Lincomycin and tetracycline resistance in poultry. Review. Matrix Sci. Pharma. 2017;1(1):33–38. [Google Scholar]
- Khan K., Shah S., Afzal M. Revue Scientifique et Technique de l'OIE; France: 1988. Observations on Gumboro disease (infectious bursal disease) in Pakistan. [DOI] [PubMed] [Google Scholar]
- Khan K., Shah S., Afzal M. Observations on Gumboro disease in Pakistan. Rev. Sci. Tech. Int. Epi. 1988;7:625–629. doi: 10.20506/rst.7.3.366. [DOI] [PubMed] [Google Scholar]
- Khan M., Hussain I., Siddique M., Rahman S., Arshad M. Adaptation of a local wild Infectious bursal disease virus on chicken embryo fibroblast cell culture. Int. J. Agric. Biol. 2007;9:925–927. [Google Scholar]
- Khan R.W., Khan F.A., Farid K., Khan I., Tariq M., Sheikh F., Banga S., Banga S., Najeeb S., Lone B. Prevalence of infectious bursal disease in broiler in district Peshawar. J. Agric. Biol. Sci. 2009;4:1–5. [Google Scholar]
- Khan G.D. Infectious Bursal Disease in Pakistan. Pak. Poultry J. Karachi. 1991:6–9. [Google Scholar]
- Khan S.A., Aslam A., Muhammad K., Khan K.A. Effect of post vaccination on medication on layer chicks vaccinated with Gumboro Vaccine Nobilis D-78. Pakistan Veterin. J. 2003;23:192–196. [Google Scholar]
- Mahmood M., Siddique M. Comparative efficacy of RT-PCR, AGPT and reverse passive haemagglutination assay for the detection of infectious bursal disease virus in broilers. Pakistan Veterin. J. 2006;26:167. [Google Scholar]
- Mahmood S., Saleem M.F., Ahmad F., Abbas G., Mahmood A., Qamar S.H., Rehman M.Z. Comparative effect of different commercial herbal growth promotors on performance, minor body parts weight and immune response in broilers. Adv. Zool. Botany. 2014;2:69–74. [Google Scholar]
- Malik M.W., Ayub N., Qureshi I.Z. Passive immunization using purified IgYs against infectious bursal disease of chickens in Pakistan. J. Vet. Sci. 2006;7:43–46. doi: 10.4142/jvs.2006.7.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannan S., Ihsan A., Kanwal S. Comparative analysis of protein profile of vaccine strain and local isolates of avian infectious bursal disease virus by western blot. JAPS, J. Anim. Plant Sci. 2015;25:1297–1302. [Google Scholar]
- Muhammad G., Rashid I., Firyal S. Practical aspects of treatment of organophosphate and carbamate insecticide poisoning in animals. Matrix Sci. Pharma. 2017;1(1):10–11. [Google Scholar]
- Müller H., Islam M.R., Raue R. Research on infectious bursal disease—the past, the present and the future. Vet. Microbiol. 2003;97:153–165. doi: 10.1016/j.vetmic.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Mushtaq F., Khan S., Aslam A., Saeed K., Saleem G., Mushtaq H. Effects of immunostimulants on broilers suffering from Infectious bursal disease. Pakistan Veterin. J. 2003;23:36–40. [Google Scholar]
- Mushtaq M., Durrani F., Imtiaz N., Sadique U., Hafeez A., Akhtar S., Ahmad S. Effect of administration of Withania somnifera on some hematological and immunological profile of broiler chicks. Pak. Vet. J. 2012;32:70–72. [Google Scholar]
- Qayyum A., Yousaf A., Ahmad T., Rehman Z., Farooq U. Immunomodulatory effects of Lisovit® in response to Newcastle disease and infectious bursal disease vaccines in broilers. J. Anim. Plant Sci. 2012;22:11–14. [Google Scholar]
- Qureshi A. FEATURES-Gumboro Disease In Pakistan First diagnosed in 1971, this disease has now become widespread. Poultry Int. 1999;38:42–43. [Google Scholar]
- Qureshi M.S. Poultry production in Pakistan. Pakistan Veterin. J. 1981;1:131–134. [Google Scholar]
- Rashid M., Saleem M.I., Deeba F., Khan M.S., Mahfooz S.A., Butt A.A., Abbas M.W. Effect of season on occurrence of caprine mastitis in beetal in Faisalabad premises. Matrix Sci. Med. 2017;1(1):19–21. [Google Scholar]
- Rasool M.H. Preparation and evaluation of an experimental iscom-based infectious bursal disease vaccine. Indian J. Microbiol. 2008;48:401–404. doi: 10.1007/s12088-008-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool M.H., Hussain I. Preparation and evaluation of Vero-cell infectious bursal disease vaccine in Pakistan. Vaccine. 2006;24:2810–2814. doi: 10.1016/j.vaccine.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Raziq F., Chand N., Sultan A., Mushtaq M., Suhail R.S.M., Zeb A. Effect of water based infusion of Aloe barbedensis, Pimpinella anisum, Berberis lycium, Trigonella foenum-graecum and Allium sativum on The Performance of Broiler Chicks. Pakistan Veterin. J. 2012;32:593–596. [Google Scholar]
- Rehman K.A., Raza M., Ahmad M.A., Joiya M.J., Haq M.H., Qazi I., Bachaya K.N., Allah H. Effects of Infectious Bursal Disease vaccine on the immunity induced by Newcastle Disease vaccine in broiler birds. Sci. Int. 2014;26 [Google Scholar]
- Rehman S.H. Current respiratory disease problem and the probes in chicken. Pakistan Veterin. J. 2001;22:17–20. [Google Scholar]
- Riaz M., Hussain I., Akhtar M., Rasool M., Mansoor N., Haq S. Evaluation of in ovo vaccination against infectious bursal disease virus in commercial broilers in Pakistan. Int. J. Agric. Biol. 2004;6:984–986. [Google Scholar]
- Saleem M.H., Mehmood K. Effect of a herbal supplement Livol on the growth performance and antibody response against infectious bursal disease virus in broiler chicks. Pakistan J. Zool. 2013;45:1387–1391. [Google Scholar]
- Sarfraz M., Ashraf Y., Ashraf S. A Review: Prevalence and antimicrobial susceptibility profile of listeria species in milk products. Matrix Sci. Medica. 2017;1(1):03–09. [Google Scholar]
- Shareef M., Jamal M., Sarfraz M. A review of anti-bacterial activity of Nigella sativa in gut of broiler chicks. Matrix Sci. Pharma. 2017;1(1):27–32. [Google Scholar]
- Siddique M., Javed T., Sabri M. Incidence and pathology of various poultry diseases prevalent in Faisalabad and surrounding districts [Pakistan] Pakistan Veterin. J. (Pakistan) 1987 [Google Scholar]
- Sindhu Z.D., Shafiq Z., Naseer M.U., Khan M.N., Saleemi M.K., Aslam B., Abbas R.Z., Khan M.K. Prevalence of ectoparasitic fauna and efficacy of two commercial acaricides against argus persicus in layer poultry. Matrix Sci. Pharma. 2017;1(1):39–40. [Google Scholar]
- Sultana J., Hussain M., Malik S.A., Naeem K. Use of DOT-ELISA for the diagnosis of infectious bursal disease in chickens. Pakistan Veterin. J. 1999;19:158–160. [Google Scholar]
- Sultana, R., S.A.H., Ilyas, S., Anjum, A., Zaidi, R., Hussain, F., 2008. Epidemiology of Infectious Bursal Disease in Broilers in and around Lahore. Pakistan J. Zool. 23, 067–072.
- Tsai H., Lu Y. Epidemiology of infectious bursal disease in Taiwan in 1992. J. Chinese Soc. Vet. Sci. 1993;19:249–258. [Google Scholar]
- Yunus A., Nasir M., Aziz T., Böhm J. Prevalence of poultry diseases in district chakwal and their interaction with mycotoxicosis: 2. Effects of season and feed. J. Anim. Plant Sci. 2009;19:1–5. [Google Scholar]
- Yunus A., Nasir M., Farooq U., Böhm J. Prevalence of poultry diseases and their interaction with mycotoxicosis in district Chakwal: 1. Effects of age and flock size. J. Anim. Pl. Sci. 2008;18:107–113. [Google Scholar]
- Zahid B., Saleem G., Aslam A., Imran M., Younas M. Effect of immunostimulants on humoral response against infectious bursal disease in broilers. Pakistan Veterin. J. 2015;35:227–230. [Google Scholar]
- Zahoor M., Abubakar M., Naim S., Khan Q., Arshed M. Molecular Typing of Field Isolates from two outbreaks of Infectious Bursal Disease Virus from Pakistan. Veterinary World. 2011;4:297–300. [Google Scholar]
- Zaheer Z., Rahman S., Zaheer I., Abbas G., Younas T. Methicillin-resistant staphylococcus aureus in poultry- an emerging concern related to future epidemic. Matrix Sci. Medica. 2017;1(1):15–18. [Google Scholar]