Abstract
Acitretin has been a valuable option for the treatment of psoriasis, however, the molecular events of acitretin leading to the normalization of keratinocytes differentiation on psoriasis patients have not been fully explored. To investigate whether there were certain relationship between keratinocytes proliferation and JAK/STAT signaling pathways in psoriasis, and how acitretin modulated the signaling pathways. HaCaT cells, an in vitro immortal human keratinocyte cell line, was chosen as a in vitro model of psoriasis. The small interfering RNA targeting STAT1 (siRNA-STAT1) and STAT3 (siRNA-STAT3) were subsequently transfected into the HaCaT cells which were treated with or without acitretin. We found that HaCaT cells proliferation and the expression of STAT1 or STAT3 were inhibited by acitretin, siRNA-STAT1 and siRNA-STAT3. Our experimental data shows that acitretin might inhibit HaCaT cells proliferation in psoriasis by decreasing the expression of STAT- and STAT3-dependent mechanism.
Keywords: Acitretin, HaCaT cells, STAT1, STAT3, SOCS1, SOCS3
1. Introduction
Psoriasis is a chronic, common, and immune–mediated inflammatory skin disorder (Vassantachart et al., 2016, Torres et al., 2016). Although its pathogenesis remains unclear, psoriasis is now considered as a mixed Th1/Th17 cell-mediated autoimmune disease because the activated T lymphocytes release numerous cytokines, such as interferon-γ (IFN-γ) and IL-17, to stimulate the proliferation of keratinocytes and recruit neutrophils. The cytokines-activated keratinocytes can also express a broad array of cytokines, chemokines and membrane molecules in return that induce the recruitment and activated of T lymphocytes in the skin (Wang et al., 2015, Iftakhar et al., 2015, Kupetsky et al., 2013). In addition, those key cytokines including IFN-γ, IL-6, IL-8, IFN-α, IL-12 and IL-17 were up-regulated in psoriasis patients (Johnson-Huang et al., 2012, Martin et al., 2013, Zhao and Ashraf, 2016). Thus, the T cell/cytokines/keratinocytes network is formed and partake in the pathogenesis of psoriasis.
Those key cytokines of psoriasis such as IL-6, IL-17, IL-22 and INF-γ are mediated by JAK-STAT signaling pathway. STAT1 and STAT3 can be activated by IFN-γ and IL-6 signaling, respectively. Many researches have demonstrated that the JAK-STAT signaling pathway is involved in melanoma, atopic dermatitis and psoriasis (Luo et al., 2016, Liongue and Ward, 2013, Palanivel et al., 2014). Therefore, selective blockading the JAK-STAT signaling pathway could be a potential strategy for those common skin disorders. Acitretin plays an critical role in the treatment of psoriasis thanks to its non-immunosuppressive risks and its ability to complete a long-term response (Yine et al., 2015, Sarfraz et al., 2016, Carretero et al., 2013). So far, the effects of acitretin on JAK-STAT signaling pathway of keratinocytes have been rarely reported in literatures. In this study, we explored the relationship between keratinocytes proliferation and STAT1, STAT3 expression and how acitretin modulates the JAK/STAT signaling pathways.
2. Methodology
2.1. Cell culture
HaCaT cells was obtained from institute of Biochemistry and Cell Biology (Shanghai, China) and cultured in DEMN supplemented with 100 U/ml streptomycin and 10% heat-inactivated fetal bovine serum (Hyclone). HaCaT cells were kept at 37 °C and 5% CO2 in humidified atmosphere.
2.2. siRNA transfection
The three pairs of siRNA (sigma) duplexes targeting STAT1 and STAT3 gene, and a pair of negative control siRNA (NC-siRNA) with no complementary target sequence were obtained commercially. HaCaT cells were seed into 6-well plates at a density of 5 × 104 cells/well approximately 48 h before transfection. When HaCaT cells were 40–50% confluent, 5 μL lipofectamine-2000 (Invitrogen) was added to 500 μL opti-MEM Serum Medium (GIBICO), mixed gently, and incubated for 5 min at room temperature. In parallel, 2.5 μL, 5 μL, 10 μL siRNA (20 μmol/L) were separately added to 500ul opti-MEM Serum Medium, mixed with the lipofectamine-2000. Then STAT1-siRNA, STAT3-siRNA and control siRNA with the final concentrations of 25 nmol/L, 50 nmol/L and 100 nmol/L were respectively introduced into HaCaT. The efficiency of gene knockdown was evaluated by Q-PCR and Western-blot after incubation in normal cell culture conditions for 24 h.
2.3. Acitretin effection
2.3.1. MTS assay of cell viability
HaCaT cell viability was assessed using 3-(4,5-di-methyl-thiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Briefly, HaCaT cells induced with the chosen siRNA-STAT1 and siRNA-STAT3 were seeded at their optimal cell density (1 × 105 cells/well) in 96-well plates. Meanwhile, eight groups containing cells group (A), lipofectamine-2000 group (B), NC-siRNA group (C), acitretin group (D), STAT1-siRNA group (E), STAT3-siRNA group (F), STAT1-siRNA + acitretin group (G), STAT3-siRNA + acitretin group (H) were set up. The cells were treated with 5 μmol/L acitretin (selleckchem) for 12, 24, 48 and 72 h respectively. At end of each incubation period, cells were mixed with 10ul MTS (Promega) and incubated for 4 h at 37 °C in CO2 incubator. Cell viability was metered by measuring the optical density (OD) at 490 nm.
2.3.2. Q-PCR and Western blot analysis of acitretin affection on STAT1/3 and SOCS1/3
The selected STAT1-siRNA and STAT3-siRNA were transfected to HaCaT cells which were treated with or without 5 mol/L acitretin. The eight groups’ expression of STAT 1/3, SOCS 1/3 mRNA and protein were determined by Q-PCR and western after incubation for 24 h. Detailed primer sequences for STAT1, STAT3, SOCS1, SOCS3 and GAPDH were given as follows: STAT1 forward 5′ATT ACA AAG TCA TGG CTG CT3′, reverse 5′ATA TCC AGT TCC TTT AGG GC3′; STAT3 forward 5′ CAT CTT GAG CAC TAA GCC T 3′, reverse 5′ GAG ATA GAC CAG TGG AGA CA 3′; SOCS1 forward 5′AGC TTC GAC TGC CTC TTC3′, reverse 5′GGA AGG AGC TCA GGT AGT C3′; SOCS3 forward 5′GAC GGA GAC TTC GAT TCG3′, reverse 5′AAA CTT GCT GTG GGT GAC3′; GAPDH forward 5′ TCC ACT GGC GTC TTC ACC ACC AT 3′, reverse 5′ GGA GGC ATT GCT GAT GAT CTT GAG G 3′. Relative expression of STAT1/3 and SOCS1/3 were normalized to GAPDH.
2.4. Statistical analysis
Dates were expressed as mean ± SD for three independent experiments. Comparisons among groups were carried out using One-Way ANOVA. In all analyses, P value < 0.05 was deem to demonstrate statistical significance.
3. Results & discussion
Three pairs of STAT1-siRNA and STAT3-siRNA were independently introduced into HaCaT cells at 25, 50, 100 nmol/L. As shown in the Q-PCR analysis, compared with the control group, STAT1 and STAT3 mRNA were down-regulated by STAT1-siRNA and STAT3-siRNA, respectively (Fig. 1a, Fig. 2a). As for the protein levels, STAT1 and STAT3 protein expression in the treated groups were lower than the control group (Fig. 1b, Fig. 2b). Therefore, 50 nmol/L siRNA-STAT1-003 and siRNA-STAT3-001 were optimal for the subsequent experiments.
Fig. 1.
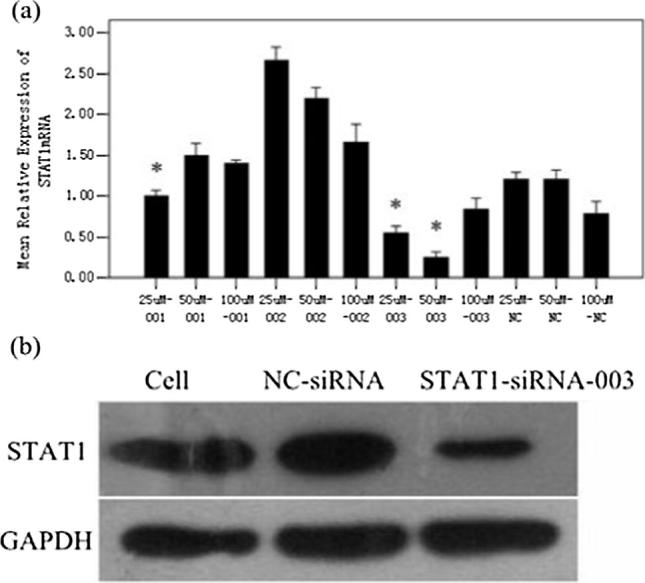
Relative expression of STAT1. The level of STAT1mRNA in HaCaT cells were detected by Q-PCR (a). (*p < 0.05 vs NC-siRNA). The protein content of STAT1 in HaCaT cells which was transfected with 50 nMol/L STAT1-siRNA-003 (b).
Fig. 2.
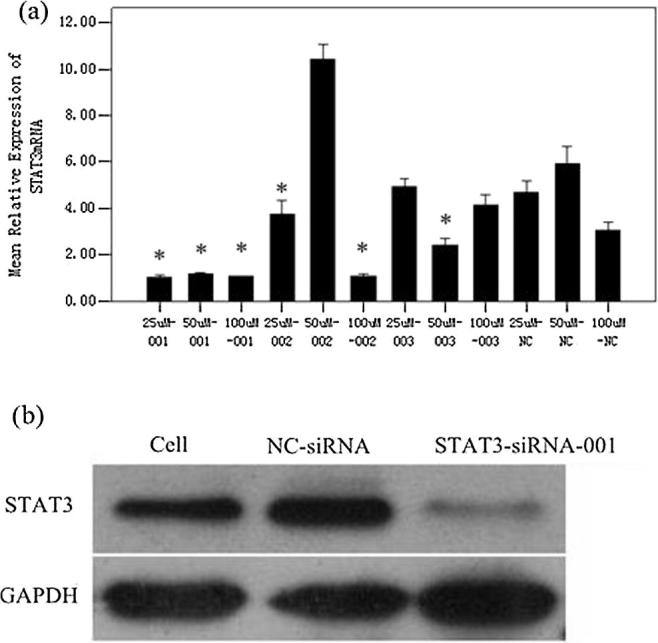
Relative expression of STAT3. The level of STAT3mRNA in HaCaT cells were detected by Q-PCR (a). (*p < 0.05 vs NC-siRNA). The protein content of STAT3 in HaCaT cells which was transfected with 50 nMol/L STAT3-siRNA-001 (b).
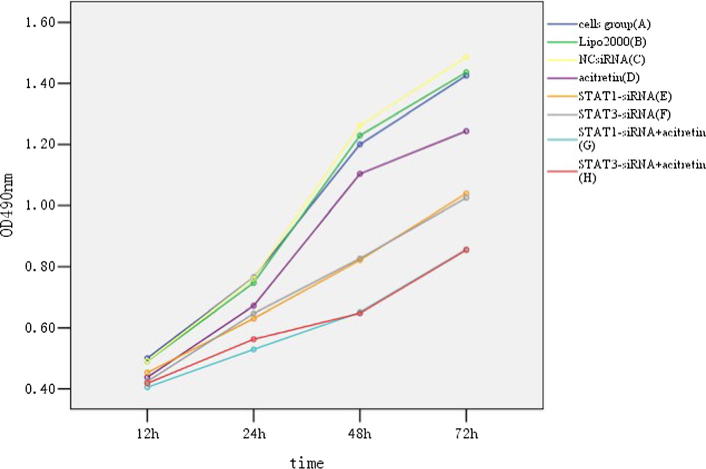
HaCaT cells transfected with 50 nmol/L of siRNA-STAT1-003 and siRNA-STAT3-001 were treated with or without 5 μmol/L acitretin. Cell proliferation was assessed by MTS assays. The comparison of eight groups indicated that acitretin, STAT1-siRNA and STAT3-siRNA alone, or combination with each other could inhibit HaCaT cells proliferation (Fig. 3).
Fig. 3.
Growth curve of HaCaT cells. MTS assay of the viability of HaCaT cells treated with STAT1-siRNA or STAT3-siRNA (50 nM) in presence or absence of acitretin (5 μM) for 12, 24, 48 and 72 h.
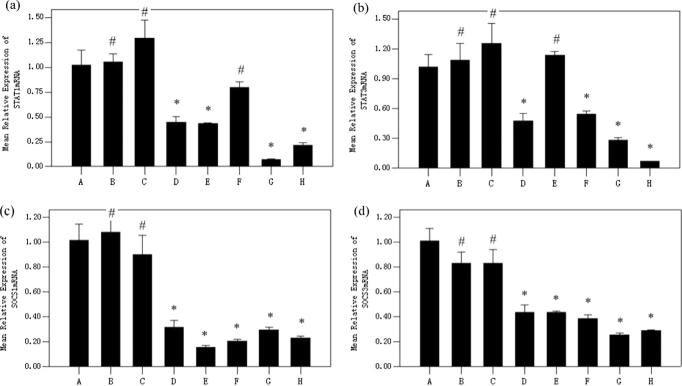
HaCaT cells transfected with 50 nmol/L siRNA-STAT1-003 and siRNA-STAT3-001 were treated with or without 5 μmol/L acitretin. STAT1, STAT3, SOCS1 and SOCS3 mRNA were assessed by Q-PCR. Those results shows that STAT1-siRNA could inhibit the expression of STAT1 and SOCS1 mRNA, while STAT1-siRNA is unable to down-regulate the STAT3 mRNA. Meanwhile, STAT3-siRNA could inhibit the expression of STAT3 and SOCS3 mRNA but cannot down-regulate the STAT1 mRNA. However, acitretin could down-regulated STAT1, STAT3, SOCS1 and SOCS3 mRNA (Fig. 4).
Fig. 4.
Relative expression of STAT1 (a), STAT3 (b), SOCS1 (c) and SOCS3 (d) mRNA in HaCaT cells of eight groups. STAT1-siRNA inhibited the expression of STAT1 and SOCS1 mRNA in HaCaT cells. STAT3-siRNA inhibited the expression of STAT3 and SOCS3 mRNA. Acitretin could down-regulated STAT1, STAT3, SOCS1 and SOCS3 mRNA. (*p < 0.05 vs Cells group, #p > 0.05 vs Cells group). Cells group (A), lipofectamine-2000 group (B), NC-siRNA group (C), acitretin group (D), STAT1-siRNA group (E), STAT3-siRNA group (F), STAT1-siRNA + acitretin group (G), STAT3-siRNA + acitretin group (H).
The alteration of protein is in agreement with the trends of mRNA. The content of STAT1 protein was decreased in acitretin and STAT1-siRNA treated groups, which has the same trend of STAT3 protein in acitretin or STAT3-siRNA treated groups. As for the SOCS1 and SOCS3 protein, both of which in acitretin, STAT1-siRNA or STAT3-siRNA treated groups was less than in blank, lipo-2000 or NC-siRNA groups (Fig. 5).
Fig. 5.
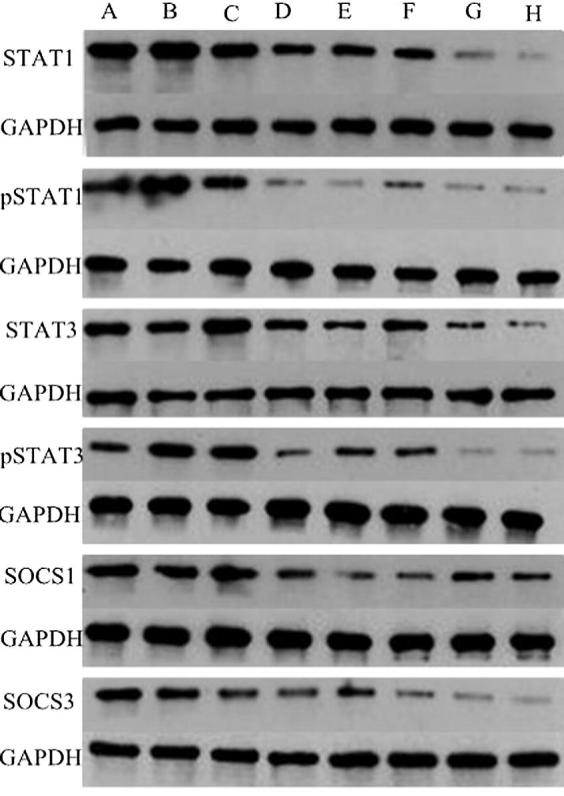
Expression of STAT1, pSTAT1, STAT3, pSTAT1, SOCS1 and SOCS3 protein in HaCaT cells of eight groups. HaCaT cells were treated with STAT1-siRNA or STAT3-siRNA (50 nM) in presence or absence of acitretin (5 μM).
Our previous studies have shown the increased level of STAT1 and STAT3 in psoriatic skin. Activation of the STAT1 signaling pathway is initiated upon IFN-γ which has long been considered the central cytokine involved in psoriasis pathogenesis. IFN-γ can up-regulate Keratin17 (K17) expression by JAK/STAT1 pathway. K17 is regarded as a hallmark of psoriasis because it is overexpressed in psoriatic lesional epidermis but not found in healthy epidermis. High expression of K17 in psoriatic lesional can stimulate the abnormal activation of T cells in return, form the “IFN-γ-K17-T lymphocytes” vicious spiral, thus contribute to the pathophysiology of psoriasis (Hald et al., 2013, Fu and Wang, 2012). STAT3 signaling pathway can be activated by IL-6, which together with IL-17, IL-23 to constitute complex cytokine regulation network. Sano et al. demonstrated that transgenic mice with keratinocytes over expressing a constitutively active Stat3 (K5.Stat3C mice) develop a skin phenotype either spontaneously, or in response to wounding, that closely resembles psoriasis. Meanwhile, the level of STAT3 in human psoriatic lesions, particularly in the nuclei of keratinocytes was increased, and there was no difference in lesions from three nonpsoriatic inflammatory skin disorders with characteristic epidermal hyperplasia (prurigo, chronic dermatitis and lichen planus) compare to normal epidermis (Liu et al., 2015, Liu et al., 2016, Kojima et al., 2013).
The SOCS proteins are functionally correlated by their ability to negatively regulate cytokine signaling. Actually, SOCS1 and SOCS3 act in a classical negative-feedback loop to cytokine signal transduction manners. SOCS1 was shown to associate with JAKs to inhibit their activity and SOCS3 appears to inhibit signaling by interacting with activated cytokine receptors. There is no one-to-one correspondence between SOCS and cytokines. In fact, SOCS1 can be induced by INF-γ, IL-2 and IL-6, while SOCS3 can be induced by IL-6, IL-2, IL-12 and TNF-α (Linossi et al., 2013). SOCS1 plays an important role in maintaining immune homeostasis and the delelation of SOCS1 gene leads to a catastrophic monocytic inflammation of the liver in mice largely caused by excessive INF-γ signaling. SOCS3 affects not only the generous of the IL-6 signaling response, but also its quality. SOCS1 and SOCS3 mRNA are not highly expressed in unstimulated cells, instead, they are encoded rapidly in cytokines treated cells with an activated-STAT-dependent manner. The expression of SOCS1 and SOCS3, to a certain extent, reflects the activation of STAT (Chen et al., 2016, Madonna et al., 2012).
4. Conclusion
Psoriasis is now considered as a chronic skin disorder featured by cutaneous inflammation and keratinocyte hyperproliferation. Acitretin is an oral second-generation synthetic retinoid which is valid in the systemic treatment of psoriasis, either in monotherapy or in combination. Previous studies have shown that acitretin can reduce the level of INF-γ and IL-17 in psoriasis patients, and modulate keratinocyte proliferation and differentiation, although the behind mechanism is still unclear (Niu et al., 2012). In this study, we found that the HaCaT cells proliferation was inhibited significantly with the decreasing expression of STAT1 and STAT3 which can be down-regulated by acitretin. We infer that acitretin modulates HaCaT cells proliferation through STAT1 and STAT3-dependent mechanisms.
Acknowledgements
This study was supported by Science and Technology Planning Project of Guangdong Province, China (Grant no. 2011B031800106). Scientific research of traditional Chinese medical of Guangdong Province (Grant no. 20141164).
Footnotes
Peer review under responsibility of King Saud University.
References
- Carretero G., Ribera M., Carrascosa J.M. Guidelines for the use of acitretin in psoriasis. Actas. Dermosifiliogr. 2013;104(7):598–616. doi: 10.1016/j.adengl.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Chen Y., Gao Y., Ashraf M.A., Gao W. Effects of the traditional chinese medicine dilong on airway remodeling in rats with OVA-induced-Asthma. Open Life Sci. 2016;11(1):498–505. [Google Scholar]
- Fu M., Wang G. Keratin 17 as a therapeutic target for the treatment of psoriasis. J. Dermatol. Sci. 2012;67(3):161–165. doi: 10.1016/j.jdermsci.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Hald A., Andres R.M., Salskov-Iversen M.L. STAT1 expression and activation is increased in lesional psoriatic skin. Br. J. Dermatol. 2013;168(2):302–310. doi: 10.1111/bjd.12049. [DOI] [PubMed] [Google Scholar]
- Iftakhar A., Hasan I.J., Sarfraz M., Jafri L., Ashraf M.A. Nephroprotective effect of the leaves of Aloe barbadensis (Aloe Vera) against toxicity induced by diclofenac sodium in albino rabbits. West Indian Med. J. 2015;64(5):462–467. doi: 10.7727/wimj.2016.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Huang L.M., Pierson K.C., Fuentes-Duculan J. A single intradermal injection of IFN-γ induces an inflammatory state in both non-lesional psoriatic and healthy skin. Invest. Dermatol. 2012;132(4):1177–1187. doi: 10.1038/jid.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Inoue T., Kunimoto H. IL-6-STAT3 signaling and premature senescence. JAKSTAT. 2013;2(4):e25763. doi: 10.4161/jkst.25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupetsky E.A., Mathers A.R., Ferris L.K. Anti-cytokine therapy in the treatment of psoriasis. Cytokine. 2013;61(3):704–712. doi: 10.1016/j.cyto.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Linossi E.M., Babon J.J., Hilton D.J. Suppression of cytokine signaling: the SOCS perspective. Cytokine Growth Factor Rev. 2013;24(3):241–248. doi: 10.1016/j.cytogfr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liongue C., Ward A.C. Evolution of the JAK-STAT pathway. JAKSTAT. 2013;2(1):e22756. doi: 10.4161/jkst.22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.K., Gao P., Ashraf M.A., Wen J.B. The complete mitochondrial genomes of two weevils, Eucryptorrhynchus chinensis and E. brandti: Conserved genome arrangement in Curculionidae and deficiency of tRNA-Ile gene. Open. Life Sci. 2016;11(1):458–469. [Google Scholar]
- Liu P., Meng W., Wang S., Sun Y., Ashraf M.A. Quaternary ammonium salt of chitosan: preparation and antimicrobial property for paper. Open Med. 2015;2015(10):473–478. doi: 10.1515/med-2015-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Wang X., Yang Y., Lan T., Ashraf M.A., Mao Q. Successful treatment of cerebral aspergillosis in a patient with acquired immune deficiency syndrome. West Indian Med. J. 2016;64(5):540–542. doi: 10.7727/wimj.2016.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna S., Scarponi C., Pallotta S. Anti-apoptotic effects of suppressor of cytokine signaling 3 and 1 in psoriasis. J. Cell. Death. Dis. 2012;3:e334. doi: 10.1038/cddis.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D.A., Towne J.E., Kricorian G. The emerging role of IL-17 in the pathogenesis of psoriasis: preclinical and clinical findings. J. Invest. Dermatol. 2013;133(1):17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X., Cao W., Ma H. Acitretin exerted a greater influence on T-helper (Th) 1 and Th17 than on Th2 cells in treatment of psoriasis vulgaris. J. Dermatol. 2012;39(11):916–921. doi: 10.1111/j.1346-8138.2012.01637.x. [DOI] [PubMed] [Google Scholar]
- Palanivel J.A., Macbeth A.E., Chetty N.C. An insight into JAK-STAT signalling in dermatology. Clin. Exp. Dermatol. 2014;39(4):513–518. doi: 10.1111/ced.12273. [DOI] [PubMed] [Google Scholar]
- Sarfraz M., Ashraf Y., Sajid S., Ashraf M.A. Testosterone level in testicular cancer patients after chemotherapy. West Indian Med. J. 2016;64(5):487–494. doi: 10.7727/wimj.2016.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres T., Raposo I., Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am. J. Clin. Dermatol. 2016;17(2):107–112. doi: 10.1007/s40257-015-0166-0. [DOI] [PubMed] [Google Scholar]
- Vassantachart J.M., Soleymani T., Wu J.J. Comparison of phototherapy guidelines for psoriasis: a critical appraisal and comprehensive review. J. Drugs. Dermatol. 2016;15(8):995–1000. [PubMed] [Google Scholar]
- Wang D., Fu Y.B., Ashraf M.A. Artifacts reduction in strain maps of tagged magnetic resonance imaging using harmonic phase. Open Med. 2015;10:425–433. doi: 10.1515/med-2015-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yine H., Shufang D., Bin W., Wei Q., Ashraf M.A., Junling G. Application of food-specific IgG antibody detection in allergy dermatosis. Open Med. 2015;2015(10):377–381. doi: 10.1515/med-2015-0067. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao L., Ashraf M.A. Influence of Ag/HA nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med. J. 2016;64(5):506–513. doi: 10.7727/wimj.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]