Abstract
The effective treatment of patients suffering from neuropathic pain remains challenging. Dexmedetomidine (DEX) possesses anti-inflammatory activity. However, the role of DEX in neuropathic pain is still unclear. The aim of the present study was to examine DEX an α2-adrenoceptor agonist could improve pain hypersensitivity and reduce inflammatory in a chronic constriction injury (CCI) model of the sciatic nerve in Sprague-Dawley rats. Dex was intrathecally administrated 1-h after operation. The paw mechanical withdrawal threshold (MWT) and paw withdrawal thermal latency (PWTL) were measured on day 1 before operation and on days 1, 7, 14 and 21 after operation, respectively. On day 21, all the rats were decapitated to collect the L4-6 segments of the spinal cord to examine IL-1, TNF-α, IL-6, NR2B, NF-κB, and iNOS mRNA levels using RT-PCR. The postoperative MWT and PWTL were significantly decreased in CCI, and DEX groups as compared to those before surgery and Sham group (P < 0.05). And DEX reversed this trend (P < 0.05). Interleukin 1 (IL-1), tumor necrosis factor α (TNF-α), IL-6 mRNA expression significantly increased postsurgery in CCI group as compared to that of Sham group (P < 0.05); DEX blocked increased IL-1, TNF-α, IL-6, N-methyl-D-aspartate (NMDA) receptor 2B (NR2B), nuclear factor κB (NF-κB), and inducible isoform of nitric oxide synthase (iNOS) mRNA levels (P < 0.05). DEX may alleviate neuropathic hypersensitivity and inflammation partially by inhibiting NR2B, NF-κB, and iNOS expression in the spinal cord of rats with neuropathic pain resulting from CCI of the sciatic nerve.
Keywords: Neuropathic pain, Dexmedetomidine, Inflammation, Rat
1. Introduction
Neuropathic pain is a common syndrome, which may result from many disorders including diabetic polyneuropathy, radicular back pain, postherpetic neuralgia, stroke, and spinal cord injury (Farghaly et al., 2014). It is characterized by hyperalgesia, allodynia and spontaneous pain (Zhou et al., 2014, Sun et al., 2006). The CCI of the sciatic nerve is a widely used model of neuropathic pain which evokes a series of molecular, biochemical and cytoarchitectural changes in primary sensory neurons and produces neuropathic pain sensations similar to those observed in humans (Farghaly et al., 2014). Ligation of the sciatic nerve also leads to the cardinal symptoms of neuropathic pain, namely apparent spontaneous pain, allodynia, and hyperalgesia (Farghaly et al., 2014, Bennett and Xie, 1988a, Bennett and Xie, 1988b).
Treatment of neuropathic pain remains difficult, due to an inadequate understanding of the mechanisms involved in the development and maintenance of neuropathic pain, and a relative absence of clinically effective treatments (Liu et al., 2012). Several animal studies have implicated the α2-adrenoceptors (α2-AR) in experimental neuropathic models of pain (Kalso et al., 1991, Brummett et al., 2009). In the 1990s, DEX, a newly developed α2-AR agonist was first introduced into clinical practice as a short-term intravenous sedative in the intensive care unit (Zhang et al., 2013). And also a study has confirmed Dex has the potential effect for pain treatment (Grosu and Lavand’homme, 2010), but the involved mechanism still remains to be elucidated.
Recently, a growing body of literature demonstrates that enhanced spinal neuroimmune and neuroinflammatory activities initiate and maintain inflammatory and neuropathic pain (He et al., 2014). Specifically, proinflammatory cytokines such as TNF-α, IL-1, IL-6 and iNOS have been strongly implicated in the initiation and development of inflammatory and neuropathic pain and can activate, or be activated by NF-κB (He et al., 2014).
In this study, we provide the evidence that DEX affords significant relief of inflammatory and neuropathic pain, and that this effect is likely related to inhibition of cytokines in the spinal cord.
2. Methods
2.1. Animals
Forty-five adult male Sprague-Dawley rats, weighting 180–220 g, were housed in a 12-h light-dark cycle with free access to food and water. Before experiments, animals were allowed to habituate to the housing facilities for a week. The present study was conducted with approval from the Animal Ethics Committee of the University.
2.2. Surgical procedure
The CCI model was performed as previously described by Bennett and Xie, 1988a, Bennett and Xie, 1988b. Briefly, rats were anesthetized with 10% chlorate hydrate (350 mg/kg, i.p.), the left sciatic nerve was exposed at the mid-thigh level through a small incision, and four loose ligatures of chromic gut (4.0) tied around the nerve, with a 1 mm spacing between them.
The wound was then closed and secured using suture clips. The surgical procedure was identical for the sham operated animals except that the sciatic nerve was not ligated. The wound was treated with antibiotics.
2.3. Intrathecal injection
The intrathecal injection procedure followed the method of Hylden and Wilcoxon in 1980 (Zhang et al., 2013, Hylden and Wilcox, 1980). Briefly, a stainless needle attached to a 25 ml micro-syringe was inserted between the L5 and L6 vertebrae of conscious rat. A sudden slight flick of the tail indicated the needle entered into the subarachnoid space. Ten microliters of Dex (1 μg/kg) solution or saline was injected over a period of more than 20 s. After complete injection of drug, the needle was removed after a 10 s wait, to ensure retention.
2.4. Assessment of mechanical allodynia
Mechanical allodynia, as a behavioral measure of neuropathic pain, was assessed by the paw withdrawal threshold. The mechanical withdrawal threshold (MWT) was evaluated by von Frey filaments as previously described (Bennett and Xie, 1988a, Bennett and Xie, 1988b). The animals were habituated in a wire mesh cage 30 min before the experiment. An automated dynamic plantar aesthesiometer (Ugo Basile, Varese, Italy) was used to measure the MWT, which was recorded as the lowest force (g) causing a rapid withdrawal of the right hind paw. Each measurement was repeated three times at intervals of 5 min, and the average force evoking reliable withdrawals was taken as threshold. Quick withdraw or licking of the paw in response to the stimulus was considered a positive response.
2.5. Assessment of thermal hyperalgesia
Thermal hyperalgesia of hind paws was evaluated using a plantar test (7370, UgoBasile, Comeria, Italy) according to the method described by Hargreaves et al., 1988, Qu et al., 2009. After acclimation of 30 min, the heat source was positioned under the glass floor directly beneath the hind paw. The intensity of the thermal stimulus was adjusted to achieve an average baseline paw withdrawal latency of approximately 9–11 s. A digital timer automatically recorded the duration between the start of stimuli and the paw withdrawal thermal latency (PWTL). A maximal cutoff time of 20 s was used to prevent unnecessary tissue damage. Each paw was measured alternatively after more than 5 min.
2.6. RT-PCR
The expression levels of IL-1, TNF-α, IL-6, NR2B, NF-κB, and iNOS mRNA were determined by RT-PCR. Animals were decapitated and total RNA from L4-L5 spinal cord tissues was extracted on day 21 after the CCI surgery using the RNAiso plus kit (Takara Biotechnology, Dalian, China) according to the manufacturer’s instructions. Then, the isolated RNA was reverse-transcribed to synthesize the first strand cDNA with the oligo (dT) 18 primer using the cDNA synthesis kit (Takara Biotechnology, Dalian, China), and the obtained cDNA was used as a template to perform PCR amplification using SYBR_ Premix Ex TaqTM II kit (Takara Biotechnology, Dalian, China).
The sequences of the primers for RT-PCR are as follows,
IL-1β, Forward, 5′- CAGCAATGGTCGGGACATAGTT-3′, and Reverse, 5′- GCATTAGGAATAGTGCAGCCATCT-3′;
IL-6, Forward, 5′-CCAACTTCCAATGCTCTCCTAATG -3′, and Reverse, 5′- TTCAAGTGCTTTCAAGAGTTGGAT-3′;
TNF-α, Forward, 5′-GGCTGCCTTGGTTCAGATGT-3′ and Reverse, 5′-CAGGTGGGAGCAACCTACAGTT -3′;
NR2B, Forward, 5′-TATGTGTGGCCTCGGATGTGT -3′, and Reverse, 5′- TCTTCCCGTGCTTGCCATT-3′;
NF-κB, Forward, 5′- TGGGGACCAGGAAGAGGTGGC 3′ and Reverse, 5′- GCTGTGGCCCTGACAGTAGCC -3′;
iNOS, Forward, 5′- TGTGCTAATGCGGAAGGTCAT-3′ and Reverse, 5′-CGACTTTCCTGTCTCAGTAGCAAA -3′;
β-actin, Forward, 5′-CATCACCATTGGCAATGAGCG -3′ and Reverse, 5′-CTAGAAGCATTTGCGGTCGGAC -3′.
Each 20 μl reaction system comprised 2 μl of cDNA, 10 μl SYBR Premix Ex Taq II, 10 μmol/l of both sense and antisense primers. For normalization, β-actin was used to normalize mRNA.
2.7. Statistical analysis
Statistical analyses were performed using SPSS 19.0. Data were expressed as mean ± standard deviation (SD). Comparison between groups was performed with analysis of variance (ANOVA) and Student-Newman-Keuls test (q test). Statistical significance was assumed at a value of P < 0.05.
3. Results
3.1. General behavioral observations
All experimental animals maintained good health throughout the experimental period, with no animals demonstrating significant weight loss, wounds or scars, or failure to groom. Furthermore, no infection, autonomy, or motor weakness was observed in any of the animals. The general health of rats with nerve injuries did not differ from sham-operated controls. No abnormal behavior or foot deformity was seen in the sham group. However, all CCI rats in the experimental groups developed varying degrees of abnormality of foot, gait and posture after surgery, including refrained from weight bearing on the affected paw and modified their stance accordingly, with eversion of the foot. Dex treatment alleviated those abnormal behaviors.
3.2. The effect of Dexmedetomidine (Dex) on mechanical allodynia
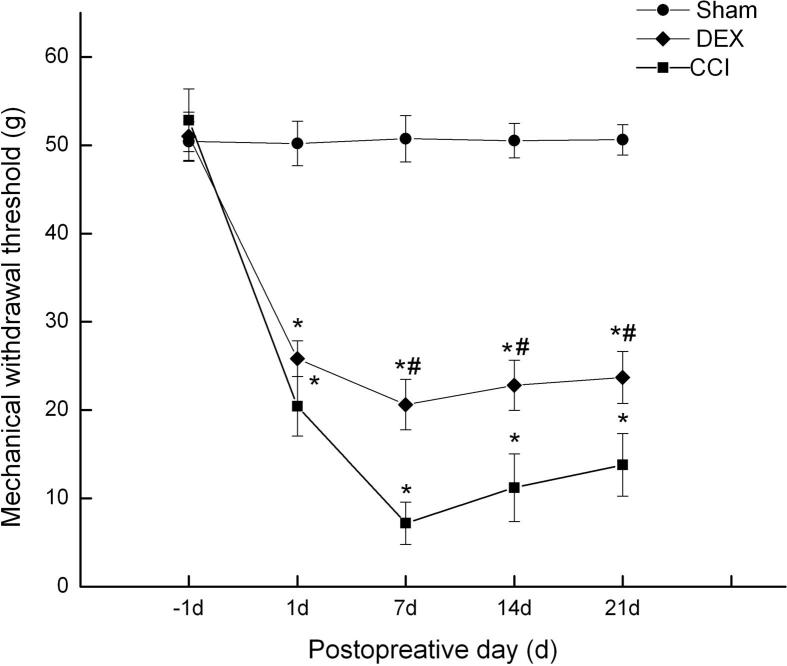
After CCI, rats were administered Dex (1 μg/kg intraperitoneal, once daily) for a period of 21 days. Mechanical withdrawal thresholds were assessed before operation and on days 1, 7, 14, and 21 after CCI. After surgery, animals (CCI group and Dex-treated group) developed a significant hypersensitivity to pain when compared with the sham group as previously described (Bennett and Xie, 1988a, Bennett and Xie, 1988b). As shown in Fig. 1, the decrease in the paw withdraw threshold was significant from day 1 to 21. Dex reversed CCI-induced mechanical allodynia from day 1, which was significant as compared with CCI group from day 7 to 21 (p < 0.05).
Fig. 1.
Effects of Dex on the mechanical allodynia in neuropathic pain rats induced by spinal nerve ligation (mean ± SD). *P < 0.05, compared with Sham group; #P < 0.05, compared with CCI group at the corresponding time points.
3.3. The effect of Dexmedetomidine (Dex) on thermal hyperalgesia
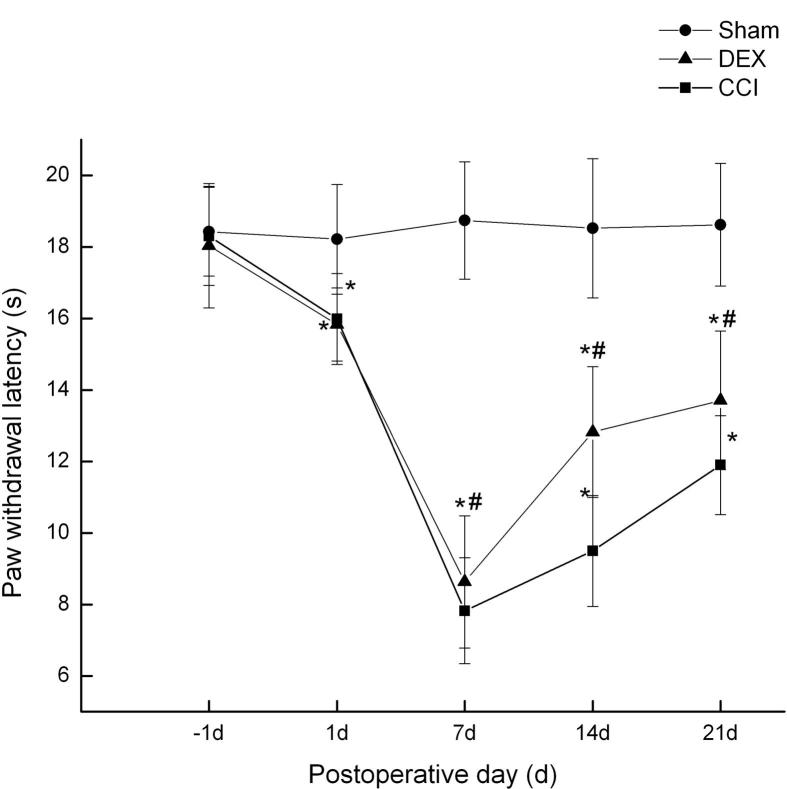
Similar to these results of mechanical withdrawal threshold, as shown in Fig. 2, CCI operation elicited a rapid-onset and long-lasting thermal hyperalgesia. The decreases were significant in CCI group and Dex group at day 1 after surgery, compared with sham group (p < 0.05). In contrast, treatment with Dex, significantly attenuated thermal hyperalgesia as compared with the CCI group from day 7 to 21 (p < 0.05). These results suggested Dex attenuated CCI induced nociceptive response.
Fig. 2.
Effects of Dex on thermal hyperalgesia in neuropathic pain rats induced by spinal nerve ligation (mean ± SD). *P < 0.05, compared with Sham group; #P < 0.05, compared with CCI group at the corresponding time points.
3.4. Effects of Dex on pro-inflammatory cytokines in spinal cord
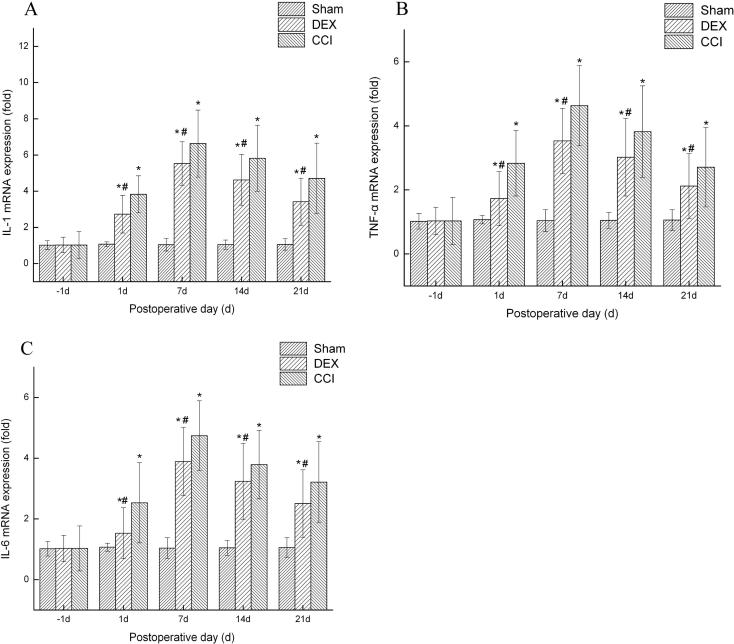
Some studies demonstrated the pro-inflammatory cytokines are involved in the neuropathic pain process. Therefore, we investigated the effects of Dex on pro-inflammatory cytokines in spinal cord. As shown in Fig. 3, there were significant increases in the mRNA levels of TNF-α (Fig. 3a), IL-1β (Fig. 3b) and IL-6 (Fig. 3c) in spinal cord of CCI rats, as compared with the sham group. However, Dex reversed CCI-increased the levels of TNF-α, IL-1β and IL-6 (p < 0.05). Taken together, these results suggest that Dex attenuated the expression of TNF-α, IL-1β and IL-6 in spinal cord of CCI rats.
Fig. 3.
Dexmedetomidine reduces the expression of IL-1, TNF-α and IL-6 following CCI. The total RNA was extracted from spinal cord tissues using RNAiso kit. RT-PCR quantitation of IL-1, TNF-α and IL-6 expression mRNA in spinal cord. CCI significantly increased the expression of IL-1 (A) TNF-α (B) and IL-6 (C) mRNA in the spinal cord, whereas DEX injection inhibited the upregulation of these mRNAs. Data shown as mean ± SD. *P < 0.05 versus sham group; #P < 0.05 versus CCI group.
3.5. Effects of Dex on CCI-induced NR2B expression in spinal cord
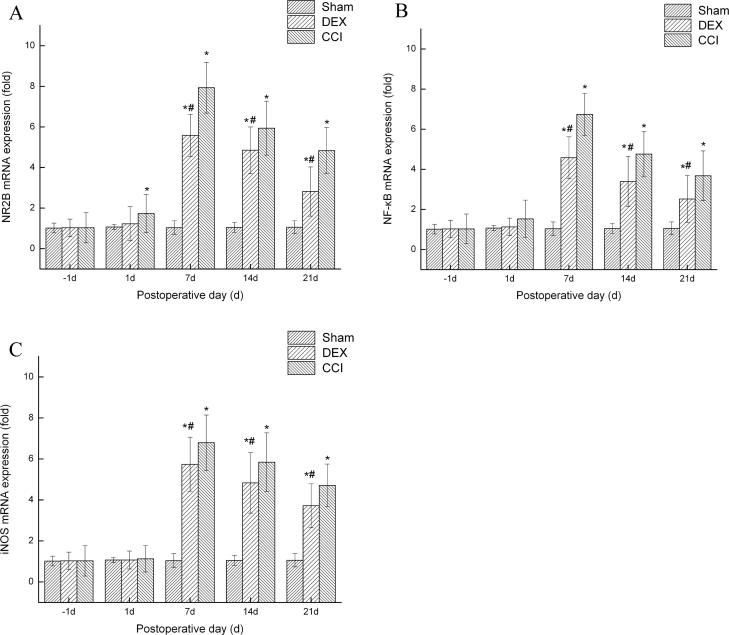
CCI induced the higher-expression of NR2B at days 7, 14 and 21 in CCI group and Dex group compared with the sham group (p < 0.05), whereas the Dex-treated group significantly attenuated the NR2B mRNA expression (p < 0.05, Fig. 4a).
Fig. 4.
Dexmedetomidine decreases the expression of NR2B, NF-κB and iNOS following CCI. The total RNA was extracted from spinal cord tissues using RNAiso kit. RT-PCR quantitation of NR2B, NF-κB and iNOS expression mRNA in spinal cord. CCI significantly increased the expression of NR2B (a) NF-κB (b) and iNOS(c) mRNA in the spinal cord, whereas DEX injection inhibited the upregulation of these mRNAs. Data shown as mean ± SD. *P < 0.05 versus sham group; #P < 0.05 versus CCI group.
3.6. Effects of Dex on CCI-induced NF-κB expression in spinal cord
NF-κB is a key transcription factor complex that controls the expressions of pro-inflammatory and pain mediators (Zhou et al., 2014, Ahn et al., 2007). As shown in Fig. 4b, compared with the sham group, CCI significantly increased the expression of NF-κB at days 7, 14 and 21 (p < 0.05). But Dex significantly attenuated CCI-induced NF-κB expression level from at days 7, 14 and 21 (p < 0.05).
3.7. Effects of Dex on CCI-induced iNOS expression in spinal cord
In the CCI model, iNOS expression was also local in the sciatic nerve. Previous studies in the CCI models have shown that iNOS expression is concentrated mainly away from the ligatures and that Schwann cells are the main type of cell expressing iNOS in the early stages, with macrophages being more important later on (Lv et al., 2015). In the current study though, as shown in Fig. 4c, in comparison with the sham group, CCI significantly increased the expression of iNOS at days 7, 14 and 21 (p < 0.05). However, the levels of iNOS was significantly decreased compared to the CCI treated group from day 7 to day 21 after DEX treated groups (p < 0.05).
4. Discussion
The main findings of this study are that dexmedetomidine (Dex) reversed CCI-decreased mechanical withdrawal threshold and thermal withdrawal latency; Dex inhibited CCI-induced the levels of pro-inflammatory cytokines TNF-a, IL-1 and IL-6 in spinal cord. Furthermore, Dex reduced the elevated expression of NR2B, NF-κB and iNOS in spinal cord induced by CCI.
Several animal models have been developed to study neuropathic pain mechanisms. The chronic constriction injury model has provided a better understanding of nociception and the pathogenesis of chronic pain, which is probably the most frequently used model (Bennett and Xie, 1988a, Bennett and Xie, 1988b). In this study, we used CCI model to evaluate the effects of Dex on neuropathic pain, and we found that CCI produced obvious mechanical allodynia and thermal hyperalgesia, which suggest that the model was successful (Bennett and Xie, 1988a, Bennett and Xie, 1988b). In addition, we found that Dex attenuated mechanical allodynia and thermal hyperalgesia for 21 days, suggesting the possible of therapeutic efficacy of Dex.
In addition, proinflammatory cytokines have been involved in demyelination and degeneration of peripheral nerves, increase in sensory afferent excitability, and induction of neuropathic pain (Jancalek et al., 2010). Following activation, proinflammatory cytokines, such as TNF-α, IL-1, IL-6 and iNOS are expressed and facilitate activation of immune responses. TNF-α interacts with a diverse family of TNF receptors and serves as an important intermediate in the innate immune response. TNF-α mRNA expression was correlated with neuropathic pain development in CCI rat model. Proinflammatory cytokine IL-1 is known to modulate pain sensitivity and is commonly involved in the inflammatory response following CNS injury. In animal models, administration of cytokine inhibitors before nerve injury reduces neuropathology and pain-related behaviors. For example, intrathecal injection of IL-1β induces mechanical allodynia, and IL-1 receptor antagonist can inhibit hyperalgesic responses to IL-1β (Marchand and Perretti, 2005). Another cytokine IL-6 has been correlated with mechanical allodynia after nerve constriction injury (Marchand and Perretti, 2005). iNOS is the primary form active during the chronic inflammatory process. Two of the primary cytokines responsible for iNOS upregulation are TNF-α and IL-1, both of which act through the NF-κB pathway within several hours following injury.
In this study, we found that Dex significantly decreased the over-expression of spinal IL-1, TNF-α, and IL-6 in CCI model. We conclude that the analgesic effect of Dex might be mediated, at least partly, through the prevention of IL-1, TNF-α, and IL-6 products.
NR2B has a relatively restricted distribution in nociceptive transmission and pain regulatory pathways such as in the forebrain and in the superficial dorsal horn of the spinal cord (Moalem and Tracey, 2006, Baamonde et al., 2007). This kind of distribution suggests that NR2B subunit may play a role in pain transmission and in the development of chronic pain. Consistent with previous studies, the expression of NR2B was dramatically increased in spinal cord in CCI models, and DEX treatment significantly inhibited this upregulation.
This study demonstrates the possibility of using Dex for therapeutic intervention of neuropathic pain in CCI rat model, which resembles closely the clinical peripheral mononeuropathy. We suggest that the anti-neuroinflammatory actions of Dex could be attributed to the inhibition of IL-1, TNF-α, IL-6 release, which would result in the reduction of NR2B, NF-κB, and iNOS signaling pathways in spinal cord.
5. Conclusion
Although precise mechanism of neuropathic pain was not exactly characterized in our study DEX may alleviate neuropathic hypersensitivity and inflammation partially by inhibiting NR2B, NF-КB, and iNOS expression in the spinal cord of rats with neuropathic pain resulting from CCI of the sciatic nerve. The present study suggests the potential use of Dex in the treatment of neuropathic pain.
Grant support & financial disclosures
None.
Declaration of interest
All authors declared there was no conflict of interests involved.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahn K.S., Sethi G., Aggarwal Nuclear factor-kappa B: from clone to clinic. Curr. Mol. Med. 2007;7:619–637. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- Baamonde A., Curto-Reyes V., Juárez L., Meana A., Hidalgo A., Menéndez L. Antihyperalgesic effects induced by the IL-1 receptor antagonist anakinra and increased IL-1b levels in inflamed and osteosarcoma-bearing mice. Life Sci. 2007;81:673–682. doi: 10.1016/j.lfs.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Bennett G.J., Xie Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–89. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Brummett C.M., Trivedi K.A., Dubovoy A.V., Berland D.W. Dexmedetomidine as a novel therapeutic for postoperative pain in a patient treated with buprenorphine. J. Opioid. Manage. 2009;5:175–179. doi: 10.5055/jom.2009.0018. [DOI] [PubMed] [Google Scholar]
- Farghaly H.S., Abd-Ellatief R.B., Moftah M.Z., Mostafa M.G., Khedr E.M., Kotb H.I. The effects of dexmedetomidine alone and in combination with tramadol or amitriptyline in a neuropathic pain model. Pain Physician. 2014;17:187–195. [PubMed] [Google Scholar]
- Grosu I., Lavand’homme P. Use of dexmedetomidine for pain control, F1000. Med. Rep. 2010;2:90. doi: 10.3410/M2-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves K., Dubner R., Brown F., Flores C., Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- He Z.W., Wei W., Li S.P., Ling Q., Liao K.J., Wang X. Anti-allodynic effects of obtusifolin and gluco-obtusifolin against inflammatory and neuropathic pain. Biol. Pharm. Bull. 2014;37:1606–1616. doi: 10.1248/bpb.c14-00307. [DOI] [PubMed] [Google Scholar]
- Hylden J.L., Wilcox G.L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Jancalek R., Dubovy P., Svizenska I., Klusáková I. Bilateral changes of TNF-alpha and IL-10 protein in the lumbar and cervical dorsal root ganglia following a unilateral chronic constriction injury of the sciatic nerve. J. Neuroinflammation. 2010;7:11. doi: 10.1186/1742-2094-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso E.A., Poyhia R., Rosenberg P.H. Spinal antinociception by dexmedetomidine, a highly selective alpha 2-adrenergic agonist. Pharmacol. Toxicol. 1991;68:140–143. doi: 10.1111/j.1600-0773.1991.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Liu M., Zhou L.L., Chen Z.W., Hu C. Analgesic effect of iridoid glycosides from Paederia scandens (LOUR.) MERRILL (Rubiaceae) on spared nerve injury rat model of neuropathic pain. Pharmacol. Biochem. Behav. 2012;102:465–470. doi: 10.1016/j.pbb.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Lv C., Hu H.Y., Zhao L., Zheng H., Luo X.Z., Zhang J. Intrathecal SRT1720, a SIRT1 agonist, exerts anti-hyperalgesic and anti-inflammatory effects on chronic constriction injury-induced neuropathic pain in rats. Int. J. Clin. Exp. Med. 2015;8:7152–7159. [PMC free article] [PubMed] [Google Scholar]
- Marchand F., Perretti M., McMahon S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- Moalem G., Tracey D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006;51:240–264. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Qu X.X., Cai J. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp. Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Sun T., Song W.G., Fu Z.J., Liu Z.H., Liu Y.M., Yao S.L. Alleviation of neuropathic pain by intrathecal injection of antisense oligonucleotides to p65 subunit of NF-κB. Br. J. Anaesth. 2006;97:553–558. doi: 10.1093/bja/ael209. [DOI] [PubMed] [Google Scholar]
- Zhang H., Zhou F., Li C., Kong M., Liu H., Zhang P., Zhang S., Cao J., Zhang L., Ma H. Molecular mechanisms underlying the analgesic property of intrathecal dexmedetomidine and its neurotoxicity evaluation: an in vivo and in vitro experimental study. PLoS ONE. 2013;8:e55556. doi: 10.1371/journal.pone.0055556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Cheng H., Xu D., Yin Q., Cheng L., Wang L., Song S., Zhang M. Attenuation of neuropathic pain by saikosaponin a in a rat model of chronic constriction injury. Neurochem. Res. 2014;39:2136–2142. doi: 10.1007/s11064-014-1407-y. [DOI] [PubMed] [Google Scholar]