Abstract
Background: Oridonin is a diterpenoid isolated from Rabdosia rubescens with potent anticancer activity. The aim of our study is to investigate the role of oridonin to inhibit growth and metastasis of human breast cancer cells. Methods: The effect of oridonin on proliferation was evaluated by MTT assay, cell migration and invasion were evaluated by transwell migration and invasion assays in human breast cancer cells. The inhibitive effect of oridonin in vivo was determined by using xenografted nude mice. In addition, the expression of Notch receptors (Notch 1–4) was detected by western blot. Results: Oridonin inhibited human breast cancer cells in vitro and in vivo. In addition, oridonin significantly induced human breast cancer cells apoptosis. Furthermore, the oridonin treatment not only inhibited cancer cell migration and invasion, but more significantly, decreased the expression of Notch 1-4 protein. Conclusion: Our results suggest that the inhibitive effect of oridonin is likely to be driven by the inhibition of Notch signaling pathway and the resulting increased apoptosis.
Keywords: Oridonin, Breast cancer, Cell apoptosis, Notch signaling pathway
1. Introduction
Breast cancer is one of the most lethal malignancies among women (PDQ Adult Treatment Editorial Board, 2002–2016). Approximately 70% of patients are diagnosed at an advanced stage with lymph node metastasis (Naidoo and Pinder, 2016). 70% of patients were diagnosed with advanced lymph node metastases (Naidoo and Pinder, 2016). Only 25% of patients with early diagnosis, advanced breast cancer patients with 5-year survival rate of 20%–25% (Dai et al., 2016, Parsa et al., 2016). It is difficult to find early in the early years, mainly due to its inherent metastatic nature and poor prognosis (Xie et al., 2016, Bozorgi et al., 2015). The widespread use of chemotherapy has poor gastrointestinal toxicity, including severe nausea and vomiting, nephrotoxicity and neurotoxicity (Feng et al., 2016; Joo et al., 2009). More importantly, long-term use of chemotherapeutic drugs is bound to lead to drug resistance, which is a major obstacle to cancer chemotherapy (Kim et al., 2009). Then, to explore more effective methods to reduce side effects or to address drug resistance has become the most important issue for breast cancer.
Recently, natural products extracted from medicinal plants have received increasing attention in cancer treatment. Vitamin is extracted from Chinese herbal medicine and it is a natural compound of tetracycline diterpenoids (Wang et al., 2013, Gao et al., 2010) (structure shown in Fig. 1). Qi et al. (2012) reported that oridonin can effectively induce the apoptosis of pancreatic cancer cells. Oridonin nano-suspension is more effective than free lysine on G2/M cell cycle arrest and apoptosis in human pancreatic cancer PANC-1 cells. Gao et al. (2012) found oridonin induces apoptosis and senescence by increasing the consumption of hydrogen peroxide and glutathione in colorectal cancer cells. At the same time, some researchers report that autologous engulfed in the treatment of human breast cancer MCF-7 cells treated with oridonin (Cui et al., 2007). Among patients with lung cancer, oridonin also inhibits mTOR signaling and growth of lung cancer tumors, and mTORC1 inhibition may be an effective target for promoting clinical outcomes with oridonin treatment (Wang et al., 2014).
Fig. 1.
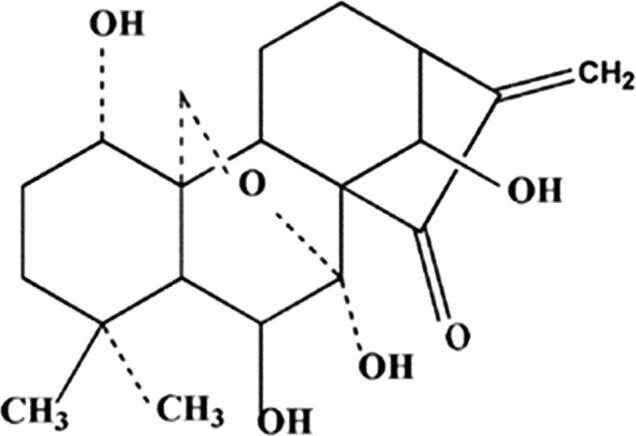
Chemical structures of oridonin.
There are accumulating clinical evidences showing that, as a potential target, Notch could prevent the progress of tumor metastasis (Garcia and Kandel, 2012). Notch mediates vascular endothelial cells and tumor cells communication and promotes tumor angiogenesis of tumor (Villa et al., 2001). The tumor-related growth factors such as VEGF can induce jagged ligands in the microenvironment of tumor (Shawber and Kitajewski, 2004) and then activate Notch (Folkman, 2002) expressed in tumor endothelial cells. Surprisingly, Jagged 2 is the most Notch ligand associated with the overall and non-metastatic survival of breast cancer patients (Xing et al., 2011). Vascular endothelial cells express Notch receptors 1, 2 and 3, and Notch signaling is critical for the proper formation of functional vasculature (Iso et al., 2003). In addition, Notch activity was specifically upregulated in the tumor endothelium, suggesting that interference with Notch activity may have a negative effect on tumor neovascularization. Several Notch inhibitors such as RO4929097 (Tolcher et al., 2012) and MK-0752 (Krop et al., 2012) have been used in clinical trials. Thus, targeting the Notch pathway in endothelial cells may provide an effective strategy for antiangiogenic therapy (Zhou et al., 2007).
In the present study, we used human breast cancer cell line 4T1, and explored the mechanism of oridonin therapy. Furthermore, we identified Notch signaling pathway as a direct target of oridonin, and oridonin exerted the tumor suppressive effect via inhibiting Notch receptors expression in breast cancer cells.
2. Material and methods
2.1. Agents and cell lines
Oridonin (purity ≥ 98%) was purchased from Sigma-Aldrich China Inc. (Shanghai, China). The human breast cancer cell line 4T1 were obtained from the American Type Culture Collection (Rockville, Maryland, USA). Cell counting kit-8 (CCK-8) was provided by Dojindo Laboratories (Kumamoto, Japan). Hoechst 33258, the ApoDETECT annexin V–fluorescein isothiocyanate (FITC) apoptosis detection kit, the Light Shift chemiluminescent electrophoretic mobility shift assay (EMSA) kit, and NE-PER nuclear and cytoplasmic extraction reagents were obtained from ThermoFisher Scientific Inc. (Waltham, Massachusetts, USA). The cells were cultured in DMEM medium (Hyclone, Beijing, China) enriched with 10% fetal bovine serum (Hyclone) at 37 °C and 5% CO2.
2.2. MTT assay
The cells were cultured in 96-well cell culture plates and treated as shown on the second day. According to the manufacturer’s protocol, MTT assay (Promega) was used to assess the number of viable cells treated with drugs. At a reference wavelength of 630 nm, the absorbance (A) was measured at 570 nm.
2.3. Tumor inhibition assay in vivo
The protocol of the animal study was approved by the Animal Ethics Committee of Sichuan University. All experiments were conducted in accordance with institutional guidelines. Briefly, 56 male BALB/C athymic nude mice (Shanghai Laboratory Animal Center, Shanghai, China) weighing between 18 and 23 g (4–6 weeks old) were subcutaneously engrafted with 1 × 107 4T1 cells. Tumors were allowed to establish and grow to a volume of 100–150 mm3. The rodents were then randomly divided into seven groups of eight mice; they received the following treatments by an intraperitoneal injection: 0.9% saline, two doses of oridonin (10 and 20 mg/kg). The treatment for each group was administered once daily for 21 days, with a 1-day interval every 6 days. Tumor size was measured every 3 days starting on the day of first treatment (day 0) and ending 28 days after the first treatment (day 28) according to the published literature (27). At day 28, the animals were killed and tumor weight was determined.
2.4. Flow cytometry
Quantification of apoptotic cells by flow cytometry was performed using an ApoDETECT annexin V–FITC apoptosis detection kit according to the manufacturer’s instructions. Briefly, 1 × 105 4T1 cells subjected to the various treatments were harvested, washed twice with cold PBS, and then resuspended in 0.5 ml of binding buffer. Subsequently, 10 μl of FITC-labeled annexin V and 10 μl propidium iodide (PI) solution (20 μg/ml) were added to 190 μl of cell suspension. After incubation in the dark for 10 min at RT, the cells were analyzed on a flow cytometer (model FACS Calibur; BD Biosciences, Franklin Lakes, New Jersey, USA). Early apoptotic cells were defined as FITC+/PI− cells and late apoptotic cells were defined as FITC+/PI+ cells. Assays were repeated independently three times.
2.5. Cell migration and invasion assays
For the transwell migration assay, 5 × 104 BGC-823 cells were placed in the upper chamber (Corning). For the invasion assay, 1 × 105 BGC-823 cells were placed in the upper chamber containing 40 µl of matrigel. A medium supplemented with 20% FBS was added to the lower chamber. After incubation for several hours, the cells which were attached to the lower surface were stained with crystal violet for 20 min. The cells were counted in 5 random magnitudes of 100× magnification.
2.6. RNA isolation and quantitative real-time PCR (qRT-PCR)
Trizol reagent (Invitrogen) was used to isolate total RNA from tissue and cell samples. The spectrophotometry and electrophoresis were used to determine total RNA concentration and quality. The SuperScript III first-strand synthesis system was used to synthesize cDNA. The ABI 7900 system using SYBR Premix Ex Taq was applied to conduct RT-PCR. The 2-ΔΔCT method was used to calculate relative expression levels of miR-338-3p and Notch1.
2.7. Transient transfection
The pEGFP-Notch 1 plasmid was generated using the following primers: forward, 5′-CGCAGTTGTGCTCCTGAA-3′ and reverse, 5′-ACCTTGGCGGTCTCGTAGCT-3′. The Lipofectamine 2000 (Invitrogen) was used to conduct transfection based on the manufacturer’s protocol. The qRT-PCR was conducted to monitor the transfection efficiency.
2.8. Western blot
The total protein from the cells was dissolved by RIPA buffer. The same amount of protein was isolated by 12% SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad). After blocking with 5% fat-free milk, the membrane was incubated with anti-Notch 1–4 antibody or anti-GAPDH (Abcam). The membranes were then washed well and incubated with goat anti-mouse secondary antibody (Pierce). The ECL reagent (Pierce) was used to detect proteins.
2.9. Statistical analysis
The SPSS 17.0 software (IBM) was used to perform statistical analyses. The data are expressed as mean ± SD from at least three separate experiments. Differences were analyzed using Student t test or one-way analysis of variance. A P value < 0.05 was considered statistically significant.
3. Results
3.1. 4T1 cells are sensitive to oridonin
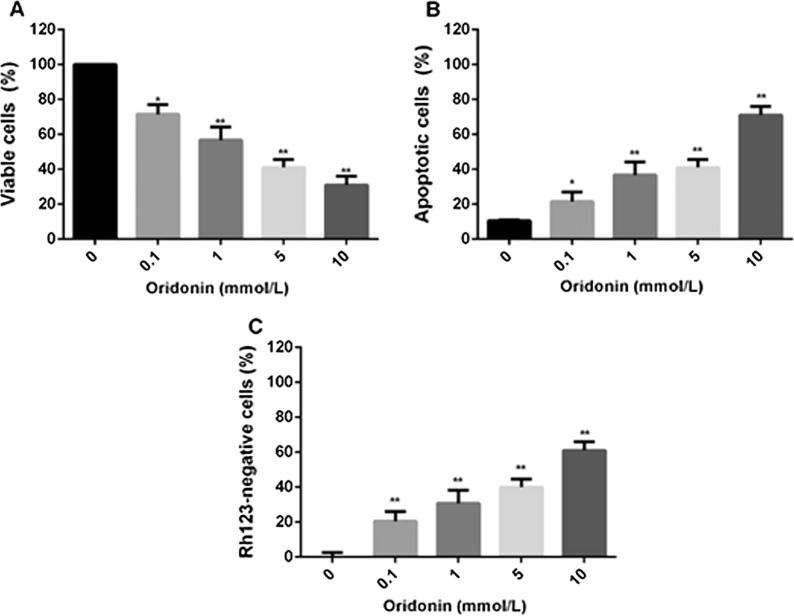
We first tested the efficacy of oridonin in the 4T1 cell line by MTT assay. After 24 h of treatment, the percentage of viable cells was significantly decreased in a concentration-dependent manner (P < 0.01; Fig. 2A).
Fig. 2.
Oridonin display potent antiproliferative and proapoptotic effects on human breast cancer cells. A: MTT assays showing inhibition of viable cells after a 24-h treatment with different concentrations of oridonin. B and C: cells were treated with oridonin (0, 0.1, 1, 5, and 10 mmol/L) for 24 h, then the percentages of cells undergoing apoptosis were determined by FITC–Annexin V/PI staining and flow cytometry and shown in B, and the percentage of Rh123-negative cells are shown in C. *P < 0.05, **P < 0.01 compared with the 0 mmol/L group.
Next, we examined the effect of oridonin on cell apoptosis. FITC-Annexin V/PI double staining showed that oridonin was effective in inducing 4T1 cell apoptosis (Fig. 2B). In addition, oridonin significantly increased the proportion of Rh123-negative cells (Fig. 2C), indicating the occurrence of mitochondrial-dependent apoptosis.
3.2. Oridonin inhibits the growth of xenografted T41 cells in nude mice
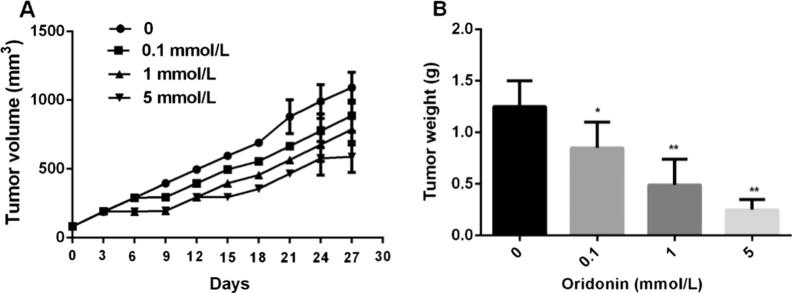
Next, we confirmed the antitumor activity of oridonin in nude mice with xenografted 4T1 cells. As shown in Fig. 3A and B, 1 mg/kg oridonin demonstrated significant inhibitive effects (33 decrease in tumor volume and 31 decrease in tumor weight, respectively) after 28 days of treatment. However, compared with 1 mg/kg oridonin alone, 5 mg/kg oridonin induced a considerably higher reduction in tumor volume (72%) and tumor weight (84%). Taken together, these findings indicate an antitumor effect of oridonin in a dose-dependent manner in vivo.
Fig. 3.
Oridonin inhibits the growth of xenografted 4T1 cells in nude mice. (A) Tumor growth after treatment with oridonin. The treatment for each group was administered once daily for 21 days, with a 1-day interval every 6 days. (B) Tumor weight at 28 days after treatment. Data are expressed as means ± SD (n = 8). *P < 0.05, **P < 0.01, compared with the 0 mmol/L group.
3.3. Cisplatin inhibit breast cancer cell migration and invasion in vitro
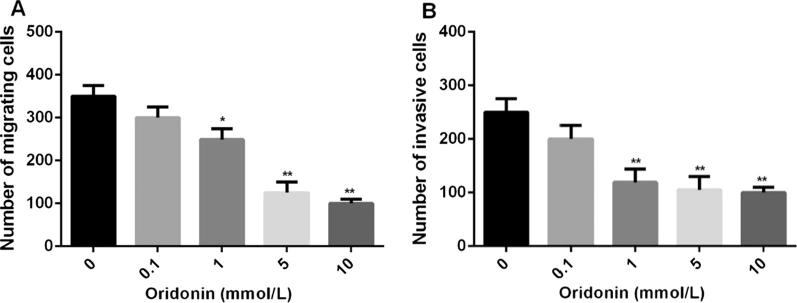
Transwell migration assay was conducted to show that oridonin demonstrated significant inhibitive effects in a concentration-dependent manner (Fig. 4A). In addition, transwell invasion assay was conducted to show that oridonin significantly suppress 4T1 cell invasive capacity (Fig. 4B).
Fig. 4.
Oridonin inhibits human breast cancer cell migration and invasion in vitro. A. Transwell migration assay of T41 cells treated with oridonin. B. Transwell invasion assay of T41 cells treated with oridonin. *P < 0.05, **P < 0.01, compared with the 0 mmol/L group.
3.4. Notch signaling pathway is a direct target of oridonin
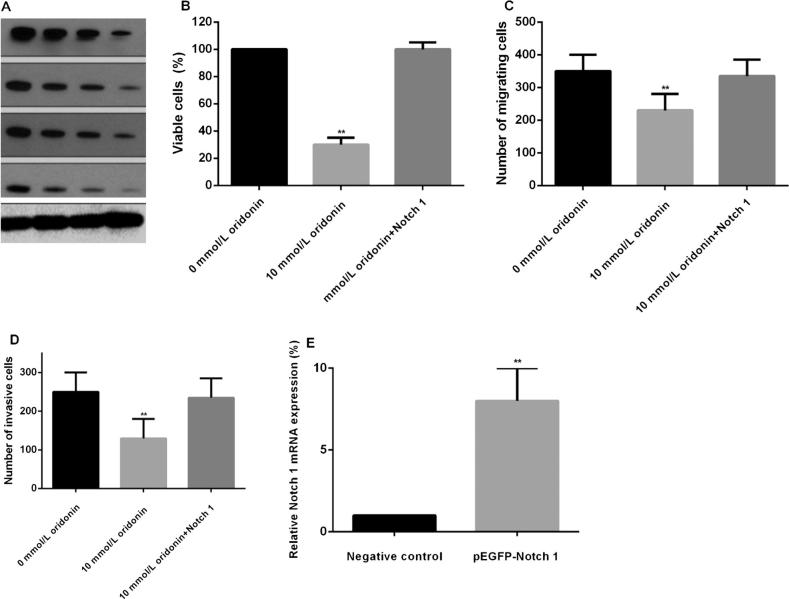
We hypothesize that Notch signaling pathway is the potential target of oridonin. Treatment of oridonin significantly inhibited Notch receptors (Notch 1–4) protein expression in 4T1 cells (Fig. 5A). In addition, we further investigated whether overexpression of Notch 1 could reverse the inhibitive effects of oridonin. The increased expression of Notch 1 significantly reversed the inhibitive effect of oridonin on 4T1 cells (Fig. 5B–D). The qRT-PCR was used to confirm pEGFP-Notch 1 effects (Fig. 5E).
Fig. 5.
Notch signaling pathway is the oridonin target in ovarian cancer. A. Notch 1–4 expression in 4T1 cells was inhibited by oridonin. B. Notch 1 attenuated the suppressive effect of oridonin on ovarian cancer cell proliferation. C. The Notch 1 transfected 4T1 cell transwell migration assay. D. The Notch 1 transfected 4T1 cell transwell invasion assay. E. The qRT-PCR was conducted to detect Notch 1 expression. **P < 0.01.
4. Discussion
In the current study, we report for the first time an antitumor effect of oridonin on human breast cancer cell 4T1 cells both in vitro and in vivo. In addition, we found that oridonin significantly induces apoptosis and also inhibits the expression of Notch 1–4. Taken together, our results suggest that the inhibitory effect of oridonin is likely to be driven by the inhibition of Notch signaling pathway and the resulting increased apoptosis.
Growing evidence showed that oridonin holds great promise for novel therapeutic approaches for treating human cancers. Even though recent evidence indicated the inhibitory effect of oridonin on human cancers, such as NSCLC, neuroblastoma and hepatocellular carcinoma (Zhen et al., 2012), there is little knowledge about oridonin and its targets in human breast cancer.
There are accumulating clinical evidences showing that oridonin has great hope for a new treatment for the human malignant neoplasms. Although recent evidence suggests that oridonin inhibits human malignancies including NSCLC, neuroblastoma and hepatocellular carcinoma (Zhen et al., 2012), but regarding human breast cancer, oridonin effects and its underlying mechanism are not fully investigated.
In this study, our findings indicate that oridonin can significantly enhance the antitumor activity against 4T1 cells both in an in vitro growth inhibition assay and in xenografted mice. Furthermore, the oridonin treatment induced significantly greater levels of apoptosis than the control group. Hence, the inhibitory effect is considered to be the result of induction of apoptosis. Further studies revealed that oridonin inhibited 4T1 cell migration and invasion.
In molecular mechanisms involving tumor angiogenesis and metastasis, evidence of accumulation indicates that Notch is a key regulator of tumor angiogenesis and metastasis (Joo et al., 2009). A variety of evidences suggested that, in breast cancer, Notch plays a major carcinogenic role (Dong et al., 2014), indicating the importance of Notch signaling in tumorigenesis. VEGF and Notch are signaling interactions in tumor angiogenesis (Dong et al., 2014). It is well known that VEGF acts as an effective activation stimulus for angiogenesis. These reports indicate that VEGF and Notch may be an effective therapeutic target in human malignant neoplasms (Dong et al., 2014). In this study, we have shown that, similar to its inhibitor DAFT, oridonin inhibits Jagged2, Notch and its downstream genes expressions (Fig. 6A and B). In addition, we found that Oridonin downgraded Notch activity (Fig. 6C). These results indicate that the above biological function of ovarian protein inhibition of tumor angiogenesis and metastasis is achieved by blocking the serrated gap pathway.
In conclusion, this study shows a synergistic antitumor effect between cisplatin and oridonin on human ovarian cancer cell A2780/DDR cells both in vitro and in vivo. Mechanistic studies indicate that this synergistic effect is likely to be driven by the inhibition of expression of ADAM17 and the resulting increased apoptosis. These findings suggest the potential of oridonin to serve as an adjunct therapeutic agent to chemotherapy and warrant further investigation of the combination of oridonin and cisplatin for the treatment of ovarian cancer.
Disclosure of conflict of interest
None.
Footnotes
Peer review under responsibility of King Saud University.
References
- Bozorgi A., Khazaei M., Khazaei M.R. New findings on breast cancer stem cells: a review. J. Breast Cancer. 2015;18(4):303–312. doi: 10.4048/jbc.2015.18.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q., Tashiro S., Onodera S., Minami M., Ikejima T. Autophagy preceded apoptosis in oridonin-treated human breast cancer MCF-7 cells. Biol. Pharm. Bull. 2007;30:859–864. doi: 10.1248/bpb.30.859. [DOI] [PubMed] [Google Scholar]
- Dai X., Xiang L., Li T., Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J. Cancer. 2016;7(10):1281–1294. doi: 10.7150/jca.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.M., Zhang T., Li J.J., Deng H.Y., Song Y.J., Zhai D., Peng Y., Lu X.L., Liu M.Y., Zhao Y.X., Yi Z.F. Oridonin inhibits tumor growth and metastasis through anti-angiogenesis by blocking the notch signaling. PLoS One. 2014;9(12):e113830. doi: 10.1371/journal.pone.0113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., Ashraf M.A., Peng L. Characterization of particle shape, zeta potential, loading efficiency and outdoor stability for chitosan-ricinoleic acid loaded with rotenone. Open Life Sci. 2016;11(1):380–386. [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- Gao F.H., Hu X.H., Li W. Oridonin induces apoptosis and senescence in colorectal cancer cells by increasing histone hyperacetylation and regulation of p16, p21, p27 and c-myc. BMC Cancer. 2010;10:610. doi: 10.1186/1471-2407-10-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F.H., Liu F., Wei W. Oridonin induces apoptosis and senescence by increasing hydrogen peroxide and glutathione depletion in colorectal cancer cells. Int. J. Mol. Med. 2012;29:649–655. doi: 10.3892/ijmm.2012.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A., Kandel J.J. Notch: a key regulator of tumor angiogenesis and metastasis. Histol. Histopathol. 2012;27(2):151–156. doi: 10.14670/hh-27.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iso T., Hamamori Y., Kedes L. Notch signaling in vascular development. Arterioscler. Thromb. Vasc. Biol. 2003;23(4):543–553. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- Joo W.D., Lee J.Y., Kim J.H. Efficacy of taxane and platinum-based chemotherapy guided by extreme drug resistance assay in patients with epithelial ovarian cancer. Journal of Gynecologic Oncology. 2009;20:96–100. doi: 10.3802/jgo.2009.20.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S., Kim T.J., Chung H.H. In vitro extreme drug resistance assay to taxanes or platinum compounds for the prediction of clinical outcomes in epithelial ovarian cancer: a prospective cohort study. J. Cancer Res. Clin. Oncol. 2009;135:1513–1520. doi: 10.1007/s00432-009-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krop I., Demuth T., Guthrie T., Wen P.Y., Mason W.P., Chinnaiyan P., Butowski N., Groves M.D., Kesari S., Freedman S.J., Blackman S., Watters J., Loboda A., Podtelezhnikov A., Lunceford J., Chen C., Giannotti M., Hing J., Beckman R., Lorusso P. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 2012;30(19):2307–2313. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- Naidoo K., Pinder S.E. Micro- and macro-metastasis in the axillary lymph node: A review. Surgeon. 2016:30050–30056. doi: 10.1016/j.surge.2016.07.002. pii: S1479-666X(16) [DOI] [PubMed] [Google Scholar]
- Parsa Y., Mirmalek S.A., Kani F.E., Aidun A., Salimi-Tabatabaee S.A., Yadollah-Damavandi S., Jangholi E., Parsa T., Shahverdi E. A review of the clinical implications of breast cancer biology. Electron. Physician. 2016;8(5):2416–2424. doi: 10.19082/2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDQ Adult Treatment Editorial Board, 2002–2016. Breast Cancer Treatment (PDQ®): Health Professional Version. PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US).
- Qi X., Zhang D., Xu X. Oridonin nanosuspension was more effective than free oridonin on G2/M cell cycle arrest and apoptosis in the human pancreatic cancer PANC-1 cell line. Int. J. Nanomed. 2012;7:1793–1804. doi: 10.2147/IJN.S29483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawber C.J., Kitajewski J. Notch function in the vasculature: insights from zebrafish, mouse and man. BioEssays. 2004;26(3):225–234. doi: 10.1002/bies.20004. [DOI] [PubMed] [Google Scholar]
- Tolcher A.W., Messersmith W.A., Mikulski S.M., Papadopoulos K.P., Kwak E.L., Gibbon D.G., Patnaik A., Falchook G.S., Dasari A., Shapiro G.I., Boylan J.F., Xu Z.X., Wang K., Koehler A., Song J., Middleton S.A., Deutsch J., Demario M., Kurzrock R., Wheler J.J. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J. Clin. Oncol. 2012;(19):2348–2353. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N., Walker L., Lindsell C.E., Gasson J., Iruela-Arispe M.L., Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 2001;108(1–2):161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Wang S., Zhong Z., Wan J. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am. J. Chin. Med. 2013;41:177–196. doi: 10.1142/S0192415X13500134. [DOI] [PubMed] [Google Scholar]
- Wang Y.Y., Lv Y.F., Lu L., Cai L. Oridonin inhibits mTOR signaling and the growth of lung cancer tumors. Anticancer Drugs. 2014;25:1192–1200. doi: 10.1097/CAD.0000000000000154. [DOI] [PubMed] [Google Scholar]
- Xie H., Huang H., He W., Fu Z., Luo C., Ashraf M.A. Research on in vitro release of Isoniazid (INH) super paramagnetic microspheres in different magnetic fields. Pak. J. Pharm. Sci. 2016;29(6):2207–2212. [PubMed] [Google Scholar]
- Xing F., Okuda H., Watabe M., Kobayashi A., Pai S.K., Liu W., Pandey P.R., Fukuda K., Hirota S., Sugai T., Wakabayshi G., Koeda K., Kashiwaba M., Suzuki K., Chiba T., Endo M., Mo Y.Y., Watabe K. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30(39):4075–4086. doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen T., Wu C.F., Liu P., Wu H.Y., Zhou G.B., Lu Y. Targeting ofAML1-ETO in t (8; 21) leukemia by oridonin generates a tumor suppressor-like protein. Sci. Transl. Med. 2012;4:127. doi: 10.1126/scitranslmed.3003562. [DOI] [PubMed] [Google Scholar]
- Zhou G.B., Kang H., Wang L., Gao L., Liu P., Xie J. Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t (8; 21) leukemia in vitro and in vivo. Blood. 2007;109:3441–3450. doi: 10.1182/blood-2006-06-032250. [DOI] [PMC free article] [PubMed] [Google Scholar]