Abstract
Atopic dermatitis (AD) is a common inflammatory skin disease with high rates of morbidity and is associated with erythema, pruritus, scaling of affected areas of skin. It is extremely important to introduce a therapeutic agent which has significant anti-inflammatory effect with less side-effect for treatment of AD. This study evaluated the effect of a natural compound from herbal extracts, the crude polysaccharide extracted from the white wax scale (CWPS), on AD-like mice. Repeated applications of 2,4-dinitrochlorobenzene (DNCB) were performed on ear and dorsal skin of BALB/c mice to induce AD-like symptoms and skin lesions. Oral administration of CWPS decreased serum IgE level and limited the infiltration of mast cells and eosinophils to the dermal tissues in the DNCB-induced AD mice. In addition, CWPS reduced Th1 and Th17 responses, leading to an attenuated cutaneous inflammatory response. Furthermore, in vitro study also demonstrated that CWPS limited T cell activation and cytokines (i.e. IFN-γ and IL-17) production induced by DNCB. We conclude that CWPS attenuates DNCB-induced AD-like skin lesion through modulating T cell-elicited immune responses and CD4+ T cell polarization, and could be exploited as a new therapeutic approach for AD.
Keywords: Atopic dermatitis, Polysaccharide, White wax scale, Inflammatory skin lesion, Th cells
1. Introduction
Atopic dermatitis (AD) is a chronic, pruritus and relapsing inflammatory skin disease occurring commonly in infants and children (Bieber, 2008, Halim and Phang, 2017). In most cases, AD is associated with elevated serum immunoglobulin E (IgE) and infiltration of innate immune cells (different types of dendritic cells (DCs), mast cells, eosinophils, basophils, innate lymphocytes, and myeloid-derived suppressor cells) as well as adaptive immune cells (B cells and T cells) (Leung and Soter, 2001). In AD, recruitment of CD4+ T helper (Th) cells into the dermis is believed to contribute to the cutaneous inflammation. Lesions of acute AD skin showed a predominantly Th2 response characterized by an increased skin expression of Th2 cytokines IL-4, IL-13, IL-5, and IL-31 (Leung and Soter, 2001). Subsequently, the chronic phase is characterized by a predominantly local Th1 response. The increased expression of interferon-γ by Th1 cells follows a peak of interleukin-12 expression, which coincides with the appearance of inflammatory dendritic epidermal cells in skin (Trautmann et al., 2000). Th17 cells are important to the regulation of innate immunity and inflammation which involves in some allergic disorders (Oboki et al., 2008). The role of Th17 cells in the development of AD is suggested by a direct correlation between the presence of Th17 cells and severity of the disease (Koga et al., 2008). For many years, AD has been medically treated by the application of corticosteroids topically or systematically. However, these steroids often produce adverse effects such as skin atrophy, striae distensae, and perioral dermatitis in sensitive areas. Recently, it has been reported that treatment using natural compounds can decrease inflammation in skin disease (Kim et al., 2013, Kim et al., 2014, Yoon et al., 2015).
The white wax scale insect (Ericerus pela, Chavannes) is a famous insect owing to its role in the wax production and has been bred in China for over a thousand years (Yang et al., 2015, Yang et al., 2012). Historically, the white wax has been used in traditional medicine, candle production, printing, and its applications has since expanded to food, pharmaceutical, chemical, and cosmetic industries (Yang et al., 2012; Mustafa et al., 2017). Crude polysaccharide was extracted from the white wax scale with water and precipitated by alcohol. The crude polysaccharide from the white wax scale (CWPS) consists of glucose, mannose and galactose identified by the capillary zone electrophoresis (CZE) method (He et al., 2008). In recent years, it has been demonstrated that CWPS extracted from female wax scale has remarkable antioxidant, anti-inflammatory activity and anti-cancer activity (Feng et al., 2014, He et al., 2015, Nawaz et al., 2017, Rashid et al., 2017). To extend the applications of CWPS for treatment of skin inflammatory disease, we orally administrated CWPS to 2,4-dinitrochlorobenzene (DNCB)-induced AD model in BALB/c mice and investigated whether CWPS has therapeutic efficacy on AD-like symptoms, including ear swelling, AD-like skin lesions, the serum level of IgE and mast cells infiltration. We also analyzed the changes in the frequency of Th1, Th2 and Th17 cells in spleen and mRNA expression of IFN-γ, IL-4 and IL-17 in dorsal skin and ear. Furthermore, we investigated the cell proliferation and cytokine production in DNCB-restimulated splenocytes obtained from both control and CWPS-treated AD mice.
2. Material and methods
2.1. Preparation of CWPS
CWPS was provided by Research Institute of Resources Insects, Chinese Academy of Forestry according to the previous protocol (He et al., 2008). To prepare CWPS solution, CWPS powder was dissolved in 0.9% saline (50 mg/ml) and stored at 4 °C before use.
2.2. Animals
BALB/c male mice with six-week-old were purchased from the Animal Center of Southern Medical University (Guangzhou, China). Mice were housed in individual ventilated cage under specific pathogen-free conditions at 22 ± 2 °C with a 12-h light-dark cycle. After 1 week of acclimation, they were divided into two groups (n = 6 per group): (1) Vehicle: mice were sensitized with DNCB and treated with normal saline; (2) CWPS: mice were sensitized with DNCB and oral application with CWPS (1 mg/g body weight).
DNCB was applied to the dorsal skin and the back of both ears of BALB/c mice to induce AD-like symptoms. One day after complete dorsal hair removal (approximately 4 cm2), 150 μl of 2% DNCB solution (dissolved in a 3:1 mixture of acetone and olive oil) was applied on the dorsal skin, and 10 μl of 2% DNCB solution was applied on the back of both ears. After the first sensitization, mice were housed without any further treatment (for 4 days). In the secondary induction, 0.5% DNCB was dissolved in acetone: olive oil mixture (3:1 vol/vol) was applied on the dorsal skin (150 μl) as well as the back of both ears (10 μl each) once every 2 days. DNCB-treated BALB/c mice were orally administrated with CWPS (1 mg/g body weight) daily from the first day to the thirteenth day. AD mice in the vehicle group were given an equal volume of normal saline. All animal experiments in this study were approved by the Welfare and Ethical Committee for Experimental Animal Care of Southern Medical University (Guangzhou, China).
2.3. Histopathological analysis
To evaluate the epidermal thickening and mast cell infiltration, the ear and dorsal skin of each mouse were fixed with 4% paraformaldehyde and embedded in paraffin. Deparaffinized sections were stained with Hematoxylin and eosin (H&E) and toluidine blue (TB), severally. The number of mast cells per 0.95 mm2 skin was counted at 200× magnification. Tissue sections were examined using an Olympus IX71 light microscope.
2.4. Serum IgE measurement
Blood samples were collected at the end of the experiment. Serum IgE levels were measured using an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. The absorbance was measured at 450 nm using a microplate reader.
2.5. Flow cytometric analysis of Th cells
At the end of the experiment, splenocytes were prepared in single cell suspension. For intercellular cytokine staining, the prepared splenocytes (5 × 106) were cultured in flat-bottomed 24-well plates in a volume of 500 μl/well with cell stimulation cocktail and protein inhibitor (eBioscience) for 5 h according to the manufacturer’s protocol. After surface staining with FITC labeled rat anti-mouse CD4 (Clone RM4-5, BD Pharmingen), permeabilized cells were stained with PE-labeled rat anti-mouse IFN-γ mAb (Clone XMG1.2, BD Pharmingen), PE-Cy7-labeled rat anti-mouse IL-4 mAb (Clone 11B11, BD Biosciences) and APC-labeled rat anti-mouse IL-17 mAb (Clone Tc11-18H10, BD Pharmingen). Data were collected in a BD FACSCalibur™ Flow Cytometer and analyzed by FlowJo software.
2.6. Evaluation of cytokines and transcriptional factors mRNA levels in dorsal skin and ear by Real-time PCR
Total RNA is isolated from dorsal skin and ear using TRIzol (Takara, Dalian, China) according to manufacturer’s instruction. For reverse transcription, 500 ng of total RNA was used and cDNA was generated using TranScript All-in-One First-Strand cDNA Synthesis SuperMix (Transgen Biotech, Beijing, China) in a total volume of 20 μl. The mRNA level was determined using 1 μl of cDNA by quantitative real-time PCR (qRT-PCR) with SYBR using a protocol provided by the manufacturer (Takara). The levels of target gene were normalized with respect to GAPDH gene expression. The primer sequences of cytokines and transcription factors are listed as follow (Table 1).
Table 1.
Primers for cytokines and T cell-specific transcript factors.
| Forward primer (5′-3′) | Reverse primer (5′-3′) | |
|---|---|---|
| IL-6 | TACCACTTCACAAGTCGGAGGC | CTGCAAGTGCATCATCGTTGTTC |
| IFN-γ | CATCAGCAACAACATAAGCGTCA | CTCCTTTTCCGCTTCCTGA |
| IL-4 | TCG GCA TTT TGA ACG AGG TC | GAA AAG CCC GAA AGA GTC TC |
| IL-17A | CAGACTACCTCAACCGTTCCAC | TCCAGCTTTCCCTCCGCATTGA |
| T-bet | CCACCTGTTGTGGTCCAAGTTC | CCACAAACATCCTGTAATGGCTTG |
| GATA3 | GGGTTCGGAT GTAAGTCG | AGATGTGGCTCAGGGATG |
| RORγt | CCGCTGAGAGGGCTTCAC | TGCAGGAGTAGGCCACATTACA |
| GAPDH | CATCACTGCCACCCAGAAGACTG | ATGCCAGTGAGCTTCCCGTTCAG |
2.7. Determination of T cell activation stimulated by DNCB in vitro
Spleens from AD mice were removed aseptically at the last day of the experiment and gently mashed through a 70 µm nylon mesh screen. After red blood cell lysis, the splenocytes were re-suspended and cultured in round-bottomed 96-well microplates in a volume of 200 μl/well with complete RPMI-1640 medium. The cultures were incubated in the presence of 0.5 μg/ml of DNCB for 4 days. In the last 8 h of incubation, [3H] thymidine was added into each well. Then, the cells were harvested, using a 96-well plate harvester, onto fiber glass filters and radioactivity on the filter matt is counted in a liquid scintillation counter.
2.8. Statistical analysis
Statistical analysis was performed with SPSS Statistics Software (SPSS Inc., Chicago, USA). Data are presented as the mean ± standard error of the mean (SEM) and statistical comparisons between groups were performed using Independent-Sample t test and Chi-square test. Data are representative of three independent experiments. Significance was set at p < 0.05.
3. Results
3.1. CWPS attenuated the DNCB-induced AD-like symptoms in BALB/c mice
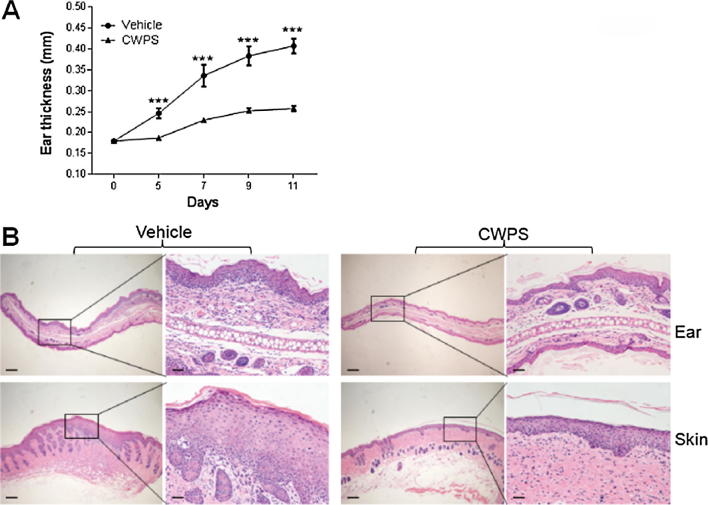
To investigate the potential therapeutic effect of CWPS on AD, a BALB/c AD model was established by topical application of DNCB on each ear and the dorsal skin (Fig. 1). Edema, excoriation, erythema, and scarring were apparent on the skin of DNCB-sensitized mice after multiple challenged with of DNCB. Strikingly, the severity of skin lesions was ameliorated in DNCB-sensitized mice treated with CWPS compared with saline-treated mice. Ear thickness was measured the next day after DNCB challenge. As shown in Fig. 2A, oral administration of CWPS significantly reduced the DNCB-induced increase in ear thickness.
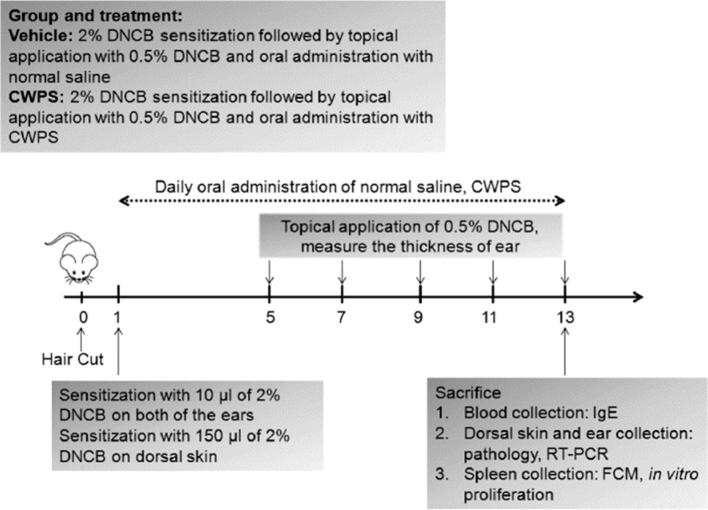
Fig. 1.
Schematic of atopic dermatitis (AD) induction and CWPS treatment. To induce AD-like symptoms, the hair-removed dorsal skin and ear of BALB/c mice (n = 6) were sensitized with 2% DNCB. The dorsal skin and ear of mice of re-challenged with 0.5% DNCB at the indicated time. CWPS dissolved in distilled water was orally administrated to BALB/c mice daily from day 1. The mice were sacrificed on day 13 to evaluate the effects of DNCB and CWPS treatment.
Fig. 2.
CWPS alleviated the AD-like symptoms induced by DNCB in BALB/c mice. (A) Ear thickness was measured from day 0 to day 11. ***p < 0.001 compared with the DNCB-treated group. Data shown represents three independent experiments with similar results. (B) The dorsal skin and ear of each mouse were biopsied, sectioned, and performed with H&E staining. Sections were evaluated under microscope. Scale bars = 1 mm. Right panels showed the higher-magnification views. Scale bars = 200 μm. Data are representative of three independent experiments with similar results.
The histopathological features of the ear and dorsal skin lesions are showed in Fig. 2B. Following H&E staining, the DNCB group showed the epidermis and dermis become thicker. By contrast, the CWPS-treated group had markedly less epidermal and dermal ear thickness. Additionally, CWPS-treated mice also showed less hypertrophy and granulocyte infiltration in the epidermis and dermis than DNCB-sensitized BALB/c mice.
3.2. CWPS reduced serum IgE elevation and alleviated mast cells infiltration in DNCB-sensitized mice
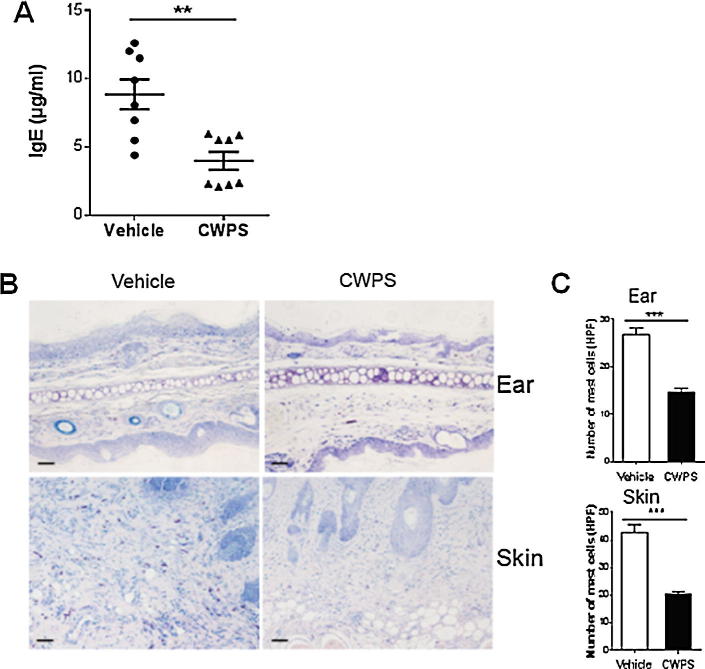
To verify the therapeutic efficacy of CWPS, we measured serum IgE concentrations in mice treated with CWPS versus saline-treated AD mice at the end of the experiment. Indeed, topical application of DNCB strikingly induced a higher level of serum IgE in BALB/c AD mice (8.85 ± 2.60 μg/ml), while CWPS treatment significantly suppressed the level of serum IgE in DNCB-treated mice (3.97 ± 1.55 μg/ml) (Fig. 3A).
Fig. 3.
CWPS inhibited DNCB-induced increasing serum IgE production and mast cells infiltration in BALB/c mice. (A) Blood was collected on the last day of the experiment (day 13). Serum levels of IgE were measured by ELISA. **p < 0.01, One of three independent experiments is shown. (B) The dorsal skin and ear sections were stained with toluidine blue for mast cell staining. Scale bars = 200 µm. (C) Infiltration of mast cells in dorsal skin and ear were quantified as means in randomly selected four fields per section. ***p < 0.001, Data shown represent three independent experiments with similar results.
Mast cells in skin were stained with toluidine blue, and the result showed that CWPS treatment decreased significantly the numbers of mast cells in the dermis from DNCB-sensitized mice compared with those from the saline-treated group (Fig. 3B). Upon stimulation with DNCB, infiltrated mast cells increased up to 23.62–29.98 per high power field (HPF) in the ear and 36.47–49.13 per HPF in dorsal skin, respectively (Fig. 3C). By contrast, less infiltrated mast cells were shown in the ear and skin from the CWPS-treated mice. As shown in Fig. 3C, the numbers of mast cells were 12.81–16.39 per HPF in the ear and 18.29–22.51 per HPF in the dorsal skin from the CWPS-treated AD mice.
3.3. Decreased percentage of Th1/Th17 cells after CWPS treatment in AD-like mice
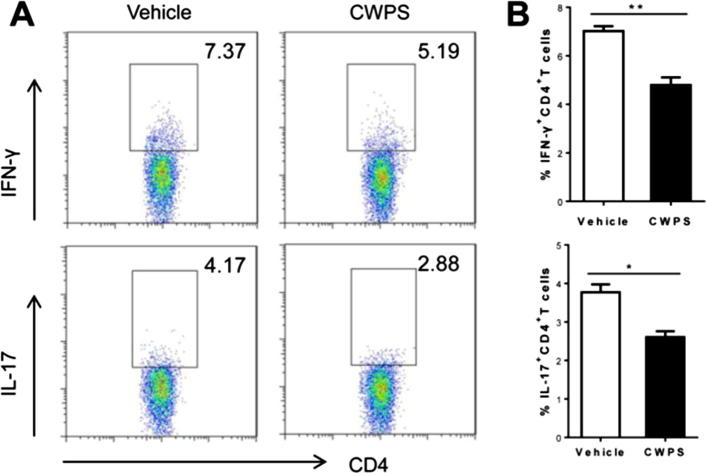
In AD, Th1 had been characterized as a sign of chronicity, while Th17 was reported close-correlated with the severity of AD. Thus, we further analyzed the differentiation of CD4+ Th cells in DNCB-treated BALB/c mice. Splenocytes obtained from DNCB-sensitized mice were tested for the expression of IFN-γ, IL-4 and IL-17 by intercellular staining and subsequently determined by FACS analysis. The percentage of IFN-γ-producing CD4+ Th1 lymphocytes was significantly lower in the CWPS-treated group (4.80 ± 1.34%) than that in the saline-treated group (7.02 ± 0.87%) (Fig. 4A and B). Likewise, the value of IL-17-producing CD4+ Th17 lymphocytes was also statistically decreased in the CWPS-treated group (2.60 ± 0.66%) compared with that in the saline-treated group (3.77 ± 0.87%) (Fig. 4A and B). However, the value of IL-4-producing CD4+ Th2 lymphocytes had no significance difference between these two groups (Fig. S1A and S1B). These data suggested that CWPS ameliorated inflammation of AD might be via modulating Th1/Th17 polarization.
Fig. 4.
CWPS treatment led to restraint of Th1/Th17 cell proliferation in AD-like mice. The spleen was extracted from AD-like BALB/c mice at the last day of the experiment, and single-cell suspension of splenocyte was prepared. The intracellular IFN-γ and IL-17 production of splenic CD4+ T cells were compared by FACS analysis (A). Summaries of the ratio of IFN+CD4+ T cells and IL-17+CD4+ T cells in normal saline-treated or CWPS-treated AD-like mice (n = 6) were presented (B). *p < 0.05, **p < 0.01, Data shown represent three independent experiments with similar results.
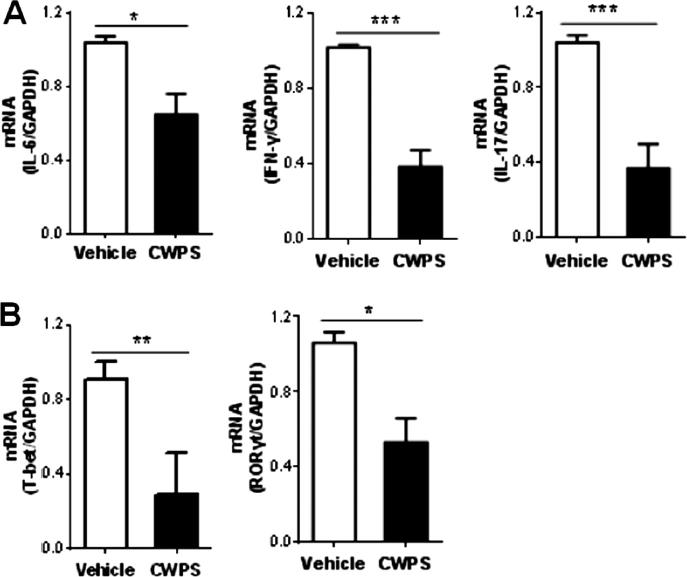
3.4. CWPS decreased DNCB-induced mRNA expression of IFN-γ, IL-17A and IL-6 in BALB/c mice
After DNCB application for the indicated time periods, the total RNA was isolated from dorsal skin. Subsequently, the mRNA expressions of IFN-γ, IL-17A, and IL-6 were measured by RT-PCR. Expressions of IFN-γ, IL-17A, and IL-6 were found to be markedly decreased in the CWPS-treated group compared with those in the saline-treated group (Fig. 5A). Besides, the CWPS-treated AD mice had significantly lower gene expressions of the Th1 transcription factor T-bet as well as Th17 transcription factor RORγt in the dorsal skin than that in the saline-treated group (Fig. 5B). In contrast, the mRNA expressions of IL-4 and its transcript factor GATA3 show no noticeable variations in the lesion of AD mice with or without CWPS treatment (Fig. S1C and S1D).
Fig. 5.
CWPS down-regulated the expression of cytokines and transcript factors in skin lesions from AD mice. Total RNA was extracted from the skin tissues. Relative mRNA expression levels of cytokines (i.e. IL-6, IFN-γ and IL-17A) (A) and T cell transcript factors (i.e. T-bet and RORγt) (B) were measured by quantitative RT-PCR analysis and expressed as a ratio to GAPDH. *p < 0.05, **p < 0.01, Data are representative of three independent experiments.
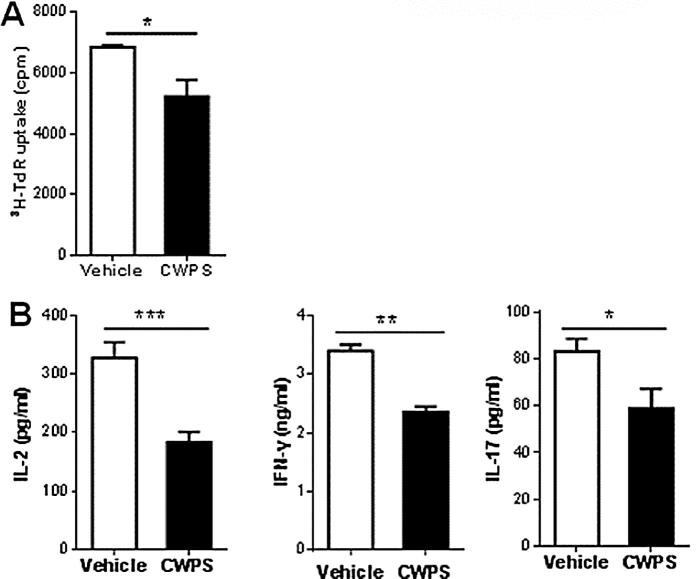
3.5. Inhibitory effect of CWPS on DNCB-specified T cell proliferation
To further demonstrate the effect of CWPS on regulating T cell function, splenocytes from DNCB-sensitized BALB/c mice was restimulated with DNCB in vitro. [3H] thymidine uptake was reduced in DNCB-stimulated splenocytes from the CWPS-treated DNCB-sensitized BALB/c mice compared to those from the saline-treated DNCB-sensitized mice after 4 days incubation (Fig. 6A). In accordance with proliferation assay, cytokine levels (i.e. IL-2, IFN-γ, and IL-17) in the culture supernatant were significantly lower in the CWPS-treated group than those in the saline-treated group (Fig. 6B). These results suggest that the treatment of CWPS suppress the DNCB-specified T cell activation.
Fig. 6.
CWPS suppressed the DNCB-specified T cell activation. Splenocytes from DNCB-induced AD mice with or without CWPS treatment were re-stimulated with 0.5 μg/ml of DNCB in vitro. (A) The DNCB-specified T cell proliferation was measured by [3H] thymidine incorporation. *p < 0.05. Data are representative of three independent experiments. (B) The levels of IL-2, IFN-γ, and IL-17 in the culture supernatants were measured by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001. Data are representative of three independent experiments.
4. Discussion
AD is a chronic inflammatory skin disease, which is characterized by severe pruritus, increased serum IgE level, and relapsing eczematous skin lesions (Bieber, 2008, Leung and Soter, 2001). Recently, emerging evidence derived from randomized clinical trials have suggested the potential clinical applications of Chinese herbal medicine in the management of AD (Gu et al., 2016, Razali and Said, 2017). The present study demonstrated that oral application of Chinese herbal mixture extract, CWPS, alleviated DNCB-induced AD-like symptoms in BALB/c mice. CWPS treatment significantly reduced excessive differentiation of keratinocytes in the epidermis as well as infiltration of inflammatory cells into the DNCB-induced AD-like skin lesions. In addition, we observed that CWPS limited the Th1 and Th17 activation during the development of AD, indicating that CWPS inhibited the AD-like pathology at least partially through shaping T helper cell responses.
Hyperplasia of stratum corneum with eosinophils and mast cells accumulation in dermal is a crucial histopathological feature of AD (Liu et al., 2011, Modena et al., 2016). Indeed, mast cells play a critical role as effector cells in IgE-mediated immediate hypersensitivity reactions via the production and secretion of proinflammatory mediators such as histamine, chemokines, cytokines, and growth factors (Theoharides and Kalogeromitros, 2006). Hyper IgE production has been considered as a hallmark of AD in human patients as well as transgenic mice and mice suffering from AD-like skin lesions (Bieber, 2008, Leung and Soter, 2001, Theoharides and Kalogeromitros, 2006). IgE binds to the FcεRI on the mast cell surface, leading to the degranulation and expression of proinflammatory mediators which subsequently trigger skin lesions. In our study, repeated oral treatment of CWPS suppressed extensively mast cell infiltration and serum IgE levels in DNCB-treated BALB/c mice. We also found that CWPS treatment suppressed IL-6 mRNA expression in epidermis tissue. Semmingly, the reduction of infiltration of mast cells is related to suppression of inflammatory cytokines.
AD is a Th cell-mediated skin disease associated with an imbalance in Th1/Th2 and contribution of Th17 (Brandt and Sivaprasad, 2011, Koga et al., 2008, Turner et al., 2012). Dense infiltration of activated CD4+ Th cells were observed in the dermis especially in the acute lesions (Bieber, 2008, Biedermann et al., 2015). Over the past few decades, Th2 cells and Th2 cytokines were believed to act as the key roles in promoting cutaneous inflammation in AD (Brandt and Sivaprasad, 2011, Shareef et al., 2017). Recent studies have pointed out the importance of both Th1 and Th17 cells for the maintenance of chronic stage of the AD-like pathology in humans and CHS in mice (Akhtar et al., 2010, Koga et al., 2008). On chronic AD, Th1 cells and Th1-type cytokine IFN-γ are assumed to contribute to skin hypertrophy in chronic AD (Spergel et al., 1999). Here, we determined that CWPS treatment decreased the percentage of Th1 and production of IFN-γ in the DNCB-induced AD mice model. DCs play a pivotal role in directing the differentiation of distinct subsets of CD4 + T helper cells. The polarization of naïve T helper cells into Th1 cells requires DCs producing large amounts of IL-12 (Trautmann et al., 2000, Walsh and Mills, 2013). Since DCs function as the bridge between innate and adaptive immunity and build a dense dermal network of immune sentinels in the skin, activation of skin-resident DC was deduced to associated with increased priming of Th1 cells in AD and leading to chronic skin lesions (Biedermann et al., 2015). Previous study demonstrated that CWPS could increase the phagocytic function of monocytes in mice (Feng et al., 2014). Therefore, it is likely that CWPS modulating Th1 cell function in vivo partially owing to the effect of CWPS on DCs, and further investigations are warranted. Th17 has been identified and shown to play a significant role in tissue inflammation (Steinman, 2007). Previous studies showed that the percentage of Th17 was associated with severity of AD. IL-17 is a potent stimulator for keratinocytes to produce skin inflammation associated molecules, such as IL-6 (Koga et al., 2008). Our study demonstrated that Th17 lymphocytes were significantly decreased after CWPS treatment in DNCB-induced AD mice. CWPS treatment also suppressed the mRNA expression of Th17 transcript factor (RORγt) and IL-17 in skin from AD mice. It has been reported that splenocytes isolated from CWPS-fed mice have stronger proliferation activity than those from normal diet mice upon Con A stimulation (Feng et al., 2006). In this study, we determined that splenocytes from CWPS-treated AD mice showed decreased T cell activation and cytokines (i.e. IFN-γ and IL-17) production re-stimulated by DNCB compared to those from normal saline-treated AD mice. Our observations support the conclusion that CWPS could limit the T cell activation and polarization into various effector subsets during AD, therefore, modulate the pathogenesis of chronic skin inflammation.
5. Conclusions
In summary, we demonstrate that CWPS treatment could alleviate AD by reducing serum IgE levels, inhibiting eosinophils and mast cells infiltration. CWPS can limit Th1 and Th17 polarization and inhibit proinflammatory cytokine expression induced by DNCB in AD mice model. These findings substantiate the concept that CWPS has therapeutic potentials as an anti-inflammatory agent to attenuate AD-like skin lesions.
Author disclosure statement
All authors have no potential conflicts of interest to declare.
Acknowledgments
This work was supported in part by Science and Technology Planning Project of Guangdong Province 2011B031800106 (to L.S.), Develop project of Distinguished Young Teacher in Southern Medical University (to L.S.), Foundation for Distinguished Young Teacher in Higher Education of Guangdong Yq2013034 (to D.Z.), Science and Technology Planning Project of Guangdong Province 2016A020215106 (to D.Z.), and Science and Technology Planning Project of Guangzhou 201607010195 (to D.Z.).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jsps.2017.04.035.
Appendix A. Supplementary material
CWPS treatment did not affect Th2 polarization in AD mice. (A and B) The spleen was isolated from AD-like BALB/c mice on the last day of the experiment. Subsequently, the intracellular IL-4 production of splenic CD4+ T cells was compared by FACS analysis (A). Summaries of the ratio of IL-4 + CD4 + T cells in normal saline-treated or CWPS-treated AD-like mice (n = 6) were presented (B). Data shown here represents three independent experiments with similar results. (C and D) Total RNA was extracted from the skin tissues. Relative mRNA expression levels of IL-4 (C) and its transcript factors GATA3 (D) were measured by quantitative RT-PCR analysis and expressed as a ratio to GAPDH. NS, not significant, Data are representative of three independent experiments.
References
- Akhtar N., Verma K.K., Sharma A. Study of pro- and anti-inflammatory cytokine profile in the patients with parthenium dermatitis. Contact Dermatitis. 2010;63:203–208. doi: 10.1111/j.1600-0536.2009.01693.x. [DOI] [PubMed] [Google Scholar]
- Bieber T. Atopic dermatitis. N. Engl. J. Med. 2008;358:1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- Biedermann T., Skabytska Y., Kaesler S., Volz T. Regulation of T cell immunity in atopic dermatitis by microbes: the Yin and Yang of cutaneous inflammation. Front. Immunol, 2015;6:353. doi: 10.3389/fimmu.2015.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt E.B., Sivaprasad U. Th2 cytokines and atopic dermatitis. J. Clin. Cell. Immunol. 2011:2. doi: 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Chen X.M., Ma Y., He Z. Experimental study on immunomodulation of white wax scale (Ericerus pela Chavannes) For. Res. 2006;19:221–224. [Google Scholar]
- Feng Y., He Z., Li X., Chen Z.Y., Sun L. Immunomodulatory and antitumor activities of polysaccharide from Chinese white wax scale. For. Res. 2014;27:388–392. [Google Scholar]
- Gu S.X., Zhang A.L., Coyle M.E., Chen D., Xue C.C. Chinese herbal medicine for atopic eczema: an overview of clinical evidence. J. Dermatol. Treat. 2016:1–5. doi: 10.1080/09546634.2016.1214673. [DOI] [PubMed] [Google Scholar]
- Halim A.N.I., Phang I.C. Salicylic acid mitigates pb stress in Nicotiana tabacum. Galeri Warisan Sains. 2017;1(1):16–19. [Google Scholar]
- He Z., Li X., Sun L., Chen Z.Y., Feng Y. Antioxidant activities of five insect polysaccharides in vitro. J. Environ. Entomol. 2015;37:61–67. [Google Scholar]
- He Z., Sun L., Feng Y., Chen X.M. The extraction of polysaccharide from white wax scale and analysis of monosaccharide compositions. For. Res. 2008;21:792–796. [Google Scholar]
- Kim J.H., Kim M.H., Yang G., Huh Y., Kim S.H., Yang W.M. Effects of topical application of Astragalus membranaceus on allergic dermatitis. Immunopharmacol. Immunotoxicol. 2013;35:151–156. doi: 10.3109/08923973.2012.733708. [DOI] [PubMed] [Google Scholar]
- Kim S.R., Choi H.S., Seo H.S., Ku J.M., Hong S.H., Yoo H.H., Shin Y.C., Ko S.G. Oral administration of herbal mixture extract inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis in BALB/c mice. Mediators Inflamm. 2014;2014:319438. doi: 10.1155/2014/319438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga C., Kabashima K., Shiraishi N., Kobayashi M., Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J. Invest. Dermatol. 2008;128:2625–2630. doi: 10.1038/jid.2008.111. [DOI] [PubMed] [Google Scholar]
- Leung D.Y., Soter N.A. Cellular and immunologic mechanisms in atopic dermatitis. J. Am. Acad. Dermatol. 2001;44:S1–S12. doi: 10.1067/mjd.2001.109815. [DOI] [PubMed] [Google Scholar]
- Liu F.T., Goodarzi H., Chen H.Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin. Rev. Allergy Immunol. 2011;41:298–310. doi: 10.1007/s12016-011-8252-4. [DOI] [PubMed] [Google Scholar]
- Modena B.D., Dazy K., White A.A. Emerging concepts: mast cell involvement in allergic diseases. Transl. Res. 2016;174:98–121. doi: 10.1016/j.trsl.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Mustafa G., Arif R., 1, Atta A., Sharif S., Jamil A. Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Matrix Sci. Pharma. 2017;1(1):17–26. [Google Scholar]
- Nawaz S., Shareef M., Shahid H., Mushtaq M., Sarfraz M. A review of antihyperlipidemic effect of synthetic phenolic compounds. Matrix Sci. Med. 2017;1(1):22–26. [Google Scholar]
- Oboki K., Ohno T., Saito H., Nakae S. Th17 and allergy. Allerg. Int.: Off. J. Jpn. Soc. Allerg. 2008;57:121–134. doi: 10.2332/allergolint.R-07-160. [DOI] [PubMed] [Google Scholar]
- Rashid M., Saleem M.I., Deeba F., Khan M.S., Mahfooz S.A., Butt A.A., Abbas M.W. Effect of season on occurrence of caprine mastitis in beetal in faisalabad premises. Matrix Sci. Med. 2017;1(1):19–21. [Google Scholar]
- Razali M.A.A., Said F.M. Red pigment production by monascus purpureus in stirred-drum bioreactor. Galeri Warisan Sains. 2017;1(1):13–15. [Google Scholar]
- Shareef M., Jamal M., Sarfraz M. A review of Anti-bacterial activity of Nigella sativa in gut of broiler chicks. Matrix Sci. Pharma. 2017;1(1):27–32. [Google Scholar]
- Spergel J.M., Mizoguchi E., Oettgen H., Bhan A.K., Geha R.S. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J. Clin. Invest. 1999;103:1103–1111. doi: 10.1172/JCI5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- Theoharides T.C., Kalogeromitros D. The critical role of mast cells in allergy and inflammation. Ann. N. Y. Acad. Sci. 2006;1088:78–99. doi: 10.1196/annals.1366.025. [DOI] [PubMed] [Google Scholar]
- Trautmann A., Akdis M., Kleemann D., Altznauer F., Simon H.U., Graeve T., Noll M., Brocker E.B., Blaser K., Akdis C.A. T cell-mediated Fas-induced keratinocyte apoptosis plays a key pathogenetic role in eczematous dermatitis. J. Clin. Invest. 2000;106:25–35. doi: 10.1172/JCI9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M.J., Travers J.B., Kaplan M.H. T helper cell subsets in the development of atopic dermatitis. J. Drugs Dermatol.: JDD. 2012;11:1174–1178. [PubMed] [Google Scholar]
- Walsh K.P., Mills K.H. Dendritic cells and other innate determinants of T helper cell polarisation. Trends Immunol. 2013;34:521–530. doi: 10.1016/j.it.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Yang P., Chen X.M., Liu W.W., Feng Y., Sun T. Transcriptome analysis of sexually dimorphic Chinese white wax scale insects reveals key differences in developmental programs and transcription factor expression. Sci. Rep. 2015;5:8141. doi: 10.1038/srep08141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Zhu J.Y., Gong Z.J., Xu D.L., Chen X.M., Liu W.W., Lin X.D., Li Y.F. Transcriptome analysis of the Chinese white wax scale Ericerus pela with focus on genes involved in wax biosynthesis. PLoS ONE. 2012;7:e35719. doi: 10.1371/journal.pone.0035719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H.J., Jang M.S., Kim H.W., Song D.U., Nam K.I., Bae C.S., Kim S.J., Lee S.R., Ku C.S., Jang D.I. Protective effect of diet supplemented with rice prolamin extract against DNCB-induced atopic dermatitis in BALB/c mice. BMC Complement. Altern. Med. 2015;15:353. doi: 10.1186/s12906-015-0892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CWPS treatment did not affect Th2 polarization in AD mice. (A and B) The spleen was isolated from AD-like BALB/c mice on the last day of the experiment. Subsequently, the intracellular IL-4 production of splenic CD4+ T cells was compared by FACS analysis (A). Summaries of the ratio of IL-4 + CD4 + T cells in normal saline-treated or CWPS-treated AD-like mice (n = 6) were presented (B). Data shown here represents three independent experiments with similar results. (C and D) Total RNA was extracted from the skin tissues. Relative mRNA expression levels of IL-4 (C) and its transcript factors GATA3 (D) were measured by quantitative RT-PCR analysis and expressed as a ratio to GAPDH. NS, not significant, Data are representative of three independent experiments.