Abstract
The effect of the Rabdosia rubescens total flavonoids on focal cerebral ischemia reperfusion model in rats was observed. The model group, nimodipine group, cerebral collateral group, and large, medium and small dose group of the Rabdosia rubescens total flavonoids were administered with corresponding drugs but sham operation group and model group were administered the same volume of 0.5%CMC, 1 times a day, continuous administration of 7 d. After 1 h at 7 d to medicine, left incision in the middle of the neck of rats after anesthesia, we can firstly expose and isolate the left common carotid artery (CCA), and then expose external carotid artery (ECA) and internal carotid artery (ICA). The common carotid artery and the external carotid artery are ligated. Then internal carotid artery with arterial clamp is temporarily clipped. Besides, cut the incision of 0.2 mm from 5 cm of the bifurcation of the common carotid artery. A thread Line bolt is inserted with more than 18–20 mm from bifurcation of CCA into the internal carotid artery until there is resistance. Then the entrance of the middle cerebral artery is blocked and internal carotid artery is ligated (the blank group only exposed the left blood vessel without Plugging wire). Finally it is gently pulled out the plug line after 2 h. Results: Compared with the model mice, Rabdosia rubescens total flavonoids can significantly relieve the injury of brain in hippocampus and cortex nerve cells; experimental rat focal cerebral ischemia was to improve again perfusion model of nerve function defect score mortality; significantly reduce brain homogenate NOS activity and no content, MDA, IL-1, TNF-a, ICAM-1 content; increase in brain homogenate SOD and ATPase activity (P < 0.05, P < 0.01); and reduce the serum S-100β protein content. Each dose group of the Rabdosia rubescens total flavonoids has a better Improvement effect on focal cerebral ischemia reperfusion model in rats.
Keywords: The Rabdosia rubescens total flavonoids, Focal cerebral ischemia reperfusion, Animal model
1. Introduction
Cerebral ischemia reperfusion injury (CIRI) refers to the cerebral artery occlusion, cerebral ischemia, hypoxia and lack of energy supply, however restoring blood perfusion and oxygen supply, tissue or organ damage and aggravating the phenomenon. Cerebral ischemia reperfusion injury has high incidence of a disease in elderly people, with the deepening of the aging population in our country, and higher morbidity and mortality have made it one of the main diseases endangering public health (Iftakhar et al., 2015, Sarfraz et al., 2016, Pendlcbury et al., 2009), effectively inhibiting the reperfusion injury has a very important clinical significance.
2. Material and method
2.1. Experimental materials
2.1.1. Drugs and reagents
The Rabdosia rubescens total flavonoids products were from Henan University of TCM analysis chemistry laboratory, and content of total flavonoids was 50.4%, batch number 20120705; nimodipine tablets, provided by Shandong Xinhua Pharmaceutical Co., Ltd. production and batch number 1105036; brain collaterals pass capsule, provided by Harbin Pharmaceutical Group Sanjing Pharmaceutical Factory Co., Ltd production, batch 201109002; sodium carboxymethyl cellulose, provided by Tianjin Hengxing chemical reagent manufacturing Co., Ltd. Production and the batch number of it is 20060728; chloral hydrate, products from Pu Shan Shanghai Chemical Co., Ltd., batch number 20120401; formaldehyde solution, provided by Tianjin Kermel Chemical Reagent Co., Ltd production, batch number 20120406; and adenosine triphosphate (ATP) enzyme test kit, provided by Nanjing Jiancheng biological engineering research production, batch number 20130111; Coomassie brilliant blue test box comes from Nanjing built Production of Material Engineering Research Institute and the batch number of it is 20130115; nitric oxide test kit was provided by Nanjing built Production of Material Engineering Research Institute, and the batch number of it is 20130111; NOS kit was provided by Nanjing built Production of Material Engineering Research Institute, and the batch number of it is 20130114; malondialdehyde (MDA) test kit was provided by Nanjing built Production of Material Engineering Research Institute, batch number 20130108; SOD test box was provided by Nanjing Jiancheng biological engineering research production, batch number 20130114; S-100 beta protein ELISA kit, the TNF-ELISA kit. ICAM-1ELISA detection kit and IL-1 ELISA kit were provided by R & D Co. batch number 20130101A.
2.1.2. Instrument
UV-2000 UV visible spectrophotometer, especially Niko (Shanghai) Instrument Co., Ltd. production; of tgl-16g desktop centrifuge, Shanghai Anting Scientific Instrument Factory; JA1103N electronic analytical balance, Ohaus (Shanghai) Co., Ltd. production; can be adjustable liquid shifter, Shanghai Lei Bo Instrument Co., Ltd. Production.
2.1.3. Laboratory animals
Wistar rats, male, weight SPF, 230-250 g, were from Shandong Lukang Pharmaceutical Limited by Share Ltd, rat Certificate No. 13889.
2.2. Experimental methods
2.2.1. Modeling and administration
112 rats of Wistar rats with 230–250 g were divided into 7 groups and 16 rats in each group, and were gavaged with big, middle and small dose of total flavonoids of Rabdosia rubescens mixed suspension (200 mg/kg, 100 mg/kg, 50 mg/kg, pro with 0.5% CMC drug concentration of 20 mg/ml, 10 mg/ml, 5 mg/ml (1 ml/100 g)), nimodipine mixed suspension (20 mg/kg, pro before use 0.5%CMC with drug concentration of 2 mg/ml 1 ml/100 g of clinical dosage of 10 times), brain collaterals Tong capsule suspension liquid (500 mg/kg, the pro before use 0.5% CMC with drug concentration of 50 mg/ml). 1 ml/100 g, the clinical dosage of 10 times and the same volume of 0.5%CMC (sham operation group, model group) were administered 1 times a day, continuous administration of 7 d. On the sixth night fasting but not water, 7 days morning weighing, administered 1 h later, and intraperitoneal injection of 10% chloral hydrate (0.3 ml/100 g) anesthesia in rats and the midline of the neck to the left side incision, layer by layer separated and exposed the left CCA, ECA, and ICA, ligation of the common carotid artery and external carotid artery, by artery clamp temporary clamp of the ICA. In common carotid artery from bifurcation at 5 mm 0.2 mm mouth was cut, suture was inserted, via the common carotid artery bifurcation into the internal carotid artery, to deeply bifurcate above 18–20 mm until resistance of that blockade of brain Artery at the entrance, ligation of the internal carotid artery (blank control group only exposed left vessels not in line processing), 2 h after gently pull out suture. Embolism on all rats was carried out on the left side of the brain artery, and middle cerebral artery occlusion and reperfusion (MCAO) animal models were established. Sham operation group was only for dissection and ligation of the common carotid artery.
Neurological scores after 24 h surgery were calculated using Zealonga scoring method (Longa et al., 1989). Scoring criteria were as follows: 0: no neurological deficit symptoms, normal activities; 1 point cannot fully extend to the contralateral forepaw; 2: crawling to hemiplegia contralateral circling; 3: when walking, body to hemiplegia side dumping them; 4: unable to walk spontaneously, consciousness loss; 5 points: death. Score 1–3 points were for successful modeling, and weed out unqualified rats. 0 and 4 points were removed; eyeball blood, half an hour later, centrifugal, and supernatant were measured by S-100 beta protein content; rats were decapitated after rapid stripping brain tissue, half brain preparation 10% of brain homogenate and according to the kit for detection of MDA, SOD, NO, NOS, TNF alpha, IL-1 beta, ICAM-1, ATP enzyme, Coomassie brilliant blue; the other half brain tissue, was fixed with 10% formaldehyde solution, for HE staining observed brain tissue morphological change.
3. Results
3.1. Effects of the Rabdosia rubescens total flavonoids on the neurological function score and mortality of rats with focal cerebral ischemia model, see Table 1
Table 1.
The effect of neurological function score and mortality rate on focal cerebral ischemia in rats ().
| Group | n | Dose (mg/kg) | Nerve function score | n (deaths) | Mortality (%) |
|---|---|---|---|---|---|
| Sham operation group | 16 | 0.0 + 0.0 | 0 | 0.0 | |
| Model group | 10 | 3.6 ± 0.5 | 6 | 37.5 | |
| Nimodipine group | 11 | 20 | 2.8 ± 0.6* | 5 | 31.2 |
| Cerebral collateral group | 12 | 500 | 2.6 ± 0.7** | 4 | 25.0 |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 1.8 ± 0.7** | 4 | 25.0 |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 2.0 ± 0.6** | 4 | 25.0 |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 2.2 ± 0.6** | 5 | 31.2 |
Contrast model group P < 0.05.
Contrast model group P < 0.01.
From the table seen, the rats in the sham operation group were no neurological deficit and other groups in rats after 24 h ischemia appeared at different degrees of neurological dysfunction; by group and sham operation ratio, model group, the neurological function score increased significantly (P < 0.01) in rats with focal cerebral ischemia and reperfusion model successfully. Compared with the model group, large, medium and small doses of the Rabdosia rubescens total flavonoids and the brain collaterals group can significantly reduce the neurological function score (P < 0.01), and nimodipine group can significantly reduce the neurological function score (P < 0.05).
3.2. Effect of the Rabdosia rubescens total flavonoids on cerebral homogenate NO and NOS activity of rats with focal cerebral ischemia model, see Table 2
Table 2.
Effect on rat model of focal cerebral ischemia cerebral homogenate NO and NOS activity ().
| Group | n | Dose (mg/kg) | NO (μmol/gprot) | NOS (U/mgprot) |
|---|---|---|---|---|
| Sham operation group | 16 | 3.11 ± 0.26** | 0.52 ± 0.09** | |
| Model group | 10 | 9.17 ± 1.37 | 1.26 ± 0.12 | |
| Nimodipine group | 11 | 20 | 6.26 ± 0.94** | 0.93 ± 0.11** |
| Cerebral collateral group | 12 | 500 | 6.00 ± 1.04** | 0.84 ± 0.10** |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 4.55 ± 0.41** | 0.69 ± 0.08** |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 5.54 ± 0.76** | 0.76 ± 0.08** |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 6.37 ± 0.72** | 0.86 ± 0.12** |
From the table seen, compared with the sham operation group, model group rat brain homogenate NO content, NOS activity significantly increased (P < 0.01), indicating that the rat focal cerebral ischemia reperfusion model was successful. Compared with the model group, the large, medium and small doses group of the Rabdosia rubescens total flavonoids, nimodipine group and the cerebral collaterals group can significantly reduce the NO content and NOS activity (P < 0.01) in the brain homogenate of rats.
3.3. Effect of the Rabdosia rubescens total flavonoids on cerebral homogenate MDA and SOD activity in focal cerebral ischemia model in rats, see Table 3
Table 3.
Effect on rat model of focal cerebral ischemia cerebral homogenate MDA and SOD activity ().
| Group | n | Dose (mg/kg) | MDA (U/mgprot) | SOD × 102 (nmol/mgprot) |
|---|---|---|---|---|
| Sham operation group | 16 | 3.74 ± 0.33** | 2.37 ± 0.21** | |
| Model group | 10 | 7.28 ± 0.59 | 1.53 ± 0.10 | |
| Nimodipine group | 11 | 20 | 5.05 ± 0.45** | 1.88 ± 0.14** |
| Cerebral collateral group | 12 | 500 | 5.46 ± 0.83** | 1.98 ± 0.21** |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 3.87 ± 0.33** | 2.10 ± 0.12** |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 4.66 ± 0.24** | 2.07 ± 0.09** |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 5.32 ± 0.49** | 1.95 ± 0.12** |
From the table seen, and the sham operation group, the ratio, the content of MDA in model group rats in ischemic brain tissue increased significantly (P < 0.01), and the level of SOD significantly reduced (P < 0.01), indicating in rats with focal cerebral ischemia and reperfusion model success; and by model group ratio, large, medium and small dose group of Rabdosia rubescens total flavonoids, nimodipine group and brain collaterals group were significantly decreased in rat brain tissue MDA content (P < 0.01), and significantly increased the level of SOD (P < 0.01).
3.4. The effect of the Rabdosia rubescens total flavonoids on the activity of ATP in brain homogenate of rats with focal cerebral ischemia model, see Table 4
Table 4.
Effect on rat model of focal cerebral ischemia brain homogenate ATP activity ().
| Group | n | Dose (mg/kg) | Na+-K+-ATPenzyme (umolPi/mgprot/h) | Mg2+-ATPenzyme (umolPi/mgprot/h) | Ca2+-ATPenzyme (umolPi/mgprot/h) |
|---|---|---|---|---|---|
| Sham operation group | 16 | 10.82 ± 0.83** | 8.13 ± 0.66** | 12.39 ± 1.07** | |
| Model group | 10 | 5.16 ± 0.74 | 4.03 ± 0.67 | 7.24 ± 0.74 | |
| Nimodipine group | 11 | 20 | 6.76 ± 0.81** | 5.46 ± 0.49** | 9.14 ± 0.64** |
| Cerebral collateral group | 12 | 500 | 7.08 ± 0.77** | 6.17 ± 0.86** | 9.40 ± 0.96** |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 9.50 ± 0.43** | 6.94 ± 0.26** | 10.86 ± 0.39** |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 8.53 ± 0.44** | 6.46 ± 0.47** | 9.97 ± 0.60** |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 7.77 ± 0.39** | 5.36 ± 0.37** | 9.23 ± 0.77** |
From table seen, and sham operation group than in model group, the rats in the brain tissue of Na+, K+-ATPase, enzyme Mg2+-ATP, Ca2+-ATP enzyme activity significantly decreased (P < 0.01), the description of rat focal cerebral ischemia and reperfusion model was done successfully; and model group ratio, large, medium and small dose of Rabdosia rubescens total flavone group, nimodipine group and brain collaterals through group rat brain Na+, K+-ATPase, enzyme Mg2+-ATP, Ca2+-ATP enzyme activity were significantly increased (P < 0.01).
3.5. Effects of the Rabdosia rubescens total flavonoids on brain homogenate TNF- alpha and IL-1 beta in rats with focal cerebral ischemia model, see Table 5
Table 5.
Effects of TNF- alpha and IL-1 beta on brain homogenate in rats model with focal cerebral ischemia ().
| Group | n | Dose (mg/kg) | TNF-α (ng/ml) | IL-1β (ng/ml) |
|---|---|---|---|---|
| Sham operation group | 16 | 9.61 ± 0.90** | 10.50 ± 0.69** | |
| Model group | 10 | 23.43 ± 1.24 | 30.19 ± 2.40 | |
| Nimodipine group | 11 | 20 | 16.26 ± 1.16** | 19.26 ± 1.26** |
| Cerebral collateral group | 12 | 500 | 14.98 ± 1.06** | 18.74 ± 1.35** |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 11.78 ± 0.89** | 12.09 ± 0.98** |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 12.97 ± 1.32** | 14.58 ± 1.10** |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 14.31 ± 0.98** | 16.20 ± 0.96** |
From the table seen, sham operation group ratio, model group rats in ischemic brain tissue TNF alpha and IL-1 beta content increased significantly (P < 0.01), indicating that in rats with focal cerebral ischemia and reperfusion model success; and model group ratio, large, medium and small dose of Rabdosia rubescens total flavonoids group, nimodipine group and brain collaterals group were significantly decreased in rat brain tissue TNF alpha and IL-1 beta in (P < 0.01).
3.6. Effects of Rabdosia rubescens total flavonoids on ICAM-1 and serum levels of S-100β in brain homogenate of rats with focal cerebral ischemia model, see Table 6
Table 6.
Effects of ICAM-1 on S-100β and serum levels of brain homogenate in rats with focal cerebral ischemia ().
| Group | n | Dose (mg/kg) | ICAM-1 (ng/ml) | S-100β (ng/ml) |
|---|---|---|---|---|
| Sham operation group | 16 | 14.17 ± 1.02** | 10.52 ± 0.57** | |
| Model group | 10 | 29.13 ± 1.13 | 29.23 ± 0.71 | |
| Nimodipine group | 11 | 20 | 20.44 ± 1.11** | 18.38 ± 1.04** |
| Cerebral collateral group | 12 | 500 | 19.55 ± 0.83** | 17.63 ± 1.08** |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 15.49 ± 0.75** | 13.91 ± 0.73** |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 18.02 ± 0.14** | 16.10 ± 0.72** |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 19.38 ± 1.17** | 18.33 ± 0.87** |
From the table seen, Compared with sham operated group, In model group, the content of ICAM-1 and the content of serum S-100β were significantly increased in the ischemic side of the rats (P < 0.01), indicating the focal cerebral ischemia and reperfusion model success; and model group ratio, large, medium and small dose groups of the Rabdosia rubescens total flavonoids, nimodipine group and brain collaterals group were significantly decreased in brain tissue ICAM-1 content and serum S-100 beta content (P < 0.01).
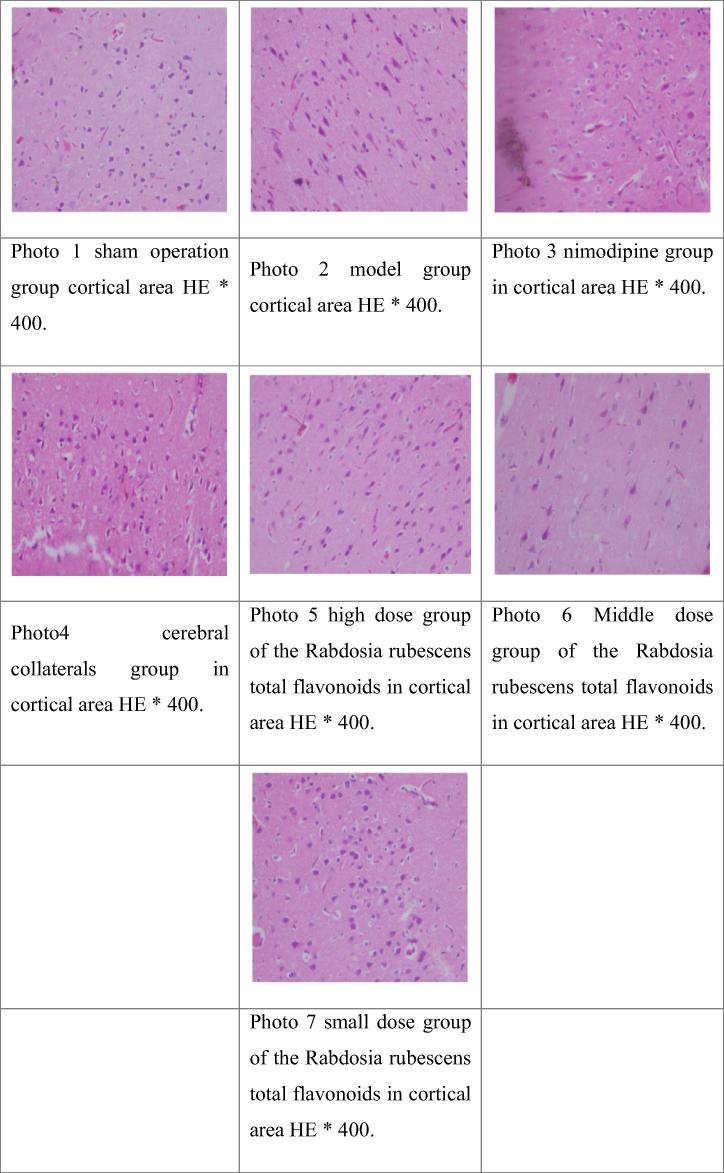
3.7. Effect of the Rabdosia rubescens total flavonoids on the pathological changes of cerebral cortex in rats with focal cerebral ischemia model in Table 7, Fig. 1
Table 7.
Pathological changes of focal cerebral ischemia reperfusion in rats.
| Group | n | Dose (mg/kg) | − | + | ++ | +++ |
|---|---|---|---|---|---|---|
| Sham operation group | 16 | 11 | 1 | 0 | 0 | |
| Model group | 10 | 0 | 0 | 0 | 1 | 9 |
| Nimodipine group | 11 | 20 | 0 | 0 | 5 | 6 |
| Cerebral collateral group | 12 | 500 | 2 | 3 | 2 | 5 |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 8 | 4 | 0 | 0 |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 8 | 4 | 0 | |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 1 | 4 | 6 | 0 |
“−” with normal nerve cells, glial cells were basically normal; “+” a few nerve cell degeneration, glial cells were normal; “++” most of the neural cells degeneration, with normal glial cells or partial condensation; “+++” all nerve cell degeneration, glial cell condensation or disappear. According to semi quantitative standards experimental animals in each group were measured.
Fig. 1.
HE staining of the cortical areas of the cerebral cortex after focal cerebral ischemia reperfusion model in rats.
The Ridit test, and sham operation group ratio, ischemia/reperfusion group have significantly statistical significance (P < 0.01), indicating a successful model. Compared with the model group, large, in small doses group of Rabdosia rubescens total flavonoids have significantly statistical significance (P < 0.01), and brain collateral group had obvious statistical significance (P < 0.05), indicating the improved brain injury.
Pathological observation of rats in each group: the sham operation group was basically normal, and the glial cells were normal. In model group, most of the cerebral cortical neurons were degenerated, and most of the glial cells were Nimodipine group rats cerebral cortex neurons decreased and degeneration of glial cells increased, and most of them were normal. In the cerebral cortex of rats, the degeneration or atrophy of the cerebral cortex neurons was normal. In the high dose of the total flavonoids of the total flavonoids in the rats of the total flavonoids of the rat brain, most of the cerebral cortex neurons were basically normal, and the glial cells were basically normal. In the middle dose of the total flavonoids of the rats in the group the total flavonoids of the cerebral cortex of the rat are basically normal, and glial cells are basically normal. In the small dose of the total flavonoids in the rats, the total flavonoids of the rat cerebral cortex nerve cells were in the state, the glial cell part of the solid shrinkage.
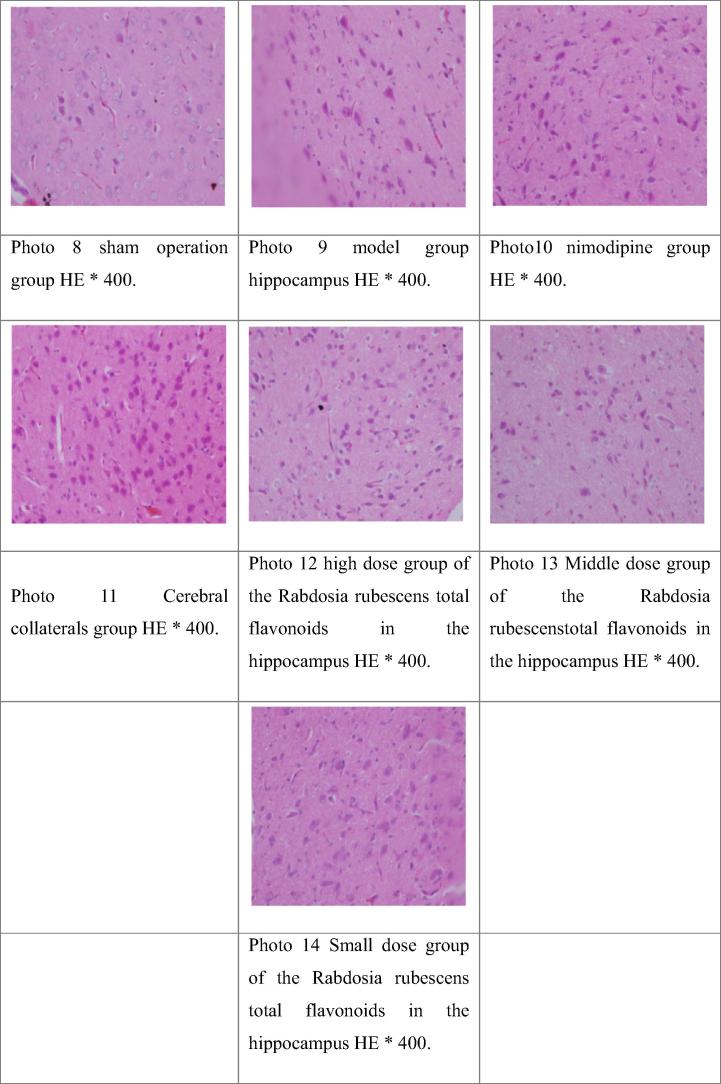
3.8. The effect of the Rabdosia rubescens total flavonoids on the pathological changes of brain tissue in rats with focal cerebral ischemia model in the hippocampal neuronal nuclei was shown in Table 8, Fig. 2
Table 8.
The results of semi quantitative analysis of the pathological changes of brain tissue in hippocampus of rat model of focal cerebral ischemia.
| Group | n | Dose (mg/kg) | − | + | ++ | +++ |
|---|---|---|---|---|---|---|
| Sham operation group | 16 | 11 | 1 | 0 | 0 | |
| Model group | 10 | 0 | 0 | 3 | 7 | |
| Nimodipine group | 11 | 20 | 0 | 1 | 2 | 8 |
| Cerebral collateral group | 12 | 500 | 0 | 1 | 2 | 9 |
| Large dose group of the Rabdosia rubescens total flavonoids | 12 | 200 | 7 | 4 | 1 | 0 |
| Middle dose group of the Rabdosia rubescens total flavonoids | 12 | 100 | 7 | 4 | 1 | 0 |
| Small dose group of the Rabdosia rubescens total flavonoids | 11 | 50 | 6 | 4 | 1 | 0 |
“−” with normal nerve cells, glial cells were basically normal; “+” a few nerve cell degeneration, glial cells were normal; “++” part of the nerve cell degeneration, with normal glial cells or partial condensation; “+++” all nerve cell degeneration, glial cell condensation or disappear.
Fig. 2.
The HE staining of the hippocampal neurons in the rat model of focal cerebral ischemia reperfusion model in rats.
The Ridit test, and sham operation group compared to ischemia reperfusion group have significantly statistical significance (P < 0.01), indicating a successful model. Compared with the model group, large, and small dose group of Rabdosia rubescens total flavonoids have significantly statistical significance (P < 0.01)..
Most of pathological observation of brain tissue of rats hippocampus nerve nuclei results: In sham operation group rats brain nerve nuclei of cells is basic and normal, and glial cells is basic and normal; in model group rats brain nerve nucleus cell degenerate, glial cells most solid shrinkage; in nimodipine group rat brain nerve nucleus cell degenerate, glial cells pyknosis; in brain collaterals group rat brain nerve nucleus cell degenerate with edema, glial cells pyknosis; winter rubescens total flavonoids in high dose group rats brain nerve nuclei of cells is basic and normal, glial cells was normal; winter rubescens total flavonoids in dose group rats brain nerve nuclei of cells is basic and normal, and glial cell is matrix, this is normal; the total flavonoids in the small dose group of the total flavonoids in the brain of the rat brain tissue nucleus cells of individual degenerate, and glial cells are basically normal.
4. Discussion
Ischemic cerebrovascular disease, as a common clinical disease, which has the characteristics of high incidence, high disability rate and high fatality rate, can seriously harm the health of the elderly population. It was rated as one of “the three major killers” in the developed countries in Europe and the United States (Jiang et al., 2016). According to the World Health Organization (WHO), China's annual new ischemic cerebral vascular disease in patients is about 200 million, about 75% of the survivors have movement, aphasia and cognitive dysfunction, and severe disability is about 40%. It is a heavy burden to the family and the society (Jia et al., 2010). Inhibiting the ischemia and reperfusion injury has become the key link in the treatment of ischemic cerebrovascular diseases.
Modern research shows that superoxide anion free radical, apoptosis, inflammation, calcium overload, are involved in the cerebral ischemia reperfusion injury incidence. But the platelet aggregation inhibitors, calcium channel block agent medicine has more side effects, while targeted was not so strong that is why the application of chemicals is limited. Chinese medicine holds that ischemia reperfusion injury is a “stroke” category, deficiency of vital energy and blood stasis is one of the main pathogeneses of ischemic stroke. Modern studies suggest that the body's blood can’t flow smoothly, resulting in thrombosis, causing the expression of inflammatory molecules and inflammatory cells aggregation, making for neurotoxicity and nerve damage. Therefore, activating blood circulation and Clearing away heat and toxic material is an important treatment for cerebral ischemic diseases in Chinese medicine (Hu et al., 2010). Rabdosia Labiatae for dry Herba Rabdosiae rubescentis on the part, was bitter, sweet, slightly cold, Activating blood circulation and Clearing away heat and toxic material effect, promoting blood circulation and relieving pain. Modern pharmacological research shows that Pubescent Holly Root with the protective effect of brain tissue can clear free radicals, inhibit inflammatory reaction, reduce the area of cerebral infarction, and reduce the brain damage.
The cerebral vascular anatomy and function of SD rats are similar to human. Cheap and easy to get, strong anti infection ability and so on. SD male rats were used as experimental animals in the preparation of cerebral ischemia reperfusion model Kong and Zhang, 2013). Currently, the commonly used thread embolism method was used in the experiment made bureau foci of cerebral ischemia reperfusion model can not only simulate different states of human permanent and transient Bureau focal cerebral ischemia, but also can accurately control ischemia and reperfusion time. In preclinical studies, (Fishcr et al., 2009) the evaluation of the animal model using the neural behavior score is an important basis for judging the efficacy of the drug, and is also one of the most important indicators of the observation of drug research (Luo et al., 2016, Peng et al., 2012). According to longa's score standard, the mark of success model for the surgery, the rats after anesthesia are sober left hemiparesis, standing instability, tail, circling to the side.
In the study by Wang et al. (2015) ischemic brain injury is a complex pathological cascade process, which is very easy to cause the disorder of energy metabolism (Huang et al., 2015, Pendlcbury et al., 2009). Cerebral ischemia and reperfusion appear microcirculation, inadequate supply of oxygen and glucose, ATP is rapidly depleted, mitochondrial ATP synthase failure, resulting in ATP dependent enzymes (including Ca2+ATP enzyme and Na + -K+-ATPase enzyme activity decreased, resulting in a massive outflow of intracellular K+, Na+, Cl- and Ca2+ large inflows of cells, resulting in intracellular and extracellular ion imbalance. Brain tissue cells are very sensitive to oxidative stress, and oxidative stress injury after ischemia–reperfusion is the key link of tissue and cell damage (Peng et al., 2012). Superoxide dismutase (SOD) is by getting rid of ischemia and reperfusion (I/R) injury of the brain is done a lot of superoxide anion free radicals, thereby protecting cells and tissue free oxidation stress. Activity in the organization, is often used as scavenging oxygen free radicals of the main indicators; MDA is inside the body, a product of lipid peroxidation, and malondialdehyde (MDA) content can reflect the level of oxygen free radicals in the generation and indirectly reflect the damage degree of the cell membrane.
Cerebral ischemic injury causes a series of cascade reactions, and the inflammatory response is one of the important mechanisms of ischemia reperfusion injury (Ahmad et al., 2014). At the early stage of ischemia, a large number of inflammatory cells are activated, releasing of cytokines, chemotactic factor and matrix metalloproteinase inflammatory mediators, thereby promoting neutrophil cells migrate to the brain ischemic area, ultimately increasing the death of neurons and the whole brain injury (Tao et al., 2016, Amantea et al., 2015). Cerebral ischemia reperfusion by triggering TNF-a, IL-1, two key cytokine releases, triggering a series of leukocyte infiltration and inflammatory cytokine expression of inflammatory reaction activation is caused by an important pathological mechanism of brain injury (Chen et al., 2016, Liu et al., 2016, Khaliq et al., 2016, Zwagerman et al., 2010). NO has the function of neurotransmitter or neuromodulator. The excessive production of NO after cerebral ischemia reperfusion can activate guanine nucleotide cyclase, which further result in DNA damage and mediated neurotoxicity.
ICAM-1 in cerebral ischemia reperfusion injury through the white blood cells and endothelial cell adhesion leads to vascular blockage, and no reflow phenomenon occurred, aggravated ischemic brain damage (Zhao and Ashraf, 2016, Xie et al., 2016, Sun et al., 2012). S100-beta protein is considered to be a marker of glial cells, which can promote neuronal differentiation, axon growth, glial proliferation and the stability of intracellular calcium (Feng et al., 2016, Kaca-Orynska et al., 2010). When ischemia and hypoxia and other factors cause the blood brain barrier damage, S100-beta can damage the blood brain barrier to enter the blood circulation. Therefore, the serum levels of S100-protein can be a reference to determine the degree of brain damage and prognosis.
Acute cell necrosis and apoptosis in brain tissue induced by cerebral ischemia reperfusion is the direct cause of acute cerebral ischemic injury. Rain hippocampus, cortical area is the most vulnerable part of the brain ischemic animals. Brain glial cells especially astrocytes were activated by proliferation, synthesis and secretion of neurotrophic factors, maintaining cell and sediment transport and acid-base balance and metabolism of neurotransmitters, on neuronal damage repair has an important role when cerebral ischemia occurred.
It is a new idea to treat focal cerebral ischemia by activating blood circulation and clearing away heat and toxic material drugs, which can reduce the inflammatory damage, the secondary reaction of cerebral ischemia, induce ischemic tolerance, and finally protect the brain tissue. Rabdosia rubescens has anti-inflammatory and detumescence, sterilization, vascularization, pharmacological activity of anticancer.
5. Conclusions
As the experimental results suggest, compared with the model mice, Rabdosia rubescens total flavonoids can significantly relieve the injury of brain in hippocampus and cortex nerve cells; experimental rat focal cerebral ischemia was to improve again perfusion model of nerve function defect score mortality; significantly reduce brain homogenate NOS activity and no content, MDA, IL-1, TNF-a, ICAM-1 content; increase in brain homogenate SOD and ATPase activity (P < 0.05, P < 0.01); reduce the serum S-100β protein content. Total flavonoids of the herb can inhibit the inflammatory response after ischemia, and improve the inflammatory response of a series of cascade reaction media and cytokines, which play a protective role in cerebral ischemia reperfusion injury. This study gives a better basis of pharmacological effects of Rabdosia rubescens treatment Bureau focal cerebral ischemia resistant disease, and offers the guidance for the deep research of clinical heat clearing, detoxifying, promoting blood circulation and removing blood stasis drugs for treatment of cerebral ischemia reperfusion injury, meanwhile providing experimental basis for the development of future rabdosia herb and product.
Acknowledgments
The authors acknowledge Fund project: Excellent science and technology innovation team in Henan Province (Grant No. TCJ2014-391); Natural Science Foundation of Henan Province (Grant No. 132300410019); Zhengzhou science and technology innovation team (131PCXTD612).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad M., Dar N.J., Bhat Z.S. Inflammation in ischemic stroke: mechanisms, consequences and possible drug targets. CNS Neurol. Dis. Drug Targets. 2014;13:1378–1396. doi: 10.2174/1871527313666141023094720. [DOI] [PubMed] [Google Scholar]
- Amantea D., Micieli G., Tassorelli C. Rational modulation of the in-nate immune system for neuroprotection in ischemic stroke. Front. Neurosci. 2015;9:147. doi: 10.3389/fnins.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Gao Y., Ashraf M.A., Gao W. Effects of the traditional Chinese medicine Dilong on airway remodeling in rats with OVA-induced-asthma. Open Life Sci. 2016;11(1):498–505. [Google Scholar]
- Feng B., Ashraf M.A., Peng L. Characterization of particle shape, zeta potential, loading efficiency and outdoor stability for chitosan-ricinoleic acid loaded with rotenone. Open Life Sci. 2016;11(1):380–386. [Google Scholar]
- Fishcr M., Fcucrstcin U., Howclls D.W. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Hu Y., Huang X. Research progress of effective components of Chinese herbal medicine on cerebral ischemia reperfusion injury. J. Pract. Cardiovasc. Cerebrovasc. Dis. 2010;1:92–95. [Google Scholar]
- Huang X., Deng L., Lu G., He C., Wu P., Xie Z., Ashraf M.A. Research on the treatment of Pseudomonas aeruginosa pneumonia in children by macrolide antibiotics. Open Med. 2015;2015(10):479–482. doi: 10.1515/med-2015-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftakhar A., Hasan I.J., Sarfraz M., Jafri L., Ashraf M.A. Nephroprotective effect of the leaves of Aloe barbadensis (Aloe Vera) against toxicity induced by diclofenac sodium in albino rabbits. West Indian Med. J. 2015;64(5):462–467. doi: 10.7727/wimj.2016.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Li J., Chen Y.S., Yang B. Study on the protective effect of seven pieces of ice on focal cerebral ischemia reperfusion injury in rats. Chinese Foreign Med. Res. 2016;1:148–149. [Google Scholar]
- Jia Q., Liu L.P., Wang Y.J. Stroke in China. J. Clin. Exp. Pharmacol. Physiol. 2010;37:259–264. doi: 10.1111/j.1440-1681.2009.05290.x. [DOI] [PubMed] [Google Scholar]
- Kong C.Z., Zhang Z. Bcl-2 overexpression inhibits generation of intracellular reactive oxygen species and blocksadriamycin-induced apoptosis in bladder cancer cells. Asian Pac. J. Cancer Prev. 2013;2:895–901. doi: 10.7314/apjcp.2013.14.2.895. [DOI] [PubMed] [Google Scholar]
- Kaca-Orynska M., Tomasiuk R., Friedman A. Neuron-specific enolase and S 100B protein as predictors of outcome in ischaemic stroke. Neurol. Neurochir. Pol. 2010;44:459–463. doi: 10.1016/s0028-3843(14)60136-5. [DOI] [PubMed] [Google Scholar]
- Khaliq T., Sarfraz M., Ashraf M.A. Recent progress for the utilization of curcuma longa, Piper nigrum and Phoenix dactylifera seeds against type 2 diabetes. West Indian Med. J. 2016;64(5):527–532. doi: 10.7727/wimj.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.K., Gao P., Ashraf M.A., Wen J.B. The complete mitochondrial genomes of two weevils, Eucryptorrhynchus chinensis and E. brandti: conserved genome arrangement in Curculionidae and deficiency of tRNA-Ile gene. Open. Life Sci. 2016;11(1):458–469. [Google Scholar]
- Luo J., Wang X., Yang Y., Lan T., Ashraf M.A., Mao Q. Successful treatment of cerebral aspergillosis in a patient with acquired immune deficiency syndrome. West Indian Med. J. 2016;64(5):540–542. doi: 10.7727/wimj.2016.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa E.Z., Weinstein P.R., Carlson S. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Peng B., Guo Q.L., He Z.J. Remote ischemic postconditioning protects the brain from global cerebral ischemia/reperfusion injury by upregulating endothelial nitric oxide synthase through the PI3K/Akt pathway. Brain Res. 2012;26:92–102. doi: 10.1016/j.brainres.2012.01.033. [DOI] [PubMed] [Google Scholar]
- Pendlcbury S.T., Rothwell P.M., Prevalence Incidence and factors associated with pre-stroke and post -stroke dementia, a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Sarfraz M., Ashraf Y., Sajid S., Ashraf M.A. Testosterone level in testicular cancer patients after chemotherapy. West Indian Med. J. 2016;64(5):487–494. doi: 10.7727/wimj.2016.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Ch., Chen Y.M., Feng H.L. Effects of insulin on NSE and behavioral changes in rats with acute global cerebral ischemia and reperfusion. Med. Res. Ed. 2012;29:5–9. [Google Scholar]
- Tao X., Ashraf M.A., Zhao Y. Paired observation on light-cured composite resin and nano-composite resin in dental caries repair. Pak. J. Pharm. Sci. 2016;29(6):2169–2172. [PubMed] [Google Scholar]
- Wang CC., Liu R., Yuan SH., Liu QX., Chen JB. CXCLl2 in the early stage of transient focal cerebral ischemia in rats the role. J. Stroke Neurol. Dis. 2015;12:1095–1098. [Google Scholar]
- Xie H., Huang H., He W., Fu Z., Luo C., Ashraf M.A. Research on in vitro release of Isoniazid (INH) super paramagnetic microspheres in different magnetic fields. Pak. J. Pharm. Sci. 2016;29(6):2207–2212. [PubMed] [Google Scholar]
- Zhao L., Ashraf M.A. Influence of Ag/HA nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med. J. 2016;64(5):506–513. doi: 10.7727/wimj.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwagerman N., Plumlee C., Guthikonda M. Toll-like receptor-4 and cytokine cascade in stroke after exercise. Neurol. Res. 2010;32:123–126. doi: 10.1179/016164109X12464612122812. [DOI] [PubMed] [Google Scholar]