Abstract
Complement dysregulation underlies several inflammatory disorders, and terminal complement inhibition has thus far afforded significant clinical gains. Nonetheless, emerging pathologies, fueled by complement imbalance and therapy-skewing genetic variance, underscore the need for more comprehensive, disease-tailored interventions. Modulation at the level of C3, a multifaceted orchestrator of the complement cascade, opens up prospects for broader therapeutic efficacy by targeting multiple pathogenic pathways modulated by C3-triggered proinflammatory crosstalk. Notably, C3 intervention is emerging as a viable therapeutic strategy for renal disorders with predominantly complement-driven etiology, such as C3 glomerulopathy (C3G). Using C3G as a paradigm, we argue that concerns about the feasibility of long-term C3 intervention need to be placed into perspective and weighed against actual therapeutic outcomes in prospective clinical trials.
Keywords: C3 inhibitors, compstatin, anti-C5 therapy, clinical efficacy, C3 glomerulopathy, AMY-101
“Do not render judgment before you hear both sides”
Hesiod, in Hesiodus Fragmenta, (c. 700 BC)
1. Complement: a Gatekeeper of Innate Immunity in a Tight Balancing Act
In an era that has propelled the systems-wide investigation of immunological processes, complement biology has witnessed a series of paradigm-shifting discoveries that have profoundly reshaped our understanding of this evolutionarily conserved innate immune system [1,2]. Perceived for many years as a linchpin of humoral innate immunity against invading pathogens, complement is now increasingly recognized as a multi-tasking protein network that engages in intricate crosstalk with diverse immunoregulatory pathways that affect not only homeostatic but also disease-promoting processes [1,3]. This versatile innate immune sensor contributes to tissue immunosurveillance and homeostasis by essentially transducing pathogen- or danger-associated molecular patterns into rapid effector responses that can neutralize infectious agents or remove potentially autoreactive cell debris. Moreover, through its extensive crosstalk with other pattern recognition systems (e.g., Toll-like receptors [TLRs]), complement modulates a variety of processes ranging from host-microbiome symbiosis to key fate-determining pathways of adaptive immunity (e.g., T helper-cell polarization and effector function) [2,3].
While complement proteins are effectually deployed under steady-state conditions as rapid responders to microbial insults or stress signals, this host protective response can also go overboard when regulatory control is lost, causing detrimental consequences by perpetuating a vicious cycle of tissue injury and thromboinflammation [4,5]. The deleterious effects of excessive complement activation can be manifested both in the fluid phase (through deregulated release of proinflammatory/chemotactic mediators that instruct immune cells to extravasate into tissues) and on opsonized surfaces, where the lack of proper regulation causes aberrant C3 deposition through the alternative pathway (AP) amplification loop (Box 1: Overview of the complement cascade). Factors that contribute to this imbalance include genetic perturbations affecting complement regulatory checkpoints (e.g., genomic rearrangements and deletions, common/rare genetic polymorphisms) [6], loss- or gain-of-function mutations in complement proteins that alter complement control [7], and autoimmune drivers (i.e., autoantibodies) [4,8].
Box 1. Overview of the Complement Activation Cascade.
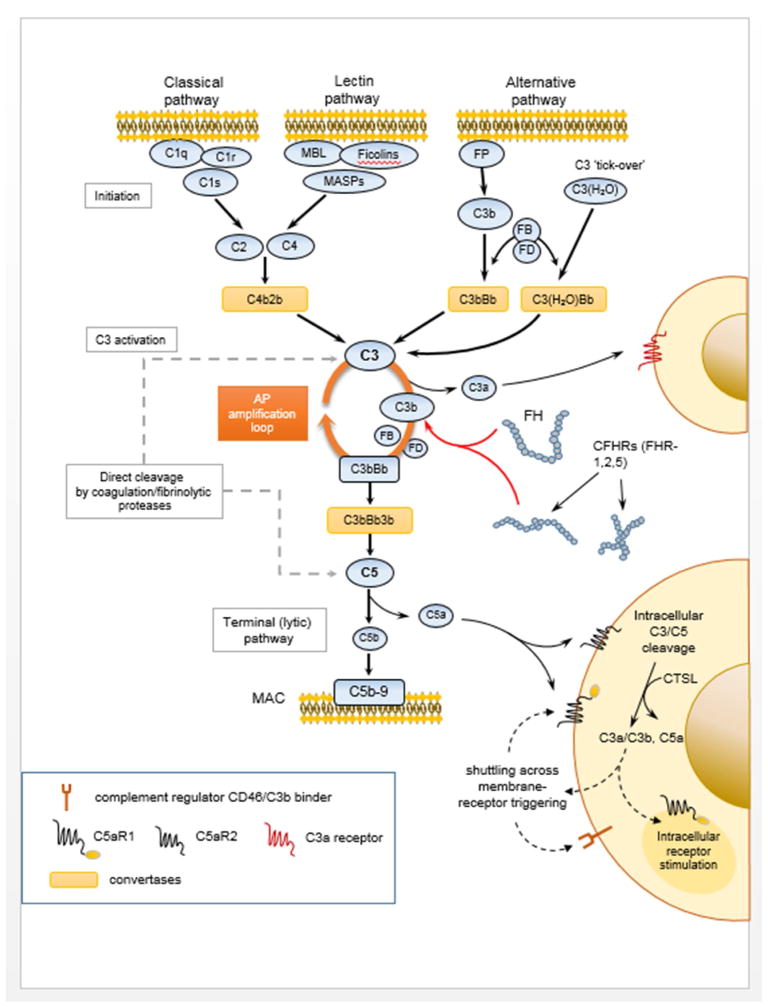
Triggered by multiple molecular cues and routes of activation, complement rapidly amplifies its effector responses through the convergence of its initiating signals on its core component, the complement protein C3 (16). Activation of C3 proceeds through a series of elaborate conformational rearrangements (48) to yield bioactive fragments that essentially drive the opsonization of target cells and amplification of the complement response on target surfaces and also modulate the chemotactic recruitment, attachment, and immune activation of effector cells (4). The complement cascade has been traditionally described as being triggered by three canonical pathways (classical, alternative, lectin) that all lead to the assembly of C3- and C5-processing enzymes termed “convertases” (Figure I). These multi-protein complexes are responsible for the proteolytic activation of the central components C3 and C5 and the release of their respective bioactive fragments, C3a and C3b, and C5a and C5b. Amplification of C3b deposition via the alternative pathway (AP) is a major pathogenic driver underlying several diseases associated with complement dysregulation (e.g., C3 glomerulopathy). AP amplification can occur regardless of the initiating route of activation and may lead to high opsonic turnover and downstream proinflammatory signaling though the interaction of C3 fragments (C3b, iC3b, C3dg), also known as opsonins, with receptor-bearing immune cells. In fact, AP-mediated amplification of complement responses has been shown to account for more than 80% of the downstream activation that is elicited following the initial triggering of the CP or LP in certain pathophysiological settings (49). C5b forms the initial component of the terminal (lytic) pathway that culminates in the assembly of the cell-perforating membrane attack complex (MAC, C5b-9). In addition to its canonical pathways of activation, accumulating evidence indicates that complement can mobilize a broader base of triggering cues, deploying a wide spectrum of proteases that efficiently cleave its central components, C3 and C5, into bioactive fragments (50,51). This “bypass activation” pathway, exemplified by proteolytic enzymes of the coagulation and fibrinolytic systems, adds another level of complexity to the landscape of complement-mediated interactions and underscores the tight linkage between these danger-triggered blood-borne defense systems. Such protease-dependent pathways may operate both in the extracellular space and within the cell interior (e.g., B and T cells), thereby defining a compartmentalized complement activation circuitry that elicits immunomodulatory responses pertinent to lineage commitment and effector differentiation (e.g., cathepsin L-mediated activation of intracellular C3 and C5) (17,19).
Figure I. An Overview of the Complement Activation Cascade.
A schematic representation of the wide spectrum of protein-protein interactions, multi-protein assemblies and effector functions mediated by complement components in the vasculature, on the surface, or in the inner compartments of targeted cells. Key interactions are discussed in greater detail in the text. Abbreviations: MBL, mannose-binding lectin; MASPs, mannose-binding lectin associated proteases; FP, properdin; FB, factor B; FD, factor D; AP, alternative pathway; FH, factor H; CFHRs, complement factor H-related proteins; MAC, membrane attack complex; C5aR1, C5a receptor 1; C5aR2, C5a receptor 2; CTSL, cathepsin-L.
The expanding landscape of complement-mediated diseases continues to fuel recent efforts to develop targeted complement inhibitors with clinical potential [9–11]. Thus far, clinical experience has been gained with the sole complement-specific drug, eculizumab (Soliris, Alexion Pharmaceuticals), a therapeutic antibody that targets C5 activation, blocking the generation of downstream effectors (C5a, the membrane attack complex (MAC)) [12]. C5 blockade has not only provided an effective therapeutic handle for treating diseases with complement-mediated pathophysiology but has also revealed new pathogenic mechanisms that remain unaddressed by this clinically available anti-complement therapy (e.g., C3-mediated extravascular lysis of erythrocytes in patients suffering from paroxysmal nocturnal hemoglobinuria [PNH], a form of acquired hemolytic anemia) [13,14]. The strategic positioning of C3 at the intersection of all complement activation pathways and its pivotal role in coordinating crosstalk with multiple immune and inflammatory networks have galvanized efforts to develop C3-based therapies [15]. Accumulating evidence suggests that C3-targeted intervention may hold clinical promise as a viable therapeutic entity alongside anti-C5 therapeutics.
2. Revisiting Complement-Targeted Therapy: Paradigm Shifts, Opportunities, and Challenges
The sheer diversity of protein-protein interactions mediated by the various components of this system and the broad spectrum of targets selected by its main effectors make complement both an attractive and also a challenging target for therapeutic manipulation [16] (see also Box 1). The tissue-destructive side of complement dysregulation has accentuated research efforts to develop potent and highly selective complement-targeted inhibitors that can tame its proinflammatory effects in human disease. An unprecedented surge of creative approaches has yielded promising complement inhibitors in various stages of clinical development, ranging from small-sized inhibitors (e.g., synthetic compounds, peptidic inhibitors, and RNAi-based therapeutics) to larger biologics (fusion proteins and antibodies) [9–11].
What has become increasingly appreciated, however, is that no single component can serve as a universal target for therapeutic intervention in every single complement-driven clinical indication. Nor can a single complement inhibitor cater for every pathology, especially as it becomes clearer that disease pathogenesis is underpinned by diverse mechanisms of complement dysregulation, contributing at different levels and to variable extents in each clinical condition [11] (see Box 2: Selection of the optimal complement-targeting strategy is dictated by disease pathophysiology). The distinct pathological drivers of each disease state and the relative penetrance of each complement pathway in the clinical phenotype both dictate, to a great extent, the mode of complement–targeted intervention that should be selected to afford the optimal therapeutic outcome.
Box 2. Selection of the Optimal Complement-Targeting Strategy is Dictated by Disease Pathophysiology.
The complement cascade offers multiple points for therapeutic intervention, at various steps and regulatory checkpoints along the cascade, and in a disease-specific context (52). Targeting approaches aimed at the initiation stage, the central hub of the system C3, or at downstream effectors (C5a, MAC), all have their merits and limitations (52). Upstream complement intervention at the initial level of C1s inhibition (e.g., using anti-C1s mAbs) would be more relevant to conditions driven by immune complexes and pronounced classical pathway activation (e.g., autoimmune-driven hemolytic anemias) (53). Likewise, therapeutic targeting of MASPs, key serine proteases that participate in the activation of the lectin pathway, might be more appropriate in conditions that are driven by high lectin pathway turnover (54). As opposed to upstream complement modulation, therapeutic targeting of the central protein, C3, is anticipated to afford a broader and more comprehensive coverage. C3-targeted intervention abrogates opsonization and amplification of complement responses on opsonized surfaces, regardless of the initial route of activation. It also blocks the generation of opsonic fragments that can drive immune cell activation and extravascular cell destruction. By virtue of its ability to block the convertase-mediated processing of C5, C3 targeting can also attenuate the activation of downstream inflammatory and cytolytic responses propagated by effectors of the terminal complement pathway (C5a, MAC). From a different standpoint, therapeutic targeting of the terminal pathway, either by C5 blockade or inhibition of C5a-triggered activation of effector responses, represents a viable approach for containing clinical manifestations that are largely driven by terminal pathway effectors (such as C5a and the MAC). Although C5aR1-mediated signaling has been identified as a main culprit in many inflammatory pathologies, a cautionary note should be appended to strategies targeting C5a receptors (C5aR1/CD88 and C5aR2/C5L2) through Ab-mediated ligand (i.e., C5a) neutralization, since these receptors appear to trigger divergent or even opposing cellular responses in a strictly context-dependent fashion (55,56). Consequently, their concomitant targeting requires a thorough assessment of their distinct involvement in disease pathogenesis.
This long-held debate revolving around the “holy grail” of therapeutic complement modulation (i.e., the optimal target) has seen a fresh twist in recent findings describing a fully operable intracellular complement activation pathway within mammalian cells [17,18] (see also Box 1). Elegant studies have revealed the homeostatic operation of an intracellular complement circuitry in lymphoid and non-lymphoid cells that can be triggered by both external and internal cues under certain pathophysiological circumstances (e.g., proteolytic enzymes that cleave intracellular C3 and C5 to release bioactive fragments with diverse immunomodulatory activities) [19]. The implications of this emerging paradigm shift, that complement-mediated immune surveillance and regulation of adaptive responses may rely on the shuttling of activated complement fragments between extra- and intracellular compartments, have only recently begun to be revealed, and they may have important consequences for complement therapeutic design.
Although these studies are still in their infancy, they have introduced intriguing prospects and charted new conceptual avenues for the design of comprehensive complement inhibitory strategies. Since intracellular C3 stores define a separate entity with broad immunological properties, it would be interesting to speculate that chronic inhibition of plasma C3 would suffice to block the inflammatory sequelae of chronic C3 dysregulation in the vasculature, while sparing the immunomodulatory functions of intracellular C3 in various immune effector cells (e.g., APCs and lymphocytes). The presence of a functionally diverse intracellular arsenal of complement proteins argues for a potential disconnect between plasma C3 (mainly derived from liver cells) and the presence of intracellular or locally expressed C3 in extra-hepatic tissues (lymphoid or myeloid). This compartmentalization of C3’s function and its tissue-specific pattern of expression could reflect an evolutionary safeguarding of crucial immunological functions of C3, exerting compensatory feedback in settings of genetic deficiency or pharmacologic inhibition of C3.
3. C3-Targeted Intervention: Making Headway with New Clinical Indications
Because it is strategically positioned at the intersection of all the complement activation pathways, C3 serves as an ideal candidate for comprehensive complement modulation [16]. Inhibition of C3 activation essentially abrogates the formation of the C3 and C5 convertases, prevents the amplification of complement deposition on target surfaces via the AP, and attenuates the generation of downstream proinflammatory effectors [20]. Although C3 poses certain challenges as a druggable target because it displays extended surfaces for protein-protein interactions, a rapid turnover rate and a high plasma concentration, recent drug design and development efforts have effectively circumvented these challenges [21]. Indeed, C3-targeted intervention is gaining considerable traction in the pipeline of pharmaceutical companies, with the first C3 inhibitors already advancing through clinical development for various indications [11,21]. A significant milestone has been the structure-guided development of highly potent, next-generation peptidic C3 inhibitors of the compstatin family [21]. These therapeutic agents show saturable, high-affinity binding to plasma C3 and afford sustainable complement inhibition in primate models with favorable pharmacokinetic behavior after subcutaneous (SQ) administration, marked by an extended half-life that is largely attributable to a target-driven, prolonged plasma elimination [22].
Surface-directed C3 convertase inhibitors that fuse complement-modulatory domains of endogenous C3 regulators with unique targeting moieties that bind to opsonized host cells (e.g., CR2-FH/TT30, mini-FH) offer other viable alternatives to C5-targeted intervention that warrant investigation [23]. Notably, a series of preclinical studies targeting C3 in primate models has strongly attested to the clinical potential of C3 intervention for a number of inflammatory diseases with distinct complement-driven etiologies [21]. Moreover, robust evidence indicates that targeting C3, either systemically or directly on C3b-opsonized surfaces, may offer broader therapeutic coverage by intercepting pathogenetic mechanisms that elude anti-C5 therapy (e.g., extravascular hemolysis of C3b-opsonized erythrocytes) [24]. C3 therapeutics have shown promise in the treatment of rare complement-mediated hemolytic anemias (i.e., paroxysmal nocturnal hemoglobinuria [PNH]), malarial anemia, sepsis, and dysbiotic periodontal diseases and appear to be making headway as a new therapeutic option for C3 glomerulopathy, a rare renal disorder driven by chronic AP dysregulation (all these studies are extensively reviewed in [21,25,26]). These findings raise our awareness of a new complement-targeting approach that warrants clinical evaluation, since it may afford broader therapeutic benefit in patients, perhaps even surpassing the efficacy of C5-targeted therapy in certain indications.
4. C3 Glomerulopathy: Bridging the Gap between Pathophysiology and Therapy?
Pinning Down the “Culprit”
In many ways, the kidney represents an Achilles’ heel that bears the clinical burden of several complement-driven pathologies [4,27]. A wide spectrum of thrombotic microangiopathies (TMAs) (i.e., atypical hemolytic-uremic syndrome, aHUS), hemolytic anemias and other inflammatory disorders often culminate in acute or chronic renal injury [28]. The unique anatomical and functional blueprint of this organ partly explains its pronounced susceptibility to autologous complement-mediated damage (Figure 1). The distinct polyanionic surface signature of the capillary endothelium and glomerular basement membrane (GBM) (i.e., the glycomatrix) skews the binding of fluid-phase complement regulators. In conjunction with the high local concentration of complement proteins resulting from hemoconcentration, glomerular ultrafiltration, and the local biosynthesis of complement proteins by tubular epithelial cells, the fenestrated nature of the glomerular endothelial cells means that up to 40% of the glomerular surface area is constituted by GBM and its overlying glycocalyx, devoid of cell-membrane bound regulators of complement and creating a polyanionic microenvironment that contributes to the pronounced complement deposition and inflammatory damage inflicted on the kidney in settings that subvert complement AP regulation [29,30] (Figure 1).
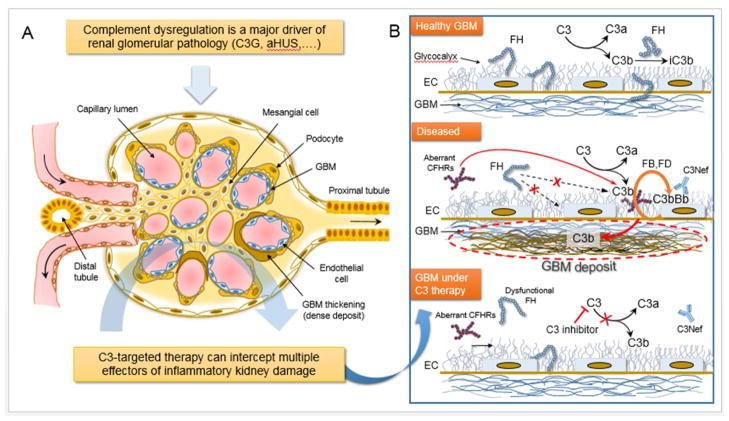
Figure 1. C3-targeted Intervention Intercepts Multiple Proinflammatory Effectors of Renal Pathology.
Panel A: Through its dense network of capillaries, the glomerulus serves as the basic blood filtration unit of the kidney. Therefore proper renal function relies largely on the structural and functional integrity of this specialized ‘unit’. Mesangial cells form the so-called mesangium, a stalk-like structure of mesenchymal origin that serves as a support for the glomerular vasculature and also contributes to the blood filtration process, thereby aiding urine formation (panel A). Podocytes are specialized perivascular epithelial cells extending long processes (foot processes, or pedicels) that wrap around glomerular capillaries and contribute to blood ultrafiltration by creating appropriate filtration slits (panel A). Capillary endothelial cells, podocytes and the glomerular basement membrane (GBM) altogether form the glomerular filtration barrier. (Panel B): A derailed complement response can target multiple anatomical sites and structures within the kidney glomerulus, such as the glomerular basement membrane (GBM), the mesangium, and the capillary endothelial cell wall. Excessive complement activation contributes to renal pathology in several clinical indications, including microangiopathies (atypical hemolytic uremic syndrome [aHUS]) and C3 glomerulopathy (C3G). These clinical disorders, although manifested in distinct anatomical compartments of the kidney, share a common profile of complement-specific genetic perturbations and serum biomarkers. Most of these genetic or acquired alterations affect the functionality of complement regulators that normally control autologous complement responses, protecting host surfaces such as the GBM from inflammatory damage (right panel, top schematic). In C3G, complement dysregulation results in autologous complement attack on the GBM and is tightly linked to the formation of electron dense deposits and impaired renal function (left panel). This deregulated response can be driven by genetic factors or autoantibodies (i.e., C3 nephritic factors, C3Nefs) that stabilize the enzymatic complex that cleaves C3 into its bioactive fragments C3a and C3b (panel B). Factor H is a fluid-phase regulator of the alternative pathway (AP) that circulates in the plasma and can attach to host surfaces through high-affinity binding to carbohydrate moieties (e.g., GAGs) (right panel, top schematic). Genetic variations in FH leading to partial or complete loss of its ability to regulate C3 convertase in the fluid phase and on host surfaces have been linked to C3G. Impaired FH function can lead to uncontrolled C3 activation and persistent C3b deposition in the GBM via AP amplification (right panel, middle). Recent studies have shown that targeted C3 inhibition can restore complement regulation in C3G, attenuating multiple drivers of inflammatory damage (right panel, bottom schematic). C3-based inhibitors offer broad control over the detrimental consequences of complement activation in the kidney by simultaneously blocking the generation of multiple effectors (e.g., C3a, C5a, MAC). Abbreviations: iC3b, inactive C3; FH, factor H; CFHRs, complement FH-related proteins; EC, glomerular endothelial cell; GBM, glomerular basement membrane; C3Nef, C3 nephritic factor.
C3 glomerulopathy (C3G) is a rare renal disorder that is primarily driven by dysregulated complement AP activation and illustrates an emerging paradigm reflecting the clinical potential of C3 intervention [29,31]. C3G encompasses a spectrum of pathologies characterized by predominant C3 deposition in the glomeruli, in the absence or scarce presence of deposited immunoglobulins [32]. Despite being heterogeneous in its presentation, C3G is identified by common pathophysiological attributes that are shared by its distinct subgroups. Histopathological classification of this disease, aided by electron microscopy, has led to the demarcation of two pathological entities: dense deposit disease (DDD), which is characterized by the presence of electron-dense deposits within the glomerular basement membrane (GBM), and C3 glomerulonephritis (C3GN), which is characterized by lighter discontinuous deposits, usually within the mesangial space [32,33]. The clinical prospects of C3G are daunting, since half of all patients progress to end-stage renal failure (ESRD) within 10 years of diagnosis. Of note, even after kidney transplantation, disease relapse occurs in more than 50% of transplant recipients, leading to allograft failure [34].
Genetic or acquired dysregulation of the alternative pathway (AP) of complement has been recognized as a key pathogenic driver in C3G [31,32,35] (Figure 1). Indeed, this disease has a distinct genetic signature, affecting key components of AP activation and regulation (e.g., C3, Factor H/FH, Factor I/FI, Factor B/FB) [31,36,37]. An illustrative example of genetic predisposition to C3G is a C3 gene mutation identified in a Spanish pedigree with a DDD phenotype [7]. A heterozygous two amino acid deletion results in the production of a mutated C3 protein that cannot be cleaved by the AP C3 convertase, thus resulting in fluid-phase restricted AP dysregulation and almost complete consumption of the normal C3 allele from plasma [7]. Most of the C3G-related genetic or acquired aberrations affect the structure and/or function of fluid-phase AP regulators, essentially disturbing the regulatory balance in the glomerular microenvironment, thereby allowing autologous complement attack and glomerular C3 deposition to proceed unhindered [36]. Importantly, pathogenic drivers of acquired complement dysregulation in C3G include autoantibodies that bind to and stabilize the AP C3 convertase, thus extending its half-life and activity. These autoantibodies, termed C3 nephritic factors (C3Nefs), can act upon both fluid-phase and cell-bound C3 convertases, increasing C3b deposition on glomerular surfaces and leading to plasma C3 or C5 comsumption or even terminal pathway activation [31,36] (see Box 3: Mechanistic aspects of AP dysregulation in C3 glomerulopathy).
Box 3. Mechanistic Aspects of AP Dysregulation in C3 Glomerulopathy.
AP dysregulation in C3G occurs predominantly in the circulation but also on the surface of glomerular cells and the GBM (31,32) (Figure 1). A unique structural feature that partially explains the pronounced susceptibility of the GBM to autologous complement attack is its inherent lack of membrane-associated complement regulators (57). Therefore, complement regulation over glomerular endothelial pores relies on the recruitment of fluid-phase AP regulators such as FH, FHL-1, and on the FH-modulatory activity of complement FH–related proteins (CFHRs) (29). Binding of these proteins to the glyocalyx overlying the GBM is mediated by polyanionic surfaces comprising carbohydrate moieties such as glycosaminoglycans (GAGs) (e.g., heparan sulfate). In this glomerular microenvironment, it has been proposed that distinct surface carbohydrate “signatures” impact binding and become altered under inflammatory stress (e.g., post-infection), impacting the recruitment of distinct C3 regulators or combinations thereof, in such a way as to alter control of complement activity. The currently accepted model of C3G pathogenesis has been enriched with findings pointing to a previously elusive role for the CFHR gene cluster (40). Genomic aberrations within the CFHR locus have been described in C3G patients that yield CFHR fusion proteins (hybrid CFHRs) and aberrant oligomeric complexes, which deregulate AP C3 convertase activity via multiple mechanisms (58). These mechanisms appear to include stabilization of the C3 convertase or direct competition of these oligomeric, hybrid CFHRs with FH for binding to C3b, most likely through high-avidity interactions (40,59,60) (Figure 1).
A pathological role in C3G has also been ascribed to key autoimmune drivers. Autoantibodies known as C3 nephritic factors (C3Nefs) bind to fluid-phase and cell-bound AP C3 convertases and hinder their FH-mediated decay (29) (Figure 1). As a result, C3Nefs stabilize C3 convertases and prolong their half-life, thereby leading to chronic, fluid-phase AP dysregulation (31). In addition to C3Nefs, other autoimmune factors have been linked to C3G (e.g., autoantibodies against FB or FH) (28,29). These drivers of C3G pathology all target AP-mediated C3 activation and regulation, strongly attesting to the key involvement of dysregulated C3 turnover in the disease process.
Key to elucidating the pathogenic role of complement dysregulation in C3G was the establishment of the FH-deficient mouse (Cfh−/− mouse mutant) [66]. This strain presents with a C3GN-like pattern of renal histology, C3 deposition in the glomeruli, and consumption of plasma C3 due to deregulated AP activation in the fluid phase. To date, the Cfh−/− mouse strain remains the primary model for C3G as it closely recapitulates the histological and pathophysiological traits of the human disease.
Solving the Conundrum of Treatment
Clinical management of C3G patients relies mostly on palliative measures, which include blood pressure control, immunosuppressive therapy, and, on occasion, plasma exchange [38]. Despite an appreciation that the terminal complement pathway is an exacerbating factor, anti-C5-therapy has not produced satisfactory results in the majority of C3G patients [39]. Although anti-C5 therapy blocks terminal pathway activation, blunting the generation of downstream effectors (C5a, MAC) that can contribute to renal inflammatory damage, it does not interfere with C3 activation or AP amplification, two crucial pathogenic drivers that fuel the disease process (Figure 1). This notion is accentuated by the failure of this anti-complement treatment to control proteinuria or prevent disease progression and relapse after kidney transplantation in over 50% of eculizumab-treated patients [40]. While the poor response of some patients to eculizumab has also been attributed to genetic variance or late therapeutic intervention at a stage when renal damage has become irreversible [30], it is reasonable to assume that C5-targeted inhibition might be therapeutically more relevant in cases in which C5 convertase dysregulation is more pronounced (i.e., marked by consumption of circulating C5, and elevated serum levels of C5a and soluble C5b-9).
Recent studies have provided interesting insights into the relative contribution of C3 and C5 convertase dysregulation in C3G. The correlation between high terminal pathway activity and low serum levels of the positive AP regulator properdin observed in some C3G patients implies that the consumption of this factor in the circulation and its likely increased binding to the injured glomeruli may favor the formation of C5 convertases, thus contributing to higher C5 turnover in a subset of C3G patients, typically those with a C3GN pattern of diffuse, mostly mesangial deposits on electron microscopy [41]. Whereas the efficacy of anti-C5 therapy in other renal pathologies such as aHUS has been consistently higher, the poor outcomes in C3G may also reflect the recruitment of distinctly different pathological mechanisms in these renal disorders. aHUS patients, for example, present with thrombotic manifestations (microthrombi formation) preferentially targeting the renal endothelium, while in C3G patients, the endothelial-pore exposed GBM is particularly vulnerable and susceptible to autologous C3 deposition. This discriminating difference may explain, in part, the inadequate response of C3G patients to eculizumab. Indeed, anti-C5 treatment attenuates proinflammatory and procoagulant reactions that are mainly driven by C5a/C5aR1, thereby containing thrombogenic responses, which are a cardinal feature of TMAs. Conversely, this therapeutic approach would not be expected to ameliorate pathology in C3G, a disorder in which AP dysregulation and aberrant C3 fragment deposition in the GBM or mesangial space often override terminal pathway activation. Not only do these clinical observations underscore the multiplicity of factors affecting complement dysregulation in C3G, but they also highlight the need for more comprehensive diagnostic algorithms for stratifying C3G patients.
The increasing awareness that C3G pathophysiology, irrespective of its distinct histopathological hallmarks, is underpinned by aberrant AP activation and amplification on the GBM, makes C3-targeted intervention a promising therapeutic modality for C3G. Considering that 60–80% of patients have autoantibodies that stabilize C3 convertases (e.g. C3Nefs) and that 20–40% of patients carry ultra-rare genetic alterations predicted to affect complement regulation (i.e., dysfunctional factor H (FH) or aberrant complement factor H-related protein (CFHR) hybrids or oligomers), it is reasonable to speculate that systemic C3 consumption resulting from deregulated C3 convertase activity and the reciprocal increase in C3b deposition in the glomeruli can be best tackled by agents targeting the C3 convertase or its substrate, C3 (Figure 1). Conversely, whereas the use of C5-inhibiting agents would be expected to prevent the generation of downstream proinflammatory mediators (C5a) and the assembly of the MAC, this approach does not address the underlying cause of disease (i.e., AP dysregulation), thereby allowing for uncontrolled AP activity and persistent opsonization of the glomeruli. It should be noted, however, that patients with C3GN tend to have a relatively greater degree of terminal pathway dysregulation than DDD patients [37]. This difference has been attributed to both genetic and acquired factors that together lead to excessive AP activity (e.g. C3Nef-stabilized C3 convertases) and densely spaced C3b deposition, which favors pronounced C5 convertase activity and downstream effector generation. The detection of elevated terminal complement components (C5a, soluble C5b-9) in the plasma of C3GN patients supports this observation [37], making it imperative to develop comprehensive biomarker panels and other molecular tools for stratifying DDD and C3GN patients for optimal therapeutic intervention (i.e., selection of C3-versus C5-targeted therapy).
The administration of sCR1 (CDX-1135, Celldex), a potent C3 regulator [42], provided proof-of-concept for the efficacy of C3-targeted therapy in C3G, restoring complement balance in a pediatric C3G patient during a 2-week therapeutic window. Further substantiating the clinical potential of C3 intervention, a next-generation C3-targeted peptidic inhibitor, Cp40, has been shown to reverse complement dysregulation in ex vivo models of C3G [43]. Cp40, a potent analog of the compstatin family, restored complement balance, intercepting both acquired and genetic factors associated with disease pathogenesis [43]. The Cp40-based therapeutic AMY-101 (Amyndas Pharmaceuticals) has displayed favorable pharmacokinetic behavior and sustained inhibitory potency in primate models and is currently being clinically developed for local or SQ dosing in various complement–mediated indications [21]. AMY-101 is the first complement-targeted drug to receive expedited regulatory approval for C3G and could point to a viable and affordable treatment for this disorder [22] (FDA orphan designation: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=494915, EMA orphan designation: http://www.ema.europa.eu/ema/index.jsp?curl=pages%2Fmedicines%2Flanding%2Forphan_search.jsp&mid=WC0b01ac058001d12b&searchkwByEnter=false&alreadyLoaded=true&isNewQuery=true&status=Positive&status=Negative&status=Withdrawn&status=Expired&keyword=C3+glomerulopathy&keywordSearch=Submit&searchType=Disease).
In the aggregate, these developments open up new prospects for testing in C3G patients a broad spectrum of C3 therapeutics that can modulate AP activity, both in the fluid phase and closer to the opsonized surface. The rational design of chimeric C3 regulators that direct C3 inhibitory modules to C3b-opsonized surfaces has expanded the therapeutic arsenal [23,44]. It is conceivable that these surface-directed approaches may offer a plausible strategy for increasing the efficacy of complement modulation in cases in which renal pathology is mainly driven by local complement imbalance on the GBM (e.g. in cases of aberrant CFHR binding to deposited C3b). The recent disclosure of new AP-inhibiting compounds that specifically target factor D, a key protease that contributes to the formation of active AP C3 convertases, provides a range of diversified approaches for tackling dysregulated C3 turnover in C3G and other AP-driven clinical disorders [45].
5. Balancing between Assertions, Risks and Clinical Evidence
Complement drug discovery has long been enlivened by a fertile debate addressing potential concerns about the safety of prolonged C3-based intervention. The emerging clinical potential of next-generation C3 therapeutics in chronic indications (e.g., C3G, PNH) has fueled discussions about potentially compromised antimicrobial surveillance and about autoimmune or IC-related complications resulting from prolonged, systemic C3 inhibition (extensively reviewed in [4,20,21]). However, clinical experience from patients with primary C3 deficiencies [46] has indicated that increased susceptibility to certain pyogenic infections tends to associate more with an early age and a less developed immune system [61, 62]. This observation likely indicates the recruitment of compensatory mechanisms that may largely sustain pathogen immune surveillance during adulthood, even in the absence of a circulating C3 pool [61]. Notably, C3 deficiency does not necessarily reflect a total absence of complement activity in terms of opsonophagocytosis (i.e., the C4b-tagging route remains intact), while pharmacologic C3-targeted intervention may be tuned to allow residual C3 activity for immune surveillance or swift recovery of plasma C3 activity in a clinical setting [21]. Lending further credence to this notion, animal studies have revealed that extrahepatic synthesis of C3 (e.g. in macrophages within lymphoid tissues) can sustain humoral immune responses to infections even in the absence of liver-derived complement [47, 63]. Interestingly, the genetic absence or pharmacologic inhibition of C3 in certain animal models can even attenuate the spread of infection, implying a more complex impact of complement on host-pathogen interactions [64,65]. Further lines of evidence supporting the safety and clinical feasibility of prolonged pharmacologic C3 intervention are extensively discussed in recent reviews [15,16, 21].
In light of these findings, we argue that the alleged negative impact of prolonged C3 intervention on pathogen surveillance and humoral immunity has been overemphasized. In view of the lack of definitive clinical data, this contention should be placed into a broader perspective that embraces emerging functions of complement within extravascular compartments. Undoubtedly, microbial prophylaxis against certain highly virulent, encapsulated bacteria (e.g. meningococci, pneumococci, H. influenzae) should be considered essential in a prolonged C3-inhibitory protocol, in line with the established vaccination scheme preceding anti-C5 therapy [26]. Overall, hypothetical assertions about compromised immunosurveillance during C3 intervention must be reconciled with actual data from future clinical trials.
6. Concluding Remarks and Outlook
Our expanding knowledge of complement’s involvement in human pathophysiology has spawned a new generation of C3-targeted therapeutics that are on the verge of clinical translation [9,11]. A thorough understanding of the underlying pathophysiology is imperative for designing tailored therapeutic interventions based on C3 modulation. Elucidating the mechanistic aspects of complement’s intricate involvement in immune regulation and its signaling crosstalk with key inflammatory pathways is a prerequisite for selecting the appropriate target, optimal therapeutic window, and dosing route in the clinical condition under question (see also Outstanding Questions). The increasing awareness that complement dysregulation can affect many facets of tissue immune surveillance, along with the appreciation that elicited responses may be dictated by complex genetic traits [8,14] will enable the development of disease-tailored therapeutics with high chances of successful clinical application. As C3-targeted therapeutics move ahead in clinical development, the time is ripe to reconcile dogmatic assertions and hypothetical discussions about safety and efficacy with evidence-based discovery and long-awaited clinical data.
“Outstanding questions” box.
Whereas several lines of evidence from animal studies and clinical cases of C3 deficiency argue that prolonged pharmacologic C3 intervention in adults should not fuel susceptibility to infections, autoimmune manifestations, or immune complex-driven inflammation, will this be corroborated in clinical trials evaluating the first C3-targeted complement inhibitors?
The pathology in certain C3G patients is consistent with more pronounced complement AP dysregulation locally in the GBM than in the circulation, partly because of rare FH variants or CFHR aberrations that preferentially affect complement regulation in the glomerular microenvironment. Will systemic C3 intervention confer sufficient clinical benefit on such patients?
Given the clinical heterogeneity and variable degree of complement dysregulation in different C3G subgroups (i.e., DDD and C3GN), is C3-targeted therapy expected to have a uniformly favorable therapeutic outcome in all patients? How predictive of this response to anti-C3 agents will the levels of C3Nefs or other autoantibodies be?
Patients with C3GN tend to be associated with a relatively greater degree of terminal complement dysregulation, as compared to C3 dysregulation at the C3 convertase level. To what extent will this phenotype affect the outcome of C3-targeted intervention?
Anti-C5 therapy should still be considered a viable treatment option for C3G patients with underlying predominant C5 convertase dysregulation. Can biomarkers reliably stratify C3G patients into “good” or “poor” responders to anti-C3 therapy vs anti-C5 therapy?
The recent discovery of an intracellular complement network in lymphoid and non-lymphoid cells argues for a compartmentalization of complement’s homeostatic function in the mammalian immune system, with broader physiological implications. What is the pathophysiological involvement of this intracellular C3 pool, and would pharmacologic C3 intervention and blockage of circulating C3 affect the basal activity and immunomodulatory functions of this intracellular system?
Trends.
Proteins of the complement cascade together form a key innate immune sensor that mediates immunosurveillance and tissue homeostasis through extensive crosstalk with other pattern recognition systems. However, deregulation of complement responses can fuel a vicious cycle of tissue injury and inflammation.
C5 blockade has proven effective for treating some complement-mediated diseases but also revealed new pathogenic mechanisms that remain unaddressed.
C3 glomerulopathy (C3G) is a rare renal disorder driven by aberrant complement activation and dysregulation of complement responses on the glomerular basement membrane (GBM).
Blockade of the central component C3 is emerging as a therapeutic modality for C3G. Assertions related to potential risks and complications during prolonged C3 intervention need to be replaced by evidence-based discovery and clinical data.
Acknowledgments
We thank Dr. Deborah McClellan for her excellent editorial assistance. The authors are supported by grants from the U.S. National Institutes of Health (AI003040, AI068730 to J.D.L.; DK110023 to R.J.S.), the National Science Foundation (No. 1423304 to D.R. and J.D.L.), and the European Community’s Seventh Framework Programme under grant agreement number 602699 (DIREKT to J.D.L.).
Footnotes
Conflicts of interest
J.D.L., D.R. and R.J.S. are the inventors of patents and/or patent applications that describe complement inhibitors and/or their use for therapeutic purposes. J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. The remaining authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ricklin D, et al. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolev M, et al. Complement - tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–20. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 3.Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nat Rev Immunol. 2011;11: 187–200. doi: 10.1038/nri2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricklin D, et al. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12: 383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ekdahl KN, et al. Thromboinflammation in Therapeutic Medicine. Adv Exp Med Biol. 2015;865: 3–17. doi: 10.1007/978-3-319-18603-0_1. [DOI] [PubMed] [Google Scholar]
- 6.Schramm EC, et al. Genetic variants in the complement system predisposing to age-related macular degeneration: a review. Mol Immunol. 2014;61: 118–125. doi: 10.1016/j.molimm.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Barricarte R, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120: 3702–3712. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris CL, et al. The complotype: dictating risk for inflammation and infection. Trends Immunol. 2012;33: 513–521. doi: 10.1016/j.it.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reis ES, et al. Applying complement therapeutics to rare diseases. Clin Immunol. 2015;161: 225–240. doi: 10.1016/j.clim.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan BP, Harris CL. Complement, a target for therapy in inflammatory and degenerative diseases. Nat Rev Drug Discov. 2015;14: 857–877. doi: 10.1038/nrd4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricklin D, Lambris JD. New milestones ahead in complement-targeted therapy. Semin Immunol. 2016;28: 208–222. doi: 10.1016/j.smim.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rother RP, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25: 1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 13.Risitano AM, et al. Complement fraction 3 binding on erythrocytes as additional mechanism of disease in paroxysmal nocturnal hemoglobinuria patients treated by eculizumab. Blood. 2009;113: 4094–4100. doi: 10.1182/blood-2008-11-189944. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura J, et al. Genetic variants in C5 and poor response to eculizumab. N Engl J Med. 2014;370: 632–639. doi: 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]
- 15.Mastellos DC, et al. From orphan drugs to adopted therapies: Advancing C3-targeted intervention to the clinical stage. Immunobiology. 2016;221: 1046–1057. doi: 10.1016/j.imbio.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ricklin D, et al. Complement component C3 - The “Swiss Army Knife” of innate immunity and host defense. Immunol Rev. 2016;274: 33–58. doi: 10.1111/imr.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liszewski MK, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39: 1143–1157. doi: 10.1016/j.immuni.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeley S, et al. The “ins and outs” of complement-driven immune responses. Immunol Rev. 2016;274: 16–32. doi: 10.1111/imr.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arbore G, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4(+) T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricklin D, Lambris JD. Therapeutic control of complement activation at the level of the central component C3. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mastellos DC, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. Eur J Clin Invest. 2015;45: 423–440. doi: 10.1111/eci.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu H, et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218: 496–505. doi: 10.1016/j.imbio.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt CQ, et al. Protection of host cells by complement regulators. Immunol Rev. 2016;274: 152–171. doi: 10.1111/imr.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risitano AM, et al. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014 doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hajishengallis G, et al. Complement inhibition in pre-clinical models of periodontitis and prospects for clinical application. Semin Immunol. 2016 doi: 10.1016/j.smim.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastellos DC, et al. Complement in paroxysmal nocturnal hemoglobinuria: exploiting our current knowledge to improve the treatment landscape. Expert Rev Hematol. 2014;7: 583–598. doi: 10.1586/17474086.2014.953926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sacks S, Zhou W. New boundaries for complement in renal disease. J Am Soc Nephrol. 2008;19: 1865–1869. doi: 10.1681/ASN.2007101121. [DOI] [PubMed] [Google Scholar]
- 28.Durey MA, et al. Anti-complement-factor H-associated glomerulopathies. Nat Rev Nephrol. 2016;12: 563–578. doi: 10.1038/nrneph.2016.99. [DOI] [PubMed] [Google Scholar]
- 29.Zipfel PF, et al. The role of complement in C3 glomerulopathy. Mol Immunol. 2015;67: 21–30. doi: 10.1016/j.molimm.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Thurman JM, Nester CM. All Things Complement. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.01710216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao X, et al. C3 glomerulopathy: the genetic and clinical findings in dense deposit disease and C3 glomerulonephritis. Semin Thromb Hemost. 2014;40: 465–471. doi: 10.1055/s-0034-1376334. [DOI] [PubMed] [Google Scholar]
- 32.Pickering MC, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84: 1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi S, Fervenza FC. Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS) Semin Thromb Hemost. 2014;40: 416–421. doi: 10.1055/s-0034-1375701. [DOI] [PubMed] [Google Scholar]
- 34.Medjeral-Thomas NR, et al. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9: 46–53. doi: 10.2215/CJN.04700513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Servais A, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82: 454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
- 36.Nester CM, Smith RJ. Complement inhibition in C3 glomerulopathy. Semin Immunol. 2016;28: 241–249. doi: 10.1016/j.smim.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, et al. Defining the complement biomarker profile of C3 glomerulopathy. Clin J Am Soc Nephrol. 2014;9: 1876–1882. doi: 10.2215/CJN.01820214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nester CM, Smith RJ. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22: 231–237. doi: 10.1097/MNH.0b013e32835da24c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bomback AS, et al. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7: 748–756. doi: 10.2215/CJN.12901211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbour TD, et al. Update on C3 glomerulopathy. Nephrol Dial Transplant. 2016;31: 717–725. doi: 10.1093/ndt/gfu317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corvillo F, et al. Serum properdin consumption as a biomarker of C5 convertase dysregulation in C3 glomerulopathy. Clin Exp Immunol. 2016;184: 118–125. doi: 10.1111/cei.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, et al. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol. 2013;24: 1820–1829. doi: 10.1681/ASN.2013010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220: 993–998. doi: 10.1016/j.imbio.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt CQ, et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J Immunol. 2013;190: 5712–5721. doi: 10.4049/jimmunol.1203548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maibaum J, et al. Small-molecule factor D inhibitors targeting the alternative complement pathway. Nat Chem Biol. 2016;12: 1105–1110. doi: 10.1038/nchembio.2208. [DOI] [PubMed] [Google Scholar]
- 46.Reis S, et al. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand J Immunol. 2006;63: 155–168. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 47.Verschoor A, et al. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J Immunol. 2003;171: 5363–5371. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 48.Gros P, et al. Complement driven by conformational changes. Nat Rev Immunol. 2008;8: 48–58. doi: 10.1038/nri2231. [DOI] [PubMed] [Google Scholar]
- 49.Harboe M, et al. The down-stream effects of mannan-induced lectin complement pathway activation depend quantitatively on alternative pathway amplification. Mol Immunol. 2009;47: 373–380. doi: 10.1016/j.molimm.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Huber-Lang M, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006;12: 682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- 51.Amara U, et al. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185: 5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190: 3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berentsen S. Role of Complement in Autoimmune Hemolytic Anemia. Transfus Med Hemother. 2015;42: 303–310. doi: 10.1159/000438964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garred P, et al. A journey through the lectin pathway of complement-MBL and beyond. Immunol Rev. 2016;274: 74–97. doi: 10.1111/imr.12468. [DOI] [PubMed] [Google Scholar]
- 55.Woodruff TM, et al. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48: 1631–1642. doi: 10.1016/j.molimm.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 56.Li R, et al. C5L2: a controversial receptor of complement anaphylatoxin, C5a. FASEB J. 2013;27: 855–864. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 57.Smith RJ, et al. New approaches to the treatment of dense deposit disease. J Am Soc Nephrol. 2007;18: 2447–2456. doi: 10.1681/ASN.2007030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skerka C, et al. Complement factor H related proteins (CFHRs) Mol Immunol. 2013;56: 170–180. doi: 10.1016/j.molimm.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 59.Chen Q, et al. Complement factor H-related hybrid protein deregulates complement in dense deposit disease. J Clin Invest. 2014;124: 145–155. doi: 10.1172/JCI71866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gale DP, et al. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376: 794–801. doi: 10.1016/S0140-6736(10)60670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Botto M, et al. Homozygous hereditary C3 deficiency due to a partial gene deletion. Proc Natl Acad Sci USA. 1992;89:4957–4961. doi: 10.1073/pnas.89.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grumach AS, Kirschfink M. Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol Immunol. 2014;61:110–117. doi: 10.1016/j.molimm.2014.06.030. [DOI] [PubMed] [Google Scholar]
- 63.Fischer MB, et al. Local synthesis of C3 within the splenic lymphoid compartment can reconstitute the impaired immune response in C3-deficient mice. J Immunol. 1998;160:2619–25. [PubMed] [Google Scholar]
- 64.Mabbott NA, et al. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat Med. 2001;7: 485–487. doi: 10.1038/86562. [DOI] [PubMed] [Google Scholar]
- 65.Maekawa T, et al. Genetic and intervention studies implicating complement c3 as a major target for the treatment of periodontitis. J Immunol. 2014;192:6020–6027. doi: 10.4049/jimmunol.1400569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickering MC, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–8. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]