Abstract
The PSD-95 family of proteins, known as MAGUKs, have long been recognized to be central building blocks of the PSD. They are categorized as scaffolding proteins, which link surface-expressed receptors to the intracellular signaling molecules. Although the four members of the PSD-95 family (PSD-95, PSD-93, SAP102, and SAP97) have many shared roles in regulating synaptic function, recent studies have begun to delineate specific binding partners and roles in plasticity. In the current review, we will highlight the conserved and unique roles of these proteins.
Introduction
Excitatory synapses are most often localized on dendritic spines, which are abundant small membrane protrusions that decorate dendrites. Excitatory synapses include the postsynaptic density (PSD), a specialized electron dense structure positioned at the distal tip of spine heads. Receptors, adhesion molecules and postsynaptic scaffolding proteins accumulate at the PSD to allow efficient synaptic responses to glutamate released from the presynaptic terminal. Scaffolding proteins serve as a platform to hold together the PSD by binding to postsynaptic receptors, adhesion molecules, and cytoplasmic signaling proteins like protein kinases, phosphatases, and GTPases [1–3]. Membrane-associated guanylate kinases (MAGUKs) are the best studied scaffolding proteins, and findings over the last few years demonstrate that PSD-95 and other MAGUKs play diverse roles in regulating synaptic expression of receptors, synaptic plasticity, and are essential for the basic structure of the PSD itself.
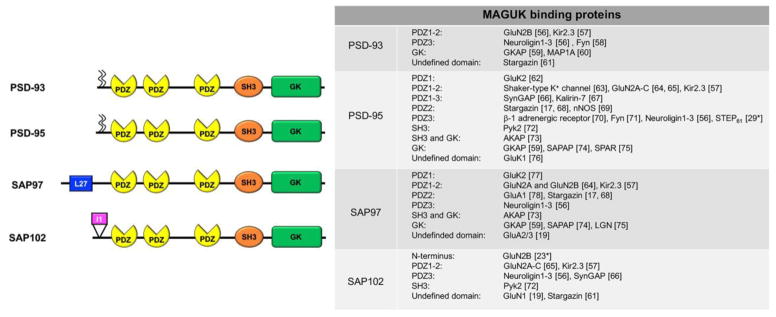
In this review, we will confine our discussion to the PSD-95 family of MAGUKs, which includes PSD-95, PSD-93, SAP102, and SAP97. Structurally, the four members contain three PSD-95/Discs large/Zona occludens-1 (PDZ) domains, followed by a Src-homology-3 (SH3) domain and a catalytically inactive guanylate kinase (GK) domain [Fig. 1]. While these domains are highly conserved among the various MAGUKs, there are also divergent regions, most notably the distinct N-termini. These multiple protein interaction domains allow MAGUKs to act as a bridge between surface-expressed receptors, channels and adhesion molecules and intracellular signaling proteins, enzymes and cytoskeletal elements. Although some binding partners are conserved among the MAGUKs, others are specific to one family member [Fig. 1]. The prototypic MAGUK is PSD-95, named based on its enrichment at the PSD. Early biochemical studies identified PSD-95 as a primary constituent of the PSD [4] and recent findings show that deleting PSD-95 results in a fragmentation of the PSD [5]. Knockdown of PSD-95, PSD-93 and SAP102 family members causes a profound disintegration of the PSD, solidifying the critical role of MAGUKs in the formation and maintenance of the structure [6*].
Figure 1. MAGUK family members and their binding proteins.
PSD-93 and PSD-95 have two palmitoylation motifs in their N-terminus. L27: Lin2-Lin7; PDZ: PSD-95/Discs large/Zona occludens-1; SH3: Src-homology-3; GK: Guanylate Kinase. SAP102 has a splice variant without the I1 region in the N-terminus.
Light and electron microscopy data [7, 8] indicate that a significant percentage of synapses contain both PSD-93 and PSD-95. Further exploration with super-resolution microscopy has found that many synapses contain sub-synaptic nanodomains enriched in PSD-95 [9*–12**]. Interestingly, these nanodomains are enriched in both PSD-95 and AMPARs. While several studies have found PSD-93 and PSD-95 to play equivalent roles in trafficking AMPARs [13, 14**], similar PSD-93-enriched nanodomains have not been reported. Given the correlation between PSD-95 nanodomains and AMPAR enrichment, it would be interesting to explore whether other MAGUKs infiltrate PSD-95 nanodomains, and whether similar nanodomains populated by other MAGUKs exist and are enriched in AMPARs.
MAGUKs and synaptic expression of glutamate receptors
A primary function of MAGUKs is to bind to and stabilize proteins at synapses. Many lines of evidence show that MAGUKs regulate the synaptic expression of glutamate receptors. All family members bind directly to GluN2 subunits of NMDARs thereby stabilizing NMDARs at the cell surface [15]. Both GluN2A and GluN2B have a conserved PDZ ligand (−ESDV), which regulates direct high affinity binding to MAGUK family members. Deletion of the – ESDV motif or mutations in that domain within GluN2B disrupt surface and synaptic expression of NMDARs [15, 16]. MAGUKs also regulate the synaptic expression of AMPARs; however, unlike NMDAR binding, PSD-95 indirectly interacts with AMPARs through the auxiliary subunit stargazin (stg) and its related family members, TARPs (Transmembrane AMPA Regulatory Proteins), which are critical for synaptic expression of AMPARs [17, 18]. However, another family member, SAP97, binds directly to the GluA1 AMPAR subunit [19]. Whereas SAP97 can rescue the deficits in AMPAR currents in PSD-93/-95 double-knockout neurons, deleting SAP97 has no effect on synaptic transmission [20].
Early work demonstrated that the phosphorylation of potassium channels in the PDZ ligand [21] disrupted PSD-95 binding. The same is true with the NMDAR/PSD-95 interaction, which is inhibited by phosphorylation. Indeed, there is an intricate interplay between phosphorylation and synaptic expression of NMDARs. Synaptic activity results in CaMKII binding to GluN2B and recruitment of casein kinase 2 (CK2) into a trimolecular complex [22]. CK2 phosphorylation of the PDZ ligand on S1480 of GluN2B disrupts PSD-95 binding resulting in dramatic reduction in NMDAR surface and synaptic expression [16]. CK2 phosphorylation of the PDZ ligand is an important step in the GluN2B to GluN2A synaptic switch. A nearby endocytic motif on GluN2B (−YEKL) is a target for tyrosine kinases and phosphatases and when phosphorylated, NMDARs are not internalized and surface/synaptic expression is increased. The CK2 phosphorylation of the PDZ ligand and the phosphorylation of the endocytic motif on GluN2B play opposing roles in modulating synaptic NMDARs.
Although MAGUKs are most often thought of as stabilizing synaptic proteins, recent findings show that SAP102 can also play an unanticipated role in clearing NMDARs from synaptic sites [23*]. It is widely accepted that SAP102 is associated with glutamate receptor targeting to synapses during neuronal development [7, 24, 25] and that PSD-95 directly binds to the PDZ ligand of GluN2B and stabilizes its surface expression at synapses [16]. All MAGUKs bind to the –ESDV motif on GluN2 subunits of NMDARs. However, non-PDZ interactions have also been reported [Fig. 1; 23*, 26]. SAP102 binds to the GluN2B C-terminus upstream of the PDZ ligand. This binding event is dependent on the SAP102 unique N-terminal domain, and is regulated by alternative splicing. When GluN2B phosphorylation blocks binding to MAGUKs via its PDZ ligand, it can still bind to SAP102 via this non-conventional binding site, which facilitates the removal of synaptic NMDARs. Because SAP102, unlike PSD-93 and PSD-95, is not palmitoylated [Fig. 1; 27] and has been shown to move in and out of spines [28], it is an ideal protein to shuttle NMDARs in and out of the synapse.
Role for PSD-95 in sculpting protein content at the PSD
In addition to the many roles that MAGUKs play in receptor trafficking, a recent study demonstrates that PSD-95 acts in an unexpected way to regulate the expression of the tyrosine phosphatase PTPN5, also known as STEP (STriatal-Enriched protein tyrosine Phosphatase), in the PSD [29**]. STEP is known to regulate surface expression of NMDARs by dephosphorylating GluN2B Y1472 within the endocytic motif, thereby increasing internalization [30]. Recent data show that PSD-95, but not other MAGUKs, binds to STEP via its PDZ3 domain [Fig. 1; 29**] in a palmitoylation-dependent manner. PSD-95 triggers the degradation of STEP and restricts STEP to a low level in the PSD. Furthermore, PSD-95 knock-down results in a marked increase in synaptic STEP and a decrease in synaptic GluN2B. Similar findings were observed in vivo in PSD-95 KO mice. Therefore, there is a PSD-95 specific effect on synaptic NMDARs that is independent of the well-established stabilizing role as a scaffolding protein. It is likely that as we learn more about MAGUKs, additional non-scaffolding roles will be identified.
MAGUK involvement in synaptic transmission
What factors influence MAGUK localization of AMPARs to synapses? There is a linear relationship between PSD diameter and AMPAR number [31], and genetic deletion of AMPARs does not affect PSD size [32]. This suggests that, on average, the size of the PSD is the primary determinant of AMPAR content. There are, however, other factors that modulate AMPAR synaptic strength. One factor that can be modulated is the affinity of TARPs for MAGUKs. It has been known for some time that TARPs, such as stg, bind AMPARs and link them to MAGUKs via an interaction between MAGUK PDZ-domains and a TARP cytoplasmic tail (c-tail) PDZ-binding motif [17, 33, 34]. One factor limiting this interaction is electrostatic attraction between the TARP c-tail and the lipid membrane, which inhibits binding of stg to PSD-95 [35]. Although phosphorylation of the stg c-tail via CaMKII and PKC has been shown to increase AMPAR EPSCs [36], the mechanism is not fully understood. It has recently been shown that phosphorylation of the stg c-tail disrupts its electrostatic interactions with the membrane [35], dissociating it and extending it into the cytoplasm. Interestingly, this facilitates binding to MAGUK PDZ domains, specifically PDZ domain 3, which had not been thought to play a large role in basal transmission [34]. Binding to PDZ domain 3 leads to increases in the percent of AMPARs at synapses, and in AMPAR-mediated EPSCs [37*]. Since phosphorylation of the stg c-tail occurs via CaMKII and PKC [36], it is tempting to speculate that one component of the AMPAR EPSC increase during LTP is CaMKII-mediated phosphorylation of the stg c-tail. However, recent evidence shows that kainate receptors, which do not interact with TARPs, are competent to mediate LTP [38], indicating that LTP can occur in the absence of this mechanism.
MAGUKs play an established role localizing AMPARs and NMDARs to synapses. RNAi-mediated knockdown of PSD-93, PSD-95 and SAP102 together reduces the size of AMPAR and NMDAR-containing synaptic responses by roughly 75%. Knockdown of PSD-93, PSD-95, or SAP102 individually causes similar reductions in baseline synaptic currents in each case: ~50% for AMPAR-EPSCs and ~25% for NMDAR EPSCs [14**]. Thus, the 3 MAGUKs contribute to basal trafficking of AMPARs and NMDARs to a similar degree.
Although glutamatergic EPSCs are greatly reduced after MAGUK knockdown, dendritic spine density is unchanged, suggesting MAGUKs are responsible for localizing glutamate receptors to synapses, but not for processes involved in spine formation or maintenance [14**]. Furthermore, these and other results indicate that compensatory mechanisms do not change spine density in response to reduced excitatory activity [32]. Together, these data are consistent with a large population of ‘silent’ spines that lack both AMPARs and NMDARs that emerge after MAGUK loss. These data, however, do not rule out changes in spine stability that could occur, for example if an activity-dependent step stabilizes new spines [39]. In particular, smaller-diameter spines, such as those seen after MAGUK loss, have previously been shown to be less stable than larger spines [40].
MAGUK loss triggers a homeostatic process
One long-standing curiosity in the field has been that removal of each MAGUK family member results in an all-or-none loss of AMPARs at individual synapses [13, 41], meaning synapses either lose their entire complement of AMPARs or are unaffected. This manifests itself as a reduction in AMPAR-containing synapses without a reduction in AMPAR synaptic strength at the remaining synapses. Since MAGUKs are at all synapses, it is puzzling how loss of a single family member causes loss of all AMPARs at a subset of synapses. Previously, this result has been variously interpreted to mean that individual synapses are reliant on either PSD-95 or PSD-93 but not both [13], or that MAGUKs are required for synaptogenesis [41].
Recent research has shown that following MAGUK loss, a compensatory program dependent on signaling though L-type calcium channels maintains synaptic strength at individual synapses [14**]. This synapse-specific homeostatic program cannibalizes a subset of synapses to localize AMPARs to the remaining synapses. Interestingly, AMPARs are added to the remaining synapses until they perfectly match the synaptic strength of unmanipulated controls, despite the large overall reduction in AMPAR EPSCs. One possibility is the shortage of MAGUKs inhibits further increases at individual synapses [8]. These results strongly suggest that individual synapses have a program that determines ‘default’ strength, which is executed at the single-synapse level and complements known examples of cell-level homeostasis. Existing examples of cell-level homeostasis have been proposed to respond to perturbations in L-type channel calcium influx by scaling overall synaptic input while maintaining relative synaptic strengths. These homeostatic programs counteract deviations from a cell-wide activity ‘set-point’ caused, for example, by Hebbian processes such as LTP. Single-synapse homeostasis, in contrast, acts to maintain a set-point at individual synapses, independent of cellular activity levels. Cell-level and single-synapse homeostasis use many of the same proteins, such as GluA2 [42] and CaMKK [43, 44], and potentially are separate consequences of activation of a common non-Hebbian pathway.
A comparison of MAGUK loss to other forms of homeostasis
Are all non-Hebbian ‘homeostatic’ plasticity programs glimpses of a common pathway that regulates synaptic strength? Other homeostatic programs at single synapses have been described that oppose changes in presynaptic input by manipulating AMPAR synaptic strength [45, 46], and have been found to use components of the cell-level homeostasis program [45]. One final process that regulates synaptic strength and is reminiscent of single-synapse homeostasis is distance-dependent scaling (DDS), a process which, like single-synapse and cell-level homeostasis, is dependent on the GluA2 subunit [47]. DDS translates global signaling cues about relative synaptic location, potentially conveyed at least in part by backpropagating action potentials [48], into a synaptic strength gradient to counter the electrotonic effect on distant synapses. Thus synapse strength increases as synapses get further from the cell body, and precisely counteracts increased electrotonic filtering of EPSCs from these distal synapses. Block of DDS results in strong synapses close to the soma with synapses growing weaker as distance from the soma increases. Given that DDS and single-synapse plasticity share reliance on the same signaling pathways and both serve to set synapse strength, it would appear that these phenomena are different facets of the same homeostatic process. The GluA2 subunit is essential for DDS, and backpropagating action potentials trigger dendritic calcium influx [48–50], through voltage-gated calcium channels including L-type channels [51]. Backpropagating action potentials decrease in size as they penetrate the dendritic arbor and the decreasing calcium influx could serve to indicate increasing distance from the soma and result in stronger synapses. However, further experimentation is required to determine the roles that L-type calcium channel signaling and CaMKK potentially play in DDS.
Multiple lines of evidence have established that homeostatic plasticity actively sets baseline synaptic strength at individual post-synaptic specializations. Although the pathways underlying this set point have been seen in multiple contexts and conceptually split into multiple processes, future research into the detailed molecular pathway of homeostatic plasticity is required to determine whether these processes are independent, or rely on one common pathway that can be initiated in many separate contexts.
Conclusions
The MAGUK family of scaffolding proteins performs a complex array of synaptic functions. The developmental differences between PSD-93/PSD-95 and SAP102, with the latter uniquely expressed early in development, were first described over 15 years ago [7]. Current studies are now elucidating the functional differences between MAGUKs hinted at by this differential expression. Examples include SAP102’s unanticipated role in clearing NMDARs [23*], PSD-95’s role in triggering STEP degradation [29**], and the divergent roles of PSD-93 and PSD-95 in both Hebbian [52] and non-Hebbian plasticity [9]. Ongoing research will almost certainly continue to reveal the mechanisms underlying differential roles [53–55*] of the MAGUKs.
Although there are many examples of heterogeneity within the MAGUK family, all MAGUKs play a basic role at the synapse: localization of glutamate receptors. In this role, each family member plays an equal part [14**]. Even this fundamental shared property has many unresolved questions to explore. What role do MAGUKs play during Hebbian plasticity, and what factors influence this role? How do the non-Hebbian pathways that control AMPAR localization use MAGUKs to modulate synaptic strength? The MAGUKs’ central role in sculpting synaptic strength will continue to provide insight into the underlying mechanisms of glutamatergic transmission for years to come.
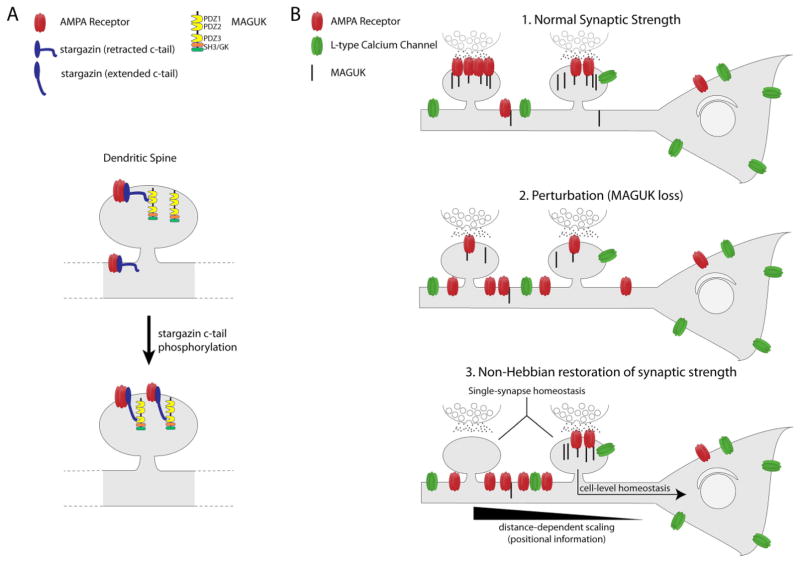
Figure 2. Role of the MAGUKs in Hebbian and non-Hebbian plasticity.
The MAGUKs are involved in pathways that set synaptic strength during Hebbian and non-Hebbian plasticity. (A) Phosphorylation of the stargazin c-tail by PKC and CaMKII allows robust binding of the c-tail to the PDZ3 domain of PSD-95. This additional binding site increases the number of synaptically localized AMPARs. (B) MAGUKs set default synaptic strength during non-Hebbian homeostasis. 1. PSD size (and MAGUK content) set baseline ‘default’ synaptic strength. 2. Reduction in MAGUK protein fragments and weakens synapses. 3. Non-Hebbian processes work to restore synaptic strength to a pre-existing set point. These processes include distance-dependent scaling, synapse-level homeostasis, and cell-level homeostatic processes.
Highlights.
PSD-95 and PSD-93 are essential for PSD structure.
SAP102 plays a role in clearing NMDARs from the synapse.
PSD-95 sculpts protein content at the PSD.
PSD-93 and PSD-95 have divergent roles in both Hebbian and non-Hebbian plasticity.
Acknowledgments
This work was supported by NINDS Intramural Research Program (S.W. and K.W.R.) and US NIMH (R.A.N. MH-38256).
Footnotes
Conflict of interest statement
Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Lowenthal MS, Markey SP, Dosemeci A. Quantitative mass spectrometry measurements reveal stoichiometry of principal postsynaptic density proteins. J Proteome Res. 2015;14:2528–2538. doi: 10.1021/acs.jproteome.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheng M, Hoogenraad CC. The postsynaptic architecture of excitatory synapses: a more quantitative view. Annu Rev Biochem. 2007;76:823–847. doi: 10.1146/annurev.biochem.76.060805.160029. [DOI] [PubMed] [Google Scholar]
- 3.Bayes A, Grant SG. Neuroproteomics: understanding the molecular organization and complexity of the brain. Nat Rev Neurosci. 2009;10:635–646. doi: 10.1038/nrn2701. [DOI] [PubMed] [Google Scholar]
- 4.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31:6329–6338. doi: 10.1523/JNEUROSCI.5968-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc Natl Acad Sci U S A. 2015;112:E6983–6992. doi: 10.1073/pnas.1517045112. This paper shows that simultaneous knockdown of PSD-95, PSD-93, and SAP102 leads to a significant rise in the number of silent synapses and decreases the size of PSDs, as evaluated by combining electrophysiology and transmission electron microscopy (TEM) tomography. The authors demonstrate that MAGUKs are required for anchoring both AMPARs and NMDARs at the PSD and govern the overall molecular organization of the PSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sans N, Petralia RS, Wang YX, Blahos J, 2nd, Hell JW, Wenthold RJ. A developmental change in NMDA receptor-associated proteins at hippocampal synapses. J Neurosci. 2000;20:1260–1271. doi: 10.1523/JNEUROSCI.20-03-01260.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Q, Turrigiano GG. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci. 2011;31:6800–6808. doi: 10.1523/JNEUROSCI.5616-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Fukata Y, Dimitrov A, Boncompain G, Vielemeyer O, Perez F, Fukata M. Local palmitoylation cycles define activity-regulated postsynaptic subdomains. J Cell Biol. 2013;202:145–161. doi: 10.1083/jcb.201302071. This study shows that PSD-95 palmitoylation, a critical step for synaptic localization of MAGUKs, occurs locally and is modulated by activity. These findings suggest the modulation of PSD-95 nanodomains by activity could occur through activity-dependent palmitoylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGillavry HD, Song Y, Raghavachari S, Blanpied TA. Nanoscale scaffolding domains within the postsynaptic density concentrate synaptic AMPA receptors. Neuron. 2013;78:615–622. doi: 10.1016/j.neuron.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nair D, Hosy E, Petersen JD, Constals A, Giannone G, Choquet D, Sibarita JB. Super-resolution imaging reveals that AMPA receptors inside synapses are dynamically organized in nanodomains regulated by PSD95. J Neurosci. 2013;33:13204–13224. doi: 10.1523/JNEUROSCI.2381-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **12.Tang AH, Chen H, Li TP, Metzbower SR, MacGillavry HD, Blanpied TA. A trans-synaptic nanocolumn aligns neurotransmitter release to receptors. Nature. 2016;536:210–214. doi: 10.1038/nature19058. This paper shows that key proteins mediating vesicle priming and fusion are mutually co-enriched and clustered within nanometer scale subregions of the presynaptic zone using STORM imaging. These presynaptic nanoclusters closely align with postsynaptic receptors and scaffolding proteins and they suggest the existence of a synaptic molecular nanocolumn to maintain and modulate synaptic efficiency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52:307–320. doi: 10.1016/j.neuron.2006.09.012. [DOI] [PubMed] [Google Scholar]
- **14.Levy JM, Chen X, Reese TS, Nicoll RA. Synaptic Consolidation Normalizes AMPAR Quantal Size following MAGUK Loss. Neuron. 2015;87:534–548. doi: 10.1016/j.neuron.2015.07.015. The authors show here that MAGUK loss triggers an activity-dependent nonuniform reorganization of synapses, indicating that synaptic strength is regulated at the level of individual synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 16.Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–996. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- 18.Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J Biol Chem. 1998;273:19518–19524. doi: 10.1074/jbc.273.31.19518. [DOI] [PubMed] [Google Scholar]
- 20.Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA. The role of SAP97 in synaptic glutamate receptor dynamics. Proc Natl Acad Sci U S A. 2010;107:3805–3810. doi: 10.1073/pnas.0914422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 22.Sanz-Clemente A, Gray JA, Ogilvie KA, Nicoll RA, Roche KW. Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 2013;3:607–614. doi: 10.1016/j.celrep.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *23.Chen BS, Gray JA, Sanz-Clemente A, Wei Z, Thomas EV, Nicoll RA, Roche KW. SAP102 mediates synaptic clearance of NMDA receptors. Cell Rep. 2012;2:1120–1128. doi: 10.1016/j.celrep.2012.09.024. SAP102 is a MAGUK family member, which is associated with NMDAR trafficking. The authors show that there is a secondary interaction between SAP102 and GluN2B in addition to the PDZ interaction. They identify two critical residues on GluN2B, which are responsible for the non-PDZ binding to SAP102, and demonstrate a nonscaffolding role for SAP102 in removing GluN2B-containing NMDARs from synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petralia RS, Sans N, Wang YX, Wenthold RJ. Ontogeny of postsynaptic density proteins at glutamatergic synapses. Mol Cell Neurosci. 2005;29:436–452. doi: 10.1016/j.mcn.2005.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci U S A. 2008;105:20953–20958. doi: 10.1073/pnas.0811025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cousins SL, Stephenson FA. Identification of N-methyl-D-aspartic acid (NMDA) receptor subtype-specific binding sites that mediate direct interactions with scaffold protein PSD-95. J Biol Chem. 2012;287:13465–13476. doi: 10.1074/jbc.M111.292862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Husseini AE, Topinka JR, Lehrer-Graiwer JE, Firestein BL, Craven SE, Aoki C, Bredt DS. Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs. J Biol Chem. 2000;275:23904–23910. doi: 10.1074/jbc.M909919199. [DOI] [PubMed] [Google Scholar]
- 28.Zheng CY, Petralia RS, Wang YX, Kachar B, Wenthold RJ. SAP102 is a highly mobile MAGUK in spines. J Neurosci. 2010;30:4757–4766. doi: 10.1523/JNEUROSCI.6108-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Won S, Incontro S, Nicoll RA, Roche KW. PSD-95 stabilizes NMDA receptors by inducing the degradation of STEP61. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1609702113. This study demonstrates that STEP61, a brain-specific protein tyrosine phosphatase, binds specifically to PSD-95 and that PSD-95 triggers the degradation of STEP61 via the ubiquitin/proteasomal pathway. In PSD-95 KO mouse brain, STEP61 expression is increased at synapses revealing precise interplay in vivo. STEP knockdown increases extrasynaptic NMDARs as PSD-95 excludes synaptic STEP under WT conditions. This paper shows a dual role for PSD-95 in regulating synaptic protein content as well as in stabilizing synaptic NMDARs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takumi Y, Ramirez-Leon V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 32.Lu W, Bushong EA, Shih TP, Ellisman MH, Nicoll RA. The cell-autonomous role of excitatory synaptic transmission in the regulation of neuronal structure and function. Neuron. 2013;78:433–439. doi: 10.1016/j.neuron.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bats C, Groc L, Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci U S A. 2002;99:13902–13907. doi: 10.1073/pnas.172511199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sumioka A, Yan D, Tomita S. TARP phosphorylation regulates synaptic AMPA receptors through lipid bilayers. Neuron. 2010;66:755–767. doi: 10.1016/j.neuron.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- *37.Hafner AS, Penn AC, Grillo-Bosch D, Retailleau N, Poujol C, Philippat A, Coussen F, Sainlos M, Opazo P, Choquet D. Lengthening of the Stargazin Cytoplasmic Tail Increases Synaptic Transmission by Promoting Interaction to Deeper Domains of PSD-95. Neuron. 2015;86:475–489. doi: 10.1016/j.neuron.2015.03.013. The mechanism by which phosphorylation of the stg c-tail might increase synaptic strength has been a longstanding curiosity in the field. Here, the authors show that phosphorylation extends the stg c-tail into the cytoplasm and facilitates its binding to PSD-95 PDZ3 domain. [DOI] [PubMed] [Google Scholar]
- 38.Granger AJ, Shi Y, Lu W, Cerpas M, Nicoll RA. LTP requires a reserve pool of glutamate receptors independent of subunit type. Nature. 2013;493:495–500. doi: 10.1038/nature11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Roo M, Klauser P, Mendez P, Poglia L, Muller D. Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb Cortex. 2008;18:151–161. doi: 10.1093/cercor/bhm041. [DOI] [PubMed] [Google Scholar]
- 40.Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich I, Klein M, Rumpel S, Malinow R. PSD-95 is required for activity-driven synapse stabilization. Proc Natl Acad Sci U S A. 2007;104:4176–4181. doi: 10.1073/pnas.0609307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci. 2009;29:6479–6489. doi: 10.1523/JNEUROSCI.3753-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 45.Beique JC, Na Y, Kuhl D, Worley PF, Huganir RL. Arc-dependent synapse-specific homeostatic plasticity. Proc Natl Acad Sci U S A. 2011;108:816–821. doi: 10.1073/pnas.1017914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72:806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shipman SL, Herring BE, Suh YH, Roche KW, Nicoll RA. Distance-dependent scaling of AMPARs is cell-autonomous and GluA2 dependent. J Neurosci. 2013;33:13312–13319. doi: 10.1523/JNEUROSCI.0678-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 49.Jaffe DB, Johnston D, Lasser-Ross N, Lisman JE, Miyakawa H, Ross WN. The spread of Na+ spikes determines the pattern of dendritic Ca2+ entry into hippocampal neurons. Nature. 1992;357:244–246. doi: 10.1038/357244a0. [DOI] [PubMed] [Google Scholar]
- 50.Yuste R, Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995;375:682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]
- 51.Markram H, Helm PJ, Sakmann B. Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. J Physiol. 1995;485(Pt 1):1–20. doi: 10.1113/jphysiol.1995.sp020708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlisle HJ, Fink AE, Grant SG, O’Dell TJ. Opposing effects of PSD-93 and PSD-95 on long-term potentiation and spike timing-dependent plasticity. J Physiol. 2008;586:5885–5900. doi: 10.1113/jphysiol.2008.163469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murata Y, Constantine-Paton M. Postsynaptic density scaffold SAP102 regulates cortical synapse development through EphB and PAK signaling pathway. J Neurosci. 2013;33:5040–5052. doi: 10.1523/JNEUROSCI.2896-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hruska M, Henderson NT, Xia NL, Le Marchand SJ, Dalva MB. Anchoring and synaptic stability of PSD-95 is driven by ephrin-B3. Nat Neurosci. 2015;18:1594–1605. doi: 10.1038/nn.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *55.Zeng M, Shang Y, Araki Y, Guo T, Huganir RL, Zhang M. Phase Transition in Postsynaptic Densities Underlies Formation of Synaptic Complexes and Synaptic Plasticity. Cell. 2016;166:1163–1175. e1112. doi: 10.1016/j.cell.2016.07.008. SynGAP is one of the most abundant proteins in the PSD. This paper demonstrates that SynGAP makes a coiled-coil trimer and binds to multimers of PSD-95. This binding of SynGAP to PSD-95 induces phase separation of the complex in PSD. They show that the multivalent nature of the SynGAP/PSD-95 complex is critical for the phase separation and suggest a model for phase-transition-mediated formation of PSD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 57.Inanobe A, Fujita A, Ito M, Tomoike H, Inageda K, Kurachi Y. Inward rectifier K+ channel Kir2.3 is localized at the postsynaptic membrane of excitatory synapses. Am J Physiol Cell Physiol. 2002;282:C1396–1403. doi: 10.1152/ajpcell.00615.2001. [DOI] [PubMed] [Google Scholar]
- 58.Nada S, Shima T, Yanai H, Husi H, Grant SG, Okada M, Akiyama T. Identification of PSD-93 as a substrate for the Src family tyrosine kinase Fyn. J Biol Chem. 2003;278:47610–47621. doi: 10.1074/jbc.M303873200. [DOI] [PubMed] [Google Scholar]
- 59.Kim E, Naisbitt S, Hsueh YP, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, Ralston HJ, Bredt DS. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J Neurosci. 1998;18:8805–8813. doi: 10.1523/JNEUROSCI.18-21-08805.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dakoji S, Tomita S, Karimzadegan S, Nicoll RA, Bredt DS. Interaction of transmembrane AMPA receptor regulatory proteins with multiple membrane associated guanylate kinases. Neuropharmacology. 2003;45:849–856. doi: 10.1016/s0028-3908(03)00267-3. [DOI] [PubMed] [Google Scholar]
- 62.Garcia EP, Mehta S, Blair LA, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 63.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 64.Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim IA, Hall DD, Hell JW. Selectivity and promiscuity of the first and second PDZ domains of PSD-95 and synapse-associated protein 102. J Biol Chem. 2002;277:21697–21711. doi: 10.1074/jbc.M112339200. [DOI] [PubMed] [Google Scholar]
- 66.Kim JH, Liao D, Lau LF, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 67.Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- 68.Choi J, Ko J, Park E, Lee JR, Yoon J, Lim S, Kim E. Phosphorylation of stargazin by protein kinase A regulates its interaction with PSD-95. J Biol Chem. 2002;277:12359–12363. doi: 10.1074/jbc.M200528200. [DOI] [PubMed] [Google Scholar]
- 69.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 70.Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA. beta 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of beta 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 71.Tezuka T, Umemori H, Akiyama T, Nakanishi S, Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc Natl Acad Sci U S A. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seabold GK, Burette A, Lim IA, Weinberg RJ, Hell JW. Interaction of the tyrosine kinase Pyk2 with the N-methyl-D-aspartate receptor complex via the Src homology 3 domains of PSD-95 and SAP102. J Biol Chem. 2003;278:15040–15048. doi: 10.1074/jbc.M212825200. [DOI] [PubMed] [Google Scholar]
- 73.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 74.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPs. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 75.Zhu J, Shang Y, Xia C, Wang W, Wen W, Zhang M. Guanylate kinase domains of the MAGUK family scaffold proteins as specific phospho-protein-binding modules. EMBO J. 2011;30:4986–4997. doi: 10.1038/emboj.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron. 2003;37:625–638. doi: 10.1016/s0896-6273(02)01191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta S, Wu H, Garner CC, Marshall J. Molecular mechanisms regulating the differential association of kainate receptor subunits with SAP90/PSD-95 and SAP97. J Biol Chem. 2001;276:16092–16099. doi: 10.1074/jbc.M100643200. [DOI] [PubMed] [Google Scholar]
- 78.Cai C, Coleman SK, Niemi K, Keinanen K. Selective binding of synapse-associated protein 97 to GluR-A alpha-amino-5-hydroxy-3-methyl-4-isoxazole propionate receptor subunit is determined by a novel sequence motif. J Biol Chem. 2002;277:31484–31490. doi: 10.1074/jbc.M204354200. [DOI] [PubMed] [Google Scholar]