Abstract
Objective
Natural killer (NK) cells represent a powerful immunotherapeutic target as they lyse tumors directly, do not require differentiation, and can elicit potent inflammatory responses. The objective of these studies was to use an IL-15 super-agonist complex, ALT-803 (Altor BioScience Corporation), to enhance the function of both normal and ovarian cancer patient derived NK cells by increasing cytotoxicity and cytokine production.
Methods
NK cell function from normal donor peripheral blood mononuclear cells (PBMCs) and ovarian cancer patient ascites was assessed using flow cytometry and chromium release assays +/− ALT-803 stimulation. To evaluate the ability of ALT-803 to enhance NK cell function in vivo against ovarian cancer, we used a MA148-luc ovarian cancer NOD scid gamma (NSG) xenogeneic mouse model with transferred human NK cells.
Results
ALT-803 potently enhanced functionality of NK cells against all ovarian cancer cell lines with significant increases seen in CD107a, IFNγ and TNFα expression depending on target cell line. Function was also rescued in NK cells derived from ovarian cancer patient ascites. Finally, only animals treated with intraperitoneal ALT-803 displayed an NK dependent significant decrease in tumor.
Conclusions
ALT-803 enhances NK cell cytotoxicity against ovarian cancer in vitro and in vivo and is able to rescue functionality of NK cells derived from ovarian cancer patient ascites. These findings suggest that ALT-803 has the potential to enhance NK-cell-based immunotherapeutic approaches for the treatment of ovarian cancer.
Keywords: Immunotherapy, ovarian cancer, IL-15 super-agonist, Natural Killer cells
1. Introduction
Ovarian cancer is the most lethal gynecologic malignancy. The estimated 5-year survival is 46% for all stages of ovarian cancer, and 28% for distant disease. Notably, 62% of women with ovarian cancer present with Stage III or IV disease, for which the rate of recurrence is 60–70% (1). Women who recur cannot be cured with current therapies.
Studies have demonstrated that ovarian cancers are immunogenic and elicit spontaneous antitumor immune responses (2, 3). Some of the strongest evidence linking anti-tumor immunity and cancer has been made in ovarian cancer (4–6). The first evidence of the role of immunosurveillance against human ovarian cancer was the presence of tumor-infiltrating lymphocytes (TILs), which correlated positively and strongly with patient survival (4). Patients whose tumors contained TILs had five-year overall survival (OS) rates of 38%, whereas the OS for patients whose tumors lacked TILs was 4.5%.
Natural killer (NK) cells are of the innate immune system and do not rely on HLA-mediated recognition of tumor targets. We and others have shown that NK cells derived from healthy donors can recognize and kill in vitro ovarian carcinoma cells (7, 8). NK cells are involved in innate immunity and tumor surveillance; they recognize major histocompatibility complex (MHC) class I or class I-like molecules on target cells through a unique class of receptors, NK cell receptors (NKR), that inhibit or activate NK cell function (9). NK cells represent about 5–10 % of circulating lymphocytes, with a CD56+CD3− mature phenotype and are active via major histocompatibility complex (MHC)-independent mechanisms (10). NK cells can be divided into a CD56brightCD16− population, characterized by low cytotoxicity but capable of producing high levels of cytokines (11), and a CD56dimCD16+ population, which better mediate direct cell killing through natural cytotoxicity receptors or antibody-dependent cell-mediated cytotoxicity (ADCC) via CD16 without the need for antigen priming (12, 13). Whereas autologous NK cells from cancer patients may have functional defects (14), allogeneic NK cells from healthy donors have normal function and can be safely administered to cancer patients as we have shown in an ovarian cancer population (15). Although found in the peritoneal cavity of patients with ovarian cancer, NK cells demonstrate reduced function based on tumor induced suppression in the peritoneal microenvironment (16, 17). In vitro studies confirm that the intraperitoneal cavity containing malignant ascites is an immune suppressive environment (18–22).
Methods to induce the activation and proliferation of NK cells are under investigation. Based on preclinical non-human primate and early phase clinical trial data, the cytokine IL-15 can potently increase NK-cell numbers to augment immunotherapy (23–26). Endogenous IL-15 binds to IL-15Rα (on or shed by monocytes and dendritic cells) to form a natural complex to bind IL-2/15Rβ/γChain on NK cells and CD8+ T cells through a process called IL-15 trans-presentation (27, 28). Altor Bioscience Corporation (Altor, Miramar, FL) has developed an IL-15N72D/IL-15Rα-Fc super-agonist complex, termed ALT-803, the design of which inhibits complement activation, includes the addition of a sushi domain to mediate IL-15/IL-15Rα trans-presentation to NK cells, and increases the half-life and stability by inclusion of the Fc domain to mediate more ideal pharmacokinetics with prolonged cytokine function (29). In the present study we tested ALT-803 to determine whether it could improve NK cell function in the ovarian cancer setting.
2. Materials and Methods
2.1. Cells and Isolation
Cell lines were maintained as described previously (30). Buffy coats collected from healthy donors were obtained from Memorial Blood Bank (Minneapolis, MN). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque (GE Healthcare) and used directly in experiments or were cryopreserved in 10% DMSO/90% FBS for later use. Cells for in vivo experiments were enriched through CD3/CD19 depletion with magnetic beads (StemCell Technologies). After depletion, cells were activated overnight with 10ng/ml IL-15 (R&D Systems). Samples were then treated with or without ALT-803 (Altor). Use of peripheral blood mononuclear cells from donors was approved by the Committee on the Use of Human Subjects in Research at the University of Minnesota.
Ascites samples from ten patients were obtained through the University of Minnesota Cancer Center Tissue Procurement Facility with the approval of the University of Minnesota Institutional Review Board. All samples were obtained at the time of a primary cytoreductive surgery from patients diagnosed with Stage III or IV serous ovarian or primary peritoneal carcinoma. Of these, all were high grade serous histology except one was low grade (grade 1). Cells were pelleted, underwent red blood cell lysis and were then cryopreserved in 10% DMSO/90% FBS and stored in Liquid Nitrogen. Ascites fluid supernatant was stored at −80 C (31).
2.2. Proliferation Assays
PBMCs from healthy donors or ascites cells from ovarian cancer patients were labeled with CellTrace Violet Cell Proliferation Dye (ThermoFisher Scientific) per manufacturer’s protocol, and incubated for 7d with noted treatment. NK cells (CD56+CD3−) were then analyzed for dilution of dye by flow cytometry.
2.3. Flow Cytometry Analysis
PBMCs and ascites cells were phenotyped with the following fluorescent-labeled mAbs: BV421 or PE-Cy7 conjugated CD56 (HCD56; Biolegend), PE-CF594-conjugated CD3 (UCHT1; BD Biosciences), APC-Cy7-conjugated CD16 (3G8; Biolegend), PE-Cy7-conjugated KIR2DL1/S1/S3/S5 (HP-MA4; eBioscience), FITC-conjugated KIR2DL2/L3 (DX27; Biolegend), PE-conjugated KIR3DL1 (DX9: Biolegend), APCconjugated NKG2A (Z199; Beckman Coulter), BV605-conjugated CD57 (NK-1: BD), BV421-conjugated CD122 (TU27; Biolegend), PE-conjugated CD132 (TUGh4; BD), FITC-conjugated CD25 (M-A251; BD), PE-conjugated CD69 (FN50; Biolegend), FITC-conjugated CD107a (LAMP-1; H4A3; Biolegend), BV421-conjugated IFN-γ (4S.B3; Biolegend), AF647-conjugated TNFα (MAb11; Biolegend). Cells were stained, run on an LSRII (BD Biosciences), and analyzed with FlowJo software (Tree Star Inc.) as described previously (32).
2.4. CD107a and IFNγ/TNFα Assays
Healthy donor PBMCs or unselected ovarian cancer ascites cells were incubated overnight +/− 1 nM ALT-803 and then co-cultured for 4hrs with the noted tumor targets at 2:1 effector to target (E:T) ratios. PBMCs were also incubated for 24hrs in a 75% solution of cell free ascites fluid from 10 different patients with or without ALT803, and then incubated 4hrs +/− K562 targets. Within the 4hr incubation a CD107a-FITC antibody was added during the first hr, followed by GolgiStop and GolgiPlug (BD Biosciences) incubation for the following 3hrs. Cells were washed, stained with LiveDead viability dye (ThermoFisher Scientific), and then stained with surface antibodies and fixed with 2% paraformaldehyde. Permeabilization buffer (BioLegend) was used to permeabilize cells and then stain cells with noted intracellular antibodies (32).
2.5. Chromium-51 Release Assays
Chromium-51 was incubated with human ovarian cancer cell lines (A1847, MA-148, or SKOV3) for 1hr at 37 C, thrice washed and co-cultured with NK cells at three E:T ratios (20:1, 6.66:1, 2.22:1). 5% Triton-X 100 was used to achieve total cell lysis. After 4hrs, supernatant was harvested and analyzed in a PerkinElmer 1470 Automatic Gamma Counter. Specific 51Cr lysis was calculated using the following equation: Percentage of specific lysis = 100 × (Test release − Spontaneous release)/(Maximal release − Spontaneous release).
2.6. In Vivo Mouse Study and Imaging
NOD/SCID/γc−/− (NSG) mice (Jackson Labs) were xenografted intraperitoneally (IP) with 2×105 firefly luciferase expressing MA-148 tumor cells (Day -3) (8). Mice were sublethally irradiated (225 cGy) and analyzed for presence of tumor cells by Bioluminescent imaging (BLI) using the Xenogen IVIS imaging system (Caliper Life Science, Hopkinton, MA) on day 0. On day 1 mice were IP injected 1×106 overnight activated NK cells, calculated from a CD3/CD19 depleted product. Shortly after NK injection, a separate ALT-803 (50ug/kg) injection was given either IP or subcutaneously (SubQ). One group of mice was harvested 3 days later and peritoneally lavaged postmortem for activation marker evaluation. The second group of mice were given ALT-803 twice weekly (50ug/kg), either IP or SubQ, and analyzed for tumor cells at days 7, 14, 21, and 28. At day 29, the animals were euthanized and a postmortem peritoneal lavage was performed. NK cell function was evaluated in a 4hr assay with K562 targets.
2.7. Statistical Analysis
All statistical tests (noted in figure legends) were carried out with Graphpad Prism software. Bars represent the mean ± SEM. Statistical significance is indicated as *P≤0.05; **P<0.01; ***P<0.001; ****P<0.0001.
3. Results
3.1. NK cells within the ascites of ovarian cancer patients display changes in phenotype
To investigate methodologies to improve NK cell based immunotherapies in ovarian cancer, cells were obtained from the peritoneal cavity of a cohort of ovarian cancer patients immediately prior to undergoing cytoreductive surgery. Peripheral blood mononuclear cells (PBMCs) from healthy donors were used for comparison. A phenotypic panel of NK cell markers was run to characterize differences present between normal peripheral blood NK cells (PBNK) and ascites NK cells from the peritoneal cavity of ovarian cancer patients (ASC NK). Ascites NK cells displayed a higher proportion of the less mature CD56bright cells accompanied with a decrease in proportion of more mature CD56dim cells (Figs. 1A–B). In comparison to the normal PBNK, ASC NK had decreases in both the proportion and per cell surface density of CD16 activating receptor, needed for antibody dependent cell mediated cytotoxicity (Figs. 1C–D). Inhibitory and activating KIR family receptors remained mostly constant (Figs. 1E–F) with the exception of inhibitory KIR3DL1, which was notably lower in the ASC NK (Fig. 1G). No clear differences were noted in NKG2A, an inhibitory receptor that can be used to track maturation, while a trend was seen for decreased proportion of ASC NK cells expressing CD57 (Fig. 1H), a marker of terminal maturation (Figs. 1I). No significant differences were seen in IL-15 signaling components, IL-2/15Rβ chain (CD122) and the common-γ chain (CD132), between PBNK and ASC NK (Figs. 1J–K). IL-2Rα (CD25), which can be used to track activation of the IL-15 pathway, was also similarly expressed basally in both groups (Fig. 1L). No differences were found in PD-1 and Tim-3, two receptors thought to be involved in immune exhaustion (data not shown). These data indicate that although some maturation differences can be seen between PBNK and ASC NK, the NK cells present in the peritoneal cavity of patients are able to respond to ALT-803 treatment.
Figure 1. Phenotypic assessment of NK cells derived from ascites of ovarian cancer patients.
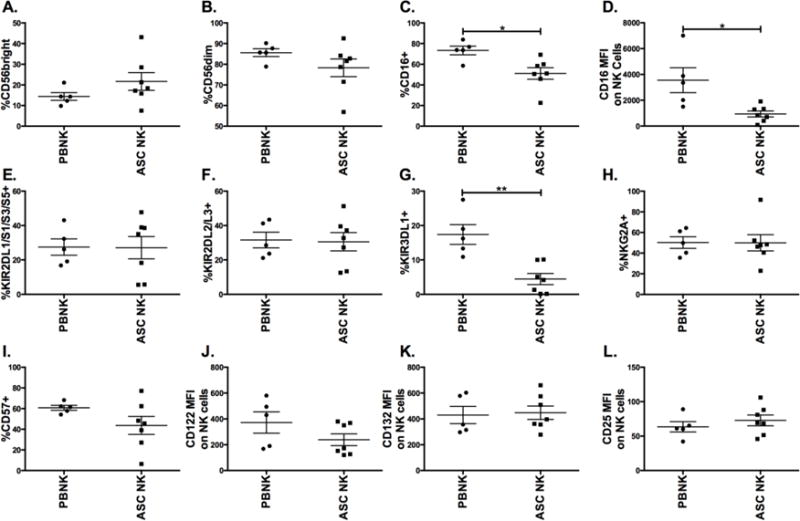
Cells from the ascites of ovarian cancer patients were compared to PBMCs from healthy donors. Cells were stained and gated on CD56+CD3− NK cells from peripheral blood (PBNK) and patient ascites (ASC NK) to evaluate the percentage of NK cells expressing (A) CD56bright, (B) CD56dim, (C) CD16, (E) KIR2DL1/S1/S3/S5, (F) KIR2DL2/L3, (G) KIR3DL1, (H) NKG2A, and (I) CD57. NK cells were evaluated for median fluorescent intensity (MFI) of (D) CD16, (J) CD122 (K) CD132, and (L) CD25. Filled circle (PBNK) and square (ASC NK) represents an individual (n = 5 for PBNK and n = 7 for ASC NK). Unpaired t test was utilized to compare samples.
3.2. ALT-803 induces proliferation in ovarian cancer patient ascites NK cells
The effect of ALT-803 on ASC NK cell proliferation was assessed by labeling PBMCs from healthy donors or whole ascites cells from ovarian cancer patients with CellTrace Violet dye and culturing the cells for 1 week with media (RPMI-10%) alone, media with ALT-803, or media with monomeric IL-15. Upon harvest, NK cells were evaluated for dilution of dye, which is indicative of proliferation (Figs. 2A–B). Untreated cells did not proliferate as much and were present in substantially lower proportions and numbers, presumably through increased cell death and decreased proliferation in the absence of IL-15 signaling (data not shown). Though the proliferation induced by ALT-803 and monomeric IL-15 was more variable in the ASC NK cell group when compared to PBNK, both molecules induced proliferation.
Figure 2. ALT-803 mediates proliferation of NK cells from ascites of ovarian cancer patients.
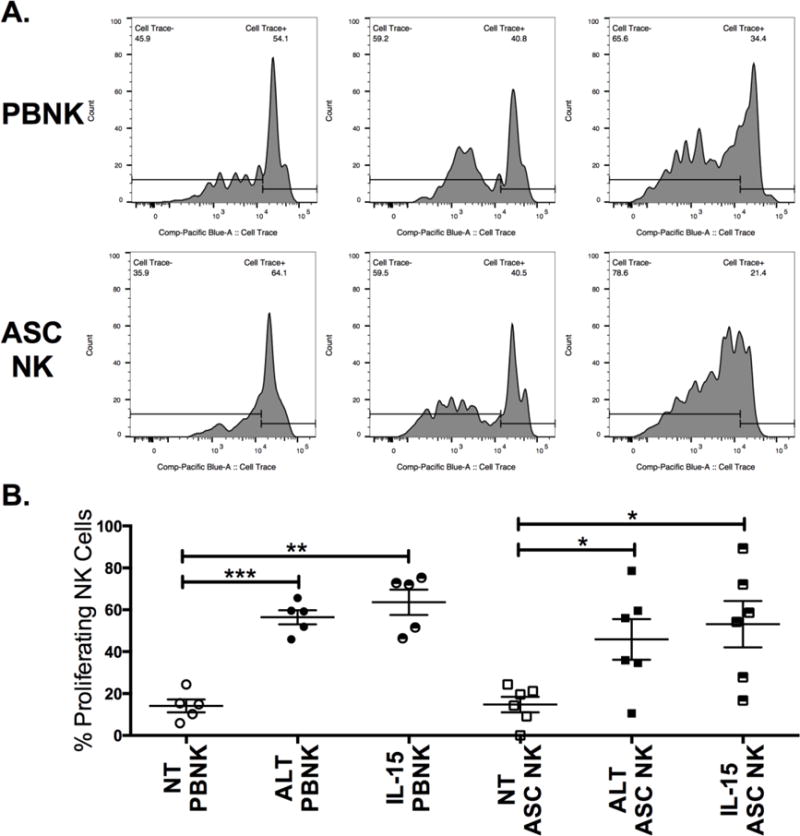
PBMCs from healthy donors or ascites cells from ovarian cancer patients were labeled with CellTrace Violet dye and placed in culture for 1 week with media alone (NT), 1 nM ALT-803 (ALT) or 1 nM IL-15. Cells were then harvested and stained for surface antigens. (A) Representative histograms showing CellTrace dilution on healthy PBNK or patient ASC NK treated with ALT-803. (B) Pooled data showing proliferation (% cells that have diluted dye) on PBNK and ASC NK. Circles and squares represent different individuals within groups (n = 5 for PBNK and n =7 for ASC NK). One-way ANOVA was used to calculate statistics within the PBNK and ASC NK groups.
3.3. ALT-803 amplifies NK cell function against ovarian cancer tumor targets
Next, the ability of ALT-803 to enhance PBNK cell activation against ovarian cancer cell lines was tested. Fresh PBMCs from healthy donors were incubated overnight with ALT-803 and then placed in culture with the following ovarian cancer cell lines: OVCAR3, MA-148, OVCAR5, SKOV3, and A1847. After a 4hr incubation, NK cell function was measured by flow cytometry. Incubation without prior ALT-803 treatment induced only minor degranulation, measured by surface CD107a expression, and inflammatory cytokine production, measured by intracellular IFNγ and TNFα expression, on NK cells when co-incubated with all tested ovarian cancer cell lines (Fig. 3A–C). In contrast, ALT-803 treatment potently induced CD107a, IFNγ and TNFα expression on NK cells incubated with ovarian cancer cells. To test if the increase in NK cell function mediated by ALT-803 translated to an increase in ovarian cancer cell killing, a chromium release assay was performed whereby ovarian cancer cells were labeled with radioactive chromium. Chromium release, as a measure of target killing, was assessed when the ovarian cancer cells were incubated with PBMCs treated with or without ALT-803 overnight. NK cells mediate killing in this assay. ALT-803 treatment enhanced killing of A1847, MA-148, and SKOV3 ovarian cancer cells, particularly at the higher effector to target ratios (Fig. 3D–F). These experiments highlight the capability of ALT-803 to enhance PBNK cell mediated ovarian cancer cell kiling.
Figure 3. ALT-803 induces enhanced NK cell functionality against ovarian tumor targets.
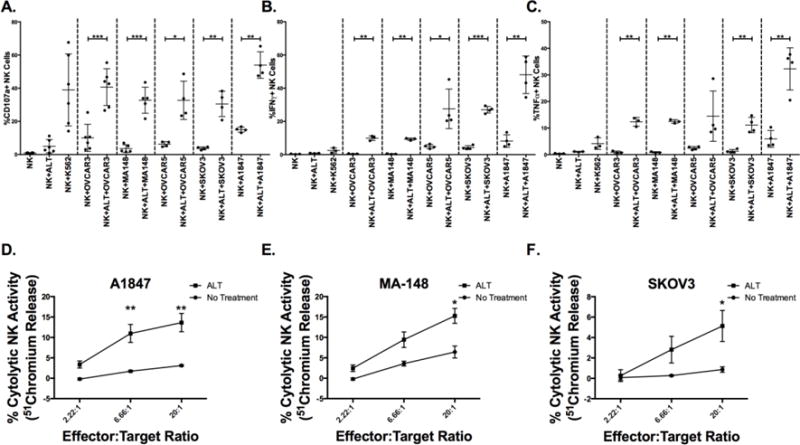
Fresh PBMCs from healthy donors were incubated overnight ALT-803 and placed in culture with noted ovarian tumor targets at a 2:1 effector (PBMC) to (tumor) target ratio for 4hrs. (A) CD107a, (B) IFNγ, and (C) TNFα were evaluated on NK cells by flow cytometry. Circles represent individuals (n = 4–6). Chromium-51 loaded (D) A1847, (E) MA-148, and (C) SKOV3 ovarian cancer cells were incubated with PBMCs, pretreated overnight +/− ALT-803 (ALT), for 4hrs at the noted effector to target ratios. The percentage of cytolytic activity of NK cells was calculated based on the amount of chromium released by dying ovarian cancer cells. Squares (+ ALT-803) and circles (no treatment) represent pooled data (n = 3, representative of two experiments). Paired t test (A–C) and 2way ANOVA test (D–F) were utilized to compare statistical differences.
3.4. ALT-803 potently rescues NK cell function in the ovarian cancer setting
We next tested whether NK cells exposed to the ovarian cancer tumor microenvironment would react similarly with ALT-803. We used K562 targets as a tool to induce NK cell function in these assays. Without any ALT-803 treatment, ASC NK cells degranulate less and produce less IFNγ than healthy donor PBNK, indicating a defect in ASC NK cell function (Fig. 4). However, when cells are pre-treated with ALT-803 overnight, CD107a, IFNγ, and TNFα are all potently induced beyond what we see on healthy donor PBNK without ALT-803 (Figs. 4A–F).
Figure 4. ALT-803 rescues NK cell function on ovarian cancer patient ascites NK cells.
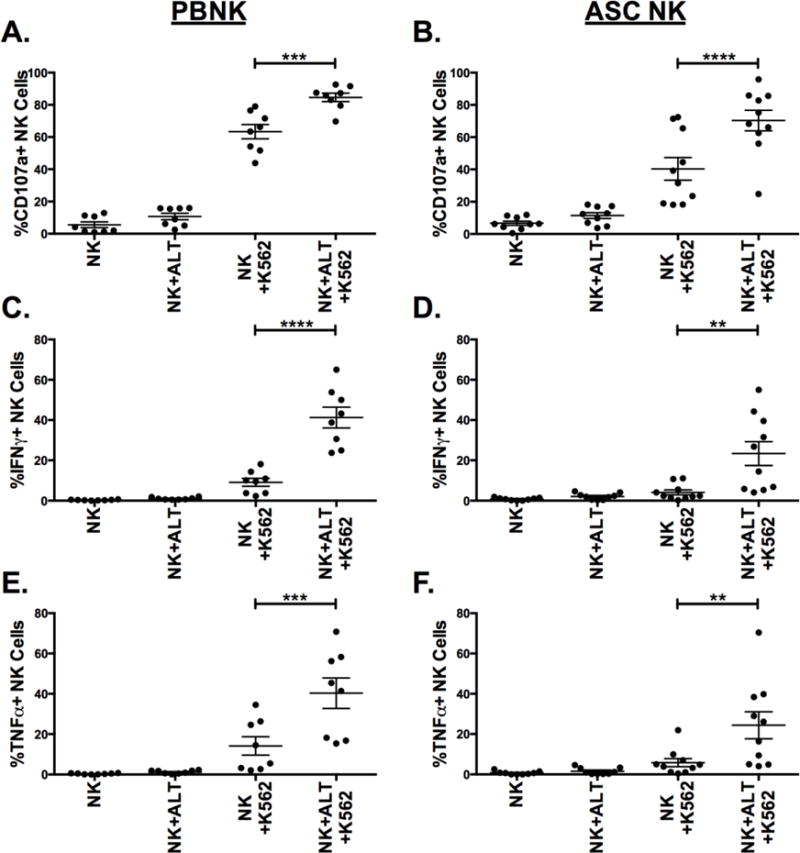
Healthy donor PBMCs (left) or ovarian cancer patient ascites cells (right) were incubated overnight +/− ALT-803 and then incubated +/− K562 targets for 4hrs. (A–B) CD107a, (C–D) IFNγ and (E–F) TNFα were then measured on NK cells by flow cytometry. Circles represent individuals within the treatment groups (n = 8 for PBNK and n = 10 for ASC NK). Paired t test was utilized to compare statistical differences.
The effects of the soluble ovarian tumor microenvironment on healthy donor PBNK cells, and whether ALT-803 can rescue such effects, were evaluated next. To test NK cell function, PBNK cells were incubated with a solution containing ascites fluid from 10 different ovarian cancer patients for 24hrs in the presence or absence of ALT-803 prior to activation with K562 tumor targets. The soluble ovarian tumor microenvironment substantially impacted PBNK cell degranulation and resulted in decreased cytokine production (Figs. 5A–C). However, ALT-803 co-treatment potently rescued degranulation and induced substantial IFNγ and TNFα production upon K562 tumor target encounter. Taken together, these data indicate that ALT-803 can overcome NK cell functional deficiencies incurred by the ovarian cancer tumor microenvironment.
Figure 5. Defects in NK cell function induced by ovarian cancer patient soluble microenvironment are corrected with ALT-803 treatment.
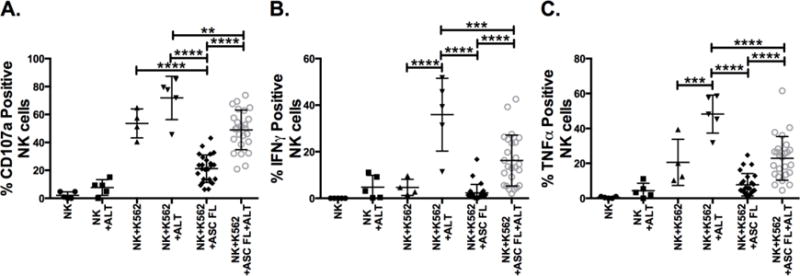
Healthy donor PBMCs were incubated in a 75% solution of the ascites from 10 ovarian cancer patients for 24hrs +/− ALT-803 (ALT). Controls were incubated in RPMI-10 +/− ALT-803. Cells were then incubated with +/− K562 targets for 4hrs to assess (A) CD107a, (B) IFNγ and (C) TNFα on PBNK cells. Symbols represent individuals within the treatment groups (n = 4–5 for no ascites fluid groups, n = 25 for ascites fluid groups (2 donors treated with ascites fluid from first 5 patients, 3 separate donors treated with ascites fluid from second 5 patients)). One-way ANOVA was used to compare statistical differences.
3.5. ALT-803 improves human NK cell activation in an in vivo xenogeneic model of ovarian cancer
Having shown that ALT-803 potently induces NK cell function against ovarian cancer cell lines and rescues function on ASC NK cells or PBNK submitted to ovarian cancer patient ascites fluid, we tested these findings in vivo. To ensure that ALT-803 mediated priming could be replicated in the peritoneal cavity of the NSG mice, which do not contain murine NK/T/NKT/γδ T/B cells, enriched human PBNK cells, from a CD3/CD19 depleted product, were injected intraperitoneally (IP) +/− a separate injection of ALT-803. Mice were sacrificed after 3 days to evaluate human NK cell activation in the peritoneal cavity. NK cells treated with ALT-803 in vivo displayed greater CD69, CD25, and CD56 expression, indicating that ALT-803 was inducing activation in vivo (Figures 6A–C). We next tested NK cell function +/− ALT-803 in an ovarian cancer xenogeneic mouse model in which MA-148 ovarian cancer cells express luciferase, allowing for luminescent measurement of the tumor real time (Fig. 6D). ALT-803 was injected directly IP or subcutaneously (SubQ) to test different possible routes of clinical administration. Measurements were taken at several time points with the greatest effect seen at day 28 (data not shown). While all NK treatments decreased tumor growth significantly, IP ALT-803 improved the control of tumor growth the most, showing a significant difference with the NK only group (Fig. 6E). With the exception of one outlier, the IP and SubQ routes behaved very similarly in terms of tumor growth. At day 29, peritoneal cells from these mice were harvested to evaluate function. Both IP and SubQ ALT-803 treatments induced robust NK cell expansion when compared to the NK only group, with the SubQ treatment generating the strongest expansion (Fig 6F). The outlier noted in Figure 6E correlated with the only mouse that did not expand NK cells in the SubQ group. Incubation with K562 targets induced NK cell degranulation, indicating that NK cells in these mice remained functional, with the greatest functionality noted in the SubQ group (Fig 6G). In summary, these data highlight the ability of ALT-803 to induce NK cell expansion and function in a relevant pre-clinical in vivo model.
Figure 6. ALT-803 induces human NK cell activation in peritoneal cavity of NSG mice and increase ovarian cancer tumor control in vivo.
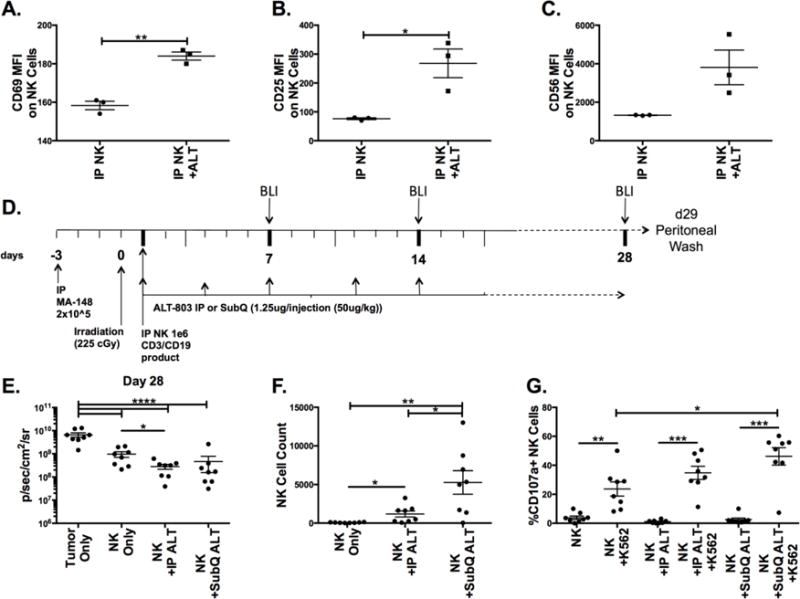
NSG mice were injected IP with one million NK cells, from a CD3/CD19 depleted product, followed by a IP injection containing +/− ALT-803 (ALT). Mice were harvested 3 days after injection to obtain peritoneal cells. (A) CD69, (B) CD25, and (C) CD56 MFI was assessed on NK cells after CD45 gating, to gate on human cells only (n = 3). (D) Schematic of xenogeneic ovarian cancer tumor model. (E) Mice were injected with Luciferin and imaged at d28 to evaluate tumor load via luminescence (p/sec/cm2/sr). Mice were sacrificed at d29 and peritoneal cells were harvested. (F) Numbers of NK cell events were assessed by flow cytometry in a 60-second acquisition. (G) Cells were activated in a 4hr assay with K562 cells and stained for surface CD107a. Circles represent individual mice in the treatment groups (n = 8). Unpaired t test (A–C), Oneway ANOVA (E–G), unpaired test (E–F, line without brackets) and paired t test (G, individual comparisons with no targets) were used compare statistical differences.
4. Discussion
There is limited data on the role of NK cells in the peritoneal cavity of women with ovarian cancer. NK cells derived from the peritoneal ascites of these women display a more immature phenotype with increased expression of CD56 and decreased expression of several activating and inhibitory receptors (16, 17). These cells exhibit reduced functionality mediated through direct or secreted inhibitory components within the tumor microenvironment (33, 34). There are a number of strategies to improve NK cell function in the tumor microenvironment that are being tested for other indications that might be useful in ovarian cancer. For instance, utilization of therapeutic antibodies or bi- and trispecific immune killer engagers have the potential to drive antigen-specific tumor lysis (35). However, utilization of these therapeutic reagents might be limited in the peritoneal cavity of ovarian cancer patients by low CD16 expression on NK cells, needed to drive ADCC. To address this several groups are exploring utilization of inhibitors of ADAM17, a matrix metalloproteinase involved in CD16 clipping, to maintain NK cell ADCC activity (36). We and others are currently exploring utilization and generation of adaptive NK cells, induced after CMV infection, or cytokine induced NK cells, via IL-12/18/15 priming, to enhance NK cell function in the ovarian cancer setting. However, in this study, for the first time, we show that an IL-15 super-agonist complex, ALT-803, is capable of rescuing the function of NK cells derived from the ascites of women with ovarian cancer. We provide additional information on the stimulatory activity of ALT-803 when delivered IP or SubQ in an in vivo ovarian cancer xenogenic model. This data suggests that ALT-803 could be used to exploit innate immune cells in ovarian cancer therapy.
The host immune system constantly monitors and detects mutated carcinogenic cells and eliminates them through the mechanism referred to as “cancer immunosurveillance” (37, 38). Unfortunately cancer cells can acquire the capacity to evade immunosurveillance. To counter this, IL-2 cytokine therapy has been used since the 1980s to amplify cytotoxic responses, showing promising efficacy against large invasive tumors (39). However, toxicities and induction of Treg expansion limit the use of IL-2, paving the way for utilization of other cytokines, such as IL-15, which display safer profiles and do not induce expansion of Treg cells (29). The ovarian cancer ascites is an environment that promotes inhibition of the innate immune system (18–22, 33, 34); an inhibition that we show here can be overcome by ALT-803 treatment. Nevertheless, when we evaluate our in vivo model, we note that although ALT-803 provides better protection than NK cells alone, the effect is not as impressive as that seen in vitro. It should be noted that our in vivo model contains only the NK cell segment of the immune response, thus the inflammatory effect of enhanced IFNγ secretion by NK cells to prime other components of the immune response is lost. An immune competent mouse model of ovarian cancer, in which ovaries are implanted with ovarian cancer cells from a transgenic mouse that develops spontaneous tumors (40), could be used to test this as ALT-803 signals on mouse NK cells too. However, it would be harder to segregate the impact of ALT-803 on the NK and CD8 T cell responses. Also, differences between mouse and human NK cells might decrease the pre-clinical applicability of this model. We are currently developing a humanized mouse model of ovarian cancer in which a more complete human immune system is engrafted into NSG-SGM3 mice via transfer of CD34 progenitors at an early age. This model may represent a better pre-clinical option to test ALT-803 and other immune based therapies. It should also be noted that the significant enhancement of NK cell IFNγ secretion mediated by ALT-803 treatment may have a negative role in our in vivo model. While inflammatory IFNγ in a fully competent immune system might provide better immune infiltration, in our xenogeneic system containing only NK cells, there is potential to induce expression of checkpoint ligands like PD-L1 (41), perhaps countering the beneficial effects of ALT-803. In fact, PD-1 blockade is currently being explored in treatment of platinum-resistant ovarian cancer patients (42). Future studies will address this issue through utilization of an ALT-803 and PD-1 blockade strategy. Another possible culprit perhaps mediating decreased NK cell function both in vitro and in vivo in the ovarian cancer indication might be HLA-KIR interactions. For instance HLA-G, which is expressed at high levels in a large proportion of primary ovarian cancer tissues, has been shown to counteract NK cell function, likely via KIR2DL4, ILT-2 or CD160 (43–45). To address whether or not ALT-803 treatment can bypass HLA–G mediated restriction of NK cell function, or whether disrupting HLAG interactions could further increase ALT-803 efficacy, upcoming studies will use commercial HLA-G blocking antibodies in conjunction with ALT-803 signaling to induce NK cell function against ovarian cancer.
Ongoing intramural trials at the NCI are testing whether continuous infusion of monomeric IL-15 (without IL-15Rα) provides optimal in vivo stimulation to lymphocytes. At our institution, we are testing subcutaneous (SubQ) outpatient dosing of the NCI rhIL-15 product in patients with advanced renal, lung, melanoma and head and neck cancers. Based on pre-clinical studies, other studies using rhIL-15, and published studies with IL-2, subcutaneous dosing appears to be safer and provides better tolerability. Similarly, eight open clinical trials are currently testing ALT-803 in the treatment of a variety of disease settings, including hematological malignancies and solid tumors. As data emerges from these clinical studies, and with our pre-clinical data findings, ALT-803 may become a viable outpatient immunotherapeutic to enhance immune cell proliferation and function for women with ovarian cancer. Our xenogeneic model seems to indicate better expansion of NK cells through subcutaneous administration of ALT-803, though there were no clear differences in tumor control when compared to intraperitoneal administration. Perhaps intraperitoneal administration causes better homing of cells to the tumors, creating a discrepancy in NK cell numbers when assessing peritoneal fluids. Furthermore, we plan to study route of delivery of ALT-803 (subcutaneous vs. intraperitoneal) in a clinical study of ovarian cancer. To this end we have designed a clinical trial in which women with epithelial ovarian, fallopian tube or primary peritoneal carcinoma receiving at least 3 cycles of first line IV/IP cisplatin and paclitaxel chemotherapy who have achieved a complete response will be eligible to receive ALT-803. 3 weeks after chemotherapy ALT-803 will be administered IP weekly for 4 weeks, followed by a 2 week rest, and weekly ALT-803 SubQ maintenance therapy 4 weeks on and 4 week off (X3). The objective of this initial study is to determine a safe dose to be used IP and SubQ, but a Phase II extension will aim to determine progression free survival. We hypothesize that ALT-803 delivered directly IP and SubQ will allow T cells and NK cells to persist, expand, and function in vivo. Immune cell activation, inflammation and immune modulation markers will be analyzed in peripheral blood and from peritoneal washings to determine immune cell infiltration and activation.
Highlights.
ALT-803 enhances NK cell cytotoxicity against ovarian cancer in vitro and in vivo
ALT-803 rescues functionality of NK cells from ovarian cancer patients
ALT-803 may enhance immunotherapeutic approaches for treatment of ovarian cancer
Acknowledgments
Research was supported by funding from the National Cancer Institute (R35 CA197292), the Minnesota Ovarian Cancer Alliance (MOCA) (Geller, M) “NK Cell Immunotherapy for Ovarian Cancer”, Mayo Clinic Ovarian Cancer SPORE (P50 CA136393), and by a Research Scholar Grant, (RSG-14-151-01-CCE) from the American Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Hing C. Wong is an employee and shareholder of Altor BioScience Corporation, but was only involved in editorial review of the manuscript and procurement of ALT-803. The remaining authors have no conflicts of interest to report.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Kandalaft LE, Powell DJ, Jr, Singh N, Coukos G. Immunotherapy for ovarian cancer: what’s next? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29(7):925–33. doi: 10.1200/JCO.2009.27.2369. Epub 2010/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galic V, Coleman RL, Herzog TJ. Unmet needs in ovarian cancer: dividing histologic subtypes to exploit novel targets and pathways. Current cancer drug targets. 2013;13(6):698–707. doi: 10.2174/15680096113139990002. Epub 2013/05/17. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LC-GJ, Katsaros D. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England journal of medicine. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 5.Tomsova M, Melichar B, Sedlakova I, Steiner I. Prognostic significance of CD3+ tumor-infiltrating lymphocytes in ovarian carcinoma. Gynecologic oncology. 2008;108(2):415–20. doi: 10.1016/j.ygyno.2007.10.016. Epub 2007/11/27. [DOI] [PubMed] [Google Scholar]
- 6.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. Epub 2005/12/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsten M, Bjorkstrom NK, Norell H, Bryceson Y, van Hall T, Baumann BC, Hanson M, Schedvins K, Kiessling R, Ljunggren HG, Malmberg KJ. DNAX accessory molecule-1 mediated recognition of freshly isolated ovarian carcinoma by resting natural killer cells. Cancer research. 2007;67(3):1317–25. doi: 10.1158/0008-5472.CAN-06-2264. [DOI] [PubMed] [Google Scholar]
- 8.Geller MA, Knorr DA, Hermanson DA, Pribyl L, Bendzick L, McCullar V, Miller JS, Kaufman DS. Intraperitoneal delivery of human natural killer cells for treatment of ovarian cancer in a mouse xenograft model. Cytotherapy. 2013;15(10):1297–306. doi: 10.1016/j.jcyt.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood reviews. 2006;20(3):123–37. doi: 10.1016/j.blre.2005.10.001. Epub 2005/12/21. [DOI] [PubMed] [Google Scholar]
- 10.Gammaitoni L, Leuci V, Mesiano G, Giraudo L, Todorovic M, Carnevale-Schianca F, Aglietta M, Sangiolo D. Immunotherapy of cancer stem cells in solid tumors: initial findings and future prospective. Expert opinion on biological therapy. 2014;14(9):1259–70. doi: 10.1517/14712598.2014.918099. Epub 2014/05/20. [DOI] [PubMed] [Google Scholar]
- 11.Patankar MS, Jing Y, Morrison JC, Belisle JA, Lattanzio FA, Deng Y, Wong NK, Morris HR, Dell A, Clark GF. Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecologic oncology. 2005;99(3):704–13. doi: 10.1016/j.ygyno.2005.07.030. Epub 2005/08/30. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nature reviews Immunology. 2012;12(4):239–52. doi: 10.1038/nri3174. Epub 2012/03/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley B, Felices M, Cichocki F, Cooley S, Verneris MR, Miller JS. The biology of NK cells and their receptors affects clinical outcomes after hematopoietic cell transplantation (HCT) Immunological reviews. 2014;258(1):45–63. doi: 10.1111/imr.12157. Epub 2014/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R, Pavlu J, Brisley G, de Lavallade H, Sarvaria A, Marin D, Mielke S, Apperley JF, Shpall EJ, Barrett AJ, Rezvani K. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica. 2014;99(5):836–47. doi: 10.3324/haematol.2013.087536. Epub 2014/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D, Dusenbery K, Bliss R, Downs LS, Miller JS. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13(1):98–107. doi: 10.3109/14653249.2010.515582. Epub 2010/09/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukesova S, Vroblova V, Tosner J, Kopecky J, Sedlakova I, Cermakova E, Vokurkova D, Kopecky O. Comparative study of various subpopulations of cytotoxic cells in blood and ascites from patients with ovarian carcinoma. Contemp Oncol (Pozn) 2015;19(4):290–9. doi: 10.5114/wo.2015.54388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belisle JA, Gubbels JA, Raphael CA, Migneault M, Rancourt C, Connor JP, Patankar MS. Peritoneal natural killer cells from epithelial ovarian cancer patients show an altered phenotype and bind to the tumour marker MUC16 (CA125) Immunology. 2007;122(3):418–29. doi: 10.11/j.1365-2567.2007.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fumita Y, Tanaka F, Saji F, Nakamuro K. Immunosuppressive factors in ascites fluids from ovarian cancer patients. Am J Reprod Immunol. 1984;6(4):175–8. doi: 10.1111/j.1600-0897.1984.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 19.Onsrud M. Immunosuppressive effects of peritoneal fluids from ovarian cancer patients. Gynecol Oncol. 1986;23(3):316–22. doi: 10.1016/0090-8258(86)90132-0. [DOI] [PubMed] [Google Scholar]
- 20.Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman JJ, Smith CV, Pecorelli S, Radominska-Pandya A, Cannon MJ, Parham GP. Phenotypic and functional analysis of tumor-infiltrating lymphocytes compared with tumor-associated lymphocytes from ascitic fluid and peripheral blood lymphocytes in patients with advanced ovarian cancer. Gynecol Obstet Invest. 2001;51(4):254–61. doi: 10.1159/000058060. doi: 58060. [DOI] [PubMed] [Google Scholar]
- 21.Giuntoli RL, 2nd, Webb TJ, Zoso A, Rogers O, Diaz-Montes TP, Bristow RE, Oelke M. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29(8):2875–84. [PubMed] [Google Scholar]
- 22.Tran E, Nielsen JS, Wick DA, Ng AV, Johnson LD, Nesslinger NJ, McMurtrie E, Webb JR, Nelson BH. Polyfunctional T-cell responses are disrupted by the ovarian cancer ascites environment and only partially restored by clinically relevant cytokines. PloS one. 2010;5(12):e15625. doi: 10.1371/journal.pone.0015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, Fleisher TA, Dubois SP, Perera LP, Stewart DM, Goldman CK, Bryant BR, Decker JM, Chen J, Worthy TA, Figg WD, Sr, Peer CJ, Sneller MC, Lane HC, Yovandich JL, Creekmore SP, Roederer M, Waldmann TA. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(1):74–82. doi: 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patidar M, Yadav N, Dalai SK. Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016;31:49–59. doi: 10.1016/j.cytogfr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncog. 2014;19(1–2):133–41. doi: 10.1615/critrevoncog.2014011091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilipow K, Roberto A, Roederer M, Waldmann TA, Mavilio D, Lugli E. IL15 and T-cell Stemness in T-cell-Based Cancer Immunotherapy. Cancer research. 2015;75(24):5187–93. doi: 10.1158/0008-5472.CAN-15-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralphamediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101(42):15154–9. doi: 10.1073/pnas.0406649101. Epub 2004/10/13. doi: 0406649101 [pii] 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi H, Dubois S, Sato N, Sabzevari H, Sakai Y, Waldmann TA, Tagaya Y. Role of trans-cellular IL-15 presentation in the activation of NK cell-mediated killing, which leads to enhanced tumor immunosurveillance. Blood. 2005;105(2):721–7. doi: 10.1182/blood-2003-12-4187. Epub 2004/09/16. doi: 10.1182/blood-2003-12-4187 2003-12-4187 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Rautela J, Huntington ND. IL-15 signaling in NK cell cancer immunotherapy. Current opinion in immunology. 2016;44:1–6. doi: 10.1016/j.coi.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Geller MA, Bui-Nguyen TM, Rogers LM, Ramakrishnan S. Chemotherapy induces macrophage chemoattractant protein-1 production in ovarian cancer. Int J Gynecol Cancer. 2010;20(6):918–25. doi: 10.1111/IGC.0b013e3181e5c442. [DOI] [PubMed] [Google Scholar]
- 31.Burleson KM, Boente MP, Pambuccian SE, Skubitz AP. Disaggregation and invasion of ovarian carcinoma ascites spheroids. J Transl Med. 2006;4:6. doi: 10.1186/1479-5876-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118(10):2784–92. doi: 10.1182/blood-2011-04-347070. Epub 2011/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gubbels JA, Felder M, Horibata S, Belisle JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP, Patankar MS. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol Cancer. 2010;9:11. doi: 10.1186/1476-4598-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Honig A, Hausler S, Voigt H, Becker JC, Leng L, Steinle A, Weller M, Bucala R, Dietl J, Wischhusen J. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol. 2008;180(11):7338–48. doi: 10.4049/jimmunol.180.11.7338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felices M, Lenvik TR, Davis ZB, Miller JS, Vallera DA. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol Biol. 2016;1441:333–46. doi: 10.1007/978-1-4939-3684-7_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis ZB, Felices M, Verneris MR, Miller JS. Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense Against Cancer. Cancer J. 2015;21(6):486–91. doi: 10.1097/PP0.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annual review of immunology. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. Epub 2004/03/23. [DOI] [PubMed] [Google Scholar]
- 38.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–48. doi: 10.1016/j.immuni.2004.07.017. Epub 2004/08/17. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192(12):5451–8. doi: 10.4049/jimmunol.1490019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nunez-Cruz S, Scholler N. Immunocompetent mouse model of ovarian cancer for in vivo imaging. Methods Mol Biol. 2013;1049:425–33. doi: 10.1007/978-1-62703-547-7_32. [DOI] [PubMed] [Google Scholar]
- 41.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual Faces of IFNgamma in Cancer Progression: A Role of PD-L1 Induction in the Determination of Pro- and Antitumor Immunity. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22(10):2329–34. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 42.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, Kanai M, Mori Y, Matsumoto S, Chikuma S, Matsumura N, Abiko K, Baba T, Yamaguchi K, Ueda A, Hosoe Y, Morita S, Yokode M, Shimizu A, Honjo T, Konishi I. Safety and Antitumor Activity of Anti-PD-1 Antibody, Nivolumab, in Patients With Platinum-Resistant Ovarian Cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(34):4015–22. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 43.Lin A, Yan WH, Xu HH, Gan MF, Cai JF, Zhu M, Zhou MY. HLA-G expression in human ovarian carcinoma counteracts NK cell function. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2007;18(11):1804–9. doi: 10.1093/annonc/mdm356. [DOI] [PubMed] [Google Scholar]
- 44.Rajagopalan S, Long EO. A human histocompatibility leukocyte antigen (HLA)-G-specific receptor expressed on all natural killer cells. The Journal of experimental medicine. 1999;189(7):1093–100. doi: 10.1084/jem.189.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajagopalan S. HLA-G-mediated NK cell senescence promotes vascular remodeling: implications for reproduction. Cell Mol Immunol. 2014;11(5):460–6. doi: 10.1038/cmi.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]


