Abstract
Background
Exposures to cholinesterase inhibitor pesticides (e.g. organophosphates) have been associated with children's neurobehavioral alterations, including attention deficit and impulsivity. Animal studies have observed transient alterations in neurobehavioral performance in relation to cholinesterase inhibitor pesticide exposures; however, limited evidence exists regarding transient effects in humans.
Methods
We estimated the associations between neurobehavioral performance and time after Mother's Day flower harvest (the end of a heightened pesticide usage period) among 308 4-to 9-year-old children living in floricultural communities in Ecuador in 2008 who participated in the ESPINA study. Children's neurobehavior was examined once (NEPSY-II: 11 subtests covering 5 domains), between 63-100 days (SD: 10.8 days) after Mother's Day harvest (blood acetylcholinesterase activity levels can take 82 days to normalize after irreversible inhibition with organophosphates).
Results
The mean (SD) neurobehavioral scaled scores across domains ranged from 6.6 (2.4) to 9.9 (3.3); higher values reflect greater performance. Children examined sooner after Mother's Day had lower neurobehavioral scores than children examined later, in the domains of (score difference per 10.8 days, 95%CI): Attention/Inhibitory Control (0.38, 0.10-0.65), Visuospatial Processing (0.60, 0.25-0.95) and Sensorimotor (0.43, 0.10-0.77). Scores were higher with longer time post-harvest among girls (vs. boys) in Attention/Inhibitory Control.
Conclusions
Our findings, although cross-sectional, are among the first in non-worker children to suggest that a peak pesticide use period may transiently affect neurobehavioral performance, as children examined sooner after the flower harvest had lower neurobehavioral performance than children examined later. Studies assessing pre- and post-exposure measures are needed.
Keywords: pesticides, neurobehavior, transient, cholinesterase, children, acute
Introduction
Early life exposures to commonly applied agricultural pesticides have been associated with neurobehavioral delays in children. In particular, organophosphate exposures have been associated with attention deficit hyperactivity disorder symptoms, including decreased attention and inhibitory control (Bouchard et al., 2011; Eskenazi et al., 2007; Horton et al., 2012; Kofman et al., 2006; Marks et al., 2010; Rauh et al., 2011, 2006), and there is growing evidence in children and animals that males may be more susceptible to the harmful effects of pesticide exposures than females (Dam et al., 2000; Horton et al., 2012; Johnson et al., 2009; Levin et al., 2001; Marks et al., 2010; Suarez-Lopez et al., 2013). Organophosphate insecticides exert their toxicity through inhibition of acetylcholinesterase (AChE) activity, which is an important regulator of the neurotransmitter acetylcholine, and likely through direct toxicity to neurons and glia (Abou-Donia, 2003; Aldridge et al., 2005; Qiao et al., 2003; Slotkin, 2004). We previously reported that lower AChE activity was associated with lower attention, inhibitory control and memory scores, among boys but not girls, within the Secondary Exposure to Pesticides among Children and Adolescents (ESPINA: Estudio de la Exposición Secundaria a Plaguicidas en Niños y Adolescentes) study, which examined children living in Ecuadorian floricultural communities (Suarez-Lopez et al., 2013).
A limited number of experimental studies indicate that pesticide exposures can also induce transient (subacute) decreases in neurobehavioral performance. In rats and zebrafish, single or recurrent exposures to organophosphates have been associated with initial decreases in neurobehavioral performance, followed by neurobehavioral improvement with greater time after removal of the exposure (Levin et al., 2003; Maurissen et al., 2000; Middlemore-Risher et al., 2010). Although limited information exists, human evidence appears to be congruent with experimental findings: seasonal use of pesticides has been found to increase pesticide exposures and to decrease neurobehavioral performance of agricultural workers (Khan et al., 2014; Rohlman et al., 2015). Furthermore, adults intoxicated with pesticides had lower neurobehavioral performance which improved over time (Delgado et al., 2004).
The objective of the present study was to estimate the associations between time after a peak pesticide spray season (Mother's Day flower harvest) and neurobehavioral performance among participants of the ESPINA study. This study examined children who lived in agricultural communities in Ecuador, but who did not work in agriculture. ESPINA study participants were examined during a low flower production season, but within approximately 100 days after Mother's Day (May). Mother's Day is one of the holidays with the most flower sales worldwide, and it is celebrated in May in 63% of countries in the world including the populous countries of China, India, USA, Brazil and Pakistan. Although the half-lives of organophosphates pesticides are short, normalization of erythrocytic AChE activity levels after irreversible inhibition (enzymatic aging) by organophosphates may take up to 3 months (Mason, 2000) and seasonal alterations of neurobehavioral performance may last for months after the end of pesticide applications (Rohlman et al., 2015). In the present study, we hypothesized that children assessed earlier in the examination period (closer to the end of the Mother's Day flower harvest) had lower neurobehavioral scores than children examined later.
Material and Methods
In 2008, we examined 313 healthy 4- to 9-year-old children and surveyed their parents as part of the ESPINA study in Pedro Moncayo County, Pichincha, Ecuador. Most participants of the ESPINA study (73%) were recruited from their participation in the “2004 Survey of Access and Demand of Health Services in Pedro Moncayo County”, collected by Fundacion Cimas del Ecuador in collaboration with the communities of Pedro Moncayo County. This was a representative survey of the county which obtained information of 71% of the population of Pedro Moncayo County, Ecuador and measured height and weight of 33% of children under the age of 5 years.
To supplement recruitment, new volunteers (27% of total sample) living in Pedro Moncayo County were also recruited through community announcements performed by leaders and governing councils, and by word of mouth. The ESPINA study aimed to have a balanced distribution of participants living with floricultural workers and non-agricultural workers. Participation of children was sought if they met the following criteria: cohabitation with a floricultural worker for at least one year for the group of children living with a floricultural worker; among children living with non-agricultural workers, they must have never cohabited with an agricultural worker, never inhabited a house where agricultural pesticides were stored and having no previous direct contact with pesticides. The ESPINA study comprises participants living in all 5 parishes of Pedro Moncayo county and has similar socio-economic and racial distributions as the general population of the county. Detailed participant recruitment information has been described elsewhere (Suarez-Lopez et al., 2012). Parents provided informed consent for themselves and for permission of participation of their children. Participants who were at least 7 years old provided assent for participation in the study. In total, 308 children had complete data for this study. This study was approved by the Institutional Review Boards of Fundación Cimas del Ecuador, the University of Minnesota and the University of California San Diego.
Floriculture in Pedro Moncayo County
The floriculture industry is central to the economy of Pedro Moncayo county using 5.3% of the geographic area (Gobierno Municipal del Canton Pedro Moncayo, 2011) (1800 hectares) and employing 21% of all adults in the county (Suarez-Lopez et al., 2012). The Ecuadorian floriculture industry uses many different pesticides (mostly insecticides and fungicides), of which diethyldithiocarbamate fungicides and organophosphate insecticides are commonly used (Grandjean et al., 2006; Harari, 2004). There is tremendous worldwide demand for flowers for Christmas (December 25), Valentine's Day (February 14), Easter (March/April) and Mother's Day (2nd Sunday in May). Flower plantations, thus, increase their production and pesticide use around October and decrease production in May. After the Mother's Day harvest, the production slows substantially for the summer months. In response to countries' strict no-tolerance policies for the importation of crops with pests (U.S. Department of Agriculture, 2012), the usage of pesticides in floriculture increases as crops mature and continue until soon (days or hours) prior to the harvest (Harari, 2004; Narvaez et al., 2002).
Measures
Each child was examined once during the period of lowest flower production of the year, and within 100 days after the Mother's Day harvest (July 10 through August 15, 2008). During this 37-day period, examinations took place on 20 weekdays days, averaging 15 participants per day. Exams were conducted in 7 schools distributed across the 5 parishes that make-up Pedro Moncayo County during the summer months, when schools were not in session. We calculated the number of days between the approximate end of the Mother's Day harvest (5/08/2008, 00:00 am) and the date and time of the beginning of the examination.
Five trained examiners applied the NEPSY-II test (Korkman et al., 2007a), a standardized test to assess neuropsychological development in children ages 3–16 years. Children were tested in 11 age-appropriate subtests in 5 domains: Attention and Inhibitory Control (also known as Attention and Executive Functioning; subtests: auditory attention and response set, inhibition, statue), Language (comprehension of instructions, speeded naming), Memory and Learning (memory-for-faces immediate and delayed, narrative memory), Sensorimotor (visuomotor precision) and Visuospatial Processing (design copying, geometric puzzles). Descriptions of each subtest have been described elsewhere (Kemp and Korkman, 2010; Korkman et al., 2007b; Suarez-Lopez et al., 2013). Three subtests required translation into Spanish using terminology appropriate for the local population (auditory attention and response set, comprehension of instructions and narrative memory). Translation of the NEPSY test has been found to be relatively unaffected by language and culture (Garratt and Kelly, 2008; Kofman et al., 2006; Mulenga et al., 2001). Participants were examined alone except when children experienced separation anxiety from their parents. In such cases (5 participants), one relative was allowed to be in the examination room and was instructed to remain silent and to sit between 2 and 4 meters away and outside of the child's line of sight.
Children's height was measured to the nearest mm following standard procedures (World Health Organization, 2008), and height-for-age z-score calculations were based on the World Health Organization normative sample (WHO Multicentre Growth Reference Study Group, 2006).
We conducted an in-person home survey of the children's parents or guardians to obtain information on socio-economic status, demographics, health and pesticide exposure information of household members.
Distance from participants' homes to the nearest flower plantation was assessed. Using portable global positioning system receivers, geographical coordinates of Pedro Moncayo County homes were collected in 2004, 2006 and 2010 by Fundacion Cimas del Ecuador as part of the System of Local and Community Information (Sistema de Información Local y Comunitario). Flower plantation edges (areal polygons) were created by measuring the geographic coordinates of each corner of each plantation's perimeter and plotting them on maps. The distance of each participant's home was calculated to the nearest 1-meter segment of flower plantation edge using ArcGIS 9.3 (Esri, Redlands, CA).
Statistical Analysis
Neurobehavioral subtest scaled scores were calculated using the NEPSY-II scoring assistant software (NCS Pearson Inc., San Antonio, TX). Most subtest scores consisted of primary scaled scores, age-adjusted values based on a national normative sample of US children (Korkman et al., 2007b). Higher scores represent higher performance. Domain scores were computed as the average of subtest primary scaled scores within a domain. For subtests that were composed of more than one primary scaled score (i.e., auditory attention and response set, inhibition and word list interference), we used the average of all available primary scaled scores per subtest for the domain score calculation. For subtests that included both correct and error components (i.e. auditory attention and response set) or time and error components (i.e. inhibition, speeded naming, visuomotor precision), we used the combined scaled scores (scores that combined both components) as primary scaled scores. Visuomotor precision was the only subtest within the sensorimotor domain; therefore, the sensorimotor domain score is equal to the visuomotor precision scaled score. We calculated a total neurobehavioral summary score which was the average of primary scores of all eleven subtests. Domain scores and the neurobehavioral summary score were used as the measures of neurobehavioral performance.
Associations between time after the Mother's Day harvest and neurobehavioral scores were analyzed using linear regression models in SAS Version 9.4 (SAS Institute Inc., Cary, NC). We used minimal and full adjustment models. The minimal adjustment models included age, sex, race and neurobehavioral examiner. The fully adjusted model, defined a-priori, also included height-for-age z-score (to estimate long-term nutritional status), income, distance to the nearest plantation edge and flower worker cohabitation status. The latter two variables are potential confounders to the association. In our study, children living with floricultural workers were found to have lower AChE activity compared to children living with non-agricultural workers (Suarez-Lopez et al., 2012); furthermore, AChE activity was positively associated with neurobehavioral scores in boys in this population (Suarez-Lopez et al., 2013). Distance to the nearest plantation edge and flower worker cohabitation are not considered to be in the causal pathway between the exposure (a construct of time) and the outcome (neurobehavior) when analyzing the data cross-sectionally. The fully adjusted model for neurobehavioral outcomes also included maternal education because it is a predictor of children's neurobehavioral performance (Brooks et al., 2010). Additionally, we analyzed data in a third model which included the full adjustment variables plus AChE activity and hemoglobin as mediation analyses.
We assessed effect modification by sex by testing interaction terms. All statistical models used the continuous variable of time after Mother's Day. We plotted adjusted means of neurobehavior by quintiles of time after Mother's Day harvest; associations that had significant interaction coefficients with sex were tested and plotted separately for boys and girls.
In order to include all children in multivariable analyses, we created a “missing” race category to account for 14 children with missing information. Because only 5 children in this study were white and 2 were black, we incorporated these 7 children in the mestizo (mix of white and indigenous) category to improve model stability when adjusting for race. We imputed missing information of maternal education for 15 children based on the household head's education in 2004. Income was imputed for 17 children according to 3 variables significantly associated with income: maternal education, type of housing in 2004 (i.e. house, apartment, room, shack) and residential building materials in 2004 (i.e. brick, adobe, wood). For children not examined in 2004, maternal education (n=3), income (n=4) and residential distance to the nearest flower plantation (n=3) were imputed from a random selection of values generated from a random normal distribution based on the concurrent ESPINA mean ± standard deviation (SD) values of the corresponding variable.
Sensitivity Analyses
Age-standardization of the neurobehavioral scaled scores (Korkman et al., 2007b) successfully removed the effect of age overall and in the domains of Sensorimotor, Language and Visuospatial processing. However, age remained associated negatively with the domain scores of Attention and Inhibitory control (β per year= -0.29, p<0.001), and Memory and Learning (β= -0.53, p<0.001) in full adjustment models. Because children examined earlier in the exam period were younger than children examined later (Table 1), we considered the possibility of residual confounding of age after its adjustment. For this reason, we tested all exposure-outcome associations among a randomly-selected subset of participants with an approximately equal distribution of age (in quartiles) across quartiles of time after Mother's Day harvest as sensitivity analyses. The random selection of participants was conducted using SAS 9.4 (‘surveyselect’ procedure). A total of 64 participants (21%) were excluded to achieve this “age-balanced subgroup” (n=244).
Table 1. Participant characteristics by exam date. N=308.
| Days after Mother's Day harvest (quintiles) | ||||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | P-Trend | |
| N | 61 | 62 | 62 | 62 | 61 | |
| Range (days) | 63.4-75.5 | 75.6-82.7 | 82.8-90.4 | 90.5-96.5 | 96.6- 99.6 | |
| Age, years | 6.2 (1.5) | 6.5 (1.4) | 6.6 (1.5) | 7.0 (1.7) | 6.9 (1.7) | 0.01 |
| Sex, male | 51 | 45 | 55 | 60 | 46 | 0.93 |
| Race, mestizo | 90 | 69 | 52 | 71 | 98 | 0.87 |
| Race, indigenous | 7 | 29 | 45 | 27 | 2 | 0.79 |
| Monthly household incomea | 3.0 (0.8) | 3.7 (0.7) | 3.3 (0.6) | 2.8 (0.7) | 2.8 (0.9) | 0.01 |
| Maternal education, years | 7.6 (4.4) | 8.6 (4.4) | 5.7 (2.8) | 6.8 (3.2) | 7.6 (3.7) | 0.81 |
| Flower worker cohabitation | 52 | 56 | 73 | 16 | 49 | 0.01 |
| Home distance to nearest flower plantation, m | 669 (472) | 245 (145) | 347 (248) | 461 (324) | 521 (282) | 0.24 |
| Height-for-age z-score, SD | -1.11 (0.78) | -1.05 (0.89) | -1.74 (0.94) | -1.22 (0.98) | -1.08 (1.04) | 0.98 |
| Hemoglobin, g/L | 12.0 (1.3) | 12.7 (0.9) | 12.8 (1.3) | 12.6 (1.0) | 13.1 (1.1) | <0.01 |
| Acetylcholinesterase, U/mL | 2.97 (0.51) | 3.10 (0.45) | 3.12 (0.50) | 3.13 (0.45) | 3.38 (0.44) | <0.01 |
Table entries are percentage or mean (SD)
Monthly income categories (USD): 1= 0-50, 2= 51-150, 3=151-300, 4=301-500, 5= 501-1000, 6=>1000
Results
Participant characteristics
The mean age of children at the time of assessment was 6.6 years (SD = 1.6); 51% were male, 76% mestizo, 22% indigenous, and 49% lived concurrently with at least one floricultural worker. The overall mean height-for-age z-score was -1.25 (SD: 0.98). Children were examined between 63 and 100 days after Mother's Day harvest [mean: 84.5 days, SD: 10.8]. Participants examined sooner after the harvest were younger, had greater household income, were more likely to live with a floricultural worker and had lower hemoglobin concentrations than those examined later. Additionally, AChE activity was lower in children examined earlier in the examination period compared to those examined later (p<0.01). Further analysis of this association is part of a separate manuscript. Participant characteristics are listed in Table 1 and distributions of neurobehavioral scores are listed in Table 2. Neurodevelopment scores in our study sample were lower but with similar variability than those of the NEPSY-II normative sample (Suarez-Lopez et al., 2013), which were designed to have a mean of 10 ± 3 for each subtest. The age-balanced subgroup had practically unchanged distributions across quintiles of days after Mother's Day harvest as the full sample, with the exception of age, in which a balanced distribution was achieved (Supplementary Table 1).
Table 2. Age-standardized neurobehavioral domain summary score means.
| Mean (SD) | Percentiles: 25th, 75th | |
|---|---|---|
| Total neurobehavior (n=308) | 8.6 (1.6) | 7.5, 9.8 |
| Attention and Inhibitory Control domain (N=302) | 8.5 (2.5) | 7, 10 |
| Memory and learning domain (N=305) | 8.8 (2.1) | 7.3, 10 |
| Visuospatial processing domain (N=308) | 9.6 (3.1) | 7.5, 11.5 |
| Language domain (N=307) | 6.6 (2.4) | 5, 8 |
| Sensorimotor domain (N=304) | 9.9 (3.3) | 7, 12 |
Time after the Mother's Day harvest and neurobehavior
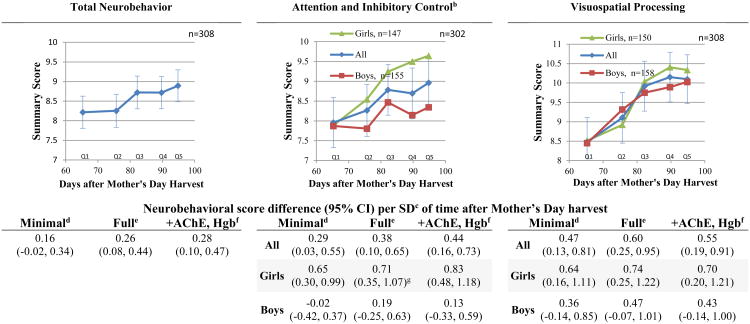
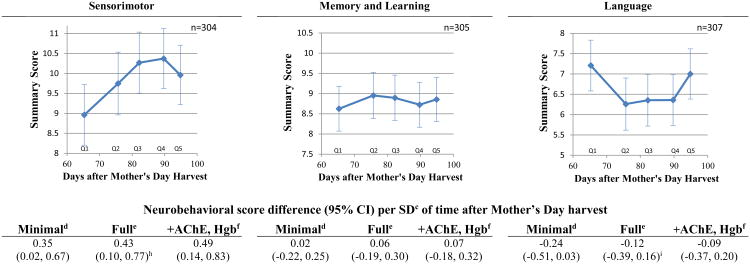
Neurobehavioral scores were lower among children tested sooner after Mother's Day than children examined later. Time after the Mother's Day harvest was positively associated with the following scores (score difference per SD of time [10.8 days], 95%CI): Total Neurobehavior (0.26, 0.08 to 0.44), Attention and Inhibitory Control (0.38, 0.10 to 0.65), Visuospatial Processing (0.60, 0.25 to 0.95) and Sensorimotor (0.43, 0.10 to 0.77); see Figure 1. There was evidence of effect modification by sex in the association between time after the Mother's Day harvest and Attention and Inhibitory Control performance in the minimal adjustment model only (p=0.03). The magnitude of the positive association between time after Mother's Day harvest and Attention and Inhibitory Control was substantially greater in girls compared to boys in both the minimally and fully adjusted models (Figure 1). Although the association in boys was also positive, it did not reach statistical significance. Although the interaction term was non-significant, we present similar associations stratified by sex with the outcome of Visuospatial processing. The differences of associations between boys and girls are less marked than those seen in Attention and Inhibitory control; however, they are roughly 1.5 times greater for girls.
Figure 1.
Children's neurobehavioral domain scores in relation to days after Mother's Day harvesta
a Figures depict full adjustment models
b P-sex interaction for each model= 0.03 (minimal), 0.11 (full)
c SD: 10.8 days
d Minimal adjustment model: age, sex, race and examiner. Quadratic associations are not shown.
e Full adjustment model: age, sex, race, examiner, height-for-age z-score, income, maternal education, distance to the nearest plantation edge and flower worker cohabitation.
f Full adjustment model plus hemoglobin and acetylcholinesterase activity. Quadratic associations are not shown.
g Quadratic model: β= 9.67, β-quadratic= -0.59 (95% CI: -1.10, -0.08)
h Quadratic model: β= 8.10, β-quadratic= -0.50 (95% CI: -0.95, -0.06)
i Quadratic model: β= -5.82, β-quadratic= 0.37 (95% CI: 0.002, 0.74)
The strength of the associations (slopes) decreased after quintile 3 (80-87 days after Mother's Day harvest) for the domains of Attention and Inhibitory Control, Visuospatial Processing, and Sensorimotor, as observed in Figure 1 and corroborated by significant quadratic terms in the Sensorimotor, and Attention and Inhibitory Control Domains (the latter was significant only among girls). We observed a significant a U-shaped association (pquadratic= 0.05) between time after Mother's Day harvest and Language domain score. Language performance of children was highest among children examined in quartile 1 (63-75 days after Mother's Day harvest), followed by a period of lower performance levels in children examined during quartiles 2-4 (76-96 days), and finalizing with greater performance among children examined in quartile 5 (97-100 days). We observed no associations between time after Mother's Day harvest and Memory and Learning domain performance. Further adjustment for AChE activity and hemoglobin concentration had overall a small effect on the association. However, in the Attention and Inhibitory Control domain it strengthened the associations by roughly 16%.
Time after the Mother's Day harvest and neurobehavior: sensitivity analyses (age-balanced subgroup)
The associations between time after the Mother's Day harvest and all neurobehavioral scores among the age-balanced subgroup were similar and slightly stronger than those of the full sample (Supplementary Figure 1). The fully adjusted neurobehavioral score difference (β, 95% CI) per standard deviation of time after Mother's Day harvest were: total neurobehavior (0.29, 0.09 to 0.49), Attention and Inhibitory Control (0.35, 0.04 to 0.65), Visuospatial Processing (0.63, 0.25 to 1.01), Sensorimotor (0.53, 0.16 to 0.90), Memory and Learning (0.07, -0.19 to 0.34) and Language (-0.13, -0.43 to 0.17). Similar to the full sample, effect modification by sex for the outcome of Attention and Inhibitory Control was observed in the minimal adjustment model only (p=0.03); significant associations in fully adjusted models were observed for girls (linear model: 0.64, 0.26 to 1.02; quadratic model: β= 10.07, β-quadratic= -0.62, 95% CI(quadratic)= -1.47 to -0.05), and for boys (β=0.19, 95% CI: -0.31 to 0.70).
Discussion
Mother's Day flower harvest is a known period of heightened pesticide use. It is an important source of pesticide exposures in this population considering that 1 in 5 adults worked in floriculture (Suarez-Lopez et al., 2012) and large areas of plantations exist near residential zones. As observed in Table 1, time after Mother's Day harvest was positively associated with AChE activity in unadjusted analyses, which indicates that it is an adequate indicator of pesticide exposures in this cohort of children. These findings were congruent with AChE and butyrylcholinesterase (BChE) decreases associated with pesticide spray seasons observed in longitudinal studies of farmworkers (Crane et al., 2013; Quandt et al., 2015). A more exhaustive analysis of the association between time after Mother's Day and AChE activity is part of a separate manuscript.
In the present cross-sectional study, children who were assessed later after Mother's Day had higher neurobehavioral scores than children assessed earlier, supporting the hypothesis that pesticide exposures induce short-term alterations in neurobehavioral performance in children. This hypothesis is also supported by a limited number of existing investigations on this topic (described below) (Delgado et al., 2004; Khan et al., 2014; Rohlman et al., 2015), and previously described positive associations between concurrent AChE activity and neurobehavioral performance in our cohort (Suarez-Lopez et al., 2013). These are important findings that highlight the vulnerability of children to non-occupational pesticide exposures, considering that children in this study did not work in agriculture but did live in agricultural communities. In this study, we conducted neurobehavioral examinations at 20 points in time averaging 15 participants per time point, within 63 and 100 days after the Mother's Day flower harvest. This allowed us to estimate neurobehavioral performance in relation to time since the harvest, although with cross-sectional data.
In the present study we observed lower overall neurobehavioral scores, particularly in the domains of Attention and Inhibitory Control, Visuospatial Processing, and Sensorimotor among participants examined earlier in the examination period (closer to Mother's Day) compared to those examined later. Our results are in agreement with animal studies that demonstrated reversible neurobehavioral alterations associated with pesticide exposures. A study of rats also reported an initial decline in sustained attention during a 14-day period of repeated exposures to chlorpyrifos, followed by linear improvements in sustained attention back to baseline throughout a 30-day washout period (Middlemore-Risher et al., 2010). Another study of rat pups with early life exposures to chlorpyrifos reported a decrease in automated motor activity at post-natal day 13 which was worse among pups that received greater doses of the pesticide. Even though the findings were not statistically significant viewed only in the context of the original study design, the decrease among exposed pups was followed by age-related improvements in motor activity at days 17, 21 and 60, in a pattern which was similar to that of unexposed pups (Maurissen et al., 2000). The motor scores at days 17, 21 and 60 were not different between the unexposed controls and the exposed PUPs. An 18-week study of zebrafish reported significant decreases in spatial discrimination within the first 6 weeks after a low-dose exposure to the organophosphate chlorpyrifos (compared to unexposed fish), and their performance in the test improved thereafter (Levin et al., 2003). However, a higher dose was associated with more pervasive performance decreases.
A limited number of studies in humans have assessed neurobehavioral performance alterations over time since a known pesticide exposure. Neurobehavioral alterations in relation to a pesticide spray season have been observed in a detailed 10-month study of 89 adolescent agricultural workers that measured pesticide exposures and neurobehavioral performance before, during and after a pesticide application season (Crane et al., 2013; Khan et al., 2014; Rohlman et al., 2015). In this study, urinary pesticide markers increased during the application season and decreased shortly thereafter; an inverse effect was observed with AChE and BChE activity (Crane et al., 2013). Additionally, there was an increased number of self-reported altered neurological/motor/behavioral symptoms (Khan et al., 2014), and decreased performance in some neurobehavioral tasks during the pesticide application period (Rohlman et al., 2015); both the altered symptoms and neurobehavioral performance persisted for some months after the end of the application season. In a study in Nicaragua, pesticide intoxicated adults had lower neurobehavioral performance (mostly in visuospatial processing) compared to other hospitalized patients without history of exposure. The deficits were most salient soon after the poisoning, followed by a prolonged recovery period during the 2-year follow-up for most neurobehavioral outcomes (Delgado et al., 2004). Among a medium exposure group, a visuomotor deficit was observed at 7 weeks after poisoning which disappeared thereafter. In the same Ecuadorian population as our study, a pilot study also observed delays in children's reaction time among children with concurrent pesticide exposures (Grandjean et al., 2006). Contrasting with our findings, a pilot study of children found no effect of pesticide application seasons on neurobehavioral performance (Fiedler et al., 2015). The lack of association may have occurred due to its small sample size (n=53). The limited number of studies that directly assessed the acute effects of agricultural seasons on children living in agricultural populations highlights the importance of conducting such studies.
We observed that the slopes of the associations of time after Mother's Day harvest with the domains of Attention and Inhibitory Control, Visuospatial Processing and Sensorimotor diminished between 80-87 days (third quintile) after the harvest. This possibly indicates that the transient “decrease” in neurobehavioral performance may begin to be resolved at around this time. It is reassuring that this timeframe coincides with the 82 days needed for full recovery of systemic erythrocytic AChE activity after irreversible inhibition by organophosphates (Mason, 2000). We previously observed strong associations between AChE activity and neurobehavior among boys of this study population (Suarez-Lopez et al., 2013). Erythrocytic AChE activity may pertain most to neurobehavioral outcomes as it is most similar to neuronal AChE activity (Aaron CK, 2007) and brain AChE has been reported to be diminished after organophosphate exposures in rats (Dam et al., 2000; Johnson et al., 2009; Qiao et al., 2002; Richardson and Chambers, 2005; Song et al., 1997).
The positive association between time after the harvest and Attention and Inhibitory Control performance was stronger among girls than boys. It is noteworthy that the scores of boys and girls in this domain were equal during quartile 1 of time after Mother's Day harvest (Figure 1). Although we lack pre-exposure performance information (an important limitation) we discuss 2 potential interpretations of our findings: A) there was a slower recovery of neurobehavioral performance in boys after removing the exposure. This is concordant with prior findings in this study population and in two other US studies where boys were more susceptible than girls to neurobehavioral alterations (particularly in attention, inhibitory control and memory) in relation to pesticide exposures (Horton et al., 2012; Marks et al., 2010; Suarez-Lopez et al., 2013); B) assuming that the basal (pre-exposure) attention and inhibitory control scores are higher for girls than boys, for the reasons described below, the transient decrease in neurobehavioral performance was greater among girls than boys; then, performance improved back to basal levels with greater time after the end of the harvest. In this study population, girls had higher mean score of Attention and Inhibitory Control than boys (8.95 vs 8.13, p=0.004) (Suarez-Lopez et al., 2013). Also, it is well established that girls and boys show differences in profiles of attention, impulsivity and susceptibility to disorders of attention (Davies, 2014), and girls in the general US population are half as likely as boys to be diagnosed with attention deficit disorder (Visser et al., 2014).
It will be important for future studies assessing short-term effects of pesticide exposures to include measures of neurobehavioral performance during pre-exposure, exposure and post-exposure periods, where possible.
It is plausible that the described positive associations between time after the harvest and neurobehavioral performance are due to examiner improvements over time. Because positive associations were not observed in all domains, we think this would be an unlikely explanation. The NEPSY-2 is designed to reduce potential biases introduced by the examiners (Kemp and Korkman, 2010). Many subtests do not involve direct interaction with the examiners (i.e. auditory attention and response set, visuomotor precision, design copy), whereas most other subtests require minimal to moderate interaction.
Although our findings are suggestive of transient neurobehavioral alterations in children, children living in areas with long agricultural seasons may have neurobehavioral alterations present during most of the year. The neurobehavioral alterations we observed can affect learning, cognition, social interactions and overall well-being of these children. In this study population, the peak flower growing seasons coincide with the school year and may be affecting the children's academic success. In addition to the effect of pesticide exposures, chronically altered neurobehavior in childhood can have negative long-term effects on children's development (Beckett et al., 2007; Pollak et al.; Windsor et al., 2007).
Considering that pesticide exposures may induce transient neurobehavioral changes and that prenatal exposures may be predictors of future exposures (e.g. children born to agricultural families are likely to have greater exposures both in-utero and throughout childhood than children born to non-agricultural families), it would be beneficial that longitudinal studies assessing chronic mental health effects of pesticide exposures involve follow-up information of exposure and neurobehavioral performance collected concomitantly.
We considered the possibility of residual confounding by age in our adjusted results given that a greater proportion of younger children were examined earlier in the examination period. Because of this, we conducted the same analyses in a randomly-selected subset of participants that had a balanced distribution of age across time after Mother's Day harvest (see Sensitivity Analyses: age-balanced subgroups). The associations observed in this age-balanced subset were similar (slightly stronger) to those of the full sample, which is an indication that statistical correction of age adequately removed confounding by age thus, supports the validity of our findings. The NEPSY-II age-corrected scaled scores, which reflect the relative performance of a child to a normative sample of children of the same age in the US (Kemp and Korkman, 2010), successfully removed the effect of age overall. However, this was not so for 2 of the 5 domains (Attention and Inhibitory control, and Memory and Learning), in which older children had lower scores than younger children. Considering the growing body of scientific evidence showing that pesticide exposures in early childhood can have long-lasting neurobehavioral impact in children, we can speculate that the basal neurobehavioral levels in these two domains may be lower in this study population due to chronic pesticide exposures. Although we did not observe significant short-term effects of time after Mother's Day with the Memory and Learning domain, we did observe previously strong positive associations with AChE activity (Suarez-Lopez et al., 2013). This may also reflect some deficiencies of using non-Ecuadorian normative samples, although we consider this less likely as this was not observed across all domains, and the NEPSY has been found to be relatively unaffected by language and culture (Garratt and Kelly, 2008; Kofman et al., 2006; Mulenga et al., 2001).
This study lacks neurobehavioral assessments in the same individuals at more than one point in time (e.g. both during and after the heightened exposure period), which does not allow us to assess change in neurobehavioral performance within individuals. This is an important limitation. The ESPINA study was not specifically designed to assess neurobehavioral performance change in relation to a peak pesticide exposure period. The present analyses are only possible due to the short examination window achieved in ESPINA, which occurred at a time relatively soon after Mother's Day. However, our study findings may have been more informative had we examined participants in a wider window of time. Nonetheless, the present examination window coupled with the relatively large sample size of our study allowed us to estimate associations from examinations conducted at 20 points in time with an average of 15 participants per time point. This allowed us to have a much smoother modeling of associations across time (although cross-sectionally) than previous studies which estimated associations using comparisons between 2 and 4 time periods.
Our cross-sectional design which compared neurobehavioral performance across children examined at different points in time avoids the psychometric biases (measurement and predictive) associated with re-testing participants using psychometric tests (Lievens et al., 2007). These biases are related to the ability of participants to change or improve their performance after repeat testing.
It is worth noting that the findings of this investigation may be reflecting the influence of a composite of various classes of insecticides, fungicides and herbicides used concomitantly in floriculture. A lack of measures of different classes of pesticides is a limitation. However, the described positive associations between time after Mother's Day harvest and AChE activity (Table 1) is an indication that cholinesterase inhibitors (organophosphates and carbamates) are used, and are reaching children in sufficient amounts to induce a physiological change.
Conclusions
Among non-worker children living in agricultural communities in Ecuador, we observed a direct association between time after the end of a period of heightened pesticide usage (Mother's Day flower harvest) and neurobehavioral performance with cross-sectional data. Although our study design does not allow us to assess change in performance, our findings are consistent with the concept that a peak pesticide use period may transiently affect neurobehavioral performance. These associations were observed for the domains of Attention and Inhibitory Control, Sensorimotor and Visuospatial processing. Our findings need to be replicated in studies of children with assessments conducted before, during and after peak exposure periods where possible.
Children living in areas with long agricultural seasons may have alterations in neurobehavioral performance during most of the year, which may affect their short- and long-term learning abilities, cognition, social interactions and overall well-being. Added precaution is advised.
Supplementary Material
Highlights.
Little is known about acute neurobehavior change related to pesticide spray periods
Ecuador's Mother's Day (MD) flower production is a period of high pesticide use
We examined 308 non-worker children aged 4-9y, once between 63-100 days after MD
Neurobehavioral scores were worse in children examined sooner (vs later) after MD
Associations were strongest with attention/inhibition, visuospatial, and sensorimotor
Acknowledgments
We thank Dr. Jose Suarez Torres, Dolores Lopez Paredes and Fundación Cimas del Ecuador for providing the infrastructure, logistical support, access to the Local and Community Information System and their long history of collaboration with Pedro Moncayo County communities, all of which were key to the success of this study. We also thank the Tabacundo Health Center of the Ministry of Public Health of Ecuador, for their assistance, and especially the people of Pedro Moncayo County and their local governments for their collaboration and support of this project. We also thank Dr. Megan Gunnar for her contributions to this manuscript.
Funding Sources: Research reported in this publication was supported by the National Institute of Occupational Safety and Health (1R36OH009402-01) and the National Institute of Environmental Health Sciences of the National Institutes of Health under Awards R01ES025792-01, R21ES026084-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of funding source: None
Abbreviations
- AChE
acetylcholinesterase
- BChE
butyrylcholinesterase
- ESPINA study
The Secondary Exposure to Pesticides among Children and Adolescents Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaron CK. Organophosphates and carbamates. In: Haddad LM, Borron SW, Burns MJ, W SM, editors. Haddad and Winchester's Clinical Management of Poisoning and Drug Overdose. Saunders/Elsevier; Philadelphia: 2007. [Google Scholar]
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–97. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, Hawkins A, Kreppner J, O'Connor TG, Stevens S, Sonuga-Barke EJS. Scholastic attainment following severe early institutional deprivation: a study of children adopted from Romania. J Abnorm Child Psychol. 2007;35:1063–73. doi: 10.1007/s10802-007-9155-y. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119:1189–95. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BBL, Sherman EMS, Iverson GL. Healthy children get low scores too: prevalence of low scores on the NEPSY-II in preschoolers, children, and adolescents. Arch Clin Neuropsychol. 2010;25:182–90. doi: 10.1093/arclin/acq005. [DOI] [PubMed] [Google Scholar]
- Crane AL, Abdel Rasoul G, Ismail AA, Hendy O, Bonner MR, Lasarev MR, Al-Batanony M, Singleton ST, Khan K, Olson JR, Rohlman DS. Longitudinal assessment of chlorpyrifos exposure and effect biomarkers in adolescent Egyptian agricultural workers. J Expo Sci Environ Epidemiol. 2013;23:356–62. doi: 10.1038/jes.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Davies W. Sex differences in Attention Deficit Hyperactivity Disorder: Candidate genetic and endocrine mechanisms. Front Neuroendocrinol. 2014 doi: 10.1016/j.yfrne.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Delgado E, McConnell R, Miranda J, Keifer M, Lundberg I, Partanen T, Wesseling C. Central nervous system effects of acute organophosphate poisoning in a two-year follow-up. Scand J Work Environ Health. 2004;30:362–70. doi: 10.5271/sjweh.824. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler N, Rohitrattana J, Siriwong W, Suttiwan P, Ohman Strickland P, Ryan PB, Rohlman DS, Panuwet P, Barr DB, Robson MG. Neurobehavioral effects of exposure to organophosphates and pyrethroid pesticides among Thai children. Neurotoxicology. 2015;48:90–99. doi: 10.1016/j.neuro.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garratt LC, Kelly TP. To what extent does bilingualism affect children's performance on the NEPSY? Child Neuropsychol. 2008;14:71–81. doi: 10.1080/09297040701218405. [DOI] [PubMed] [Google Scholar]
- Gobierno Municipal del Canton Pedro Moncayo. El Cantón [WWW Document] :2011. URL http://www.pedromoncayo.gob.ec.
- Grandjean P, Harari R, Barr DB, Debes F. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117:e546–56. doi: 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- Harari R. Seguridad, salud y ambiente en la floricultura. IFA, PROMSA; Quito: 2004. [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol Teratol. 2012;34:534–41. doi: 10.1016/j.ntt.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FO, Chambers JE, Nail Ca, Givaruangsawat S, Carr RL. Developmental chlorpyrifos and methyl parathion exposure alters radial-arm maze performance in juvenile and adult rats. Toxicol Sci. 2009;109:132–42. doi: 10.1093/toxsci/kfp053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp SL, Korkman M. Essentials of NEPSY-II assessment, Essentials of psychological assessment series. John Wiley & Sons; Hoboken, N.J: 2010. [Google Scholar]
- Khan K, Ismail AA, Abdel Rasoul G, Bonner MR, Lasarev MR, Hendy O, Al-Batanony M, Crane AL, Singleton ST, Olson JR, Rohlman DS. Longitudinal assessment of chlorpyrifos exposure and self-reported neurological symptoms in adolescent pesticide applicators. BMJ Open. 2014;4:e004177. doi: 10.1136/bmjopen-2013-004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O, Berger A, Massarwa A, Friedman A, Jaffar AA. Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatr Res. 2006;60:88–92. doi: 10.1203/01.pdr.0000219467.47013.35. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp SL. NEPSY-II: A developmental neuropsychologial assessment 2007a [Google Scholar]
- Korkman M, Kirk U, Kemp SL. NEPSY II: Clinical and Interpretive Manual. 2nd. The Psychological Corporation; San Antonio, TX: 2007b. [Google Scholar]
- Levin ED, Addy N, Nakajima a, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Brain Res Dev Brain Res. 2001;130:83–9. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–57. doi: 10.1016/S0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Lievens F, Reeve CL, Heggestad ED. An examination of psychometric bias due to retesting on cognitive ability tests in selection settings. J Appl Psychol. 2007;92:1672–1682. doi: 10.1037/0021-9010.92.6.1672. [DOI] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, Calderon N, Eskenazi B. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environ Health Perspect. 2010;118:1768–74. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason HJ. The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after over-exposure to dichlorvos. Occup Med (Lond) 2000;50:343–7. doi: 10.1093/occmed/50.5.343. [DOI] [PubMed] [Google Scholar]
- Maurissen JP, Hoberman aM, Garman RH, Hanley TR. Lack of selective developmental neurotoxicity in rat pups from dams treated by gavage with chlorpyrifos. Toxicol Sci. 2000;57:250–63. doi: 10.1093/toxsci/57.2.250. [DOI] [PubMed] [Google Scholar]
- Middlemore-Risher ML, Buccafusco JJ, Terry aV. Repeated exposures to low-level chlorpyrifos results in impairments in sustained attention and increased impulsivity in rats. Neurotoxicol Teratol. 2010;32:415–24. doi: 10.1016/j.ntt.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga K, Ahonen T, Aro M. Performance of Zambian children on the NEPSY: a pilot study. Dev Neuropsychol. 2001;20:375–83. doi: 10.1207/S15326942DN2001_4. [DOI] [PubMed] [Google Scholar]
- Narvaez A, Betancourt O, Vera B, Maldonado M, Zabala A, Chalen C. Línea de Base Prevención y eliminación progresiva del trabajo infantil en la floricultura en los cantones Cayambe y Pedro Moncayo. Organizacion Internacional de Trabajo; Lima: 2002. [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Frenn KA, Loman MM, Gunnar MR. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 81:224–36. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: what is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Env Heal Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt SA, Pope CN, Chen H, Summers P, Arcury TA. Longitudinal Assessment of Blood Cholinesterase Activities Over 2 Consecutive Years Among Latino Nonfarmworkers and Pesticide-Exposed Farmworkers in North Carolina. J Occup Environ Med. 2015;57:851–7. doi: 10.1097/JOM.0000000000000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera Fp, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats. Toxicol Sci. 2005;84:352–9. doi: 10.1093/toxsci/kfi081. [DOI] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Rasoul GA, Bonner MR, Hendy O, Mara K, Wang K, Olson JR. A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex. 2015;74:383–395. doi: 10.1016/j.cortex.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin Ta. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–74. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Himes JH, Jacobs DR, Alexander BH, Gunnar MR. Acetylcholinesterase activity and neurodevelopment in boys and girls. Pediatrics. 2013;132:e1649–58. doi: 10.1542/peds.2013-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, Alexander BH, Lazovich D, Gunnar M. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ Res. 2012;114:53–9. doi: 10.1016/j.envres.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. Cut Flowers and Greenery Import Manual. Washington, D.C: p. 2012. [Google Scholar]
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR, Kogan MD, Ghandour RM, Perou R, Blumberg SJ. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53:34–46.e2. doi: 10.1016/j.jaac.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Windsor J, Glaze LE, Koga SF. Language acquisition with limited input: Romanian institution and foster care. J Speech Lang Hear Res. 2007;50:1365–81. doi: 10.1044/1092-4388(2007/095). [DOI] [PubMed] [Google Scholar]
- World Health Organization. Training Course on Child Growth Assessment. 2008;7:25–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.