Abstract
Objective
Ovarian carcinomas that originate from fallopian epithelial cells are suggested to arise due to repeated exposure to ovulatory follicular fluid (FF). Mechanistic explanation(s) for how this occurs are unknown. Here, we sought to understand if FF exposure to fallopian epithelial cells could induce DNA damage and expression of a known family of DNA mutators, apolipoprotein B mRNA editing enzyme, catalytic polypeptide (APOBEC) cytidine deaminases.
Methods
Follicular fluid and matched patient plasma samples were obtained from donors. Fallopian epithelial cells (FT33-TAg, FT189, FT190, and FT194) were cultured with FF or plasma for 24 hr, and cell proliferation and DNA damage were assessed. Effects of FF on Apobec gene expression was determined by qRT-PCR and western blot analyses. Fallopian epithelial cells were transfected with an APOBEC3A expression vector and DNA damage was assessed.
Results
Follicular fluid exposure increased epithelial cell proliferation as measured by three independent methods, and DNA damage accumulation as assessed using three independent measures. This effect was specific to FF, as matched patient plasma did not have the same effects. Increased expression of Apobec3a was observed in fallopian epithelial cells following exposure to 5 of 8 patient FF samples, and transient overexpression of APOBEC3A was sufficient to induce double strand DNA breaks.
Conclusions
Follicular fluid can induce cell proliferation and DNA damage accumulation in cultured fallopian epithelial cells. Increased expression of APOBEC3A, a known DNA mutator, may explain the high incidence of DNA damage after FF exposure. The role of Apobec3a in ovulation-induced inflammation warrants further investigation.
Keywords: Follicular fluid, fallopian tube, Apobec, ovulation, DNA damage, ovarian cancer
Introduction
High-grade serous carcinomas make up the greatest proportion of ovarian neoplasms and have the worst prognosis, with long-term survival rates of < 30% [1]. Non-invasive precursor lesions have been identified at the distal ends of the fallopian tube fimbria, leading to the hypothesis that the fallopian tube serves as the origin of a majority of high-grade serous carcinomas, rather than the ovarian surface epithelium [2–4]. Additionally, precursor lesions in fallopian fimbria frequently contain mutations in the tumor suppressor gene TP53 identical to the TP53 mutations occurring in the carcinoma, supporting a clonal relationship [5–7]. The non-genetic risk factor most positively associated with ovarian cancer is ovulation, although the causative factors of this association are unknown [8, 9]. During ovulation, rupture of the preovulatory ovarian follicle releases the cumulus-oocyte-complex and the follicular fluid (FF). These materials are immediately juxtaposed to the fimbria of the fallopian tube and are actively funneled into the infundibulum in order to facilitate fertilization of the egg. It is hypothesized that the continual repetitive exposure to certain factors within FF may have detrimental effects on adjacent epithelial cells [10].
The physiological process of ovulation is inflammatory, and coincides with dramatic fluctuations in steroidal estrogen and progesterone secretion from the maturing ovulatory follicle following the surge of LH [11]. Inflammation is known to drive carcinogenesis processes [12], whereas hormonal signaling has been implicated in tumor progression [13]. Inflammatory factors increase in FF as ovulation approaches [14]. Previous studies have shown that human FF can induce an inflammatory signature in bovine oviductal epithelial cells [15]. This has led to the idea that FF may be tumorigenic, and that repetitive exposure of fallopian epithelial cells to this complex fluid may cause mutations and alterations leading to neoplastic transformation [10]. Three studies support the hypothesis that FF can cause DNA damage marks and cell proliferation in cultured fallopian epithelial cells [16–18]. In a mouse model, ovulation induced γH2A.X foci in oviductal epithelial cells in vivo after gonadotropin stimulation in a proliferation independent manner [16]. In another study, incubation of fallopian epithelial cells with FF for 24 hr significantly increased γH2A.X foci compared with culture medium alone [17]. The third study used human FF and immortalized human fallopian epithelial cells to show that individual FF stratified into two groups: one with high reactive oxygen species (ROS) that increased the number of cells positive for γH2A.X, and one with low ROS that did not increase γH2A.X [18]. Although these studies suggest FF is responsible for inducing DNA damage, studies designed to control for the plasma constituents in FF, and a hypothesis driving the DNA-damaging properties of FF are essential to understand early molecular changes that occur during fallopian carcinogenesis related to ovulation.
In our study, we compared the effects of exposure of FF and matched patient plasma on the induction DNA damage, and investigated a group of apolipoprotein B mRNA editing enzyme, catalytic polypeptide (APOBEC) cytidine deaminases as a possible mechanism for DNA damage accumulation. Activity of APOBEC enzymes can deaminate single stranded DNA during DNA replication, and ultimately induce double strand breaks via repair of the deoxyuridine by base excision repair [19, 20]. One family member called activation-induced cytidine deaminase (AID) has been identified as increased following exposure to FF, however a comprehensive study of the APOBEC family has not yet been performed [21].
Materials and Methods
Cell lines and cell cultures
Immortalized fallopian epithelial cell lines (FT33-TAg, FT189, FT190, and FT194) were cultured as previously described [22] and were generously provided by Dr. Ronny Drapkin (University of Pennsylvania, Philadelphia, PA). The FT33-TAg cell line was authenticated by short tandem repeat profiling (www.ddcmedical.com) and exhibited no evidence of cross-contamination with any known ATCC cell lines nor mycoplasma. Fallopian epithelial cells were cultured with 2.5% Ultraser G (2X serum, USG, Pall Corporation, Port Washington, NY). All media conditions were supplemented with 10 units/mL penicillin and 10 μg/ml streptomycin and cells were maintained in a humidified incubator with 5% CO2 at 37 °C.
Follicular fluid and plasma patient material
Follicular fluid samples were obtained from eight patients undergoing in vitro fertilization (IVF) at the University of Iowa Hospitals and Clinics as previously described [23]. Patient material was obtained from women ≤ 38 years old, with male factor or tubal infertility. Patients demonstrated normal ovulatory function clinically and the etiology of infertility was not ovarian in nature. Data was collected on body mass index (BMI) in kg/m2 and parity. Patients were stratified into a “Low BMI” group (n=4; BMI<25) and a “High BMI” group (n=4; BMI>25), however we did not observe significant differences between these groups in our results, therefore all patients were combined into one group. These two groups had the same numbers of live births.
All IVF cycles were preceded by one month of oral contraceptive pills and patients received the same gonadotropin formulations (follitropin beta, Follistim AQ: Merck & Co., Inc.; human menopausal gonadotropin/hMG, Menopur: Ferring Pharmaceuticals Inc., USA). Ovulation was triggered with 10,000 IU of chorionic gonadotropin (APP Pharmaceuticals, LLC, USA) when at least 2 follicles of ≥18 mm in diameter were achieved. Fluid was aspirated from the first follicle into a single sterile tube without culture medium and centrifuged at 3,000 rpm for 15 min at 4 °C to eliminate potential cell contaminations. The FF sample was flash-frozen in liquid nitrogen prior to storage at −80 °C. The remaining FF collected during the retrieval was pooled, centrifuged at 3,000 rpm for 15 min at 4 °C, and stored at −80 °C. Plasma was collected from seven of the eight patients at the time of FF aspiration according to standard procedure into Acid Citrate Dextrose (ACD) tubes.
Protein analysis
Cells (20 μg protein) were lysed in SDS sample buffer with 5% β-mercaptoethanol, boiled for 5 min at 95 °C, and subjected to electrophoresis using 10% SDS-PAGE in running buffer at constant 120 V for 1 hr. Proteins were electro-transferred onto nitrocellulose membranes, and blocked with 5% (w/v) skim milk in Tris-buffered saline with 0.05% (v/v) Tween-20 (TBST) for 1 hr at room temperature. Membranes were then probed with primary anti-APOBEC3A (1:250; HPA043237, Sigma, St. Louis, MO) or anti-Actin (1:500; sc-1616 Santa Cruz, Dallas, TX) antibody overnight at 4 °C TBST/BSA (50 mM Tris, 150 mM NaCl, 0.05% Tween20, 1.5% BSA) followed by incubation with the secondary rabbit IgG HRP linked antibodies (GENA934, Sigma) for 2 hr at room temperature in blocking buffer. Membranes were washed three times in TBST and detected by enhanced chemiluminescence (Thermo Fisher Scientific, Waltham, MA).
The Genotype-Tissue Expression (GTEx) Project database was utilized to identify Apobec and AID expression in normal fallopian tube epithelium (obtained from the GTEx Portal on 11/03/16). GTEx was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. RNA-Seq data from 6 fallopian tube epithelial cells are reported as average RPKM (reads per kilobase per million mapped reads).
RNA isolation and quantitative RT-PCR analysis
RNA was isolated using TRI Reagent Solution (Thermo Fisher Scientific), with a DNase I digestion step. Concentration and purity of RNA was evaluated using a Nanodrop spectrophotometer ND-1000 (Thermo Fisher Scientific), and absorbance ratios of A260/230 ratios of 2.0 – 2.2 and A260/280 ratios of 1.8 – 2.0 were considered pure. Total RNA was reverse-transcribed using SuperScript II Reverse Transcriptase (Life Technologies, Carlsbad, CA) with random hexamer primers. Quantitative PCR (qPCR) was performed with 1:5 dilution of cDNA on an Applied Biosystems HT7900 sequence detector. Primer sets used to detect Cyclin D1 (Ccnd1), APOBECs, tubulin binding protein (TBP) and 18S rRNA are shown is Supplemental Table 1. Samples were run in triplicate, and the ΔΔCt method was used to calculate the relative fold change between the samples after normalization with 18S rRNA or TBP [24]. The presence of a single dissociation curve confirmed the amplification of a single transcript and lack of primer dimers.
Proliferation assay
Cellular DNA synthesis was determined using a BrdU colorimetric ELISA assay (Roche Diagnostics, Indianapolis, IN) as a measure of cellular proliferation. Briefly, cells were cultured in a 96-well plate at 37°C for 24 hr in the presence of individual FF samples, matched patient plasma, USG, or in the absence of USG. After 24 hr, BrdU was added and incubated for 3 hr at 37 °C. DNA was denatured and cells were incubated with anti-BrdU antibody followed by addition of substrate. The reaction product was quantified at 450 nm wavelength using a scanning multi-well spectrophotometer (FlexStation 3 Multi-Mode Microplate Reader, Sunnyvale, CA).
DNA damage foci analysis
All reagents were purchased form Thermo Fisher Scientific unless otherwise stated. Fallopian epithelial cells were grown on glass coverslips (25 × 75 × 1.0 mm) in the presence of individual FF samples, matched patient plasma, USG, or carboplatin (1μM). Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton/PBS, and blocked with 2.5% bovine serum albumin. Cells were treated with primary antibody to detect γ-H2AX (1:1000 Abcam ab2893), 53BP1 (1:500 Abcam ab36823), washed, treated with secondary antibody conjugated with Alexa Fluor 488, and mounted in a 1:1 mixture of glycerol and PBS. Immunofluorescence preparations were imaged on an upright Nikon Eclipse 80i fluorescence microscope at ×20. The number of foci per nucleus were counted using an automated counter in Mathematica (Wolfram, Champaign, IL).
Comet Assay
The neutral comet assay was performed using the Trevigen Comet Assay kit (Trevigen, Gaithersburg, MD). Fallopian epithelial cells (FT33-TAg) were grown in the presence of individual FF samples, matched patient plasma, USG, no USG, or carboplatin (1 μM). After 24 hr incubation, the cells were suspended in cold PBS, and an aliquot (1000 cells/10 μl) was added to 100 μl of LMA agarose maintained at 39 °C and spread onto a comet slide. The slide was incubated at 4 °C for 10 min and transferred to cold lysis solution for 60 min at 4 °C. A denaturation step was performed in 50 mM Tris base, 150 mM pH 9, for 30 min at 4 °C. The slides were then subjected to electrophoresis with cold TAE buffer, pH 8.2 at 25 V for 30 min at 4 °C, and immersed in DNA precipitation solution (100 mM NH4Ac in 95% ethanol) and then in 100% ethanol for 30 min and air dried. DNA was stained with 100 μl SYBR Gold (Trevigen, 1:30,000) for 20 min and immediately rinsed with dH2O and air dried. The slides were imaged using an upright Nikon Eclipse 80i fluorescence microscope at ×20, and analyzed using Casplab software (Casplab.com) [25].
Micronuclei and mitotic figure quantification
Fallopian epithelial cells were grown on glass coverslips (25 × 75 × 1.0 mm) in the presence of pooled or individual FF and plasma samples, or USG for 24 hr. The cells were fixed for 10 min with 4% paraformaldehyde, stained with Hoechst dye (Thermo Fisher Scientific), and analyzed using an upright Nikon Eclipse 80i fluorescence microscope. Micronuclei were scored as having a diameter that was less than one-third of the main nuclei. The number of micronuclei in at least 1000 cells was determined in five randomly chosen fields of view.
Transfection
Fallopian epithelial cells (FT194) were transiently transfected with plasmids containing human Apobec3a [26] and expressed using the pcDNA3.1(+) vector using lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Statistics
Results of multiple repeats were presented as means ± SEM. One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison tests were used to determine statistical differences between groups. Bartlett’s tests were done to ensure equal variance among treatment groups and none exhibited a significant difference, indicating that the data was normally distributed. In cases where only two treatment groups existed, differences were determined by a non-parametric, un-paired T-Test (Mann Whitney). Values of P<0.05 were considered significant.
Results
Proliferative effects of follicular fluid and plasma on fallopian epithelial cells
Follicular fluid contains a number of growth factors and hormones, so we tested if exposure of fallopian epithelial cells to periovulatory FF causes cellular proliferation. Previous studies indicate that inclusion of FF into culture medium is proliferative using an XTT assay that measures relative cell metabolism [17]. Whether this “proliferative” activity was specific to FF was not tested, and whether it could be due to the prevalent plasma constituents of FF is unknown [27]. In our study, all four fallopian epithelial cells exposed to individual patient FF exhibited an increase in DNA synthesis at least at an equivalent level to the USG growth supplement (Figure 1A; n=8, p<0.05 ANOVA). Exposure to matched patient plasma samples did not have a significant effect on DNA synthesis (Figure 1A). Increased mitotic figures in all four cell lines after treatment with FF for 24 hr provided independent confirmation of cell proliferation (Figure 1B; p<0.05). Cyclin D1 (Ccnd1) expression, representing a proliferative index [28], was induced in the three fallopian epithelial cell lines tested (FT33-TAg, FT190, and FT194) after 24 hr of exposure to pooled FF and plasma (Figure 1C; p<0.05 ANOVA). Our results indicate that both FF and plasma contain factors that increase Ccnd1, however, only FF had the ability to increase DNA synthesis, mitotic cell counts, and overall cell number. Lastly, examination of increased cellular confluence for the cells exposed to FF also supported the increased cell proliferation results (data not shown).
Figure 1. Fallopian epithelial cell proliferation following follicular fluid or plasma exposure.
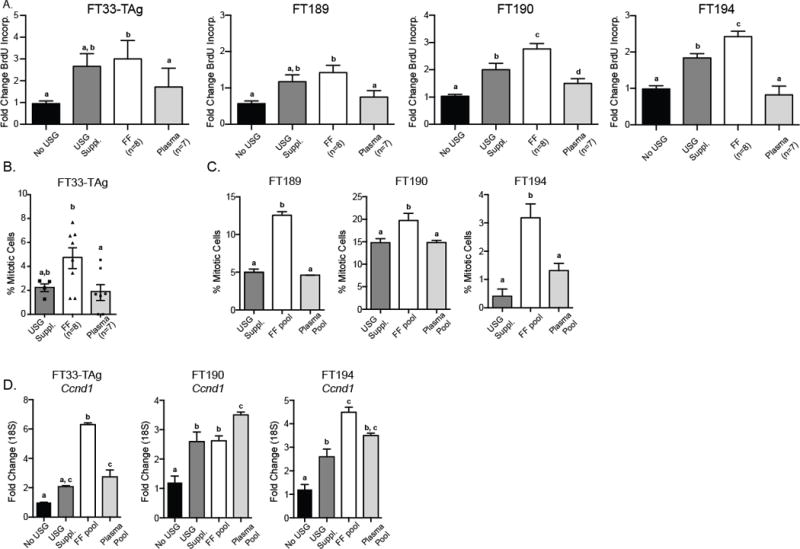
A) Cell lines were cultured in basal medium (No USG), USG supplement (USG Suppl.), 5% individual follicular fluid, or corresponding patient plasma (5%) for 24 hr and BrdU incorporation was measured. B) Microscopy image analysis of mitotic figures performed on FT33-TAg cells exposed to individual patient FF and matched plasma samples. C) Percentage of mitotic cells in the FT189, FT190 and FT194 cells grown in USG Suppl., pooled FF, or pooled plasma from all 8 patients (three independent assays). Mitotic figures were counted based on chromosome Hoescht staining. D) Proliferative index as assessed by Cyclin D1 (Ccnd1) expression following growth of FT33-TAg, FT190 and FT194 cells for 24 hr in basal medium (No USG), USG Suppl., FF pool, or matching patient plasma pool. a,b,c Means ± SEM within a panel that have different superscripts were different (p<0.05).
Follicular fluid exposure induces DNA damage accumulation
Increased proliferation from FF exposure may lead to DNA replication errors and DNA damage, or FF may have intrinsic DNA damaging factors such as ROS, so we explored whether FF induces DNA damage accumulation. Immunofluorescence staining of γH2A.X (a histone variant and marker of DNA damage) was performed after 24 hr exposure to individual FF or plasma samples (Figure 2A, 2B). FT33-TAg cells were exposed to FF from all 8 donors, and foci were counted from at least 100 cells. The chemotherapy drug carboplatin (1 μM) was used as a positive control to activate the DNA damage response. An average of 10 foci per cell were detected after exposure to FF, whereas cells grown in matched patient plasma only contained an average of 2 foci per cell (Figure 2B). Figure 2A shows representative images of γH2A.X foci in FT33-TAg cells. Foci formation of γH2A.X may be independent of DNA damage [29], so we also assessed the foci formation of 53BP1, a marker specific to double strand breaks [30]. FF exposure to the FT33-TAg cell line increased 53BP1 foci formation, while exposure to plasma did not have the same effect (Figure 2C, 2D).
Figure 2. Follicular fluid exposure induces DNA damage accumulation in fallopian epithelial cells.
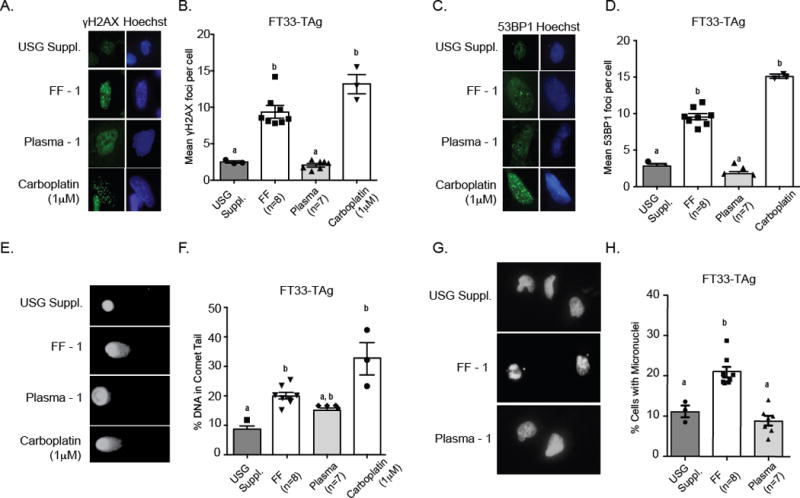
A) Representative images of FT33-TAg cells grown in USG Suppl., individual patient FF, individual patient plasma, or carboplatin with Hoescht (Blue) staining demarcating the nuclear area, and green staining showing the γH2A.X (A) and 53BP1 foci (C). Quantitative results for γH2A.X (B) and 53BP1 (D) foci in the FT33-TAg cells exposed to treatments. Representative comet assay images of nuclei E) from FT33-TAg cells following staining with SYBR Gold and quantitation of percent DNA F) in comet tail after exposure to individual patient FF, individual patient plasma, or carboplatin. Representative images of micronuclei formation (G) in FT33-TAg cells and quantitation H) of micronuclei formation in FT33-TAg cells after exposure to USG Suppl., individual patient FF and matching individual patient plasma. a,b,cMeans ± SEM within a different superscripts were different (p<0.05).
As a confirmation of the consistency of cells to acquire DNA damage following FF exposure, we examined 53BP1 foci formation in FT189, FT190, and FT194 cells following exposure to pooled FF or plasma (Supplemental Figure 1A). Only the FT194 cells displayed increased mean 53BP1 foci per cell; however, upon examination of cells with at least 2 foci per cell, FT189 cells also displayed significant increase in mean 53BP1 foci per cell. Conversely, in the FT190 cell line, exposure to FF did not induce the formation of 53BP1 foci (Supplemental Figure 1B).
To assess if cells accumulate overall DNA damage after FF exposure, we performed comet assays. Exposure to FF significantly increased total DNA damage in FT33-TAg cell line (Figure 2E, 2F). Interestingly, exposure of FT33-TAg cells to matched patient plasma slightly increased total DNA damage accumulation, although the overall increase was intermediate to that of FF-induced DNA damage and basal levels in USG-supplemented media (Figure 2F). Lastly, in our microscopy analyses, we noticed an abundance of micronuclei in each of the cell lines treated with FF. Exposure of the FT33-TAg cells to individual patient FF and plasma (Figure 2G, 2H), and exposure of the FT189, FT190 and FT194 cell lines to a pool of all 8 patient FF samples (Supplemental Figure 2C) resulted in significantly increased micronuclei formation in all cell lines except for FT190, which did not show a significant difference compared to cells grown in USG culture media or plasma (Supplemental Figure 1).
Follicular fluid treatment of fallopian epithelial cells induces APOBEC gene expression
Given the mutation signatures of early stage ovarian carcinomas and the inflammatory environment of the ovulatory follicle [11], we examined whether FF exposure could change expression of the family of APOBEC/AID cytidine deaminases, which are activated in response to interferon and by inflammation [31]. We first examined basal expression of APOBEC and AID genes in normal fallopian epithelial tissue using publically available RNA sequencing data (Figure 3A, GTEx consortium). In normal fallopian epithelium, Apobec3c was expressed the highest, followed by Apobec3G, F, D, and A, and Apobec4. The transcripts for Apobec1, Apobec2, Apobec3H, and AID were not detected. In the current cell lines (FT33-TAg, FT190, and FT194), we were able to detect all of the APOBEC family members, with exception of Apobec3f and AID, which were not present in two cell lines (Supplemental Figure 2). To analyze APOBEC/AID proteins present in fallopian epithelium, we surveyed the Human Protein Atlas to examine protein abundance in the nuclei of fallopian epithelium of pre-menopausal women [32, 33]. Out of the seven APOBEC family proteins for which data existed, only APOBEC3A was abundant in the nuclei of fallopian epithelium in 3 different normal patient samples (Figure 3B). Furthermore, only APOBEC3A demonstrated nuclear staining, consistent with possible functional influences on cellular DNA (Figure 3B).
Figure 3. Apobec genes are expressed in primary fallopian epithelial tissues and fallopian epithelial cell lines.
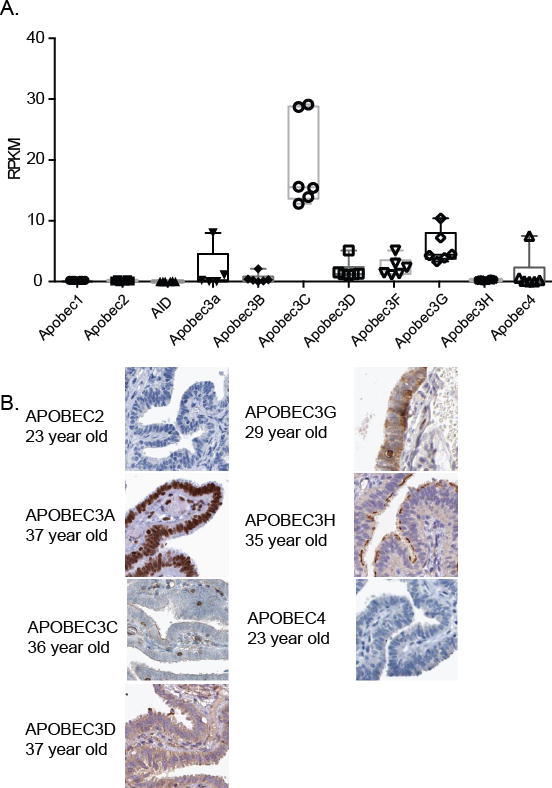
(A) The Genotype-Tissue Expression (GTEx) Project database was utilized to identify Apobec and AID expression in normal fallopian tube epithelium (n = patient samples). RNA-Seq data from XX tissues are reported as average RPKM (reads per kilobase per million mapped reads). Normal distribution across the dataset is visualized with box plots, shown as median and 25th and 75th percentiles. (B) Representative images showing abundance of APOBEC family proteins in fallopian epithelium in premenopausal women from the Human Protein Atlas.
To examine if Apobec/AID genes are altered after exposure to FF, we exposed FT33-TAg, FT190, and FT194 cells to pools of FF and plasma and performed qPCR. The expression of Apobec3A was the only Apobec/AID family gene to be consistently induced following exposure to FF (Supplemental Figure 3). Next, we examined how individual patient FF influence Apobec3A gene expression. Apobec3A was induced by at least two-fold in FT194 cells following exposure of 5 out of 8 FF samples. The increase in gene expression also resulted in an increase of protein abundance in all fallopian epithelial cell lines following exposure to pooled FF, and to a lesser extent following exposure to pooled patient plasma (Figure 4B). Finally, we transiently overexpressed APOBEC3A in FT194 cells, and examined the formation of 53BP1 foci. APOBEC3A overexpression significantly increased the mean number of 53BP1 foci in these fallopian epithelial cells (Figure 4D), demonstrating that APOBEC3A expression is sufficient to induce DNA damage in fallopian epithelial cells (p<0.05; T-test).
Figure 4. Apobec3A is induced following exposure to human FF.
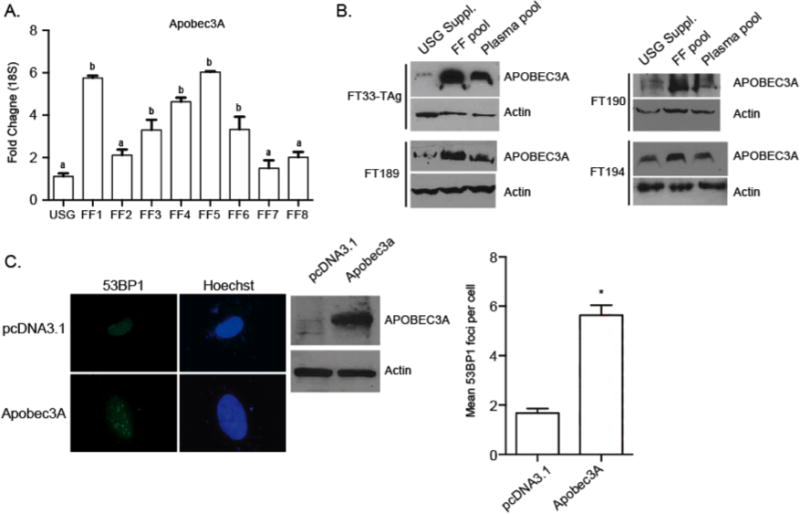
A) Apobec3A gene expression changes following exposure to individual FF from all 8 patients in FT33-TAg fallopian epithelial cell lines. B) Analysis of the effect of pooled FF and pooled plasma samples on the induction of APOBEC3A protein in all four cell lines. C) Representative image of 53BP1 foci formation in FT194 cells following transient overexpression of Apobec3A or control pCDNA3.1 plasmid DNA. Western blot demonstrating overexpression of APOBEC3A, and quantification of 53BP1 foci. a,b Means ± SEM within panel B were different (p<0.05). Means ± SEM within panels A and C were different (p<0.05) from the USG control or pcDNA3.1 control, respectively.
Discussion
The “incessant ovulation” hypothesis of ovarian cancer links repeated ovulatory cycles with the occurrence of ovarian cancer. The microenvironment of ovulation is highly pro-inflammatory and factors within FF, such as inflammatory cytokines, ROS, and steroids are exposed to the distal ends of the fallopian tubes. The main hindrance to improving the survival of ovarian cancer patients is a lack of early detection. Studying the in vitro properties of FF, and the effect FF has on fallopian epithelium is a unique model for understanding changes that occur after ovulation in the fallopian tube. Studies such as these are essential to advance our understanding of the incessant ovulation hypothesis of ovarian cancer.
Previous work studying the effect of human FF on fallopian epithelium used fetal bovine serum or USG supplemented cell culture medium for comparison (i.e., control) for understanding the effects of FF [15–17, 21]. This experimental design does not take into consideration that an individual’s FF (which is a plasma derivative [27]) may be influenced by plasma factors specific to the patient. To rectify these shortcomings and to determine the specificity of the effects of FF, we compared FF exposure to matched patient plasma. We demonstrated that only FF was capable of significantly increasing cell proliferation, mitotic figures, and increased cellular confluence. The increased expression of Ccnd1 by both FF and matched patient plasma however, did not support the unique cellular proliferation attribute of FF. This discrepancy may be explained by the role of estradiol in Ccnd1 activation [34], and the insensitivity of fallopian epithelium to proliferation after estradiol treatment [16]. Thus, the high levels of estradiol in both plasma and FF [35] of IVF patients could increase expression of Ccnd1, while allowing them to have independent effects on proliferation.
Next, we assessed the ability of FF to induce DNA damage through the formation of γH2A.X and 53BP1 foci formation. Exposure of fallopian epithelial cells to FF increased γH2A.X foci, confirming previous studies [17, 18]. However, γH2A.X increase could be independent of DNA damage, such as following activation of DNA-PKC/CHK2 in the absence of DNA damage [29]. Therefore we analyzed formation of 53BP1 DNA damage foci to specifically assess double strand breaks. Both γH2A.X and 53BP1 foci were significantly induced by FF exposure, and not by patient plasma. A global view of the DNA-damaging effects of FF on the fallopian epithelium was demonstrated using comet assays after exposure to FF. This comprehensive analysis of DNA damage indicated that FF exposure increases overall DNA damage accumulation and specifically, double strand breaks. Lastly, we examined the consistency of the DNA damage effect in 3 other cell lines. In total, three out of four fallopian epithelial cells showed an increase in 53BP1 foci formation after exposure to FF. Further analysis will need to elucidate the cause of these differences.
Given the observations of DNA damage, double strand beaks, and the known links of ovulation and inflammation, we hypothesized that APOBEC cytidine deaminases might be playing a role in FF-induced alterations in fallopian epithelium. The family of cytidine deaminase enzymes includes 10 APOBECs and AID, which are powerful DNA editors that convert cytidine to uracil (C-to-U). APOBECs can be activated in response to interferon or inflammation, and FF has been reported to contain interferon like cytokines [36]. The exchange of a C with a U triggers DNA base-excision repair enzyme uracil-DNA-glycosylase (UNG) to form apurinic/apyrimidinic (AP) sites. During replication, the base pairing of a T:A will replace what was previously a C:G [37]. Importantly, if a trans-lesion DNA polymerase is not used for repair, a stalled replication fork collapse may cause a double strand break and potentially chromosomal rearrangements. Low stage high-grade serous carcinomas exhibit significant C>T substitutions at CpG sites, suggestive of a pattern of deamination [38]. High-grade serous carcinomas exhibit clustered hypermutations, termed kataegis [38, 39]. Both the C>T substitution and kataegis mutation patterns are suggestive of a mutagenic mechanism explained by cytidine deaminase activity.
A recent study showed that AID is increased in fallopian epithelial cells after exposure to FF [21]. Activation of AID resulted in a decrease of overall DNA methylation, presumably caused by the deamination of 5mC to T. However, the activity of the other APOBEC family members was not examined. Here, we assessed changes in all APOBEC family genes after FF exposure, and identified significant elevation of Apobec3A mRNA and protein expression. When individual FF samples were examined, fluids from 5 of 8 patients induced Apobec3A gene expression, indicating a possible genetic component to the variation in the composition of each patient’s FF. Additional studies will identify the cause of Apobec3a induction and establish the causative agents within FF that lead to these changes. Lastly, we determined that Apobec3A transient overexpression was sufficient to induce double strand breaks in fallopian epithelial cells, however further experiments are necessary to determine if transient Apobec3A induction results in mutations of fallopian epithelial cells after exposure to FF.
Altogether, our data reveal a novel mechanism by which FF exposure to fallopian epithelium results in double strand breaks, adding to a body of work investigating the effect of FF on fallopian epithelium. We show a specific proliferative and DNA damaging effect of FF, compared to matched patient plasma samples. It is imperative to include patient controls in order to discern between effects of FF or the plasma constituent within FF. Limitations of our research include the use of immortalized cell lines and single exposures to FF. Future studies should consider multiple exposures of FF to fallopian epithelium, replicating the in vivo situation of repeated ovulations and replication in primary fallopian epithelial cells to account for immortalization. Our work helps to establish an understanding of molecular events occurring in the fallopian tube following ovulation, and posits a potential mechanism of action for FF induction of DNA damage within the fallopian tube.
Supplementary Material
A) FT189, FT190, and FT194 cells were exposed to pooled FF, pooled plasma, or USG Suppl. for 24 hr, and 53BP1 foci formation was analyzed. B) Cells positive for more than 2 foci per cell were selected and considered “positive” for 53BP1 foci. The mean number of 53BP1 foci in positive cells was calculated to evaluate differences in the degree of cellular response to FF. C) Micronuclei were counted in FT189, FT190, and FT194 cells were exposed to pooled FF, pooled plasma, or USG Suppl. for 24 hr, a,b, c Means ± SEM within panel B were different (p<0.05). Means ± SEM within panel A was different (p<0.05) from the USG control.
A) Basal expression (delta Ct values) of the family of Apobec genes in fallopian epithelial cell lines (FT33-TAg, FT190, and FT194). Expression was normalized to TBP.
Each Apobec family member was assessed for changes in gene expression following exposure to pooled FF in three fallopian tube cell lines (FT33-TAg, FT190, and FT194) compared to growth in USG suppl. * indicates p<0.05, T-test.
Acknowledgments
This work was supported by NIH HD082484 (LKC) and a pilot project (LKC) from the NCI P30 CA168524 University of Kansas Cancer Center, a Lalor Foundation Postdoctoral Fellowship and F32CA200357 training grant (PB). The funding agencies had no involvement in study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication.
Footnotes
Competing interests
N.S.A is the president and founder of De Novo Genomics Corporation. All other authors declare that they have no conflicting interests.
Authors’ contributions
P.B., B.J.V., and L.K.C. conceptualized the hypotheses and designed the research strategy; P.B. and N.S.A. collected data; P.B., N.S.A, and L.K.C. analyzed data; P.B. performed statistical analyses; and P.B. and L.K.C. wrote the paper. All authors read and approved the final manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 3.Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Current opinion in obstetrics & gynecology. 2007;19:3–9. doi: 10.1097/GCO.0b013e328011a21f. [DOI] [PubMed] [Google Scholar]
- 4.Auersperg N, Woo MM, Gilks CB. The origin of ovarian carcinomas: a developmental view. Gynecol Oncol. 2008;110:452–4. doi: 10.1016/j.ygyno.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma–evidence supporting the clonal relationship of the two lesions. J Pathol. 2012;226:421–6. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 7.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: Evidence for a causal relationship. The American journal of surgical pathology. 2007;31:161–9. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 8.Fathalla MF. Incessant ovulation–a factor in ovarian neoplasia? Lancet. 1971;2:163. doi: 10.1016/s0140-6736(71)92335-x. [DOI] [PubMed] [Google Scholar]
- 9.Fathalla MF. Incessant ovulation and ovarian cancer - a hypothesis re-visited. Facts, views & vision in ObGyn. 2013;5:292–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Emori MM, Drapkin R. The hormonal composition of follicular fluid and its implications for ovarian cancer pathogenesis. Reproductive biology and endocrinology : RB&E. 2014;12:60. doi: 10.1186/1477-7827-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annual review of physiology. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 12.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arumugam A, Parada J, Rajkumar L. Mammary cancer promotion by ovarian hormones involves IGFR/AKT/mTOR signaling. Steroids. 2012;77:791–7. doi: 10.1016/j.steroids.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Buscher U, Chen FC, Kentenich H, Schmiady H. Cytokines in the follicular fluid of stimulated and non-stimulated human ovaries; is ovulation a suppressed inflammatory reaction? Human reproduction. 1999;14:162–6. doi: 10.1093/humrep/14.1.162. [DOI] [PubMed] [Google Scholar]
- 15.Lau A, Kollara A, St John E, Tone AA, Virtanen C, Greenblatt EM, et al. Altered expression of inflammation-associated genes in oviductal cells following follicular fluid exposure: implications for ovarian carcinogenesis. Experimental biology and medicine. 2014;239:24–32. doi: 10.1177/1535370213508216. [DOI] [PubMed] [Google Scholar]
- 16.King SM, Hilliard TS, Wu LY, Jaffe RC, Fazleabas AT, Burdette JE. The impact of ovulation on fallopian tube epithelial cells: evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocrine-related cancer. 2011;18:627–42. doi: 10.1530/ERC-11-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahar-Shany K, Brand H, Sapoznik S, Jacob-Hirsch J, Yung Y, Korach J, et al. Exposure of fallopian tube epithelium to follicular fluid mimics carcinogenic changes in precursor lesions of serous papillary carcinoma. Gynecol Oncol. 2014;132:322–7. doi: 10.1016/j.ygyno.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 18.Huang HS, Chu SC, Hsu CF, Chen PC, Ding DC, Chang MY, et al. Mutagenic, surviving and tumorigenic effects of follicular fluid in the context of p53 loss: initiation of fimbria carcinogenesis. Carcinogenesis. 2015 doi: 10.1093/carcin/bgv132. [DOI] [PubMed] [Google Scholar]
- 19.Landry S, Narvaiza I, Linfesty DC, Weitzman MD. APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO reports. 2011;12:444–50. doi: 10.1038/embor.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Narvaiza I, Landry S, Weitzman MD. APOBEC3 proteins and genomic stability: the high cost of a good defense. Cell cycle. 2012;11:33–8. doi: 10.4161/cc.11.1.18706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sapoznik S, Bahar-Shany K, Brand H, Pinto Y, Gabay O, Glick-Saar E, et al. Activation-Induced Cytidine Deaminase Links Ovulation-Induced Inflammation and Serous Carcinogenesis. Neoplasia. 2016;18:90–9. doi: 10.1016/j.neo.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer cell. 2013;24:751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cookingham LM, Van Voorhis BJ, Ascoli M. Do alterations in follicular fluid proteases contribute to human infertility? Journal of assisted reproduction and genetics. 2015;32:737–45. doi: 10.1007/s10815-015-0447-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biology of reproduction. 2010;83:286–95. doi: 10.1095/biolreprod.109.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konca K, Lankoff A, Banasik A, Lisowska H, Kuszewski T, Gozdz S, et al. A cross-platform public domain PC image-analysis program for the comet assay. Mutat Res. 2003;534:15–20. doi: 10.1016/s1383-5718(02)00251-6. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt K, Guo K, Katuwal M, Wilson D, Prochnow C, Bransteitter R, et al. Lentivirus restriction by diverse primate APOBEC3A proteins. Virology. 2013;442:82–96. doi: 10.1016/j.virol.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamah AM, Hassis ME, Albertolle ME, Williams KE. Proteomic analysis of human follicular fluid from fertile women. Clinical proteomics. 2015;12:5. doi: 10.1186/s12014-015-9077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang WL, Li Z, Lin TY, Wang SW, Wu FJ, Luo CW. Thyrostimulin-TSHR signaling promotes the proliferation of NIH:OVCAR-3 ovarian cancer cells via trans-regulation of the EGFR pathway. Scientific reports. 2016;6:27471. doi: 10.1038/srep27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu WZ, Li B, Huang B, Wang Y, Liu XD, Guan H, et al. gammaH2AX foci formation in the absence of DNA damage: mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS letters. 2013;587:3437–43. doi: 10.1016/j.febslet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Ward IM, Minn K, van Deursen J, Chen J. p53 Binding protein 53BP1 is required for DNA damage responses and tumor suppression in mice. Mol Cell Biol. 2003;23:2556–63. doi: 10.1128/MCB.23.7.2556-2563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebhandl S, Huemer M, Greil R, Geisberger R. AID/APOBEC deaminases and cancer. Oncoscience. 2015;2:320–33. doi: 10.18632/oncoscience.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponten F, Schwenk JM, Asplund A, Edqvist PH. The Human Protein Atlas as a proteomic resource for biomarker discovery. Journal of internal medicine. 2011;270:428–46. doi: 10.1111/j.1365-2796.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 33.Kampf C, Bergman J, Oksvold P, Asplund A, Navani S, Wiking M, et al. A tool to facilitate clinical biomarker studies–a tissue dictionary based on the Human Protein Atlas. BMC medicine. 2012;10:103. doi: 10.1186/1741-7015-10-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabbah M, Courilleau D, Mester J, Redeuilh G. Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element. Proc Natl Acad Sci U S A. 1999;96:11217–22. doi: 10.1073/pnas.96.20.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de los Santos MJ, Garcia-Laez V, Beltran D, Labarta E, Zuzuarregui JL, Alama P, et al. The follicular hormonal profile in low-responder patients undergoing unstimulated cycles: Is it hypoandrogenic? Human reproduction. 2013;28:224–9. doi: 10.1093/humrep/des349. [DOI] [PubMed] [Google Scholar]
- 36.Zidovec Lepej S, Vujisic S, Stipoljev F, Mazuran R. Interferon-alpha-like biological activity in human seminal plasma, follicular fluid, embryo culture medium, amniotic fluid and fetal blood. Reproduction, fertility, and development. 2003;15:423–8. doi: 10.1071/rd03020. [DOI] [PubMed] [Google Scholar]
- 37.Roberts SA, Lawrence MS, Klimczak LJ, Grimm SA, Fargo D, Stojanov P, et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat Genet. 2013;45:970–6. doi: 10.1038/ng.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chien J, Sicotte H, Fan JB, Humphray S, Cunningham JM, Kalli KR, et al. TP53 mutations, tetraploidy and homologous recombination repair defects in early stage high-grade serous ovarian cancer. Nucleic Acids Res. 2015;43:6945–58. doi: 10.1093/nar/gkv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonard B, Hart SN, Burns MB, Carpenter MA, Temiz NA, Rathore A, et al. APOBEC3B upregulation and genomic mutation patterns in serous ovarian carcinoma. Cancer Res. 2013;73:7222–31. doi: 10.1158/0008-5472.CAN-13-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) FT189, FT190, and FT194 cells were exposed to pooled FF, pooled plasma, or USG Suppl. for 24 hr, and 53BP1 foci formation was analyzed. B) Cells positive for more than 2 foci per cell were selected and considered “positive” for 53BP1 foci. The mean number of 53BP1 foci in positive cells was calculated to evaluate differences in the degree of cellular response to FF. C) Micronuclei were counted in FT189, FT190, and FT194 cells were exposed to pooled FF, pooled plasma, or USG Suppl. for 24 hr, a,b, c Means ± SEM within panel B were different (p<0.05). Means ± SEM within panel A was different (p<0.05) from the USG control.
A) Basal expression (delta Ct values) of the family of Apobec genes in fallopian epithelial cell lines (FT33-TAg, FT190, and FT194). Expression was normalized to TBP.
Each Apobec family member was assessed for changes in gene expression following exposure to pooled FF in three fallopian tube cell lines (FT33-TAg, FT190, and FT194) compared to growth in USG suppl. * indicates p<0.05, T-test.


