Abstract
Background & Aims
Low estrogen levels could contribute to development of fecal incontinence (FI) in women after menopause by altering neuromuscular continence mechanisms. However, studies have produced conflicting results on the association between menopausal hormone therapy (MHT) and risk of FI.
Methods
We studied the association between MHT and risk of FI among 55,828 post-menopausal women (mean age 73 years) who participated in the Nurses’ Health Study, enrolled since 2008, with no report of FI. We defined incident FI as a report of at least 1 liquid or solid FI episode per month during 4 years of follow up from self-administered, biennial questionnaires, administered in 2010 and 2012. We used Cox proportional hazards models to calculate multivariate-adjusted hazard ratios (HRs) and 95% CIs for FI risk in women receiving MHT, adjusting for potential confounding factors.
Results
During more than 185,000 person-years of follow up, there were 6834 cases of incident FI. Compared with women who never used MHT, the multivariate HR for FI was 1.26 (95% CI, 1.18–1.34) for past users of MHT and 1.32 (95% CI, 1.20–1.45) for current users. The risk of FI increased with longer duration of MHT use (P trend ≤.0001) and decreased with time since discontinuation. There was an increased risk of FI among women receiving MHT that contained a combination of estrogen and progestin (HR, 1.37; 95% CI 1.10–1.70) compared to estrogen monotherapy.
Conclusions
Current or past use of MHT was associated with a modestly increased risk of FI among post-menopausal women in the Nurses’ Health Study. These results support a potential role for exogenous estrogens in the impairment of the fecal continence mechanism.
Keywords: motility, menopause, estrogen, progestin
INTRODUCTION
Fecal incontinence (FI) is a common condition, with a prevalence ranging from 7–15% among community-dwelling women, that rises with age.1 FI is associated with a substantial degree of embarrassment, hygiene issues, poor self-esteem, and significantly-impaired quality of life.2 Although there is controversy as to whether women are more likely to develop FI than men,3 women potentially have unique risk factors for FI, specifically anal sphincter trauma secondary to obstetric injury and pelvic floor changes related to menopause.
Because of the female predominance of those seeking care for FI and increased incidence with age, there has been interest in delineating the possible effect of female sex hormones on the function of the anorectum. Steroid hormone receptors have been mapped in vitro to the external anal sphincter,4 smooth muscle of internal anal sphincter,5, 6 and connective tissue of the anal canal5 with some data showing increased estrogen receptors in the anorectum of women with FI compared to controls.7 There are conflicting data linking exogenous hormones in the form of menopausal hormone therapy (MHT) with FI. Some studies report no association,8, 9 an inverse association,10 or a positive association with FI.11, 12 However, these studies are largely cross-sectional or retrospective and limited in size.
We therefore sought to prospectively examine the association between MHT and risk of incident FI in a large, ongoing study of US postmenopausal women, the Nurses’ Health Study. This cohort includes biennially-updated information on lifestyle factors, medical diagnoses, and hormone exposure (including MHT) which allows for a comprehensive examination of the possible role of MHT in the subsequent development of FI.
METHODS
Study population
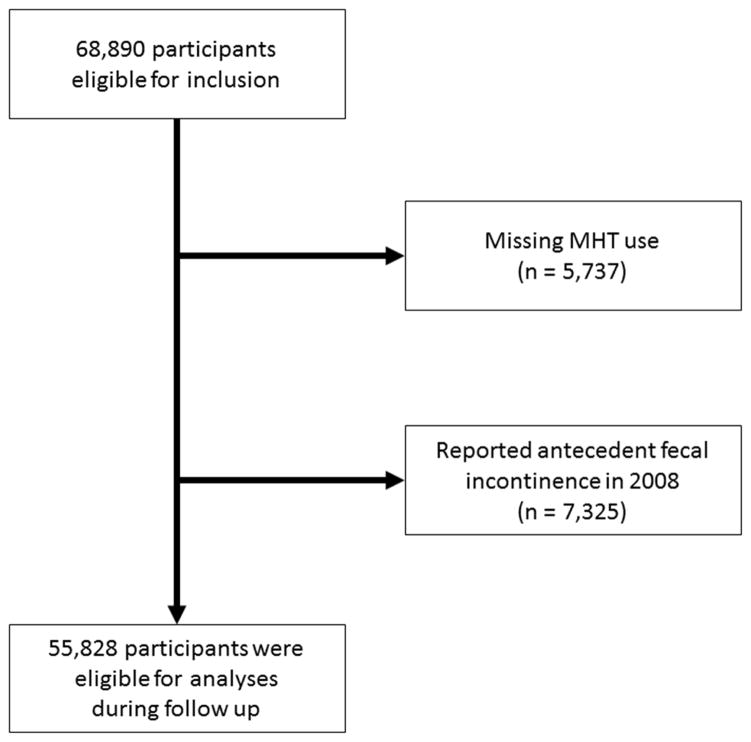
The Nurses’ Health Study is a prospective cohort that began in 1976 when 121,701 US female registered nurses, aged 30–55, completed a self-administered, mailed questionnaire regarding their health and lifestyle. Participants have received follow-up questionnaires every 2 years to update information. Questions about FI were first included on the 2008 questionnaire, when all women were postmenopausal. From the 121,700 nurses in the original cohort, 23,393 died prior to 2008, 13,587 were lost to follow up prior to 2008, 15,830 did not answer the long form of the questionnaire that included questions on FI, and 5,737 women did not report on their use of MHT. We also excluded 7,325 women who reported baseline FI on the 2008 questionnaire. Thus, the analytic population for the current analysis included 55,828 women (Figure 1). The study was approved by the Institutional Review Board at Brigham and Women’s Hospital.
Figure 1.
Flow of eligible participants into the study
Assessment of menopausal hormone therapy use
On each biennial questionnaire, participants reported the number of months during the previous two years when prescription MHT was taken, whether MHT was currently being used, and the type of MHT preparation (estrogen, progestin, or combination therapy). All women who used MHT did so using oral formulations.
Ascertainment of Fecal Incontinence
On the 2008, 2010, and 2012 questionnaires, participants were asked “On average, how often in the past year have you experience any amount of accidental bowel leakage?” Response categories included “Never,” “Less than 1/month,” “1–3x/month,” “About once/week,” “Several times/week,” or “Nearly daily.” Women were considered to have FI if they reported incontinence of liquid or solid stool at least monthly.
Assessment of covariates
On each biennial questionnaire, participants were asked about lifestyle factors pertinent to the risk of FI, including body weight, history of smoking, and physical activity. In 1988, we also inquired about number of years working night shifts. Height was reported on the 1976 questionnaire. Participants were also asked on each questionnaire about medical factors related to risk of fecal incontinence: parity, history of cholecystectomy (asked in 1982–2002), diabetes mellitus, hypertension, and presence of neurologic disease, which was defined as a diagnosis of amyotrophic lateral sclerosis (ALS), multiple sclerosis (MS), or Parkinson’s disease.
Because the presumed mechanism for MHT’s effects on risk of fecal incontinence is via hormone exposure, we also included measures that were surrogates for cumulative endogenous and exogenous estrogen exposure using available data on parity, age at menarche, age at menopause, use of oral contraceptives, and ovulatory duration (calculated by subtracting the sum of age at menarche, number of pregnancies, and duration of oral contraceptive use from the age at menopause). Because rate of estrogen loss may affect FI, we also adjusted for type of menopause (natural vs. surgical/radiation-induced) to address the hypothesis that sudden loss of estrogen (as in surgical menopause) could have a greater effect on risk of fecal incontinence than the more gradual decrease in estrogen levels seen with natural menopause.
Statistical analysis
We calculated person-time for each participant 13–15 from the date of return of the 2008 questionnaire to the date of report of FI, date of the last questionnaire returned, death from any cause, or June 1, 2012, whichever came first. We used Cox proportional hazards modeling to calculate multivariable-adjusted hazard ratios (HR) and 95% confidence intervals for incident fecal incontinence according to MHT use by current/past/never use, duration of use, time since discontinuation among past users, and type of MHT used. Because a history of hysterectomy may be associated with a differential risk of fecal incontinence and altered patterns of MHT prescribing, we performed a sub-analysis limited to women with a history of hysterectomy. We also varied the threshold for a case of FI to at least weekly episodes in a separate subanalysis. In order to determine the effects of bowel disturbances on the relationship between MHT and FI, we also performed separate analyses examining the effect of MHT on risk of liquid or solid stool FI alone. Covariates were selected a priori based on risk factors for fecal incontinence1, 16, 17 and factors affecting lifetime hormone exposure as described above. Age, MHT use, duration of MHT use, time since discontinuation of MHT, body mass index (BMI), activity level, smoking, and diagnoses of diabetes mellitus, hypertension, or neurologic disease were included as time-varying covariates to account for changes in these exposures over time. We adjusted for static variables (parity, oral contraceptive use, age at menarche, age at menopause, and ovulatory duration) based on their values in 2008. For any covariates in which data were missing from an interval questionnaire (usually only a small percentage of women), the values obtained from the previous questionnaire were carried forward. All statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, NC). Statistical significance was defined by a two-sided p-value less than 0.05. All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
From 2008 through 2012, we documented 6,834 incident cases of fecal incontinence (48% liquid, 40% solid, and 12% liquid and solid FI) among 55,828 postmenopausal women who contributed 185,794 person-years of follow up. From the study population, 2,548 women did not respond to the FI question in 2010 and an additional 3,376 women did not respond to the FI question in 2012. Compared with women who had never used MHT, current users were younger at menopause, less likely to be obese, more likely to have used oral contraceptives, more likely to have had surgical or radiation-induced menopause, and less likely to have comorbid diabetes (Table 1). The full model is available in Supplementary Table 1. A subanalysis of women with a history of hysterectomy did not demonstrate a significantly-different risk of FI among current and past users of MHT compared to the overall population.
Table 1.
Characteristics of postmenopausal women in the Nurses’ Health Study according to menopausal hormone therapy use in 2008
| Menopausal Hormone Therapy Use | |||
|---|---|---|---|
| Never (N=12,538) | Past (N=36,762) | Current (N=6,528) | |
| Age (y), mean (SD) | 73.5 (6.8) | 73.0 (6.6) | 71.0 (6.2) |
| Age range (y) | 61.5–89.0 | 61.5–89.3 | 61.5–88.6 |
|
| |||
| Race (% nonwhite) | 6 | 6 | 4 |
|
| |||
| Age (y) at menopause, mean (SD) | 50.8 (7.8) | 48.0 (7.3) | 46.6 (8.9) |
|
| |||
| BMI (%), kg/m2 | |||
| <20 | 6 | 7 | 7 |
| 20–24.9 | 34 | 38 | 46 |
| 25–29.9 | 33 | 33 | 31 |
| ≥30 | 27 | 22 | 16 |
|
| |||
| Leisure-time exercise (%) | |||
| <4 METsa. | 21 | 18 | 15 |
| 4–7 METs | 13 | 12 | 10 |
| ≥ 8 METs | 66 | 70 | 75 |
|
| |||
| Smoking (%) | |||
| Never | 47 | 45 | 47 |
| Past | 46 | 50 | 49 |
| Current | 7 | 5 | 4 |
|
| |||
| Age (y) at menarche (%) | |||
| ≤10 | 6 | 6 | 6 |
| 11 | 16 | 17 | 16 |
| 12 | 27 | 27 | 27 |
| 13 | 31 | 31 | 32 |
| ≥14 | 19 | 19 | 19 |
|
| |||
| Ever used oral contraceptives (%) | 42 | 54 | 63 |
|
| |||
| Parity (%) | |||
| 0 | 5 | 5 | 7 |
| 1 | 7 | 7 | 8 |
| 2 | 27 | 30 | 34 |
| ≥3 | 62 | 58 | 51 |
|
| |||
| Menopause type (%) | |||
| Natural | 75 | 55 | 40 |
| Surgical/radiation | 25 | 45 | 60 |
|
| |||
| Diabetes Mellitus (%) | 11 | 10 | 6 |
|
| |||
| Hypertension (%) | 50 | 52 | 48 |
|
| |||
| Neurologic diseaseb. (%) | 2 | 3 | 1 |
|
| |||
| Cholecystectomy (%) | 15 | 18 | 17 |
NOTE: Values are means (SD) or percentages.
MET= metabolic equivalent of task
Defined as a confirmed diagnosis of amyotrophic lateral sclerosis, multiple sclerosis, or Parkinson’s disease
Compared with women who never used MHT, the age-adjusted HRs for incident FI were 1.32 (95% CI, 1.20–1.45) for current users and 1.26 (95% CI, 1.18–1.34) for past users of MHT. These risk estimates were only mildly attenuated by adjustment for risk factors for FI (smoking, BMI, hypertension, diabetes, neurologic disease, cholecystectomy, parity) and factors associated with hormone exposure (oral contraceptive use, age at menarche, age at menopause, ovulatory duration, menopause type) (Table 2). In subgroup analyses of current users, the association of MHT with incident FI varied by type of MHT preparation used. Compared with current users of estrogen-only formulations, the multivariate HR for FI was 1.37 (95% CI 1.10–1.71) for current users of combined estrogen and progestin preparations.
Table 2.
Menopausal hormone therapy use and risk of incident fecal incontinence
| Menopausal Hormone Use Status | |||
|---|---|---|---|
| Never | Past | Current | |
| Person-years of follow up | 41,870 | 123,119 | 20,805 |
|
| |||
| No. of cases | 1,304 | 4,810 | 720 |
|
| |||
| Age-adjusted HR (95% CI) | 1.00 | 1.30 (1.22–1.38) | 1.32 (1.21–1.45) |
|
| |||
| Multivariate-adjusted HR (95% CI)a. | 1.00 | 1.26 (1.18–1.34) | 1.32 (1.20–1.45) |
Models adjusted for age (months), age at menopause (years), smoking (never, past, current), BMI (<20, 20–24.9, 25–29.9, >30 kg/m2), oral contraceptive use (never, ever), parity (0, 1, 2, ≥3), age at menarche (≤10, 11, 12, 13, ≥14), menopause type (natural, surgical/radiation), ovulatory duration (<24 years, 24–29 years, 29–33 years, ≥34 years), hypertension (yes/no), diabetes mellitus (yes/no), neurologic disease (yes/no), years working night shifts, and history of cholecystectomy (yes/no).
The risk of FI appeared to increase with longer duration of MHT use, although short-term MHT use remained associated with an increased risk of FI (Table 3). Compared with women who had never used MHT, the multivariate-adjusted risk of FI was 1.22 (95% CI, 1.13–1.31) for 1–5 years of use, 1.24 (95% CI, 1.15–1.35) for 6–10 years of use, and 1.32 (95% CI, 1.23–1.41) for more than 10 years of use (Plinear trend<0.0001). Because current users may have been using MHT for a considerable amount of time, we performed a sensitivity analysis examining the influence of duration of MHT use among current users only. Compared to non-users, the multivariate-adjusted risk of FI was 1.42 (95% CI, 1.00–2.01) for 1–5 years of use, 1.23 (95% CI, 0.92–1.64) for 6–10 years of use, and 1.33 (95% CI, 1.19–1.49) for more than 10 years of use (Plinear trend<0.0001).
Table 3.
Duration of menopausal hormone therapy and risk of incident fecal incontinence (2008–2012)
| Years of hormone use | |||||
|---|---|---|---|---|---|
| 0 | 1–5 | 6–10 | >10 | Ptrenda. | |
| Person-years of follow up | 41,870 | 40,061 | 33,455 | 70,409 | |
|
| |||||
| No. of cases | 1,304 | 1,501 | 1,099 | 2,930 | |
|
| |||||
| Age-adjusted HR (95% CI) | 1.00 | 1.23 (1.14–1.33) | 1.26 (1.16–1.36) | 1.36 (1.28–1.45) | <0.0001 |
|
| |||||
| Multivariate-adjusted HR (95% CI)b. | 1.00 | 1.22 (1.13–1.31) | 1.24 (1.15–1.35) | 1.32 (1.23–1.41) | <0.0001 |
Linear trend estimated by entering duration of use as a continuous variable in the model.
Models adjusted for age (months), age at menopause (years), smoking (never, past, current), BMI (<20, 20–24.9, 25–29.9, >30 kg/m2), oral contraceptive use (never, ever), parity (0, 1, 2, ≥3), age at menarche (≤10, 11, 12, 13, ≥14), menopause type (natural, surgical/radiation), ovulatory duration (<24 years, 24–29 years, 29–33 years, ≥34 years), hypertension (yes/no), diabetes mellitus (yes/no), neurologic disease (yes/no), years working night shifts, and history of cholecystectomy (yes/no).
In analyses of time since discontinuation of MHT among past users of MHT in relation to risk of incident FI (Table 4), we categorized time since discontinuation as recent (less than 2 years) or more distant (2+ years). Although compared to never users, women who had discontinued MHT less than 2 years ago had an increased risk of FI (multivariate-adjusted HR 1.25, 95% CI 1.17–1.34), women who had discontinued MHT two or more years prior did not have a significantly increased risk of FI (multivariate-adjusted HR 1.08, 95% CI 0.95–1.23).
Table 4.
Time since discontinuation of menopausal hormone therapy among women who stopped taking hormones and risk of incident fecal incontinence
| Years since discontinuation | ||||
|---|---|---|---|---|
| Never | ≥2 | <2 | Current | |
| Person-years of follow up | 41,870 | 4,780 | 110,746 | 20,805 |
|
| ||||
| No. of cases | 1304 | 251 | 4,097 | 720 |
|
| ||||
| Age-adjusted HR (95% CI) | 1.00 | 1.14 (1.00–1.30) | 1.29 (1.21–1.37) | 1.34 (1.22–1.47) |
|
| ||||
| Multivariate-adjusted HR (95% CI)a. | 1.00 | 1.08 (0.95–1.23) | 1.25 (1.17–1.34) | 1.34 (1.22–1.47) |
Models adjusted for age (months), age at menopause (years), smoking (never, past, current), BMI (<20, 20–24.9, 25–29.9, >30 kg/m2), oral contraceptive use (never, ever), parity (0, 1, 2, ≥3), age at menarche (?≤10, 11, 12, 13, ≥14), menopause type (natural, surgical/radiation), ovulatory duration (<24 years, 24–29 years, 29–33 years, ≥34 years), hypertension (yes/no), diabetes mellitus (yes/no), neurologic disease (yes/no), years working night shifts, and history of cholecystectomy (yes/no).
There were 4,828 women with missing data on time since discontinuation of MHT representing 462 cases of fecal incontinence who were not included in this analysis.
The association of MHT with risk of FI did not appear to differ according to the consistency of the stool. Compared to never users, current users of MHT had a multivariate HR of 1.27 (95% CI 1.15–1.39) for solid stool FI and 1.30 (95% CI 1.18–1.42) for liquid stool FI.
We examined the association of MHT with FI defined according to at least weekly incidents of solid or liquid stool incontinence compared to at least monthly in the primary analysis and observed consistent results. Compared with never users of MHT, the multivariate-adjusted HRs for FI occurring at least once per week was 1.38 (95% CI 1.23–1.55) for current users and 1.24 (95% CI 1.15–1.34) for past users. The association of duration of MHT use was also relatively unchanged. Compared with women who had never used MHT, the multivariate-adjusted risk of FI occurring at least once per week was 1.17 (95% CI, 1.07–1.29) for 1–5 years of use, 1.26 (95% CI, 1.14–1.39) for 6–10 years of use, and 1.31 (95% CI, 1.21–1.43) for more than 10 years of use (Plinear trend<0.0001). Finally, the multivariate HR for FI occurring at least once per week was HR 1.33 (95% CI 1.01–1.76) for combination MHT users compared to estrogen-only formulation users).
We also examined the association of women who first initiated MHT in 2008 on risk of incident FI in the subsequent 4 years. Results were largely similar to those in the primary analysis although statistical power was limited. Compared to non-users, initiation of MHT in 2008 was associated with a multivariate-adjusted HR of 1.60 (95% CI, 0.94–2.72).
DISCUSSION
In this large prospective cohort of postmenopausal women, current and past use of MHT was associated with a modestly increased risk of FI. This association persisted even after adjustment for known risk factors for FI. Additionally, the risk of FI increased with longer duration of MHT use and was progressively attenuated among past users in relation to increasing time since discontinuation of MHT. We saw a relatively greater effect of combination (estrogen plus progestin) MHT compared to estrogen MHT alone on risk of FI.
Our data are consistent with a prior, cross-sectional study that showed that current, estrogen-based MHT use was associated with 1.3 greater odds of FI.12 However, our results contrast with two studies that have shown no association between FI and MHT.8, 9 However, these studies were also smaller in size (2,800 and 283 participants), cross-sectional rather than prospective, and were unable to rigorously control for other factors related to cumulative estrogen exposure. An earlier prospective study also found an improvement in symptomatic control in addition to improved anal resting and squeeze pressures with 6 months of MHT.10 This study was severely limited by its small size (n=20) and short duration of follow up (6 months). Moreover, a plausible mechanism for the improvement in FI seen in this data compared with increased incident FI in our results could be time-dependent expression of estrogen receptors. The short-time scale of the earlier study may reflect a period in which the estrogen receptor population in the anorectum is increased by exogenous estrogen therapy.7 Conversely, long-term MHT results in significant downregulation of estrogen receptors in the same tissue and could explain the temporal tends seen in our data with increasing risk of FI with longer duration of MHT.7
Our results are also biologically plausible. Conventional wisdom and earlier studies in tissue models presumed that exogenous replacement of diminishing estrogen levels could be beneficial to tissues with significant estrogenization, such as the pelvic floor;4–6 indeed, physiologic levels of sex hormones have been proposed to be involved in the development and maintenance of the epithelial, muscular, and connective tissue components of the pelvic floor tissues where sex receptors have been localized.5, 18 However, two large, randomized controlled trials demonstrated that MHT was associated with an increased risk of urinary incontinence,19, 20 and we found similar results in a parallel cohort of younger women.21 The mechanism for this association has been hypothesized to be related to estrogen’s effects on connective tissue rather than smooth muscle. Similarly, in other studies, 6 months of estrogen-based MHT in postmenopausal women resulted in an increase in vaginal matrix metalloproteinase-2 activity with resultant degradation of collagen. A similar process may also affect the anorectum, where estrogen receptors are a known feature of the connective tissue of the internal anal sphincter and levator ani.5, 7 These structures represent two of the major involuntary mechanisms involved in the maintenance of fecal continence via resting anal tone and creation of the anorectal angle, respectively.17 Estrogen-mediated loss of the supportive connective tissue of these structures could result in ineffective contractions and/or excessive pelvic floor laxity.
Progestin is known to increase estrogen receptor expression of the anorectum. Thus, the increased risk of FI with combination MHT (estrogen plus progestin) compared with estrogen-only therapy may also suggest that the association of MHT with FI may be mediated by progestins’ ability to augment the effects of estrogen on the anorectum, In addition, progestin may influence the effects of estrogen directly at the nuclear level.7 In a series of physiologic studies, Behar and colleagues demonstrated that women with slow-transit constipation may have an increased responsiveness to circulating progesterone levels due to an overexpression of progesterone receptors in the circular muscle layer of the colon.22, 23 However, the same group also found that progesterone treatment resulted in downregulation of the serotonin reuptake transporter in non-constipated murine models—leading to increased mucosal levels of 5-hydroxytryptamine (5-HT)—an important factor in peristaltic activity of the colon.24 Thus, we could see a role for combination MHT in increasing colonic motility, leading to FI in susceptible women.
Our study has several notable strengths. Most importantly, our prospective study design avoided the potential recall and selection bias found in retrospective and case-control studies. Additionally, we were able to control for important confounders with prospectively-collected, detailed information on lifestyle factors known to be associated with fecal incontinence and lifetime exposure to estrogen. Finally, we modeled MHT use using time-varying exposures in our Cox models, which accounted for changes in hormone use over time and minimized exposure misclassification, which could bias results to the null in other studies.
Despite biologic plausibility, our results are surprising given that advancing age is the strongest risk factor for FI and menopause is one of the milestone events in aging. Thus, there should be some caution in the interpretation of our results. Importantly, our analysis did not account for the effect of bowel disturbances, an important risk factor for FI.17 However, there was no significant difference in the association of MHT with risk of either solid or liquid stool FI, with the latter a reasonable surrogate for women with chronic bowel disturbances. Finally, our study is observational and therefore we cannot exclude the effect of potential residual confounding. That said, multivariate adjustment for many known risk factors for FI did not significantly alter our age-adjusted effect estimates. Finally, our study is observational and therefore we cannot exclude the effect of potential residual confounding. That said, multivariate adjustment for many known risk factors for FI did not significantly alter our age-adjusted effect estimates. Additionally, our analysis demonstrating decreased risk of FI with greater time since discontinuation of MHT suggests that MHT was a discrete, temporal factor in the development of FI. Our HR of 1.32 for risk of FI among current users of MHT is a modest increase in risk, though previous work from the same cohort has shown that more commonly-accepted risk factors for FI have similar risks (advanced age, odd ratio (OR) 1.28; BMI>30, OR 1.06; neurologic disease OR 1.32).22
Because the Nurses’ Health Study represents a highly-educated, health-literate group of women, our results may not be representative of the population at large. Nurses notably may be more likely to report symptoms of FI compared to the general population secondary to increased vigilance and recall of symptoms. However, this may influence the absolute incidence of FI but not the relative incidence of FI according to MHT. Additionally, nurses may perform rotating shift work, which has been associated with an increased likelihood of irritable bowel syndrome (IBS) that has been shown to be independent of sleep quality23 as well as correlated with increased psychosocial stress.24 Nevertheless, previous studies from this cohort have demonstrated that the prevalence of other lifestyle factors associated with risk of FI, such as body mass index and smoking status, closely mirror that of the general population. 25, 26 Additional analyses in which we additionally adjusted for the cumulative time performing rotating shifts did not materially alter our results. Moreover, the incidence rate of FI in our large cohort is consistent with other reports of fecal incontinence in the US population.27 Nonetheless, there are racial differences in prevalence and incidence of incontinence and so our results may not be applicable to non-white women. Our analysis also only examined women using systemic MHT. We were unable to analyze the effects of topical estrogen formulations, which may have a differential effect on risk of FI in this population. It is possible that some women were using topical formulations that have not been accounted for in our analysis. However, any potential misclassification of the exposure would attenuate our risk estimates toward the null. A small randomized, placebo-controlled study demonstrated improved continence in postmenopausal women with FI who were treated with topical estrogen therapy, but the difference failed to reach statistical significance.28
In summary, in this large, prospective cohort of postmenopausal women, MHT was associated with a modestly increased risk of fecal incontinence, which was somewhat enhanced by longer duration of use and was increased by use of combination hormone preparations. Because of other risks already known to be associated with MHT,29 prescribing patterns of MHT have shifted to use in selected women with severe symptoms for short durations. If these findings are coroborated by others, our study may add to the list of potential adverse effects that should be weighed carefully by prescribers and patients considering MHT. Moreover, identifying an increased risk of fecal incontinence with MHT could help to illuminate the basic mechanisms that underlie continence and may eventually inform novel therapeutic targets.
Supplementary Material
Acknowledgments
Grant support: The infrastructure of the Nurse’s Health Study cohort is supported by NIH UM1 CA186107. KS is supported by an American Gastroenterological Association (AGA) career development award. HK is supported by an AGA career development award and NIDDK K23DK099681. ATC is supported by NIH DK098311.
Abbreviations
- FI
fecal incontinence
- MHT
menopausal hormone therapy
Footnotes
AUTHOR CONTRIBUTIONS KS, MKT, HK, and ATC planned and designed the study; KS, MKT, HK, and RM analyzed the data; KS drafted the manuscript; all authors interpreted the results and contributed to critical review of the manuscript; KS had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
DISCLOSURES KS has received clinical trial support from Astra-Zeneca. MKT, HK, RM, FG, WEW, BK, and ATC report no disclosures. WEW has received clinical trial support from Salix. CAM has received research support from Boston Scientific and Pelvalon and has served as a consultant for Pelvalon.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rao SS, Bharucha AE, Chiarioni G, et al. Functional Anorectal Disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koloski NA, Jones M, Kalantar J, et al. Psychological impact and risk factors associated with new onset fecal incontinence. J Psychosom Res. 2012;73:464–8. doi: 10.1016/j.jpsychores.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Ng KS, Sivakumaran Y, Nassar N, et al. Fecal Incontinence: Community Prevalence and Associated Factors--A Systematic Review. Dis Colon Rectum. 2015;58:1194–209. doi: 10.1097/DCR.0000000000000514. [DOI] [PubMed] [Google Scholar]
- 4.Haadem K, Ling L, Ferno M, et al. Estrogen receptors in the external anal sphincter. Am J Obstet Gynecol. 1991;164:609–10. doi: 10.1016/s0002-9378(11)80032-3. [DOI] [PubMed] [Google Scholar]
- 5.Franz HB, Wendler D, Oettling G. Immunohistochemical assessment of steroid hormone receptors in tissues of the anal canal. Implications for anal incontinence? Acta Obstet Gynecol Scand. 1996;75:892–5. doi: 10.3109/00016349609055023. [DOI] [PubMed] [Google Scholar]
- 6.Oettling G, Franz HB. Mapping of androgen, estrogen and progesterone receptors in the anal continence organ. Eur J Obstet Gynecol Reprod Biol. 1998;77:211–6. doi: 10.1016/s0301-2115(97)00212-1. [DOI] [PubMed] [Google Scholar]
- 7.Copas P, Bukovsky A, Asbury B, et al. Estrogen, progesterone, and androgen receptor expression in levator ani muscle and fascia. J Womens Health Gend Based Med. 2001;10:785–95. doi: 10.1089/15246090152636541. [DOI] [PubMed] [Google Scholar]
- 8.Bharucha AE, Zinsmeister AR, Locke GR, et al. Risk factors for fecal incontinence: a population-based study in women. Am J Gastroenterol. 2006;101:1305–12. doi: 10.1111/j.1572-0241.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 9.Gordon D, Groutz A, Goldman G, et al. Anal incontinence: prevalence among female patients attending a urogynecologic clinic. Neurourol Urodyn. 1999;18:199–204. doi: 10.1002/(sici)1520-6777(1999)18:3<199::aid-nau6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Donnelly V, O'Connell PR, O'Herlihy C. The influence of oestrogen replacement on faecal incontinence in postmenopausal women. Br J Obstet Gynaecol. 1997;104:311–5. doi: 10.1111/j.1471-0528.1997.tb11459.x. [DOI] [PubMed] [Google Scholar]
- 11.Goode PS, Burgio KL, Halli AD, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatr Soc. 2005;53:629–35. doi: 10.1111/j.1532-5415.2005.53211.x. [DOI] [PubMed] [Google Scholar]
- 12.Varma MG, Brown JS, Creasman JM, et al. Fecal incontinence in females older than aged 40 years: who is at risk? Dis Colon Rectum. 2006;49:841–51. doi: 10.1007/s10350-006-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khalili H, Higuchi LM, Ananthakrishnan AN, et al. Hormone therapy increases risk of ulcerative colitis but not Crohn's disease. Gastroenterology. 2012;143:1199–206. doi: 10.1053/j.gastro.2012.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baik CS, Strauss GM, Speizer FE, et al. Reproductive factors, hormone use, and risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2525–33. doi: 10.1158/1055-9965.EPI-10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grodstein F, Manson JE, Stampfer MJ, et al. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–6. doi: 10.1001/archinte.168.8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113–9. doi: 10.1038/ajg.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: state of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop. Am J Gastroenterol. 2015;110:127–36. doi: 10.1038/ajg.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pares D, Iglesias M, Pera M, et al. Expression of estrogen and progesterone receptors in the anal canal of women according to age and menopause. Dis Colon Rectum. 2010;53:1687–91. doi: 10.1007/DCR.0b013e3181f05422. [DOI] [PubMed] [Google Scholar]
- 19.Hendrix SL, Cochrane BB, Nygaard IE, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA. 2005;293:935–48. doi: 10.1001/jama.293.8.935. [DOI] [PubMed] [Google Scholar]
- 20.Grady D, Brown JS, Vittinghoff E, et al. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstet Gynecol. 2001;97:116–20. doi: 10.1016/s0029-7844(00)01115-7. [DOI] [PubMed] [Google Scholar]
- 21.Townsend MK, Curhan GC, Resnick NM, et al. Postmenopausal hormone therapy and incident urinary incontinence in middle-aged women. Am J Obstet Gynecol. 2009;200:86 e1–5. doi: 10.1016/j.ajog.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews CA, Whitehead WE, Townsend MK, et al. Risk factors for urinary, fecal, or dual incontinence in the Nurses' Health Study. Obstet Gynecol. 2013;122:539–45. doi: 10.1097/AOG.0b013e31829efbff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nojkov B, Rubenstein JH, Chey WD, et al. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. 2010;105:842–7. doi: 10.1038/ajg.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koh SJ, Kim M, Oh DY, et al. Psychosocial stress in nurses with shift work schedule is associated with functional gastrointestinal disorders. J Neurogastroenterol Motil. 2014;20:516–22. doi: 10.5056/jnm14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarna L, Bialous SA, Jun HJ, et al. Smoking trends in the Nurses' Health Study (1976–2003) Nurs Res. 2008;57:374–82. doi: 10.1097/NNR.0b013e31818bf38b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Dam RM, Li T, Spiegelman D, et al. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rey E, Choung RS, Schleck CD, et al. Onset and risk factors for fecal incontinence in a US community. Am J Gastroenterol. 2010;105:412–9. doi: 10.1038/ajg.2009.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinedo G, Garcia E, Zarate AJ, et al. Are topical oestrogens useful in faecal incontinence? Double-blind randomized trial. Colorectal Dis. 2009;11:390–3. doi: 10.1111/j.1463-1318.2008.01624.x. [DOI] [PubMed] [Google Scholar]
- 29.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. N Engl J Med. 2003;349:523–34. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.