Abstract
Myotonic dystrophy (DM) is a dominantly-inherited genetic disorder affecting skeletal muscle, heart, brain, and other organs. DM type 1 is caused by expansion of a CTG triplet repeat in DMPK, whereas DM type 2 is caused by expansion of a CCTG tetramer repeat in CNBP. In both cases the DM mutations lead to expression of dominant-acting RNAs. Studies of RNA toxicity have now revealed novel mechanisms and new therapeutic targets. Preclinical data have suggested that RNA dominance is responsive to therapeutic intervention and that DM therapy can be approached at several different levels. Here we review recent efforts to alleviate RNA toxicity in DM.
Introduction
Myotonic dystrophy (dystrophia myotonica, DM) is an important genetic cause of progressive neuromuscular disability. The cardinal features include muscle weakness, myotonia (slow muscle relaxation), and early cataracts. In addition, affected individuals often experience cardiac arrhythmias and changes in neuropsychological function. Genetic testing shows an expanded CTG repeat in the 3′ untranslated region of DM Protein Kinase (DMPK) in DM type 1 (DM1) [1] or an expanded CCTG repeat in intron 1 of Cellular Nucleic Acid Binding Protein (CNBP) in DM type 2 (DM2) [2]. Currently there are no disease-modifying treatments for either disorder.
In DM, mutant transcripts containing expanded CUG- or CCUG-repeats (CUGexp or CCUGexp RNA) exert a toxic gain-of-function (reviewed by Todd and Paulson[3]). Arguably, the best characterized mechanism for RNA toxicity is through sequestration of splicing factors in the Muscleblind-like (MBNL) family. Two members of the family, MBNL1 and MBNL2, are widely expressed, whereas MBNL3 is expressed mainly in placenta [4]. All MBNL proteins share a similar arrangement of four RNA-binding domains, an overlapping set of target binding sites in the transcriptome, and high affinity for CUG-repeats (KD’s of 3 to 15 nM) [5–8]. When the cellular mass of CUGexp or CCUGexp RNA rises above a disease-causing threshold, the resulting titration of MBNL proteins affects alternative splicing, polyadenylation, or expression level for hundreds of genes. Expression of CUGexp RNA also affects cell signaling, causing downstream effects on muscle metabolism and RNA processing [9–11]. Finally, expanded RNA repeats are sometimes recognized by translational machinery, in a manner that causes translation to initiate within the repeat tract, despite the absence of a canonical start codon [12]. The phenomenon of repeat-associated non-ATG (RAN) translation leads to production of neurotoxic peptides (reviewed by Ranum and colleagues in this issue).
Two additional points of DM biology deserve emphasis in the therapeutic context. The first is that toxic RNAs are retained in nuclei and form nuclear foci [13, 14]. The formation of foci is probably nucleated by CUGexp/CCUGexp – MBNL interactions, through a process that also depends on other factors and signaling pathways [15–18]. The molecular crowding in foci produces a high local concentration of MBNL binding sites. Under conditions of protein excess, a fraction of MBNL is recruited into foci, but is exchangeable with free MBNL in the nucleoplasm, and activity is retained [7, 19]. However, under conditions of CUGexp excess, when binding sites are not fully occupied, the escape of MBNL is reduced, producing loss of MBNL activity. These two conditions, protein versus RNA excess, may roughly correspond to the pre-symptomatic and full-blown stages of DM1, wherein the transition from one to the other depends on length of the CUGexp tract. Notably, the latter quantity is not fixed because expanded CTG repeats grow larger in somatic cells over time. Consistent with this model, splicing dysregulation is mild in early DM1 but in later stages it becomes quite severe, resembling mice with combinatorial loss of both Mbnl1 and Mbnl2 [20, 21].
This model, if substantially correct, has important therapeutic implications. (1) Agents acting preferentially in the nucleus, such as, antisense oligonucleotides, may have greater impact than those acting predominantly in the cytoplasm, such as, siRNAs [22, 23]. (2) Early stabilization of CTG expansions may prevent the emergence of DM symptoms. (3) Foci are not insoluble collections of denatured material. Instead, they are dynamic structures that are accessible to drugs, and they can be dispersed [7, 16, 19, 24, 25]. (4) Even a modest release of MBNL, whether by decreasing levels of toxic RNA or blocking CUGexp-interactions, may produce disproportionate reversal of RNA processing defects, and potentially improve the symptoms. (5) Early-stages of DM1 may largely reflect titration of MBNL proteins, the highest affinity poly(CUG) binding proteins [26], whereas in later stages the exposure of unoccupied CUGexp binding sites may drive a broader pathogenic cascade, which again can be corrected by reverting to conditions of MBNL excess [7].
A second point is that DM mutations, although located in noncoding regions, may reduce the expression of mutant alleles, raising questions whether loss-of-function may contribute to the phenotype, or possibly impose a safety limit on knockdown therapies that create or aggravate a DMPK or CNBP deficiency state. CNBP is a ubiquitously expressed DNA- and RNA-binding protein that functions to regulate transcription and translation [27]. One study showed that the CCTG expansion in intron 1 does not affect transcription or processing of the mutant CNBP RNA, and that CCUGexp RNA in DM2 foci is a decay intermediate of the excised intron [28]. In fact, basal expression of CNBP protein was normal even in rare examples of homozygous DM2 muscle cells. Other studies, however, showed significant reduction of CNBP protein [29, 30]. In this regard, it is important to note that disruption of Cnbp causes severe brain deformity in homozygous mice and craniofacial malformations and muscle abnormalities in heterozygotes [31]. These developmental effects do not necessarily predict that postnatal knockdown would be unsafe, but they do raise the possibility that CNBP silencing strategies for DM2 will need to be highly selective for the mutant allele.
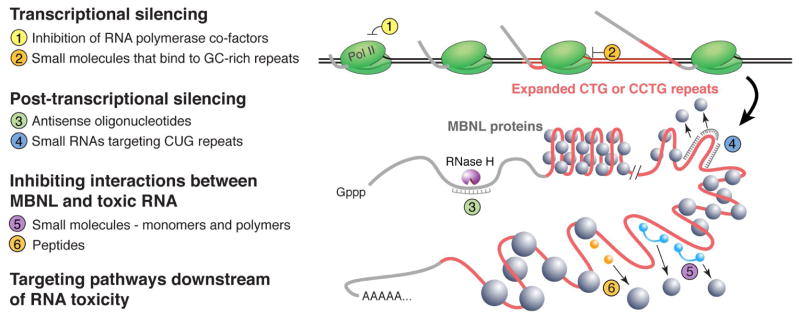
In the case of DM1, the basal expression of DM kinase protein is reduced by half due to nuclear retention of the mutant DMPK (mutDMPK) mRNA [13]. It is unclear, however, how this may affect function of skeletal muscle and heart, the two tissues with highest levels of DMPK expression. Initial reports indicated that Dmpk deletion in mice causes cardiac conduction defects in heterozygotes [32] and skeletal myopathy in homozygotes [33]. However, a recent study found no effect of DMPK knockdown or knockout on growth, survival, cardiac conduction, ventricular function, or muscle contraction in mice [34]. In addition, DMPK knockdown was well tolerated in monkeys [35]. Taken together, these data suggest that collateral knockdown of wild-type DMPK may be tolerable, at least to some extent. With these findings in mind, numerous therapeutic approaches have been designed with the goal of specifically targeting the mutant allele or its RNA product. Additional approaches have been developed to target signaling pathways downstream from CUGexp/CCUGexp expression. These strategies are discussed below and summarized in Figure 1.
Figure 1.
Strategies for treating myotonic dystrophy
Reducing RNA toxicity by silencing transcription
In general, transcription inhibition is not considered a robust generic strategy for treating gain-of-function mutations. However, for repeat expansion mutations there are indications that transcription elongation is a promising therapeutic target. The transcription elongation factor SPT4 was isolated in a screen for factors that modulate polyglutamine toxicity in yeast [36]. Subsequently, it was found that SPT4 is required for transcription of expanded CAG repeats in yeast, and that knockdown of the human paralog SUPT4H inhibits the expression of expanded CAG- or GGGGCC-repeats in patient-derived cells [36–38]. While it has not yet been determined whether SUPT4H is required for transcription of expanded repeats at the DM1 or DM2 loci, Berglund and colleagues have shown that pentamidine and related antibiotics can bind to CTG•CAG repeats and reduce the expression of CUGexp RNA [39]. Additionally, they showed that actinomycin D, a DNA intercalator that inserts preferentially at GpC dinucleotides, can reduce the expression of CUGexp RNA at doses below those necessary for general transcription inhibition [40]. It also appears that DMPK expression is upregulated by muscle regeneration [41], suggesting a feed-forward cycle in which initiation of muscle damage may drive higher expression of toxic RNA. If correct, any treatment that breaks the cycle of damage and regeneration may have the added benefit of reducing transcription of CUGexp RNA.
Post-transcriptional silencing of DMPK or CNBP
Various nucleic acid-based technologies have been used to obtain post-transcriptional knockdown of toxic RNA (Table). For example, Wansink and colleagues showed that antisense oligonucleotides (ASOs) comprised of CAG-repeats, targeted directly against the CUG-repeat, were highly effective for reducing mutDMPK mRNA in DM1 cells [42, 43]. This result was surprising since the ASOs were incapable of activating RNase H1, the usual co-factor for antisense knockdown. Although the mechanism remains a mystery, the data suggest a novel pathway for antisense knockdown that may specifically apply to expanded repeats. Lentiviral vectors expressing (CAG)15 antisense RNA were also quite effective for DMPK silencing, and again showed high selectivity for the mutant allele in cells [44]. To obtain this effect, it was necessary to drive high accumulation of therapeutic RNA in the nucleus by fusing the (CAG)15 sequence to a fragment of U7 snRNA.
Table.
Agents used for post-transcriptional silencing of toxic RNA in DM1.
| Agent | Target sequence | Comment |
|---|---|---|
| antisense oligonucleotide (RNase H1 active) | flanking sequence that lies 5′ or 3′ of the CUG-repeat tract | Successful knockdown and correction of phenotype with systemic administration in transgenic mice[45], now in clinical trials |
| Antisense oligonucleotide (RNase H1 active) | CUG-repeat tract | Local activity in transgenic mice with direct intramuscular injection[62, 63] |
| Antisense oligonucleotide (RNase H1 inactive) | CUG-repeat tract | Highly efficient knockdown in DM1 cells, and local activity in transgenic mice with direct intramuscular injection[24, 42] |
| siRNA | flanking sequence 5′ of the CUG-repeat tract | Preferential knockdown of wild-type DMPK in patient-derived cells when delivered using transfection reagent[23] |
| siRNA | CUG-repeat tract | Local activity in muscle of transgenic mice with direct intramuscular injection[64] |
| shRNA | flanking sequence 5′ of the CUG-repeat tract | Preferential knockdown of mutDMPK mRNA in patient-derived cells with expression from lentiviral construct [23]. |
| antisense RNA | CUG-repeat tract + adjacent 3′ flanking sequence | Preferential knockdown of mutDMPK mRNA in patient-derived cells using retroviral vector[65] |
| CAG-repeat antisense RNA fused to hU7-snRNA | CUG-repeat tract | Preferential knockdown of mutDMPK mRNA in patient-derived cells using lentiviral vector[44] |
The strategy that is furthest in clinical development employs RNase H1-active ASOs. In this case the targeting sequence is located outside of the repeat tract, thus preserving the chief advantage of antisense technology, its remarkable target specificity. Experiments in transgenic mice showed that ASOs targeting 5′ or 3′ of the repeat tract caused marked reduction of CUGexp RNA in skeletal muscle, accompanied by release of MBNL protein from foci, correction of splicing errors, elimination of myotonia, and improvement of muscle architecture [45]. These results were surprising given that ASO uptake in muscle is relatively low, causing failure of previous attempts to silence muscle-expressed targets. These findings prompted speculation that toxic RNA was particularly susceptible to ASOs due to prolonged dwell time in the nucleus, where RNase H1 is localized [46]. These findings spurred efforts to develop an optimized DMPK-targeting ASO [35], which recently entered clinical trials in patients with DM1.
Few studies have examined post-transcriptional silencing for DM2, which may in part reflect a concern that CNBP reduction would be deleterious. In addition, it is unclear whether targeting outside of the repeat tract would actually accelerate the clearance of CCUGexp RNA, if indeed it is already undergoing exonuclease decay [28]. Finally, there are technical limitations for monitoring knockdown efficiency, considering that the CCUG-repeats in foci are devoid of flanking sequences and therefore difficult to quantify using conventional RNA assays [28].
Small molecules to inhibit MBNL:(C)CUG-repeat interactions or disperse RNA foci
As discussed above, delivery of ASOs and siRNAs to muscle and heart poses a significant challenge, and CNS penetration is minimal unless ASOs are directly injected into CNS tissue or cerebrospinal fluid (see references [47, 48] for exceptions). A number of groups have therefore pursued the alternative approach of using rational design or high-throughput screens to identify small molecules that upregulate MBNL1, inhibit MBNL:CUGexp interaction, or disperse RNA foci, and phenotypic screens for compounds that improve function in Drosophila models have also been conducted [16, 49–54]. In this respect, the biophysical properties of expanded CUG- or CCUG-repeats are favorable for drug development (reviewed by Bernat and Disney [55]). These sequences form extended hairpins that are quite stable, despite the presence of a periodic U:U or U:C/C:U mismatch in the stem, forming a well-defined yet distinctive surface for drug binding. Furthermore, the reiterated binding motif and molecular crowding in foci may tend to drive strong RNA-ligand interactions. Several groups exploited these features by identifying CUG-binding monomers and then forming multimers using linkers of defined length so that the inter-subunit distance was tuned to the periodicity of the CUG- or CCUG-repeat duplex [56–58]. By this approach, compounds with higher specificity and affinity were obtained. More recently, Disney and colleagues achieved CUG repeat-catalyzed assembly of these molecules in situ by incorporating azide-alkyne click chemistry into the linker [53]. Others have used dynamic combinatorial libraries to achieve similar results in vitro [50]. Common across some of these molecules are aromatic groups that are predicted to intercalate between the U-U mismatches or occupy the grooves of CUG-repeat hairpins. Indeed, alterations of linker length between amidine groups was shown to modulate the potency of diamidines for displacing MBNL from CUG-repeats and rescuing mis-splicing in DM1 cell models [54]. Surprisingly, erythromycin, a commonly used rRNA-binding antibiotic, was also observed to displace MBNL from CUG repeats, and provide partial splicing rescue in a mouse model of DM1 [52].
Drug-induced release of MBNL protein from CUG repeats has been shown to restore MBNL activity and rescue splicing defects in DM1 model systems. However, unless such compounds also inhibit RAN translation, they pose a theoretical risk of promoting nuclear export, thereby increasing production of RAN peptides [59]. Another potential risk is that high-affinity poly(CUG)-binding compounds may slow the degradation of toxic RNA. For example, in a screen for compounds that inhibited binding of (CUG)12 to recombinant MBNL1 in vitro, the compound with highest activity caused slower turnover and increased accumulation of CUGexp RNA in cells [60].
Targeting signaling pathways downstream of CUG/CCUG expression
Another therapeutic strategy is to target signaling pathways activated downstream of CUGexp expression, including protein kinase C (PKC), glycogen synthase kinase 3 beta (GSK3beta), and AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR). In some DM1 mouse models and in human DM1 heart and muscle, inappropriate PKC activation leads to hyperphosphorylation of CELF family members, including CELF1 and CELF2, to cause downstream changes in CELF-dependent regulation of RNA splicing, RNA stability, and translation [9]. Inhibition of PKC by small molecules leads to normalization of CELF protein levels, rescuing muscle wasting in mouse models [61]. While a number of PKC inhibitors are in the clinic or trials, it is unclear which PKC isozyme is activated in DM1, and which symptoms would be rescued by PKC inhibition.
The stability and activity of GSK3beta in skeletal muscle is increased in transgenic mouse models and DM1 patients [10]. It has been proposed that phosphorylation of CELF1 also lies downstream of GSK3beta, in a cyclin 3-dependent manner. Inhibitors of GSK3beta normalize cyclin 3 protein levels, normalize CELF1 protein levels, and rescue muscle weakness in a transgenic mouse model. GSK3beta has been suggested as a therapeutic target for DM1, and GSK3beta inhibitors are in clinical trials for diabetes, cancer, and Alzheimer’s disease.
Deregulation of the AMPK/mTOR pathway has also been shown to occur in a mouse model of DM and DM1 cells [11]. Normalization of this pathway by administration of 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), an AMPK activator, or rapamycin, an inhibitor of mTOR, reduced muscle relaxation time following tetanic stimulation, albeit by different mechanisms. AICAR is on a list of substances banned in athletes due to performance enhancing capabilities. Rapamycin is an FDA-approved immunosuppressant medication.
Concluding remarks
Great strides have been made in identifying molecular targets for treating DM. Agents that reduce the production, accelerate the degradation, or alleviate the toxicity of repetitive RNA have shown encouraging results in preclinical models. It remains to be seen however, if any of these substances are safe and effective in DM patients. As therapies advance to human trials, it will be necessary to consider the possibility that targeting one mechanism may fail to address or even exacerbate others. This could occur, for example, if partial removal or detoxification of CUGexp RNA repeat permits ongoing growth of the expanded repeat in the genome, or if, as discussed above, the release of MBNL protein may promote increased RAN translation. On the other hand, it appears that this disease process can be simultaneously targeted at several sequential steps (Figure 1), with reasonable expectations for additive or synergistic effects. This argues for continued pursuit of all strategies discussed above and suggests that the optimal regimen may require several drugs in combination.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NS048843, NS094393, DP5-OD017865), the Muscular Dystrophy Association, and the Myotonic Dystrophy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brook JD, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- 2.Liquori CL, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293:864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 3.Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol. 2010;67:291–300. doi: 10.1002/ana.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanadia RN, et al. Developmental expression of mouse muscleblind genes Mbnl1, Mbnl2 and Mbnl3. Gene Expr Patterns. 2003;3:459–462. doi: 10.1016/s1567-133x(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 5.Warf MB, Berglund JA. MBNL binds similar RNA structures in the CUG repeats of myotonic dystrophy and its pre-mRNA substrate cardiac troponin T. Rna. 2007 doi: 10.1261/rna.610607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan Y, et al. Muscleblind-like 1 interacts with RNA hairpins in splicing target and pathogenic RNAs. Nucleic Acids Res. 2007;35:5474–5486. doi: 10.1093/nar/gkm601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••7.Sznajder LJ, et al. Mechanistic determinants of MBNL activity. Nucleic Acids Res. 2016;44:10326–10342. doi: 10.1093/nar/gkw915. In this study the authors perform systematic comparison of MBNL paralogs and their splice isoforms. In general, all MBNL isoforms recognize the same RNA motif with high affinity. MBNL1 demonstrates the highest alternative splicing activity, which correlates with the highest distribution to the nucleus. When bound to long, unsaturated CUG or CCUG repeat tracts, MBNL proteins are slow to exit from RNA foci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haghighat Jahromi A, et al. Single-molecule study of the CUG repeat-MBNL1 interaction and its inhibition by small molecules. Nucleic Acids Res. 2013;41:6687–6697. doi: 10.1093/nar/gkt330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuyumcu-Martinez NM, et al. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones K, et al. GSK3beta mediates muscle pathology in myotonic dystrophy. J Clin Invest. 2012;122:4461–4472. doi: 10.1172/JCI64081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brockhoff M, et al. Targeting deregulated AMPK/mTORC1 pathways improves muscle function in myotonic dystrophy type I. J Clin Invest. 2017 doi: 10.1172/JCI89616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zu T, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis BM, et al. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc Natl Acad Sci U S A. 1997;94:7388–7393. doi: 10.1073/pnas.94.14.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taneja KL, et al. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol. 1995;128:995–1002. doi: 10.1083/jcb.128.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dansithong W, et al. MBNL1 is the primary determinant of focus formation and aberrant insulin receptor splicing in DM1. J Biol Chem. 2005;280:5773–5780. doi: 10.1074/jbc.M410781200. [DOI] [PubMed] [Google Scholar]
- 16.Ketley A, et al. High-content screening identifies small molecules that remove nuclear foci, affect MBNL distribution and CELF1 protein levels via a PKC-independent pathway in myotonic dystrophy cell lines. Hum Mol Genet. 2014;23:1551–1562. doi: 10.1093/hmg/ddt542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent FX, et al. New function for the RNA helicase p68/DDX5 as a modifier of MBNL1 activity on expanded CUG repeats. Nucleic Acids Res. 2012;40:3159–3171. doi: 10.1093/nar/gkr1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pettersson OJ, et al. DDX6 regulates sequestered nuclear CUG-expanded DMPK-mRNA in dystrophia myotonica type 1. Nucleic Acids Res. 2014;42:7186–7200. doi: 10.1093/nar/gku352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho TH, et al. Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J Cell Sci. 2005;118:2923–2933. doi: 10.1242/jcs.02404. [DOI] [PubMed] [Google Scholar]
- 20.Nakamori M, et al. Splicing biomarkers of disease severity in myotonic dystrophy. Ann Neurol. 2013;74:862–872. doi: 10.1002/ana.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KY, et al. Compound loss of muscleblind-like function in myotonic dystrophy. EMBO molecular medicine. 2013;5:1887–1900. doi: 10.1002/emmm.201303275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vickers TA, Crooke ST. The rates of the major steps in the molecular mechanism of RNase H1-dependent antisense oligonucleotide induced degradation of RNA. Nucleic Acids Res. 2015;43:8955–8963. doi: 10.1093/nar/gkv920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langlois MA, et al. Cytoplasmic and nuclear retained DMPK mRNAs are targets for RNA interference in myotonic dystrophy cells. J Biol Chem. 2005;280:16949–16954. doi: 10.1074/jbc.M501591200. [DOI] [PubMed] [Google Scholar]
- 24.Wheeler TM, et al. Reversal of RNA dominance by displacement of protein sequestered on triplet repeat RNA. Science. 2009;325:336–339. doi: 10.1126/science.1173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Querido E, et al. Stochastic and reversible aggregation of mRNA with expanded CUG-triplet repeats. J Cell Sci. 2011;124:1703–1714. doi: 10.1242/jcs.073270. [DOI] [PubMed] [Google Scholar]
- 26.Miller JW, et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19:4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calcaterra NB, et al. CNBP: a multifunctional nucleic acid chaperone involved in cell death and proliferation control. IUBMB Life. 2010;62:707–714. doi: 10.1002/iub.379. [DOI] [PubMed] [Google Scholar]
- 28.Margolis JM, et al. DM2 intronic expansions: evidence for CCUG accumulation without flanking sequence or effects on ZNF9 mRNA processing or protein expression. Hum Mol Genet. 2006;15:1808–1815. doi: 10.1093/hmg/ddl103. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier R, et al. Absence of a differentiation defect in muscle satellite cells from DM2 patients. Neurobiol Dis. 2009;36:181–190. doi: 10.1016/j.nbd.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Raheem O, et al. Mutant (CCTG)n expansion causes abnormal expression of zinc finger protein 9 (ZNF9) in myotonic dystrophy type 2. Am J Pathol. 2010;177:3025–3036. doi: 10.2353/ajpath.2010.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W, et al. The zinc-finger protein CNBP is required for forebrain formation in the mouse. Development. 2003;130:1367–1379. doi: 10.1242/dev.00349. [DOI] [PubMed] [Google Scholar]
- 32.Berul CI, et al. DMPK dosage alterations result in atrioventricular conduction abnormalities in a mouse myotonic dystrophy model. J Clin Invest. 1999;103:R1–R7. doi: 10.1172/JCI5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy S, et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat Genet. 1996;13:325–334. doi: 10.1038/ng0796-325. [DOI] [PubMed] [Google Scholar]
- •34.Carrell ST, et al. Dmpk gene deletion or antisense knockdown does not compromise cardiac or skeletal muscle function in mice. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw266. This paper revisits DMPK deficiency as a pathomechanism of DM1. Using cardiac telemetry and ex vivo muscle mechanic measurements, the authors show that neither gene deletion, nor long-term post-developmental knockdown, leads to significant physiologic changes in cardiac or skeletal muscle function in mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •35.Pandey SK, et al. Identification and characterization of modified antisense oligonucleotides targeting DMPK in mice and nonhuman primates for the treatment of myotonic dystrophy type 1. The Journal of pharmacology and experimental therapeutics. 2015;355:329–340. doi: 10.1124/jpet.115.226969. The authors utilize a high-throughput approach to define DMPK targeting sequences that are capable of achieving robust knockdown in skeletal and cardiac muscle of rodents and non-human primates. This study highlights the capability of modern ASO chemistries when the target sequence is optimized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu CR, et al. Spt4 is selectively required for transcription of extended trinucleotide repeats. Cell. 2012;148:690–701. doi: 10.1016/j.cell.2011.12.032. [DOI] [PubMed] [Google Scholar]
- ••37.Cheng HM, et al. Effects on murine behavior and lifespan of selectively decreasing expression of mutant huntingtin allele by supt4h knockdown. PLoS Genet. 2015;11:e1005043. doi: 10.1371/journal.pgen.1005043. The authors demonstrate that reduction of Supt4h expression by heterozygous deletion leads to reduction in mutant huntingtin protein in the R6/2 mouse model of Huntington’s diease. Interestingly, homozygous Supt4h deletion animals exhibit embryonic lethality at E7.5 indicating that limits may exist in the safe, postnatal targeting of Supt4h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••38.Kramer NJ, et al. Spt4 selectively regulates the expression of C9orf72 sense and antisense mutant transcripts. Science. 2016;353:708–712. doi: 10.1126/science.aaf7791. A relatively common cause of both amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) is the expansion of a hexanucleotide (GGGGCC) repeat expansion within intron 1 of C9orf72. Kramer and colleagues show that knockdown of transcription elongation factor Stp4/SUPT4H1 specifically reduces transcription of expanded repeat RNA in both sense and antisense directions, providing further support for considering that SUPT4H1 is a shared therapeutic target for repeat expansion diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coonrod LA, et al. Reducing levels of toxic RNA with small molecules. ACS Chem Biol. 2013;8:2528–2537. doi: 10.1021/cb400431f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siboni RB, et al. Actinomycin D Specifically Reduces Expanded CUG Repeat RNA in Myotonic Dystrophy Models. Cell Rep. 2015;13:2386–2394. doi: 10.1016/j.celrep.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheeler TM, et al. Ribonuclear foci at the neuromuscular junction in myotonic dystrophy type 1. Neuromuscul Disord. 2007;17:242–247. doi: 10.1016/j.nmd.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulders SA, et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc Natl Acad Sci U S A. 2009;106:13915–13920. doi: 10.1073/pnas.0905780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez-Barriga A, et al. Design and analysis of effects of triplet repeat oligonucleotides in cell models for myotonic dystrophy. Mol Ther Nucleic Acids. 2013;2:e81. doi: 10.1038/mtna.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francois V, et al. Selective silencing of mutated mRNAs in DM1 by using modified hU7-snRNAs. Nat Struct Mol Biol. 2011;18:85–87. doi: 10.1038/nsmb.1958. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler TM, et al. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature. 2012;488:111–115. doi: 10.1038/nature11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki Y, et al. An upstream open reading frame and the context of the two AUG codons affect the abundance of mitochondrial and nuclear RNase H1. Mol Cell Biol. 2010;30:5123–5134. doi: 10.1128/MCB.00619-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •47.Goyenvalle A, et al. Functional correction in mouse models of muscular dystrophy using exon-skipping tricyclo-DNA oligomers. Nat Med. 2015;21:270–275. doi: 10.1038/nm.3765. Using new ASOs made of tricyclo-DNA (tcDNA), Goyenvalle and colleagues show enhanced uptake and effect in tissues that normally show low ASO delivery, including skeletal muscle, heart and brain. This report demonstrates the ongoing improvement of ASO technology to enhance delivery and expand the range of target organs. [DOI] [PubMed] [Google Scholar]
- 48.Hammond SM, et al. Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc Natl Acad Sci U S A. 2016;113:10962–10967. doi: 10.1073/pnas.1605731113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Lopez A, et al. In vivo discovery of a peptide that prevents CUG-RNA hairpin formation and reverses RNA toxicity in myotonic dystrophy models. Proc Natl Acad Sci U S A. 2011;108:11866–11871. doi: 10.1073/pnas.1018213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gareiss PC, et al. Dynamic Combinatorial Selection of Molecules Capable of Inhibiting the (CUG) Repeat RNA-MBNL1 Interaction In Vitro: Discovery of Lead Compounds Targeting Myotonic Dystrophy (DM1) J Am Chem Soc. 2008 doi: 10.1021/ja804398y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luu LM, et al. A Potent Inhibitor of Protein Sequestration by Expanded Triplet (CUG) Repeats that Shows Phenotypic Improvements in a Drosophila Model of Myotonic Dystrophy. Chem Med Chem. 2016;11:1428–1435. doi: 10.1002/cmdc.201600081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamori M, et al. Oral administration of erythromycin decreases RNA toxicity in myotonic dystrophy. Annals of clinical and translational neurology. 2016;3:42–54. doi: 10.1002/acn3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••53.Rzuczek SG, et al. Precise small-molecule recognition of a toxic CUG RNA repeat expansion. Nature chemical biology. 2016 doi: 10.1038/nchembio.2251. Rzuczek and colleagues used rational design to generate a small molecule targeting expanded CUG repeats. Modifications to the base molecule were made to induce covalent RNA interaction, target degradation, and on-site multimer formation. These features were used to validate accurate and specific target engagement, and their incorporation demonstrates the flexibility afforded by small molecule design in the context of expanded RNA repeats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siboni RB, et al. Biological Efficacy and Toxicity of Diamidines in Myotonic Dystrophy Type 1 Models. J Med Chem. 2015;58:5770–5780. doi: 10.1021/acs.jmedchem.5b00356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernat V, Disney MD. RNA Structures as Mediators of Neurological Diseases and as Drug Targets. Neuron. 2015;87:28–46. doi: 10.1016/j.neuron.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Childs-Disney JL, et al. Rationally designed small molecules targeting the RNA that causes myotonic dystrophy type 1 are potently bioactive. ACS Chem Biol. 2012;7:856–862. doi: 10.1021/cb200408a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee MM, et al. Controlling the specificity of modularly assembled small molecules for RNA via ligand module spacing: targeting the RNAs that cause myotonic muscular dystrophy. J Am Chem Soc. 2009;131:17464–17472. doi: 10.1021/ja906877y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jahromi AH, et al. Developing bivalent ligands to target CUG triplet repeats, the causative agent of myotonic dystrophy type 1. J Med Chem. 2013;56:9471–9481. doi: 10.1021/jm400794z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kino Y, et al. Nuclear localization of MBNL1: splicing-mediated autoregulation and repression of repeat-derived aberrant proteins. Hum Mol Genet. 2015;24:740–756. doi: 10.1093/hmg/ddu492. [DOI] [PubMed] [Google Scholar]
- 60.Hoskins JW, et al. Lomofungin and dilomofungin: inhibitors of MBNL1-CUG RNA binding with distinct cellular effects. Nucleic acids research. 2014;42:6591–6602. doi: 10.1093/nar/gku275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang GS, et al. PKC inhibition ameliorates the cardiac phenotype in a mouse model of myotonic dystrophy type 1. J Clin Invest. 2009;119:3797–3806. doi: 10.1172/JCI37976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee JE, et al. RNase H-mediated degradation of toxic RNA in myotonic dystrophy type 1. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4221–4226. doi: 10.1073/pnas.1117019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wojtkowiak-Szlachcic A, et al. Short antisense-locked nucleic acids (all-LNAs) correct alternative splicing abnormalities in myotonic dystrophy. Nucleic Acids Res. 2015;43:3318–3331. doi: 10.1093/nar/gkv163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sobczak K, et al. RNA interference targeting CUG repeats in a mouse model of myotonic dystrophy. Mol Ther. 2013;21:380–387. doi: 10.1038/mt.2012.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furling D, et al. Viral vector producing antisense RNA restores myotonic dystrophy myoblast functions. Gene Ther. 2003;10:795–802. doi: 10.1038/sj.gt.3301955. [DOI] [PubMed] [Google Scholar]