Abstract
Background
In the Learning Early About Peanut Allergy (LEAP) study, early peanut introduction in high-risk 4-11 month olds was associated with a significantly decreased risk of developing peanut allergy. However, the influences of key baseline high-risk factors on peanut tolerance are poorly understood.
Methods
Secondary analysis was conducted on the publically available LEAP dataset, exploring relationships between peanut tolerance, baseline peanut/egg sensitization, eczema severity/duration, age of introduction, gender, and race.
Results
A multiple logistic regression model predicting odds of successful oral food challenge (OFC) at 60 months noted higher odds with early introduction (OR 9.2, P<0.001, 95%CI 4.2-20.3), white race (OR 2.1, p=0.04, 95%CI 1.1-3.9), and advancing age (OR 4.8, P=0.04, 95%CI 1.1-20.8). Odds of peanut tolerance were lower with increasing peanut wheal size (OR 0.58, P<0.001, 95%CI 0.46-0.74), increased baseline SCORAD score (OR 0.98, p=0.04, 95%CI 0.97-1), and increased kUA/L of egg serum IgE (sIgE) (OR 0.99, p=0.04, 95%CI 0.98-1). The probability of peanut tolerance in the early introduction group was 83% vs. 43% in the avoidance group with SPT wheal of less than 4mm. The probability of a successful OFC was significantly higher with peanut introduction between 6-11 months than at 4-6 months. Increasing eczema severity had limited impact on the probability of peanut tolerance in the early introduction arm.
Conclusion
Increasing peanut wheal size predicted peanut tolerance only in the avoidance arm. Peanut introduction between 6-11 months of age was associated with the highest rates of peanut tolerance, questioning the “urgency” of introduction before 6 months.
Keywords: LEAP, complementary feeding, allergy prevention, early peanut introduction, peanut allergy
Introduction
Food allergy affects an estimated 8% of US children and 10% of Australian 1-year olds. 1,2 Peanut allergy in the US may affect as many as 1.4-4.6%, depending on the methodology used.1,3-5 Comparatively, peanut allergy prevalence in Australia among 1 year olds is 3%, and 1.2 -1.4% among children in the UK.2,6 While there are several therapies under investigation, there is no known treatment for peanut allergy. Studies suggest that approximately 24% of individuals will outgrow peanut allergy, meaning that for the majority it is lifelong.7
Recent focus has turned to primary prevention as a way to stem the potential rise in peanut allergy, though this rise may be more country-selective.4,5,8 A thought-provoking study from Du Toit et al. in 2008 showed that between two large observational cross-sectional samples of Ashkenazi Jewish children living in London and Tel Aviv, the London cohort had a 10-fold higher prevalence of reported peanut allergy.9 This difference was significantly associated with reported prolonged delay in peanut introduction until after 3 years of age in the London sample vs. introduction within the first year of life in the Tel Aviv sample.9 This finding led to the development of the LEAP (Learning Early about Peanut) study, a randomized-controlled trial of intentional delayed vs. early peanut introduction. The LEAP study showed a significant absolute risk reduction among those randomized to early intervention (between 4-11 months), with a number needed to treat (NNT) of 8.5 among those with no skin test sensitization and 4 among those with 1-4mm peanut wheal diameter on skin prick testing.10,11
In the ensuing months, an interim consensus document, agreed upon by 10 international allergy/immunology, pediatric, and dermatology organizations recommended that children meeting the high-risk criteria in the LEAP study start early peanut introduction between 4-6 months of life, and provided some practical recommendations for how the provider and caregiver could accomplish this task given the firm belief that the study findings would be beneficial to help potentially decrease the number of children who develop peanut allergy.12 However, to date, only Australia has issued any official change to its policies regarding the timing of infant complementary feeding/solid food introduction and early introduction of high-risk allergens, including peanut. While efforts are underway to do so in the US, spearheaded by the National Institutes for Allergy and Infectious Diseases (NIAID), an updated addendum to the 2010 Food Allergy Guidelines has just been recently published.
As part of a process for transparency in government funded research and per agreement in receiving funding from the Immune Tolerance Network, the LEAP study team has made multiple datasets available for public viewing through www.trialshare.org.13 The published LEAP analysis primarily focused on a simple skin test stratified proportional comparison between intervention arms, and longitudinal assessment of some key immunological markers of change (primarily skin test results, as well as peanut-specific sIgE and sIgG4). However, this analysis and final reporting of the LEAP trial left lingering questions regarding the ideal age for the timing of peanut intervention, specific risk inferred with incremental baseline wheal size up until the study cut off at 5mm, and any predictive associations with peanut tolerance.14-16 We therefore undertook an analysis of the publically available data from the LEAP trial to investigate a more robust understanding of what risk factors influence peanut tolerance.
Methods
The descriptions of the LEAP study, its sampling frame, inclusion/exclusion criteria, primary/secondary outcomes, and data analysis methodologies have been previously published elsewhere in full detail.8,10 The Immune Tolerance Network (ITN) TrialShare is a “clinical trials research system that provides clinical datasets for research and analysis as well as a platform for the scientific community to share data in a secure manner.” Interested users can register for a free account and can access data, data reports, and interactive data analysis tools for ITN-sponsored studies made available by a particular study team.13
The primary study authors registered an account with www.trialshare.org and downloaded the publically available LEAP data sets to a Microsoft excel spreadsheet (Microsoft Corporation, Redmond, WA). Available variables in the dataset for downloading are detailed at www.trialshare.org. Not all variables collected in the LEAP trial and analyzed in the primary publication were made publically available. Information from the available datasets were combined into a master spreadsheet and then exported into Stata SE 13 (College Station, TX), for analysis. All 640 individuals from the LEAP study included in the dataset with complete data available for enrollment and 60-month OFC outcomes to determine ultimately if the subject was peanut tolerant or peanut-allergic were included for analysis. Proportional analysis was performed using chi square/fisher exact test, and multiple logistic regression was used to model associations with a given outcome. Predictive probabilities from this regression model were assessed using the Stata margins command, and visually displayed using the marginsplot command. Strength and fit of regression models were assessed through use of receiver operator characteristic and area under the curve. This study was not subject to institutional review board oversight nor does it meet human subjects research criteria given it involves publically available, de-identified data which this authorship group did not obtain through direct intervention or interaction with the individual or identifiable private information.
Results
We first explored the relationship in the LEAP study between peanut wheal size and challenge outcome, maintaining the trial stratification of negative (0mm) peanut skin tests (n=542) versus a combined group of all infants with “positive” (1-4mm) peanut skin tests (n=98), which assumes homogeneity of wheal size from 1-4mm and clinical significance of 1 and 2mm sensitization, despite an established convention of clinical significance at 3mm or greater.17 To test the assumption of wheal size homogeneity within the 1-4mm skin test positive subgroup, we compared infants with 1-2mm wheals versus those with 3-4mm wheals for differences in the rates of successful versus unsuccessful OFCs within and between the trial arms (Table 1). Within the avoidance group there were no significant differences between 1-2mm vs. 3-4mm skin tests (p=0.7), but within the early introduction group there were significant differences seen (p=0.004) and all subjects had successful OFC's in the 1-2mm skin test subgroup. Within these wheal size subgroups between trial arms, among those with 1-2mm skin test, 12/32 (37.5%) in the avoidance group vs. 0/30 (0%) in the early introduction group had an unsuccessful OFC (p<0.001), a significant difference in favor of early introduction. Among those with 3-4mm skin tests, rates of unsuccessful OFC were very similar--6/19 (31.6%) in the avoidance arm vs. 5/17 (29.4%) in the early introduction arm, which while underpowered, represents no difference between groups. Thus, in univariate subgroup analysis, nearly 70% of those with a 3-4mm wheal had successful peanut OFC irrespective of their randomization arm, and the overall occurrence of unsuccessful OFC was lower in both the 1-2mm and 3-4mm subgroups among the early introduction arm compared to the avoidance arm.
Table 1.
Comparison of Month 60 Challenge Outcome Stratified by Skin Test Wheal Size Among LEAP Trial Participants
| Avoidance | Early Introduction | |||||
|---|---|---|---|---|---|---|
| Unsuccessful OFC | Successful OFC | Total | Unsuccessful OFC | Successful OFC | Total | |
| 1-2 mm | 12 | 20 | 32 | 0 | 30 | 30 |
| 3-4mm | 6 | 13 | 19 | 5 | 12 | 17 |
| Total | 18 | 33 | 51 | 5 | 42 | 47 |
A multiple logistic regression model was created to explore the influence of baseline peanut skin test and sIgE, egg skin test and sIgE, patient age, eczema severity and duration, race, and gender on the odds of a successful peanut OFC at month 60, the primary trial endpoint (Table 2a). We noted significantly higher odds of successful peanut OFC at month 60 with randomization to the early introduction arm, with each 1 month increase in the infant's age at introduction of the intervention, and with white race. Odds of successful OFC were significantly lower with each 1mm increase in peanut wheal skin test size, 1 kUA/L increase in egg sIgE, and 1-point increase in SCORAD score. Age demonstrated a non-linear (quadratic) interaction indicative that the age effect is parabolic, and the probability of successful peanut OFC increases with increasing age to a point, then decreases. Additional interactions (and quadratic effects) among the model variables were assessed and noted to be either non-significant or to not improve model AUROC with their inclusion. The overall regression model AUROC was 0.83, with a sensitivity of 99%, a positive predictive value of 91%, and correctly classified 90.5% of cases as tolerant of peanut. An alternative model, using a mild-moderate-severe categorical eczema severity rating variable instead of SCORAD, had an identical AUROC, nearly identical variable point estimates (and same non-significant confounders), and noted only severe eczema (versus mild or moderate eczema) was associated with decreased odds of a successful month 60 peanut challenge (OR 0.2, p=0.03, 95%CI 0.04-0.87) (Table 2b).
Table 2a.
Factors Associated with Peanut Tolerance at Age 5
| Predictors of Peanut Tolerance at Month 60 | OR | P | 95%CI |
|---|---|---|---|
| Early introduction arm | 9.2 | <0.001 | 4.2-20.3 |
| Peanut wheal size (mm) at study entry | 0.58 | <0.001 | 0.46-0.74 |
| White race | 2.1 | 0.04 | 1.1-3.9 |
| SCORAD at study entry | 0.98 | 0.04 | 0.97-1 |
| Egg sIgE (KUA/L) at study entry | 0.99 | 0.04 | 0.98-1 |
| Age (months) at study entry | 4.8 | 0.04 | 1.1-20.7 |
| Age*Age interaction | 0.91 | 0.04 | 0.83-0.99 |
Adjusted for gender, eczema duration, peanut sIgE, and egg skin test wheal size. Model AUROC 0.83
Table 2b.
Alternative Model Using Categorical Eczema Severity Rating
| Predictors of Peanut Tolerance at Month 60 | OR | P | 95%CI |
|---|---|---|---|
| Early introduction arm | 9 | <0.001 | 4.1-19.7 |
| Peanut wheal size (mm) at study entry | .6 | <0.001 | 0.47-0.77 |
| Egg wheal size (mm) at study entry | 0.92 | 0.04 | 0.85-0.99 |
| White race | 2 | 0.03 | 1.1-3.8 |
| Eczema severity at study entry (reference of mild severity) | |||
| Moderate | 0.26 | 0.09 | 0.06-1.2 |
| Severe | 0.19 | 0.03 | 0.04-0.87 |
| Egg sIgE (KUA/L) at study entry | 0.99 | 0.04 | 0.98-0.99 |
| Age (months) at study entry | 4.8 | 0.04 | 1.1-21.1 |
| Age*Age Interaction | 0.9 | 0.04 | 0.8-0.99 |
Adjusted for Peanut IgE, eczema duration, peanut sIgE and gender. Model AUROC 0.83
To better isolate subgroup effects influencing month 60 peanut tolerance, predicted probabilities were calculated from the regression model. We first tested the effect of increasing peanut wheal size on peanut tolerance at month 60 (Figure 1). This demonstrates that within the early introduction arm, the probability of a successful OFC diminishes somewhat minimally with increasing wheal size, ranging from 98% at 0mm and 83% at 4mm. In contrast, in the avoidance arm, the probability rapidly declines from 86% at 0mm to 43% at 4mm. A second predicted probability investigated the effect of increasing age at the time of peanut introduction on month 60 peanut tolerance within the early introduction group. (Figure 2a) This demonstrates an overall high predicted probability of successful OFC at month 60 across all trial ages of introduction, but a significantly lower probability between 4 to 6 months of life compared to between 6 to 11 months. The 95%CI's were widest at 4-5 months of age, and very narrow at the other ages. As a sensitivity analysis, we demonstrated the predicted probabilities at 4 and 5 months are highly susceptible to variable inclusion in the model and the 95%CI's remain constantly wide, though the probabilities and CI's for introduction between 6-11 months remain virtually unchanged. While the probability at 4-6 months is 92% in the model detailed in table 2, an alternative model which approximates the clinical considerations in the NIAID addendum (which include only peanut wheal size, age, and eczema severity as considerations) note this probability dips to 86%, with a model AUROC of 0.8 (Figure 2b). Importantly, irrespective of model, the probability of tolerance was consistently higher (and CI's the narrowest) with introduction between 6-11 months of life, relative to introduction at 4-5 months. Additional predicted probabilities were calculated for SCORAD score, eczema severity, and race, detailed in supplemental figure 1a-d. These analyses demonstrate limited impact of increasing SCORAD score or severe eczema categorization on the predicted probability of tolerating the month 60 peanut OFC in the early introduction arm. As well, these suggest a mildly diminished predicted probability of peanut tolerance for non-white race, worse at 4-5 months of introduction than at other ages, as well as a more pronounced reduced probability of peanut tolerance for non-white race based on an increased SCORAD score.
Figure 1. Predicted Probabilities of Successful Month 60 Peanut Oral Food Challenge Among LEAP Study Participants, Based on Initial Screening Peanut Skin Test.
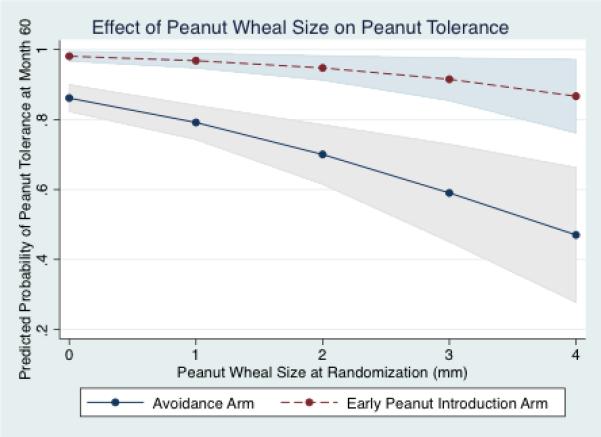
The predicted probability for successful peanut OFC at month 60 is significantly and distinctly lower at an equivalent peanut prick skin wheal size among children randomized to the delayed introduction arm compared to the early introduction arm.
Figure 2. Predicted Probabilities of Successful Month 60 Peanut Oral Food Challenge Among LEAP Study Participants, Based on the Age of Peanut Introduction.
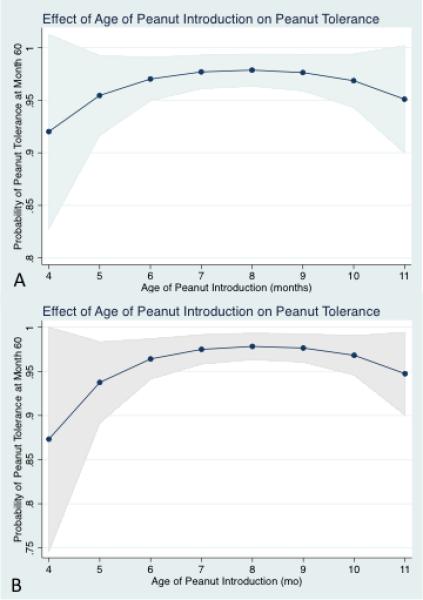
Figure 2a demonstrates the relationship between age of peanut introduction and peanut tolerance based on the regression model in table 2a, noting a maximal probability between 6-11 months. Figure 2b details a model approximating the NIAID Guideline Addendum considerations (age, eczema severity, and peanut wheal size), which demonstrates a lower probability between 4-6 months but stable probability between 6-11 months of age compared to figure 2a.
Discussion
In this secondary analysis of the publically available LEAP trial data, we describe the relationship between reported pre-randomization risk factors and the primary outcome of peanut tolerance at age 5, as well as highlight multiple distinct and highly significant subgroup effects not described in the initial DuToit et al trial publication.11 The predictive model may help the practicing allergist to better understand patient attributes that most critically influence the end-trial outcome of peanut tolerance, which per our model is predominantly early peanut introduction. The subgroup effects strongly support early peanut introduction and better highlight that the true value of the pre-randomization peanut skin test wheal size and eczema severity were of most importance in predicting OFC outcome among the avoidance group. This distinctly highlights worse and divergent outcomes for those children randomized to avoid peanut at each incremental peanut wheal size (or SCORAD score), vs. those who had early introduction. This demonstrated effect raises questions as to the value of performing skin testing in children where the intent is to give peanut early, or targeting early introduction to only those with severe eczema (as opposed to all eczema severity).
Moreover, we also demonstrate an optimal window for the specific timing of peanut introduction that is several months wide. The highest predicted probability of peanut tolerance occurred with peanut introduction between 6-11 months of life, which withstood sensitivity analysis. Arguably, the probability of tolerance is not lower than 85% with introduction at 4 and 5 months even in the most conservative model, which would be supportive of a policy suggesting peanut should be introduced starting as early as 4-6 months of life. However, peanut introduction between 6-11 months of life was consistently associated with better outcome (~95% probability of tolerance, with a very narrow 95% CI) in all models. From a policy standpoint, waiting until at least 6 months for introduction may perhaps better harmonize with WHO guidelines of 6-months exclusive breastfeeding. This would create a “win, win” situation maximizing the potential benefits of both interventions, which would not potentially elevate a rather narrow and singular benefit of early peanut introduction before 6 months over the many positive outcomes associated with 6-months of exclusive breastfeeding and will integrate well with nutritional milestones of the infant.
These additional data from this secondary analysis can be helpful to inform future policy, or fine-tune existing policy to ensure that the implementation of early peanut introduction is as feasible as possible for the clinician. It is crucial to understand the true impact of specific risk-factors, advise an appropriate starting time, and optimize any health services utilizations to help facilitate introduction.12 In this vein, we show that so long as the infant received peanut early, the stipulated risks of eczema severity (either through SCORAD score or categorical rating) and baseline peanut sensitization imparted little leverage against the development of peanut tolerance. Figures 1 and supplemental figure 1 in particular highlight this moderating effect, and it should be emphasized that 85% of infants with a peanut wheal of 4mm successfully tolerated peanut OFC, including for subjects with high SCORAD or severe eczema. We would hope this is reassuring for the practicing clinician. Properly interpreting these risks will be key for optimizing the implementation of any forthcoming policy. The more pressing concern from the trial may be the degree of potential missed benefit of early peanut introduction among those excluded from the trial with wheal sizes >4mm.15 The recommendations in the forthcoming NIAID Food Allergy Guideline Addendum recommends an 8mm peanut wheal size cut-off as opposed to 4mm, but it is unclear how the practicing allergist will apply such guidance, and if even this expanded cut off is warranted.18
These data suggesting a window of 6-11 months for potential early peanut introduction are also of clinical relevance. Introduction between 6-11 months has a ~95% probability of tolerance in all models. The issue is again how any policy would be implemented. A longer time window for successful peanut introduction, up to almost the end of the first year of life without adversely affecting predicted tolerance, should reassure providers and hesitant caregivers wary to attempt peanut introduction between 4-6 months that slight delay will make little difference to the outcome. Notwithstanding the better harmonization with WHO guidance on duration of exclusive breastfeeding, a longer window for introduction may give the infant more time to develop a fondness for eating and comfort with textures/tastes, and allow more familiarity for assessing the child's behavior with foods. This would give a clear distinction in what are normal eating behaviors (e.g., spit up, perceived gag, irritation-type rashes, etc.) and clearly abnormal ones so that peanut is not removed from the diet prematurely and “allergy” over-ascribed to insignificant events. In this vein, it is important to maintain a perspective that the introduction of solid food is part of normal development and not a medical event. A recent study noted less than 0.5% of US infants have peanut introduced by 5 months and less than 20% by 12 months according to data from the Infant Feeding Practices II study 2014, despite 2008 AAP recommendations to not delay solid food introduction past 4-6 months of life.19 These additional few months may also be of significant benefit given the contrasting experience in the Enquiring About Tolerance (EAT) trial, where only 61.9% of children under 6 months of age were able to comply with early introduction of peanut.11,20 The EAT trial may reflect the more “real-world” scenario.
Our analysis has distinct limitations. Many are limitations of the primary LEAP study. One such main limitation is lack of outcome data available on children with >4mm wheal sizes as these children were excluded from study, making the wheal size vs. outcome relationship in Figure 1 difficult to extrapolate as to where the predicted probability curve may have an inflection point towards unfavorable outcomes. Another key limitation is that the data available in the TrialShare data set does not include all potentially collected study variables. Variables of interest that were not available include maternal diet during pregnancy, lactation status, and other trends that were described in the LEAP nutrition paper, including effects of dietary diversity. Thus our models will need to be revised, and updated analysis submitted, if these additional data become available. Their future inclusion may potentially add to our understanding of what influences the development of peanut tolerance. Another limitation was that in the original LEAP analysis, egg allergy and eczema were presumed to have equal weight as a risk factor for developing peanut allergy, a factor which we could not empirically test the accuracy of in our models. Of concern is that only egg allergy and not any other food allergy was considered as a risk factor. This is important given little historical evidence children <6 months of age have robust enough routine dietary egg exposure for there to be an appreciable rate of clinical egg allergy (as opposed to having egg sensitization).20-26 Other limitations of the LEAP study applicable to our secondary analysis were that skin test cutoffs, dose/duration of the study, and use of skin testing were chosen a priori and were neither randomized nor controlled, and the study sample is clustered as it was obtained at a single food allergy referral center in the UK, and that we have no data available for outcomes of children older than 11 months. All of these limitations may limit generalizability of our findings in this analysis to different samples or populations.
In conclusion, we show novel and significant relationships that are associated with the likelihood of peanut tolerance at 60 months among infants randomized to early peanut introduction or prolonged delayed introduction, using the publically available original LEAP data. These data further emphasize the heterogeneity of the treatment effect of early vs. delayed peanut introduction, that wheal size was of far more utility in predicting less favorable OFC outcomes only in the avoidance group, and that introduction after 6 months of life was associated with a higher probability of successful peanut OFC at month 60 compared to introduction before 6 months. These data strongly emphasize a risk-reducing effect of early peanut introduction and will hopefully provide reassurance to parents and providers concerned with possible pre-existing peanut sensitization, or the difficulties of introducing peanut so quickly and in such a narrow window after complementary feeding begins. These secondary analyses should be viewed as enhancing the potential benefit of early peanut introduction, and complementary to forthcoming policy on implementing peanut allergy prevention strategies at a national or international level.
Supplementary Material
Acknowledgments
Funding: no funding was used to support effort for this study.
Abbreviations
- LEAP
Learning Early About Peanut Allergy study
- OFC
oral food challenge
- NNT
number needed to treat
- ITN
Immune Tolerance Network
- NIAID
National Institutes for Allergy and Infectious Diseases
- EAT
Enquiring About Tolerance
Footnotes
Conflicts of Interest: All authors disclose they are members of the NIAID expert panel on peanut allergy prevention. Drs. Greenhawt, Chan, Fleischer, Venter, and Spergel were contributors to the Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. The authors report there are no financial conflicts of interest pertaining to this work.
Author Contributions:
Matthew Greenhawt, MD, MBA, MSc—study design, data analysis and interpretation, manuscript drafting and revision
David Fleischer, MD: data interpretation, manuscript drafting and revision
Edmond S. Chan, MD, FRCPC: data interpretation, manuscript drafting and revisionCarina Venter, PhD, RD: data interpretation, manuscript drafting and revision
David Stukus, MD: data interpretation, manuscript drafting and revision
Ruchi Gupta, MD, MS: data interpretation, manuscript drafting and revision
Jonathan Spergel, MD, PhD: data interpretation, manuscript drafting and revision
All authors have participated in the drafting and revision of the final submitted draft of the manuscript, and have both reviewed and approved the copy of the final version prior to submission.
References
- 1.Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. 2011;128:e9–17. doi: 10.1542/peds.2011-0204. [DOI] [PubMed] [Google Scholar]
- 2.Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–76. e1–2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Sampson HA, Aceves S, Bock SA, James J, Jones S, Lang D, et al. Food allergy: a practice parameter update-2014. J Allergy Clin Immunol. 2014;134:1016–25. e43. doi: 10.1016/j.jaci.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, Workman L, Sordillo JE, Gillman MW, et al. Peanut allergy prevalence among school-age children in a US cohort not selected for any disease. J Allergy Clin Immunol. 2014;134:753–5. doi: 10.1016/j.jaci.2014.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Venter C, Hasan Arshad S, Grundy J, Pereira B, Bernie Clayton C, Voigt K, et al. Time trends in the prevalence of peanut allergy: three cohorts of children from the same geographical location in the UK. Allergy. 2010;65:103–8. doi: 10.1111/j.1398-9995.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 7.Peters RL, Allen KJ, Dharmage SC, Koplin JJ, Dang T, Tilbrook KP, et al. Natural history of peanut allergy and predictors of resolution in the first 4 years of life: A population-based assessment. J Allergy Clin Immunol. 2015;135:1257–66. e1–2. doi: 10.1016/j.jaci.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Venter C, Maslin K, Patil V, Kurukulaaratchy R, Grundy J, Glasbey G, et al. The prevalence, natural history and time trends of peanut allergy over the first 10 years of life in two cohorts born in the same geographical location 12 years apart. Pediatr Allergy Immunol. 2016 doi: 10.1111/pai.12616. In press; 10.1111/pai.12616. [DOI] [PubMed] [Google Scholar]
- 9.Du Toit G, Katz Y, Sasieni P, Mesher D, Maleki SJ, Fisher HR, et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–91. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 10.Du Toit G, Roberts G, Sayre PH, Plaut M, Bahnson HT, Mitchell H, et al. Identifying infants at high risk of peanut allergy: the Learning Early About Peanut Allergy (LEAP) screening study. J Allergy Clin Immunol. 2013;131:135–43. e1–12. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Du Toit G, Roberts G, Sayre PH, Bahnson HT, Radulovic S, Santos AF, et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:803–13. doi: 10.1056/NEJMoa1414850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleischer DM, Sicherer S, Greenhawt M, Campbell D, Chan E, Muraro A, et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. J Allergy Clin Immunol. 2015;136:258–61. doi: 10.1016/j.jaci.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 13. [December 5, 2015]; https://www.itntrialshare.org/.
- 14.Greenhawt MJ, Fleischer DM, Atkins D, Chan ES. The Complexities of Early Peanut Introduction for the Practicing Allergist. J Allergy Clin Immunol Pract. 2016;4:221–5. doi: 10.1016/j.jaip.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Greenhawt M, Chan ES, Fleischer DM. Peanut consumption in infants at risk for peanut allergy. N Engl J Med. 2015;372:2164–5. doi: 10.1056/NEJMc1504021. [DOI] [PubMed] [Google Scholar]
- 16.Greenhawt M. The Learning Early About Peanut Allergy Study: The Benefits of Early Peanut Introduction, and a New Horizon in Fighting the Food Allergy Epidemic. Pediatr Clin North Am. 2015;62:1509–21. doi: 10.1016/j.pcl.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Bernstein IL, Li JT, Bernstein DI, Hamilton R, Spector SL, Tan R, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100:S1–148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- 18.Gruchalla RS, Sampson HA. Preventing peanut allergy through early consumption--ready for prime time? N Engl J Med. 2015;372:875–7. doi: 10.1056/NEJMe1500186. [DOI] [PubMed] [Google Scholar]
- 19.Luccioli S, Zhang Y, Verrill L, Ramos-Valle M, Kwegyir-Afful E. Infant feeding practices and reported food allergies at 6 years of age. Pediatrics. 2014;134(Suppl 1):S21–8. doi: 10.1542/peds.2014-0646E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkin MR, Logan K, Marrs T, Radulovic S, Craven J, Flohr C, et al. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koplin JJ, Osborne NJ, Wake M, Martin PE, Gurrin LC, Robinson MN, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126:807–13. doi: 10.1016/j.jaci.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 22.Koplin JJ, Allen KJ. Optimal timing for solids introduction - why are the guidelines always changing? Clin Exp Allergy. 2013;43:826–34. doi: 10.1111/cea.12090. [DOI] [PubMed] [Google Scholar]
- 23.Fleischer DM, Spergel JM, Assa'ad AH, Pongracic JA. Primary prevention of allergic disease through nutritional interventions. J Allergy Clin Immunol Pract. 2013;1:29–36. doi: 10.1016/j.jaip.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 25.McKean M, Caughey AB, Leong RE, Wong A, Cabana MD. The Timing of Infant Food Introduction in Families With a History of Atopy. Clin Pediatr (Phila) 2015;54:745–51. doi: 10.1177/0009922815584927. [DOI] [PubMed] [Google Scholar]
- 26.Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, et al. Early regular egg exposure in infants with eczema: A randomized controlled trial. J Allergy Clin Immunol. 2013;132:387–92. e1. doi: 10.1016/j.jaci.2013.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


