Abstract
More than 30 incurable neurological and neuromuscular diseases are caused by simple microsatellite expansions consisted of 3–6 nucleotides. These repeats can occur in non-coding regions and often result in a dominantly inherited disease phenotype that is characteristic of a toxic RNA gain-of-function. The expanded RNA adopts unusual secondary structures, sequesters various RNA binding proteins to form insoluble nuclear foci, and causes cellular defects at a multisystem level. Nuclear foci are dynamic in size, shape and colocalization of RNA binding proteins in different expansion diseases and tissue types. This review sets to provide new insights into the disease mechanisms of RNA toxicity and foci modulation, in light of recent advancement on bi-directional transcription, antisense RNA, repeat-associated non-ATG translation and beyond.
Overview of microsatellite expansion diseases
Simple microsatellite expansions that cause neurodegenerative diseases can occur in coding regions [1–24], 5′-untranslated regions (5′-UTRs, [25–32]), intronic regions [33–51] and 3′-UTRs [24,50,52–62] (Table 1 and Fig. 1). In affected individuals, large repeat expansions show somatic and intergenerational instabilities and lead to disease phenotype. Several factors can contribute to repeat instability: sequence composition, convergent transcription, gene conversion, sister chromatid exchange, and errors in DNA replication, repair and mitotic recombination [63–66].
Table 1.
Summary of microsatellite expansion disease mechanisms. The following abbreviations are indicated: AS – antisense, t – in vitro, v – in vivo, D – downregulation, and U – upregulation. References are indicated in the disease overview section of the manuscript.
| Disease | Repeat expansion (healthy/affected) |
Location of expansion |
Antisense transcript |
RNA foci | RBPs bound to expanded RNA or foci |
RAN translation | miRNA dysregulation |
|---|---|---|---|---|---|---|---|
| C9orf72-ALS/FTD | GGGGCC (2–19/250–1600) |
Intron 1 of C9orf72 (9p21.2) |
C9orf72AS | Sense & antisense foci in both nucleus & cytoplasm |
RanGAP1, hnRNP A1/F/ H/K?/U, SRSF2, ALYREF, Purα, nucleolin, RPL7t; RanGAP1, SF2, SC35, hnRRNP A1/A3/F/ H/K?, ADARB2, Purα, SRSF2, ALYREFv |
PolyGA/GP/GR/PR/PAt; PolyGA/GP/GR/PR/PAv |
? |
| DM1 | CTG (5–37/50->4000) |
3′-UTR of DMPK (19q13.32) |
DMPKAS | Sense & antisense foci in both nucleus & cytoplasm; non- toxic cytoplasmic foci |
MBNL1/2/3, CUGBP1, DDX5/6/17t; MBNL1/2/3, DDX5, hnRNP H/Fv |
PolyQ/A/St; PolyQv | miR-29b/29c/33/100/22/ 27a/let-7d/let-7b/194/148a/ 30b/125b/23a/499/365/23b/ 328/26a/let–7 g/145/133a/ 1D; miR-1/335/9/135a/206U; mislocalization of myomiR- 1/133b/206 |
| DM2 | CCTG (<30/75–11,000) |
Intron 1 of CNBP/ ZNF9 (3q21.3) |
? | Sense foci in both nucleus& cytoplasm |
MBNL1/2/3, CUBGP1, 20S catalytic core of proteasome, eIF2t; MBNL1/2/3v |
? | miR-125b-5p/193a-3p/ 193b-3p/378a-3pD; miR- 34a-5p/34b-3p/34c-5p/ 146b-5p/208a/221-3p/381U |
| DRPLA | CAG (6–35/49–88) |
Exon 5 of ATN1 (12p13.31) |
? | ? | ? | ? | Dysregulation of miR-200b/ 429? |
| FECD | CTG (<40/50–2600) |
Intron 3 of TCF4 (18q21.2) |
? | Nuclear sense foci | MBNL1v | ? | miR-199b-5p/616-3p/29a- 3p/140-3p/184/30c-5p/30b- 5p/101-3p/502-5p/181a-5p/ 455-5p/340-5p/130b-3p/95/ 484/532-3p/15b-5p/195-5p/ 34a-3p/628-5p/330-3p/ 374b-5p/502-3p/148b-3p/ 185-5p/107/191-5p/1244/ 374a-5p/135a-5p/186-5p/ 125b-5p/181c-5p/660-5p/ 125a-5p/30d-5p/7-1-3p/ 378a-5p/29b-2-5p/671-3p/ 26a-5p/7d-5p/30a-5p/26b- 5p/340-3p/93-3p/148a-3p/ 151a-3p/151a-5p/99a-5p/ 7g-5p/532-5p/23b-3p/19a- 3p/362-5p/30e-3p/361-5p/ 149-5p/100-5p/99b-5p/483- 3p/574-3p/455-3pD |
| FRAXE | CCG (10–35/300–1200) |
5′-UTR of AFF2/ FMR2 (Xq28) |
FMR3 | ? | ? | ? | ? |
| FRDA | GAA (5–30/70->1000) |
Intron 1 of FXN (9q21.11) |
FAST-1 | No detection of foci | ? | ? | miR-500/614/944/132/9/ 125b/335/126/323/9D; miR- 886-3p/886-5p/923/518f/ 363/21/124/130a/10a/505/ 643/155/628-3p/194/20b/ 192/181aU |
| FXS | CGG (5–44/>200) |
5′-UTR of FMR1 (Xq27.3) |
ASFMR1, FMR4 & FMR6 |
? | ? | ? | miR-382/134/124/370/431/ 494/372/373D; miR-125b/ 138/128U |
| FXTAS | CGG (5–44/55–200) |
5′-UTR of FMR1 (Xq27.3) |
ASFMR1, FMR4 & FMR6 |
Nuclear sense foci | MBNL1, SRSF1/4/5/6/7/ 10, nucleophosmin, tropomycin, actin, keratine, tubulin, MAP6, SH3BP5, HBB, STRBP, NF-κB, DDX3/5/17, ILF2, MTA2, Hsp27, GMDS, ubiquitin, hnRNP A1/A2/ B1/A3/C/D/E1/G/M, Purα, ESRP1, PRP3, ZC3HA, LSM11, ZCHC8, MPP10, DAZP1, RBMS1, GLD2, DGCR8t; MBNL1, hnRNP A2/B1/G, Purα, DGCR8, lamin A/C, aB- crystallin, Sam68v |
PolyG/PolyAt; PolyGv | miR-221/455-5p/206/502- 3p/193a-5p/449a/378/486- 3p/3065-5p/135b/30b/ 550a/337-3p/502-5p/654- 5p/let-7iD; miRNA-935/ 1238/133a/582-5pU |
| HD | CAG (6–37/35–121) |
Exon 1 of HTT (4p16.3) |
HTTAS | Nuclear sense foci; no detection of antisense foci |
HTT, MBNL1v | PolyA/S/L/Ct; PolyA/S/L/ Cv |
miR-22/29c/128/132/138/ 218/222/344/674/9/125b/ 138/146a/150/218/221/222/ 100/135a/135b/181cD; miR- 1273p/145/148a/199-5p/ 199-3p/200a/205/214/335/ 34bU |
| HDL2 | CTG (6–28/>41) |
3′-UTR or exon 2A of JPH3 (16q24.2) |
JPH3AS | Nuclear sense foci containing unprocessed JPH3 RNA |
MBNL1v | PolyQ/A/St; PolyQv | ? |
| SBMA | CAG (<36/38–62) |
Exon 1 of AR (Xq12) |
? | Nuclear sense foci | ? | ? | Dysregulated miRNA-298 |
| SCA1 | CAG (6–44/>39) |
Exon 8 of ATXN1 | ATXN1AS | ? | ? | ? | miR-19/101/130D |
| SCA2 | CAG (13–31/32–79) |
Exon 1 of ATXN2 (12q24.12) |
? | ? | ? | ? | miR-12/bantam/sickleU |
| SCA3 | CAG (12–44/60–87) |
Exon 8 of ATXN3 (14q32.12) |
? | Nuclear sense foci; no detection of antisense foci |
MBNL1v | PolyQ/A/St | miR-125b/bantam/29a/let- 7f/215/342-3p/429/193a- 5p/98/223/375/500a/Plus- E1247/19b/Plus-F1074/ 374a/192/324-3p/19a/205/ 200a/18a/142-5p/125b/18a/ 27a/1297/25D; miR-34b/ 647/H8/620/Plus-E1097/ 576-5p/Plus-E1146/H6-3p/ 138-1/1260/1258/431/1261/ 574-3p/659/1264/Plus- E1110/888/935/625U |
| SCA6 | CAG (<18/20–33) |
Exon 47 of CACNA1A (19p13.13) |
? | ? | ? | ? | ? |
| SCA7 | CAG (<19/>36) |
Exon 3 of ATXN7 (3p14.1) |
SCAANT1 | ? | ? | ? | ? |
| SCA8 | CTG (15–50/71–1300) |
3′-UTR of ATXN8OS (13q21.33) |
ATXN8 & KLHL1 | Nuclear sense foci | MBNL1v | PolyQ/A/S/L/Ct; PolyAv | ? |
| SCA10 | ATTCT (10–31/800–4500) |
Intron 9 of ATXN10 (22q13.31) |
? | Nuclear & cytoplasmic sense foci |
hnRNP Ktv | ? | ? |
| SCA12 | CAG (7–32/46–78) |
5′-UTR of PPP2R2B (5q32) |
? | ? | ? | ? | ? |
| SCA17 | CAG (25–44/47–63) |
Exon 3 of TBP (6q27) |
PCD2 | ? | ? | ? | miRNA-29a/bD; miRNA- 322U |
| SCA31 | TGGAA (0/>110) |
Intron shared by BEAN & TK2 (16q21) |
TK2 | Nuclear sense foci; no detection of antisense foci |
SRSF1/9t | ? | ? |
| SCA36 | GGCCTG (5–14/650–2500) |
Intron 1 of NOP56 (20p13) |
? | Nuclear & cytoplasmic sense foci |
SRSF2tv | ? | miRNA-1292D |
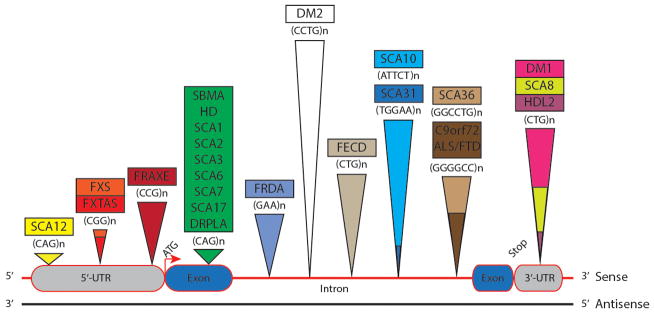
Fig. 1.
Schematic illustration of repeat expansion diseases. Repeats associated with an RNA gain-of-function are located in: (1) the 5′-UTRs, such as spinocerebellar ataxia (SCA) type 12 and Fragile X-associated tremor/ataxia syndrome (FXTAS), (2) intronic regions, such as myotonic dystrophy type 2 (DM2), Fuchs endothelial corneal dystrophy (FECD), SCA10/31/36 and C9orf72-amyotrophic lateral sclerosis/frontotemporal dementia (C9orf72-ALS/FTD), and (3) the 3′-UTRs, such as myotonic dystrophy type 1 (DM1), SCA8 and Huntington disease-like 2 (HDL2). In HDL2, the CTG repeat occurs in an alternatively spliced exon 2A, generating either a 3′-UTR CTG expansion (as shown in this figure) or homopolymers. Fragile X mental retardation syndrome (FXS), Fragile XE syndrome (FRAXE) and Friedreich ataxia (FRDA) are exceptions where an RNA loss-of-function occurs due to transcription silencing by CGG, CCG and GAA expansions respectively. The polyGlutamine (polyQ)-coding CAG expansions occur in exons and may involve RNA-mediated toxicity. These disorders include spinal and bulbar muscular atrophy (SBMA), Huntington’s disease (HD), SCA1/2/3/6/7/17 and dentatorubral-pallidoluysian atrophy (DRPLA). CAG repeat RNA-based toxicity has been suggested in some of these disorders [151,152]. The repeat length in affected individuals correlates approximately with the height of the triangle.
Repeat expansion in non-coding (and, perhaps, coding) regions often results in a toxic RNA gain-of-function (Table 1 and Fig. 1). In these disorders, the expanded RNA aggregates to form nuclear ribonucleoprotein foci which sequester RNA binding proteins (RBPs) or other essential cellular factors [67,68]. The depletion of key splicing regulatory RBPs from the cellular pool can lead to spliceopathy. Evidence to support such notion has been observed in Myotonic dystrophy type 1 (DM1), Spinocerebellar ataxia type 8 or 10 (SCA8/10), C9orf72-amyotrophic lateral sclerosis/frontotemporal dementia (C9orf72-ALS/FTD) and Fuchs endothelial corneal dystrophy (FECD) [37,51,69–71]. RBPs also participate in cellular processes such as DNA repair, transcription regulation, RNA processing/transport/localization, microRNA (miRNA) processing, protein quality control and apoptosis [72–74]. Disruption of such processes by foci sequestration may further exacerbate neuronal toxicity. As bidirectional translation occurs at most non-coding expansion regions, mechanisms such as antisense foci formation, RNA-induced silencing complex (RISC)-dependent RNA degradation, siRNA-dependent epigenetic modification and repeat-associated non-ATG (RAN) translation may also occur [34,75–78]. Theoretically, nuclear RNA foci could provide neuroprotection by acting as a toxic RNA sink, so that the generation of RAN-translated toxic polypeptides within the cytoplasm is reduced [79]. RNA Foci may also function as a ‘triage’ site – reminiscent to the role of stress granules – to determine the destination of the expanded RNA. In this review, we will focus on RNA toxicity and foci formation.
RNA toxicity and foci in DM
DM1 and DM2 (Myotonic dystrophy type 2) are multisystem diseases that target tissues including skeletal, cardiac and smooth muscles, the central nervous system (CNS) and eyes. DM2 has a milder clinical course than DM1. The genetic origin of DM stems from a CTG repeat expansion in the 3′-UTR of the DMPK gene for DM1 and a CCTG expansion in intron 1 of the CNBP/ZNF9 gene for DM2.
A hallmark of DM pathology is the formation of toxic nuclear RNA foci. In DM1, the CUG-expanded RNA forms hairpins that sequester a class of splicing regulatory RBPs – Muscleblind 1-3 (MBNL1-3) – at the periphery of the nuclear splicing speckles [80,81]. MBNL1 participates in foci formation by binding to distorted GC bases or unpaired UU bases [82–85]. Its depletion has recently been linked to mRNA mislocalization and miRNA misprocessing [86,87]. In DM1, stabilization of another splicing regulator CUG-binding protein 1 (CUGBP1 or CELF1) also occurs [88]. Changes of MBNL1 and CUGBP1/CELF1 in DM1 drive splicing of a variety of transcripts towards fetal isoforms. Many of the misspliced genes are components of the sodium/calcium current regulation, intra-/inter-cellular transport, and sarcomere/cytoskeleton structure and function [89,90]. They are thus unable to fulfill their normal cellular functions.
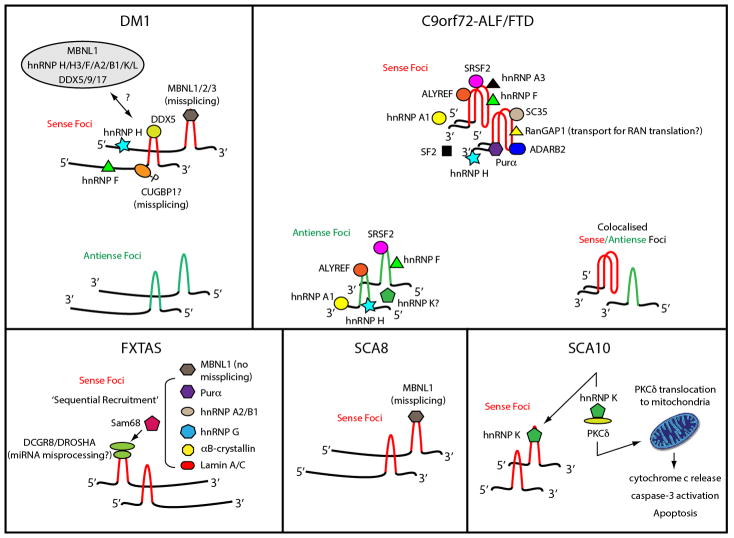
Recently, antisense CAG-expanded foci have been reported in adult and congenital DM1 patients and mice [91,92]. These foci do not appear to colocalize with sense foci in the same nucleus nor sequester any MBNL (Fig. 2). As antisense foci exist at much lower numbers than sense foci, therapies using antisense oligonucleotides that target sense foci may change the relative amount of sense vs. antisense foci. In DM2, CCUG foci appear much larger than DM1 foci and contain only intronic repeats. Several foci modifiers exist in DM. For instance, two DM1 RNA toxic foci enhancers (str-67 and ocrl-1) have been identified in Caenorhabditis elegans [93]. Nonsense-mediated decay, Staufen and DEAD-box helicase 5 (DDX5) all have an impact on toxic RNA transport and degradation [94–96]. In addition, a large ribonucleoprotein complex may transiently regulate the sense foci (Fig. 2, [97]).
Fig. 2.
Schematic illustration of RNA foci and associated RBPs in DM1, C9orf72-ALF/FTD, FXTAS, SCA8 and SCA10. This diagram only included RBPs that had been verified by in vivo colocalization experiments. Repeat expansions present in sense and antisense RNA are highlighted in red and green respectively. Hyperphosphorylated (P) CUGBP1 may bind to the base of DMPK hairpins in DM1. The expanded C9orf72 sense RNA assumes an unusual G-quadruplex structure. In FXTAS, the miRNA processing complex DGCR8/DROSHA is among the first proteins to be recruited [28]. The DGCR8/DROSHA heterodimer is partially sequestered because it can still process miRNA to some extent. Sam68 binds to DGCR8/DROSHA and recruits other RBPs [29,117].
DM foci are incredibly dynamic during cell proliferation [39,62]. Hence, caution should be taken when foci counts are used as a biomarker in non-synchronized cells. DM foci are most prominent at early prophase. The majority of foci disappear during nuclear membrane breakdown. When cells exit mitotic division, foci progressively accumulate in the nucleus.
RNA toxicity and foci in C9orf72-ALS/FTD
ALS and FTD are neurodegenerative diseases that share clinical and pathological overlap. A subset of these diseases have been linked to a common causal GGGGCC repeat expansion in intron 1 of C9orf72. An RNA toxic role has been implicated in C9orf72-ALS/FTD, because the expanded RNA binds to a variety of RBPs [78], and the expanded sense RNA alone is sufficient to recapitulate neurodegeneration in Drosophila and BAC mice [98,99]. Both sense and antisense nuclear foci have been found in patient tissues, induced pluripotent stem cells (iPS cells) and iPS-derived neurons, Drosophila and BAC mice [24,35,98,100–103]. Sense foci contain GGGGCC repeat sequences [100], and can induce apoptosis in SH-SY5Y cells and zebra fish embryos [104]. Antisense foci preferentially accumulate in vulnerable cell types [98], and correlate with a nuclear loss of TAR DNA-binding protein 43 (TDP-43) in motor neurons [35]. The above observations suggest that antisense foci play a significant role in C9orf72 pathophysiology. In contrast to DM1 foci, both sense and antisense C9orf72 foci can colocalize within the same nucleus [105].
Various interactomes containing C9orf72 RNA and RBPs have been proposed. It has been confirmed that both sense and antisense foci or RNA bind to Serine and Arginine-rich splicing factor 2 (SRSF2), AlY/REF export factor (ALYREF), Heterogeneous nuclear ribonucleoprotein F (hnRNP F) and hnRNP A1 [35,106]. It is unclear as to whether RNA secondary structure is a determinant in RBP recognition in vivo, because only the sense C9orf72 RNA has been verified to form G-quadruplexes in vitro [48], and one cannot rule out a shared secondary structure between sense and antisense C9orf72 RNA foci in vivo. Sense foci also colocalize with Purine-rich element binding protein-alpha (Purα), Ran GTPase activating protein 1 (RanGAP1) and Adenosine deaminase B2 (ADARB2) [100,107,108]. Purα may recognize partially denatured RNA in a similar way as MBNL1 [83,109]. ADARB2 may participate in foci formation or maintenance, and its sequestration leads to hypersensitivity to excitotoxicity [100]. Recently, a comparative analysis has been done on RBPs that bind to C9orf72 RNA in five studies [78]. hnRNP H shows most overlap across studies, followed by Splicing factor proline and glutamine-rich (SFPQ), Interleukin enhancer binding factor 2 (ILF2) and Myelin basic protein (MBP). Interestingly, SFPQ regulates ADARB2 expression and is essential for paraspeckle formation [110].
RNA toxicity and foci in Fragile X-associated tremor/ataxia syndrome (FXTAS)
FXTAS is an adult-onset neurodegenerative disease with clinical manifestations of tremor, gait ataxia, parkinsonism and cognitive impairment caused by a CGG intermediate expansion at the 5′-UTR of FMR1.
Several lines of evidence point to an RNA gain-of-function in FXTAS. First, expression of the expanded CGG irrespective of its genetic context is sufficient to cause neurodegeneration in mice and Drosophila [111,112]. Second, transcription efficiency of FMR1 is significantly elevated in FXTAS patients and mice, while the FMR protein (FMRP) level remains largely unchanged [113–115]. Third, co-expression of both CCG and CGG-containing RNA suppresses their independent toxicity [116]. Fourth, large ubiquitin-positive nuclear inclusions have been found in FXTAS tissues. These nuclear inclusions contain CGG expanded transcripts in post-mortem FXTAS brains but not in Drosophila [117–119]. However, the number of inclusions correlates positively with CGG repeat length in Drosophila [120].
Nuclear CGG foci/inclusions contain a number of constituents in FXTAS Drosophila, mice and patients (Fig. 2, [28,117,121–123]). Amongst these RBPs, overexpression of DGCR8, Purα or hnRNP A2/B1 has been linked to suppression of neurodegeneration or RNA toxicity [28,122,123]. Additionally, a ‘sequential recruitment’ model has been proposed via DGCR8/DROSHA and Sam68 (Fig. 2). However, the pathogenic role of DGCR8/DROSHA remains controversial [124–127]. It has recently been shown that TDP-43 suppresses the CGG repeat-induced RNA toxicity through hnRNP A2/B1 but not through direct RNA interactions [128].
RNA toxicity and foci in SCA8
SCA8 is caused by a CTG expansion in the 3′-UTR of ATXN8OS (Table 1). Clinical manifestations of SCA8 include gait and limb ataxia and cerebellar atrophy on MRI imaging. In contrast with most other non-coding expansion diseases, little evidence has linked repeat length to disease severity and progression in SCA8 [129].
RNA gain-of-function plays a significant role in SCA8. It has been shown that a (CUG)400–1000 expansion forms nuclear RNA foci in human SCA8 brains. These foci vary greatly in size, distribution and number: While multiple small CUG foci were found in Purkinje cells, single CUG foci were found in the nuclei of molecular layer interneurons and the Bergmann glia in the granule cell layer [70]. In both human and mice, MBNL1 colocalizes with CUG foci in molecular layer interneurons but not in Purkinje cells. The pathogenic significance of such tissue-specific sequestration pattern is unknown. Mutations in MBNL1 and other expansion modifiers have been shown to enhance SCA8-induced neurodegeneration synergistically in Drosophila [130]. CUG expansion also increases the expression of CUGBP1/MBNL1-regulated Gabt4, leading to a loss of GABAergic inhibition [70].
RNA toxicity and foci in SCA10
SCA10 is prevalent in Latin America and primarily impairs cerebellar Purkinje cells [131,132]. The disease-causing mutation is an ATTCT repeat expansion in intron 9 of ATXN10, which is likely to have originated in the Han Chinese population [133]. The expanded AUUCU repeats form RNA hairpins with UCU internal loops closed by AU pairs [134]. In a subset of SCA10 patients, the presence of interruption motifs within repeat expansions correlates strongly with epileptic seizures [135–137]. A similar correlation between repeat interruption and disease phenotype has been observed in DM1 [138]. In contrast, the presence of interruptions reduces pathogenicity in CAG-expanded SCA 1/17 and penetrance in SCA8 [139].
The spliced AUUCU repeat expansion is the principle pathogenic molecule that triggers neuronal death in SCA10 [51]. Both (AUUCU)1000–2000 and ectopically expressed (AUUCU)500 form nuclear and cytoplasmic foci in human cells and transgenic mouse brains. Nuclear AUUCU foci have been shown to colocalize with hnRNP K (Fig. 2). Sequestration of hnRNP K not only alters splicing regulation, but also releases Protein kinase C-delta (PKCδ) [51]. Translocation of PKCδ to mitochondria causes cytochrome c release and activation of caspase-3, leading to apoptosis [140].
RNA toxicity and foci in SCA31 and SCA36
SCA31 is caused by a TGGAA repeat expansion in the bidirectionally transcribed BEAN gene. The reverse complementary sequence of the SCA31 repeat (TTCCA.TTCCAn) matches a tandem interruption motif at the 3′-end of SCA10 subtype B [136]. The GGCCTG expansion in SCA36 matches a hexanucleotide interruption motif as seen in DM1 and differs by 1 base from the C9orf72 GGGGCC repeat (SCA36: GGCCTG.GGCCTGn) [141]. Given the above information, it is possible that the pathogenic pathways and target RBPs are shared between SCA10 and SCA31, and between C9orf72-ALS/FTD and SCA36.
Conclusions and future perspectives
In conclusion, RBPs play key roles in toxic RNA gain-of-function in non-coding expansion diseases. However, several factors warrant further investigation. (1) RAN translation has been implicated in non-coding expansion disorders including DM1, SCA8, C9orf72-ALSFTD and FXTAS (Table 1, [77]). (2) RNA foci bear extensive structural similarities to RNA transport granules, stress granules, nuclear bodies, and P bodies, as these structures all contain a large population of localized mRNA and associated RBPs [142,143]. Can RNA toxicity be extended to RNA transport, sorting and degradation pathways? (3) RBPs (such as MBNL, Staufen and FMRP) and alternative last exons near 3′-UTRs are important for guiding mRNA to neurites [87,143,144]. Disruption of mRNA localization patterns by RBP sequestration and 3′-UTR repeat expansion may contribute to disease phenotype [145]. (4) Several mechanisms may actively facilitate RNA nuclear retention. For instance, hnRNP H inhibits nuclear export of the expanded RNA in DM1 [146]. Bulged stem-loop structures that resemble repeat expansions at 5′- or 3′-UTRs have been predicted to guide mRNA nuclear localization [125]. (5) G-rich repeat expansions, such as GGGGCC, may be taken up by lysosomes via LAMP2C receptors [147], leading to over-capacitated autophagy and non-degraded toxic products. (6) Deleterious R-loops may form at CTG, CCG, CAG, CGG, GAA and GGGGCC repeat sites. When not resolved by RNase H, R-loops can affect chromosome stability, Ig class switching, mitochondrial DNA replication and telomeric transcription [148,149]. (7) The expanded antisense RNA may facilitate sense foci degradation via nuclear and cytoplasmic RISC complexes [150]. It may alternatively form antisense foci that correlate with disease severity [35,98]. The abovementioned factors may serve as additional drug targets, depending on their relative contribution to the overall pathologic mechanisms of microsatellite expansion diseases. A comprehensive analysis of these factors will also help us better understand the complexity and development of microsatellite expansion diseases and identify the linkage between toxic RNA and disease etiology at a molecular level.
Acknowledgments
This work was supported by the National Institute of Health [grant number RO1 NS083564].
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Almaguer-Mederos LE, Falcon NS, Almira YR, Zaldivar YG, Almarales DC, Gongora EM, Herrera MP, Batallan KE, Arminan RR, Manresa MV, et al. Estimation of the age at onset in spinocerebellar ataxia type 2 Cuban patients by survival analysis. Clin Genet. 2010;78:169–174. doi: 10.1111/j.1399-0004.2009.01358.x. [DOI] [PubMed] [Google Scholar]
- 2••.Banez-Coronel M, Ayhan F, Tarabochia AD, Zu T, Perez BA, Tusi SK, Pletnikova O, Borchelt DR, Ross CA, Margolis RL, et al. RAN Translation in Huntington Disease. Neuron. 2015;88:667–677. doi: 10.1016/j.neuron.2015.10.038. These authors demonstrated that both sense and antisense-associated RAN translation could occur in HD; by extension, RAN-translation may contribute to other polyQ diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilen J, Liu N, Burnett BG, Pittman RN, Bonini NM. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol Cell. 2006;24:157–163. doi: 10.1016/j.molcel.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Chung DW, Rudnicki DD, Yu L, Margolis RL. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Hum Mol Genet. 2011;20:3467–3477. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.do Costa MC, Paulson HL. Toward understanding Machado-Joseph disease. Prog Neurobiol. 2012;97:239–257. doi: 10.1016/j.pneurobio.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Mezer M, Wojciechowska M, Napierala M, Sobczak K, Krzyzosiak WJ. Mutant CAG repeats of Huntingtin transcript fold into hairpins, form nuclear foci and are targets for RNA interference. Nucleic Acids Res. 2011;39:3852–3863. doi: 10.1093/nar/gkq1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujimori KE, Hazama K, Kawasaki T, Deguchi T, Yuba S. Intergenic region between TATA-box binding protein and proteasome subunit C3 genes of Medaka function as the bidirectional promoter in vitro and in vivo. Gene. 2012;511:177–186. doi: 10.1016/j.gene.2012.09.077. [DOI] [PubMed] [Google Scholar]
- 8.Gao R, Matsuura T, Coolbaugh M, Zuhlke C, Nakamura K, Rasmussen A, Siciliano MJ, Ashizawa T, Lin X. Instability of expanded CAG/CAA repeats in spinocerebellar ataxia type 17. Eur J Hum Genet. 2008;16:215–222. doi: 10.1038/sj.ejhg.5201954. [DOI] [PubMed] [Google Scholar]
- 9.Gaughwin PM, Ciesla M, Lahiri N, Tabrizi SJ, Brundin P, Bjorkqvist M. Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntington’s disease. Hum Mol Genet. 2011;20:2225–2237. doi: 10.1093/hmg/ddr111. [DOI] [PubMed] [Google Scholar]
- 10.Hubert L, Jr, Lin Y, Dion V, Wilson JH. Xpa deficiency reduces CAG trinucleotide repeat instability in neuronal tissues in a mouse model of SCA1. Hum Mol Genet. 2011;20:4822–4830. doi: 10.1093/hmg/ddr421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karres JS, Hilgers V, Carrera I, Treisman J, Cohen SM. The conserved microRNA miR-8 tunes atrophin levels to prevent neurodegeneration in Drosophila. Cell. 2007;131:136–145. doi: 10.1016/j.cell.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Lee ST, Chu K, Im WS, Yoon HJ, Im JY, Park JE, Park KH, Jung KH, Lee SK, Kim M, et al. Altered microRNA regulation in Huntington’s disease models. Exp Neurol. 2011;227:172–179. doi: 10.1016/j.expneurol.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y, Samaco RC, Gatchel JR, Thaller C, Orr HT, Zoghbi HY. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nat Neurosci. 2008;11:1137–1139. doi: 10.1038/nn.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCann C, Holohan EE, Das S, Dervan A, Larkin A, Lee JA, Rodrigues V, Parker R, Ramaswami M. The Ataxin-2 protein is required for microRNA function and synapse-specific long-term olfactory habituation. Proc Natl Acad Sci U S A. 2011;108:E655–662. doi: 10.1073/pnas.1107198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMurray CT. Mechanisms of trinucleotide repeat instability during human development. Nat Rev Genet. 2010;11:786–799. doi: 10.1038/nrg2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mykowska A, Sobczak K, Wojciechowska M, Kozlowski P, Krzyzosiak WJ. CAG repeats mimic CUG repeats in the misregulation of alternative splicing. Nucleic Acids Res. 2011;39:8938–8951. doi: 10.1093/nar/gkr608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogue AI, Cui JG, Li YY, Zhao Y, Culicchia F, Lukiw WJ. Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett. 2010;476:18–22. doi: 10.1016/j.neulet.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 18.Pourshafie N, Lee PR, Chen KL, Harmison GG, Bott LC, Katsuno M, Sobue G, Burnett BG, Fischbeck KH, Rinaldi C. MiR-298 Counteracts Mutant Androgen Receptor Toxicity in Spinal and Bulbar Muscular Atrophy. Mol Ther. 2016;24:937–945. doi: 10.1038/mt.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roshan R, Ghosh T, Gadgil M, Pillai B. Regulation of BACE1 by miR-29a/b in a cellular model of Spinocerebellar Ataxia 17. RNA Biol. 2012;9:891–899. doi: 10.4161/rna.19876. [DOI] [PubMed] [Google Scholar]
- 20.Shi Y, Huang F, Tang B, Li J, Wang J, Shen L, Xia K, Jiang H. MicroRNA profiling in the serums of SCA3/MJD patients. Int J Neurosci. 2014;124:97–101. doi: 10.3109/00207454.2013.827679. [DOI] [PubMed] [Google Scholar]
- 21.Sinha M, Ghose J, Das E, Bhattarcharyya NP. Altered microRNAs in STHdh(Q111)/Hdh(Q111) cells: miR-146a targets TBP. Biochem Biophys Res Commun. 2010;396:742–747. doi: 10.1016/j.bbrc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Sopher BL, Ladd PD, Pineda VV, Libby RT, Sunkin SM, Hurley JB, Thienes CP, Gaasterland T, Filippova GN, La Spada AR. CTCF regulates ataxin-7 expression through promotion of a convergently transcribed, antisense noncoding RNA. Neuron. 2011;70:1071–1084. doi: 10.1016/j.neuron.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Tito AJ, Rui YN, Zhang S. Studying polyglutamine diseases in Drosophila. Exp Neurol. 2015;274:25–41. doi: 10.1016/j.expneurol.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci U S A. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y, Wu JJ, Wu ZY. Identification of 46 CAG repeats within PPP2R2B as probably the shortest pathogenic allele for SCA12. Parkinsonism Relat Disord. 2015;21:398–401. doi: 10.1016/j.parkreldis.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Halevy T, Czech C, Benvenisty N. Molecular mechanisms regulating the defects in fragile X syndrome neurons derived from human pluripotent stem cells. Stem Cell Reports. 2015;4:37–46. doi: 10.1016/j.stemcr.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santos-Reboucas CB, Abdalla CB, Fullston T, Campos M, Jr, Pimentel MM, Gecz J. Lack of FMR3 expression in a male with non-syndromic mental retardation and a microdeletion immediately distal to FRAXE CCG repeat. Neurosci Lett. 2006;397:245–248. doi: 10.1016/j.neulet.2005.12.089. [DOI] [PubMed] [Google Scholar]
- 28.Sellier C, Freyermuth F, Tabet R, Tran T, He F, Ruffenach F, Alunni V, Moine H, Thibault C, Page A, et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013;3:869–880. doi: 10.1016/j.celrep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sellier C, Usdin K, Pastori C, Peschansky VJ, Tassone F, Charlet-Berguerand N. The multiple molecular facets of fragile X-associated tremor/ataxia syndrome. J Neurodev Disord. 2014;6:23. doi: 10.1186/1866-1955-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd PK, Paulson HL. C9orf72-associated FTD/ALS: when less is more. Neuron. 2013;80:257–258. doi: 10.1016/j.neuron.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, Gu Y, Ferguson JM, Chen Q, Boatwright S, Gardiner J, Below C, Espinosa J, Nelson DL, Shaffer LG. Cytogenetic analysis of obsessive-compulsive disorder (OCD): identification of a FRAXE fragile site. Am J Med Genet A. 2003;118A:25–28. doi: 10.1002/ajmg.a.20001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zongaro S, Hukema R, D’Antoni S, Davidovic L, Barbry P, Catania MV, Willemsen R, Mari B, Bardoni B. The 3′ UTR of FMR1 mRNA is a target of miR-101, miR-129–5p and miR-221: implications for the molecular pathology of FXTAS at the synapse. Hum Mol Genet. 2013;22:1971–1982. doi: 10.1093/hmg/ddt044. [DOI] [PubMed] [Google Scholar]
- 33.Al-Mahdawi S, Pinto RM, Varshney D, Lawrence L, Lowrie MB, Hughes S, Webster Z, Blake J, Cooper JM, King R, et al. GAA repeat expansion mutation mouse models of Friedreich ataxia exhibit oxidative stress leading to progressive neuronal and cardiac pathology. Genomics. 2006;88:580–590. doi: 10.1016/j.ygeno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cleary JD, Ranum LP. Repeat associated non-ATG (RAN) translation: new starts in microsatellite expansion disorders. Curr Opin Genet Dev. 2014;26:6–15. doi: 10.1016/j.gde.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Cooper-Knock J, Higginbottom A, Stopford MJ, Highley JR, Ince PG, Wharton SB, Pickering-Brown S, Kirby J, Hautbergue GM, Shaw PJ. Antisense RNA foci in the motor neurons of C9ORF72-ALS patients are associated with TDP-43 proteinopathy. Acta Neuropathol. 2015;130:63–75. doi: 10.1007/s00401-015-1429-9. These authors demonstrated differential expression levels of sense and antisense foci in various C9orf72-ALS/FTD CNS cell types. The presence of antisense foci correlated with mislocalization of TDP-43. Several RBPs were commonly targeted by both sense and antisense foci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Biase I, Chutake YK, Rindler PM, Bidichandani SI. Epigenetic silencing in Friedreich ataxia is associated with depletion of CTCF (CCCTC-binding factor) and antisense transcription. PLoS One. 2009;4:e7914. doi: 10.1371/journal.pone.0007914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Du J, Aleff RA, Soragni E, Kalari K, Nie J, Tang X, Davila J, Kocher JP, Patel SV, Gottesfeld JM, et al. RNA toxicity and missplicing in the common eye disease fuchs endothelial corneal dystrophy. J Biol Chem. 2015;290:5979–5990. doi: 10.1074/jbc.M114.621607. This paper made the initial discovery of MBNL1 sequestration by RNA foci and missplicing events in the non-neurological/-neuromuscular FECD disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Murias M, Quintans B, Arias M, Seixas AI, Cacheiro P, Tarrio R, Pardo J, Millan MJ, Arias-Rivas S, Blanco-Arias P, et al. ‘Costa da Morte’ ataxia is spinocerebellar ataxia 36: clinical and genetic characterization. Brain. 2012;135:1423–1435. doi: 10.1093/brain/aws069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giagnacovo M, Malatesta M, Cardani R, Meola G, Pellicciari C. Nuclear ribonucleoprotein-containing foci increase in size in non-dividing cells from patients with myotonic dystrophy type 2. Histochem Cell Biol. 2012;138:699–707. doi: 10.1007/s00418-012-0984-6. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Tortosa E, Gallego J, Guerrero-Lopez R, Marcos A, Gil-Neciga E, Sainz MJ, Diaz A, Franco-Macias E, Trujillo-Tiebas MJ, Ayuso C, et al. C9ORF72 hexanucleotide expansions of 20–22 repeats are associated with frontotemporal deterioration. Neurology. 2013;80:366–370. doi: 10.1212/WNL.0b013e31827f08ea. [DOI] [PubMed] [Google Scholar]
- 41.Greco S, Perfetti A, Fasanaro P, Cardani R, Capogrossi MC, Meola G, Martelli F. Deregulated microRNAs in myotonic dystrophy type 2. PLoS One. 2012;7:e39732. doi: 10.1371/journal.pone.0039732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi H, Abe K, Matsuura T, Ikeda Y, Hitomi T, Akechi Y, Habu T, Liu W, Okuda H, Koizumi A. Expansion of intronic GGCCTG hexanucleotide repeat in NOP56 causes SCA36, a type of spinocerebellar ataxia accompanied by motor neuron involvement. Am J Hum Genet. 2011;89:121–130. doi: 10.1016/j.ajhg.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu W, Ikeda Y, Hishikawa N, Yamashita T, Deguchi K, Abe K. Characteristic RNA foci of the abnormal hexanucleotide GGCCUG repeat expansion in spinocerebellar ataxia type 36 (Asidan) Eur J Neurol. 2014;21:1377–1386. doi: 10.1111/ene.12491. [DOI] [PubMed] [Google Scholar]
- 44.Mahishi LH, Hart RP, Lynch DR, Ratan RR. miR-886–3p levels are elevated in Friedreich ataxia. J Neurosci. 2012;32:9369–9373. doi: 10.1523/JNEUROSCI.0059-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matthaei M, Hu J, Kallay L, Eberhart CG, Cursiefen C, Qian J, Lackner EM, Jun AS. Endothelial cell microRNA expression in human late-onset Fuchs’ dystrophy. Invest Ophthalmol Vis Sci. 2014;55:216–225. doi: 10.1167/iovs.13-12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mootha VV, Hussain I, Cunnusamy K, Graham E, Gong X, Neelam S, Xing C, Kittler R, Petroll WM. TCF4 Triplet Repeat Expansion and Nuclear RNA Foci in Fuchs’ Endothelial Corneal Dystrophy. Invest Ophthalmol Vis Sci. 2015;56:2003–2011. doi: 10.1167/iovs.14-16222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niimi Y, Takahashi M, Sugawara E, Umeda S, Obayashi M, Sato N, Ishiguro T, Higashi M, Eishi Y, Mizusawa H, et al. Abnormal RNA structures (RNA foci) containing a penta-nucleotide repeat (UGGAA)n in the Purkinje cell nucleus is associated with spinocerebellar ataxia type 31 pathogenesis. Neuropathology. 2013;33:600–611. doi: 10.1111/neup.12032. [DOI] [PubMed] [Google Scholar]
- 48.Reddy K, Zamiri B, Stanley SY, Macgregor RB, Jr, Pearson CE. The disease-associated r(GGGGCC)n repeat from the C9orf72 gene forms tract length-dependent uni- and multimolecular RNA G-quadruplex structures. J Biol Chem. 2013;288:9860–9866. doi: 10.1074/jbc.C113.452532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato N, Amino T, Kobayashi K, Asakawa S, Ishiguro T, Tsunemi T, Takahashi M, Matsuura T, Flanigan KM, Iwasaki S, et al. Spinocerebellar ataxia type 31 is associated with “inserted” penta-nucleotide repeats containing (TGGAA)n. Am J Hum Genet. 2009;85:544–557. doi: 10.1016/j.ajhg.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol. 2010;67:291–300. doi: 10.1002/ana.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White MC, Gao R, Xu W, Mandal SM, Lim JG, Hazra TK, Wakamiya M, Edwards SF, Raskin S, Teive HA, et al. Inactivation of hnRNP K by expanded intronic AUUCU repeat induces apoptosis via translocation of PKCdelta to mitochondria in spinocerebellar ataxia 10. PLoS Genet. 2010;6:e1000984. doi: 10.1371/journal.pgen.1000984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batra R, Charizanis K, Swanson MS. Partners in crime: bidirectional transcription in unstable microsatellite disease. Hum Mol Genet. 2010;19:R77–82. doi: 10.1093/hmg/ddq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dansithong W, Wolf CM, Sarkar P, Paul S, Chiang A, Holt I, Morris GE, Branco D, Sherwood MC, Comai L, et al. Cytoplasmic CUG RNA foci are insufficient to elicit key DM1 features. PLoS One. 2008;3:e3968. doi: 10.1371/journal.pone.0003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernandez-Costa JM, Garcia-Lopez A, Zuniga S, Fernandez-Pedrosa V, Felipo-Benavent A, Mata M, Jaka O, Aiastui A, Hernandez-Torres F, Aguado B, et al. Expanded CTG repeats trigger miRNA alterations in Drosophila that are conserved in myotonic dystrophy type 1 patients. Hum Mol Genet. 2013;22:704–716. doi: 10.1093/hmg/dds478. [DOI] [PubMed] [Google Scholar]
- 55.Gambardella S, Rinaldi F, Lepore SM, Viola A, Loro E, Angelini C, Vergani L, Novelli G, Botta A. Overexpression of microRNA-206 in the skeletal muscle from myotonic dystrophy type 1 patients. J Transl Med. 2010;8:48. doi: 10.1186/1479-5876-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalsotra A, Singh RK, Gurha P, Ward AJ, Creighton CJ, Cooper TA. The Mef2 transcription network is disrupted in myotonic dystrophy heart tissue, dramatically altering miRNA and mRNA expression. Cell Rep. 2014;6:336–345. doi: 10.1016/j.celrep.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57••.Krench M, Cho RW, Littleton JT. A Drosophila model of Huntington disease-like 2 exhibits nuclear toxicity and distinct pathogenic mechanisms from Huntington disease. Hum Mol Genet. 2016 doi: 10.1093/hmg/ddw166. This paper identified the correlation between large nuclear-aggregated polyQ proteins and neuronal toxicity in HDL2. This disease mechanism is in contrast to that of HD where polyQ proteins are predominantly localized in the cytoplasm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perbellini R, Greco S, Sarra-Ferraris G, Cardani R, Capogrossi MC, Meola G, Martelli F. Dysregulation and cellular mislocalization of specific miRNAs in myotonic dystrophy type 1. Neuromuscul Disord. 2011;21:81–88. doi: 10.1016/j.nmd.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 59.Rudnicki DD, Holmes SE, Lin MW, Thornton CA, Ross CA, Margolis RL. Huntington’s disease--like 2 is associated with CUG repeat-containing RNA foci. Ann Neurol. 2007;61:272–282. doi: 10.1002/ana.21081. [DOI] [PubMed] [Google Scholar]
- 60.Seixas AI, Holmes SE, Takeshima H, Pavlovich A, Sachs N, Pruitt JL, Silveira I, Ross CA, Margolis RL, Rudnicki DD. Loss of junctophilin-3 contributes to Huntington disease-like 2 pathogenesis. Ann Neurol. 2012;71:245–257. doi: 10.1002/ana.22598. [DOI] [PubMed] [Google Scholar]
- 61.Wilburn B, Rudnicki DD, Zhao J, Weitz TM, Cheng Y, Gu X, Greiner E, Park CS, Wang N, Sopher BL, et al. An antisense CAG repeat transcript at JPH3 locus mediates expanded polyglutamine protein toxicity in Huntington’s disease-like 2 mice. Neuron. 2011;70:427–440. doi: 10.1016/j.neuron.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62•.Xia G, Ashizawa T. Dynamic changes of nuclear RNA foci in proliferating DM1 cells. Histochem Cell Biol. 2015;143:557–564. doi: 10.1007/s00418-015-1315-5. These authors discovered the dynamic nature of DM1 RNA foci during cell proliferation and their continuous accumulation in size and number during quiescence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin Y, Leng M, Wan M, Wilson JH. Convergent transcription through a long CAG tract destabilizes repeats and induces apoptosis. Mol Cell Biol. 2010;30:4435–4451. doi: 10.1128/MCB.00332-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lopez Castel A, Cleary JD, Pearson CE. Repeat instability as the basis for human diseases and as a potential target for therapy. Nat Rev Mol Cell Biol. 2010;11:165–170. doi: 10.1038/nrm2854. [DOI] [PubMed] [Google Scholar]
- 65.Mirkin SM. Expandable DNA repeats and human disease. Nature. 2007;447:932–940. doi: 10.1038/nature05977. [DOI] [PubMed] [Google Scholar]
- 66.Richard GF, Paques F. Mini- and microsatellite expansions: the recombination connection. EMBO Rep. 2000;1:122–126. doi: 10.1093/embo-reports/kvd031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Echeverria GV, Cooper TA. RNA-binding proteins in microsatellite expansion disorders: mediators of RNA toxicity. Brain Res. 2012;1462:100–111. doi: 10.1016/j.brainres.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mohan A, Goodwin M, Swanson MS. RNA-protein interactions in unstable microsatellite diseases. Brain Res. 2014;1584:3–14. doi: 10.1016/j.brainres.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Cooper-Knock J, Bury JJ, Heath PR, Wyles M, Higginbottom A, Gelsthorpe C, Highley JR, Hautbergue G, Rattray M, Kirby J, et al. C9ORF72 GGGGCC Expanded Repeats Produce Splicing Dysregulation which Correlates with Disease Severity in Amyotrophic Lateral Sclerosis. PLoS One. 2015;10:e0127376. doi: 10.1371/journal.pone.0127376. These authors observed splicing inconsistencies in C9orf72-ALS/FTD motor neurons and lymphoblastoid cell lines, possibly as a result of RBP sequestration by RNA foci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daughters RS, Tuttle DL, Gao W, Ikeda Y, Moseley ML, Ebner TJ, Swanson MS, Ranum LP. RNA gain-of-function in spinocerebellar ataxia type 8. PLoS Genet. 2009;5:e1000600. doi: 10.1371/journal.pgen.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furling D. Misregulation of alternative splicing and microRNA processing in DM1 pathogenesis. Rinsho Shinkeigaku. 2012;52:1018–1022. doi: 10.5692/clinicalneurol.52.1018. [DOI] [PubMed] [Google Scholar]
- 72•.Batra R, Charizanis K, Manchanda M, Mohan A, Li M, Finn DJ, Goodwin M, Zhang C, Sobczak K, Thornton CA, et al. Loss of MBNL leads to disruption of developmentally regulated alternative polyadenylation in RNA-mediated disease. Mol Cell. 2014;56:311–322. doi: 10.1016/j.molcel.2014.08.027. These authors observed misregulated polyadenylation events across thousands of genes when MBNL1 was depleted in DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X, Trepanier V, Beaujois R, Viranaicken W, Drobetsky E, DesGroseillers L. The downregulation of the RNA-binding protein Staufen2 in response to DNA damage promotes apoptosis. Nucleic Acids Res. 2016;44:3695–3712. doi: 10.1093/nar/gkw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Budworth H, McMurray CT. Bidirectional transcription of trinucleotide repeats: roles for excision repair. DNA Repair (Amst) 2013;12:672–684. doi: 10.1016/j.dnarep.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dion V, Wilson JH. Instability and chromatin structure of expanded trinucleotide repeats. Trends Genet. 2009;25:288–297. doi: 10.1016/j.tig.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Green KM, Linsalata AE, Todd PK. RAN translation-What makes it run? Brain Res. 2016 doi: 10.1016/j.brainres.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haeusler AR, Donnelly CJ, Rothstein JD. The expanding biology of the C9orf72 nucleotide repeat expansion in neurodegenerative disease. Nat Rev Neurosci. 2016;17:383–395. doi: 10.1038/nrn.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79••.Kino Y, Washizu C, Kurosawa M, Oma Y, Hattori N, Ishiura S, Nukina N. Nuclear localization of MBNL1: splicing-mediated autoregulation and repression of repeat-derived aberrant proteins. Hum Mol Genet. 2015;24:740–756. doi: 10.1093/hmg/ddu492. Nuclear localization of MBNL1 was affected by inclusion of exon 7 and use of two nuclear localization signals. The sequestration of MBNL1 may function to repress the nuclear export of expanded RNA and aberrant RAN translation in the cytoplasm. [DOI] [PubMed] [Google Scholar]
- 80.Holt I, Mittal S, Furling D, Butler-Browne GS, Brook JD, Morris GE. Defective mRNA in myotonic dystrophy accumulates at the periphery of nuclear splicing speckles. Genes Cells. 2007;12:1035–1048. doi: 10.1111/j.1365-2443.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- 81.Mooers BH, Logue JS, Berglund JA. The structural basis of myotonic dystrophy from the crystal structure of CUG repeats. Proc Natl Acad Sci U S A. 2005;102:16626–16631. doi: 10.1073/pnas.0505873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ho TH, Savkur RS, Poulos MG, Mancini MA, Swanson MS, Cooper TA. Colocalization of muscleblind with RNA foci is separable from mis-regulation of alternative splicing in myotonic dystrophy. J Cell Sci. 2005;118:2923–2933. doi: 10.1242/jcs.02404. [DOI] [PubMed] [Google Scholar]
- 83.Konieczny P, Stepniak-Konieczna E, Sobczak K. MBNL proteins and their target RNAs, interaction and splicing regulation. Nucleic Acids Res. 2014;42:10873–10887. doi: 10.1093/nar/gku767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Lambert N, Robertson A, Jangi M, McGeary S, Sharp PA, Burge CB. RNA Bind-n-Seq: quantitative assessment of the sequence and structural binding specificity of RNA binding proteins. Mol Cell. 2014;54:887–900. doi: 10.1016/j.molcel.2014.04.016. These authors revealed canonical and near optimal binding motifs of RBFOX2, CUGBP1/CELF1 and MBNL1 by using RNA bind-n-seq and in vivo methods. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yildirim I, Chakraborty D, Disney MD, Wales DJ, Schatz GC. Computational investigation of RNA CUG repeats responsible for myotonic dystrophy 1. J Chem Theory Comput. 2015;11:4943–4958. doi: 10.1021/acs.jctc.5b00728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rau F, Freyermuth F, Fugier C, Villemin JP, Fischer MC, Jost B, Dembele D, Gourdon G, Nicole A, Duboc D, et al. Misregulation of miR-1 processing is associated with heart defects in myotonic dystrophy. Nat Struct Mol Biol. 2011;18:840–845. doi: 10.1038/nsmb.2067. [DOI] [PubMed] [Google Scholar]
- 87••.Taliaferro JM, Vidaki M, Oliveira R, Olson S, Zhan L, Saxena T, Wang ET, Graveley BR, Gertler FB, Swanson MS, et al. Distal Alternative Last Exons Localize mRNAs to Neural Projections. Mol Cell. 2016;61:821–833. doi: 10.1016/j.molcel.2016.01.020. These authors discovered the involvement of alternative last exons (ALEs) in mRNA isoform-specific localization. Depletion of MBNL1 and/or MBNL2 by RNA foci leads to mislocalization of hundreds of mRNA in neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kuyumcu-Martinez NM, Wang GS, Cooper TA. Increased steady-state levels of CUGBP1 in myotonic dystrophy 1 are due to PKC-mediated hyperphosphorylation. Mol Cell. 2007;28:68–78. doi: 10.1016/j.molcel.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chau A, Kalsotra A. Developmental insights into the pathology of and therapeutic strategies for DM1: Back to the basics. Dev Dyn. 2015;244:377–390. doi: 10.1002/dvdy.24240. [DOI] [PubMed] [Google Scholar]
- 90•.Dixon DM, Choi J, El-Ghazali A, Park SY, Roos KP, Jordan MC, Fishbein MC, Comai L, Reddy S. Loss of muscleblind-like 1 results in cardiac pathology and persistence of embryonic splice isoforms. Sci Rep. 2015;5:9042. doi: 10.1038/srep09042. These authors discovered missplicing events in novel RNA networks in DM1 cardiac cells as a result of MBNL1 depletion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huguet A, Medja F, Nicole A, Vignaud A, Guiraud-Dogan C, Ferry A, Decostre V, Hogrel JY, Metzger F, Hoeflich A, et al. Molecular, physiological, and motor performance defects in DMSXL mice carrying >1,000 CTG repeats from the human DM1 locus. PLoS Genet. 2012;8:e1003043. doi: 10.1371/journal.pgen.1003043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92•.Michel L, Huguet-Lachon A, Gourdon G. Sense and Antisense DMPK RNA Foci Accumulate in DM1 Tissues during Development. PLoS One. 2015;10:e0137620. doi: 10.1371/journal.pone.0137620. These authors revealed the disproportionate sense and antisense foci formation in DM1 fetal tissues, and compared their differential RNA expression levels in a tissue-specific and temporal manner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93••.Garcia SM, Tabach Y, Lourenco GF, Armakola M, Ruvkun G. Identification of genes in toxicity pathways of trinucleotide-repeat RNA in C. elegans. Nat Struct Mol Biol. 2014;21:712–720. doi: 10.1038/nsmb.2858. These authors discovered 15 genes that function as either RNA toxicity suppressors or enhancers in DM1 C. elegans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94••.Jones K, Wei C, Schoser B, Meola G, Timchenko N, Timchenko L. Reduction of toxic RNAs in myotonic dystrophies type 1 and type 2 by the RNA helicase p68/DDX5. Proc Natl Acad Sci U S A. 2015;112:8041–8045. doi: 10.1073/pnas.1422273112. The p68/DDX5 helicase reduced RNA foci formation and promoted degradation of the expanded DMPK RNA, possibly by unwinding the RNA repeat to improve its processivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pettersson OJ, Aagaard L, Andrejeva D, Thomsen R, Jensen TG, Damgaard CK. DDX6 regulates sequestered nuclear CUG-expanded DMPK-mRNA in dystrophia myotonica type 1. Nucleic Acids Res. 2014;42:7186–7200. doi: 10.1093/nar/gku352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pettersson OJ, Aagaard L, Jensen TG, Damgaard CK. Molecular mechanisms in DM1 - a focus on foci. Nucleic Acids Res. 2015;43:2433–2441. doi: 10.1093/nar/gkv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paul S, Dansithong W, Jog SP, Holt I, Mittal S, Brook JD, Morris GE, Comai L, Reddy S. Expanded CUG repeats Dysregulate RNA splicing by altering the stoichiometry of the muscleblind 1 complex. J Biol Chem. 2011;286:38427–38438. doi: 10.1074/jbc.M111.255224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98••.Liu Y, Pattamatta A, Zu T, Reid T, Bardhi O, Borchelt DR, Yachnis AT, Ranum LP. C9orf72 BAC Mouse Model with Motor Deficits and Neurodegenerative Features of ALS/FTD. Neuron. 2016;90:521–534. doi: 10.1016/j.neuron.2016.04.005. This was the first paper to describe a C9orf72-ALS/FTD BAC mouse model that exhibited both neurodegeneration and behavioral defects. These authors also showed that antisense but not sense foci correlated with the severity of neurodegeneration. [DOI] [PubMed] [Google Scholar]
- 99.Xu Z, Poidevin M, Li X, Li Y, Shu L, Nelson DL, Li H, Hales CM, Gearing M, Wingo TS, et al. Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc Natl Acad Sci U S A. 2013;110:7778–7783. doi: 10.1073/pnas.1219643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donnelly CJ, Zhang PW, Pham JT, Haeusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80:415–428. doi: 10.1016/j.neuron.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang J, Zhu Q, Gendron TF, Saberi S, McAlonis-Downes M, Seelman A, Stauffer JE, Jafar-Nejad P, Drenner K, Schulte D, et al. Gain of Toxicity from ALS/FTD-Linked Repeat Expansions in C9ORF72 Is Alleviated by Antisense Oligonucleotides Targeting GGGGCC-Containing RNAs. Neuron. 2016;90:535–550. doi: 10.1016/j.neuron.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lagier-Tourenne C, Baughn M, Rigo F, Sun S, Liu P, Li HR, Jiang J, Watt AT, Chun S, Katz M, et al. Targeted degradation of sense and antisense C9orf72 RNA foci as therapy for ALS and frontotemporal degeneration. Proc Natl Acad Sci U S A. 2013;110:E4530–4539. doi: 10.1073/pnas.1318835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peters OM, Cabrera GT, Tran H, Gendron TF, McKeon JE, Metterville J, Weiss A, Wightman N, Salameh J, Kim J, et al. Human C9ORF72 Hexanucleotide Expansion Reproduces RNA Foci and Dipeptide Repeat Proteins but Not Neurodegeneration in BAC Transgenic Mice. Neuron. 2015;88:902–909. doi: 10.1016/j.neuron.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee YB, Chen HJ, Peres JN, Gomez-Deza J, Attig J, Stalekar M, Troakes C, Nishimura AL, Scotter EL, Vance C, et al. Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep. 2013;5:1178–1186. doi: 10.1016/j.celrep.2013.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mizielinska S, Lashley T, Norona FE, Clayton EL, Ridler CE, Fratta P, Isaacs AM. C9orf72 frontotemporal lobar degeneration is characterised by frequent neuronal sense and antisense RNA foci. Acta Neuropathol. 2013;126:845–857. doi: 10.1007/s00401-013-1200-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cooper-Knock J, Walsh MJ, Higginbottom A, Robin Highley J, Dickman MJ, Edbauer D, Ince PG, Wharton SB, Wilson SA, Kirby J, et al. Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain. 2014;137:2040–2051. doi: 10.1093/brain/awu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sareen D, O’Rourke JG, Meera P, Muhammad AK, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5:208ra149. doi: 10.1126/scitranslmed.3007529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108••.Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, et al. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525:56–61. doi: 10.1038/nature14973. The expanded C9orf72 RNA disrupted nucleocytoplamic transport by inhibiting import of nuclear proteins and by binding to RanGAP in Drosophila. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109•.Weber J, Bao H, Hartlmuller C, Wang Z, Windhager A, Janowski R, Madl T, Jin P, Niessing D. Structural basis of nucleic-acid recognition and double-strand unwinding by the essential neuronal protein Pur-alpha. Elife. 2016:5. doi: 10.7554/eLife.11297. Through crystallographic and in vivo experiments, these authors revealed that both the DNA-unwinding activity and the ability to bind DNA/RNA are required for Purα to function correctly in neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee M, Sadowska A, Bekere I, Ho D, Gully BS, Lu Y, Iyer KS, Trewhella J, Fox AH, Bond CS. The structure of human SFPQ reveals a coiled-coil mediated polymer essential for functional aggregation in gene regulation. Nucleic Acids Res. 2015;43:3826–3840. doi: 10.1093/nar/gkv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Foote M, Arque G, Berman RF, Santos M. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) Motor Dysfunction Modeled in Mice. Cerebellum. 2016 doi: 10.1007/s12311-016-0797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jin P, Zarnescu DC, Zhang F, Pearson CE, Lucchesi JC, Moses K, Warren ST. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 113.Berman RF, Buijsen RA, Usdin K, Pintado E, Kooy F, Pretto D, Pessah IN, Nelson DL, Zalewski Z, Charlet-Bergeurand N, et al. Mouse models of the fragile X premutation and fragile X-associated tremor/ataxia syndrome. J Neurodev Disord. 2014;6:25. doi: 10.1186/1866-1955-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peprah E, He W, Allen E, Oliver T, Boyne A, Sherman SL. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Hum Genet. 2010;55:66–68. doi: 10.1038/jhg.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13:555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sofola OA, Jin P, Botas J, Nelson DL. Argonaute-2-dependent rescue of a Drosophila model of FXTAS by FRAXE premutation repeat. Hum Mol Genet. 2007;16:2326–2332. doi: 10.1093/hmg/ddm186. [DOI] [PubMed] [Google Scholar]
- 117.Sellier C, Rau F, Liu Y, Tassone F, Hukema RK, Gattoni R, Schneider A, Richard S, Willemsen R, Elliott DJ, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 119.Todd PK, Oh SY, Krans A, He F, Sellier C, Frazer M, Renoux AJ, Chen KC, Scaglione KM, Basrur V, et al. CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron. 2013;78:440–455. doi: 10.1016/j.neuron.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 121.Iwahashi CK, Yasui DH, An HJ, Greco CM, Tassone F, Nannen K, Babineau B, Lebrilla CB, Hagerman RJ, Hagerman PJ. Protein composition of the intranuclear inclusions of FXTAS. Brain. 2006;129:256–271. doi: 10.1093/brain/awh650. [DOI] [PubMed] [Google Scholar]
- 122.Jin P, Duan R, Qurashi A, Qin Y, Tian D, Rosser TC, Liu H, Feng Y, Warren ST. Pur alpha binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sofola OA, Jin P, Qin Y, Duan R, Liu H, de Haro M, Nelson DL, Botas J. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alvarez-Mora MI, Rodriguez-Revenga L, Madrigal I, Torres-Silva F, Mateu-Huertas E, Lizano E, Friedlander MR, Marti E, Estivill X, Mila M. MicroRNA expression profiling in blood from fragile X-associated tremor/ataxia syndrome patients. Genes Brain Behav. 2013;12:595–603. doi: 10.1111/gbb.12061. [DOI] [PubMed] [Google Scholar]
- 125.Rabani M, Kertesz M, Segal E. Computational prediction of RNA structural motifs involved in posttranscriptional regulatory processes. Proc Natl Acad Sci U S A. 2008;105:14885–14890. doi: 10.1073/pnas.0803169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Roth BM, Ishimaru D, Hennig M. The core microprocessor component DiGeorge syndrome critical region 8 (DGCR8) is a nonspecific RNA-binding protein. J Biol Chem. 2013;288:26785–26799. doi: 10.1074/jbc.M112.446880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tan H, Poidevin M, Li H, Chen D, Jin P. MicroRNA-277 modulates the neurodegeneration caused by Fragile X premutation rCGG repeats. PLoS Genet. 2012;8:e1002681. doi: 10.1371/journal.pgen.1002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128•.He F, Krans A, Freibaum BD, Taylor JP, Todd PK. TDP-43 suppresses CGG repeat-induced neurotoxicity through interactions with HnRNP A2/B1. Hum Mol Genet. 2014;23:5036–5051. doi: 10.1093/hmg/ddu216. TDP-43 rescued CGG repeat-induced toxicity in a Drosophila FXTAS model. The rescue was dependent on hnRNP A2/B1 but independent of RNA interactions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gupta A, Jankovic J. Spinocerebellar ataxia 8: variable phenotype and unique pathogenesis. Parkinsonism Relat Disord. 2009;15:621–626. doi: 10.1016/j.parkreldis.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 130.Mutsuddi M, Marshall CM, Benzow KA, Koob MD, Rebay I. The spinocerebellar ataxia 8 noncoding RNA causes neurodegeneration and associates with staufen in Drosophila. Curr Biol. 2004;14:302–308. doi: 10.1016/j.cub.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 131.Teive HA, Munhoz RP, Arruda WO, Raskin S, Werneck LC, Ashizawa T. Spinocerebellar ataxia type 10 - A review. Parkinsonism Relat Disord. 2011;17:655–661. doi: 10.1016/j.parkreldis.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 132.Xia G, McFarland KN, Wang K, Sarkar PS, Yachnis AT, Ashizawa T. Purkinje cell loss is the major brain pathology of spinocerebellar ataxia type 10. J Neurol Neurosurg Psychiatry. 2013;84:1409–1411. doi: 10.1136/jnnp-2013-305080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang K, McFarland KN, Liu J, Zeng D, Landrian I, Xia G, Hao Y, Jin M, Mulligan CJ, Gu W, et al. Spinocerebellar ataxia type 10 in Chinese Han. Neurol Genet. 2015;1:e26. doi: 10.1212/NXG.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134•.Park H, Gonzalez AL, Yildirim I, Tran T, Lohman JR, Fang P, Guo M, Disney MD. Crystallographic and Computational Analyses of AUUCU Repeating RNA That Causes Spinocerebellar Ataxia Type 10 (SCA10) Biochemistry. 2015;54:3851–3859. doi: 10.1021/acs.biochem.5b00551. These authors provided the first crystal structure of the SCA10 AUUCU repeat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matsuura T, Fang P, Pearson CE, Jayakar P, Ashizawa T, Roa BB, Nelson DL. Interruptions in the expanded ATTCT repeat of spinocerebellar ataxia type 10: repeat purity as a disease modifier? Am J Hum Genet. 2006;78:125–129. doi: 10.1086/498654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136•.McFarland KN, Liu J, Landrian I, Godiska R, Shanker S, Yu F, Farmerie WG, Ashizawa T. SMRT Sequencing of Long Tandem Nucleotide Repeats in SCA10 Reveals Unique Insight of Repeat Expansion Structure. PLoS One. 2015;10:e0135906. doi: 10.1371/journal.pone.0135906. By using SMRT sequencing, these authors demonstrated the correlation between SCA10 repeat interruption motifs and distinct clinical presentations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McFarland KN, Liu J, Landrian I, Zeng D, Raskin S, Moscovich M, Gatto EM, Ochoa A, Teive HA, Rasmussen A, et al. Repeat interruptions in spinocerebellar ataxia type 10 expansions are strongly associated with epileptic seizures. Neurogenetics. 2014;15:59–64. doi: 10.1007/s10048-013-0385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Braida C, Stefanatos RK, Adam B, Mahajan N, Smeets HJ, Niel F, Goizet C, Arveiler B, Koenig M, Lagier-Tourenne C, et al. Variant CCG and GGC repeats within the CTG expansion dramatically modify mutational dynamics and likely contribute toward unusual symptoms in some myotonic dystrophy type 1 patients. Hum Mol Genet. 2010;19:1399–1412. doi: 10.1093/hmg/ddq015. [DOI] [PubMed] [Google Scholar]
- 139.Moseley ML, Schut LJ, Bird TD, Koob MD, Day JW, Ranum LP. SCA8 CTG repeat: en masse contractions in sperm and intergenerational sequence changes may play a role in reduced penetrance. Hum Mol Genet. 2000;9:2125–2130. doi: 10.1093/hmg/9.14.2125. [DOI] [PubMed] [Google Scholar]
- 140.Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- 141.Ashizawa T, Ranum LP. GGCCTG repeats put a hex on Purkinje cells and motor neurons in SCA36. Neurology. 2012;79:302–303. doi: 10.1212/WNL.0b013e31826043d9. [DOI] [PubMed] [Google Scholar]
- 142•.Mannen T, Yamashita S, Tomita K, Goshima N, Hirose T. The Sam68 nuclear body is composed of two RNase-sensitive substructures joined by the adaptor HNRNPL. J Cell Biol. 2016;214:45–59. doi: 10.1083/jcb.201601024. These authors showed that Sam68, hnRNPD and hnRNPL were essential components of certain architectural RNA nuclear bodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Martin KC, Ephrussi A. mRNA localization: gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang SJ, Meulemans D, Vazquez L, Colaco N, Schuman E. A role for a rat homolog of staufen in the transport of RNA to neuronal dendrites. Neuron. 2001;32:463–475. doi: 10.1016/s0896-6273(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 145.Burguete AS, Almeida S, Gao FB, Kalb R, Akins MR, Bonini NM. GGGGCC microsatellite RNA is neuritically localized, induces branching defects, and perturbs transport granule function. Elife. 2015;4:e08881. doi: 10.7554/eLife.08881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim DH, Langlois MA, Lee KB, Riggs AD, Puymirat J, Rossi JJ. HnRNP H inhibits nuclear export of mRNA containing expanded CUG repeats and a distal branch point sequence. Nucleic Acids Res. 2005;33:3866–3874. doi: 10.1093/nar/gki698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147••.Hase K, Fujiwara Y, Kikuchi H, Aizawa S, Hakuno F, Takahashi S, Wada K, Kabuta T. RNautophagy/DNautophagy possesses selectivity for RNA/DNA substrates. Nucleic Acids Res. 2015;43:6439–6449. doi: 10.1093/nar/gkv579. These authors demonstrated that G-rich sequences, such as GGGGCC repeats, could bind to LAMP2C during RNautophagy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aguilera A, Garcia-Muse T. R loops: from transcription byproducts to threats to genome stability. Mol Cell. 2012;46:115–124. doi: 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 149•.Reddy K, Schmidt MH, Geist JM, Thakkar NP, Panigrahi GB, Wang YH, Pearson CE. Processing of double-R-loops in (CAG).(CTG) and C9orf72 (GGGGCC). (GGCCCC) repeats causes instability. Nucleic Acids Res. 2014;42:10473–10487. doi: 10.1093/nar/gku658. These authors showed that R-loop formation and processing to instability could occur at C9orf72 GGGGCC repeats. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kalantari R, Chiang CM, Corey DR. Regulation of mammalian transcription and splicing by Nuclear RNAi. Nucleic Acids Res. 2016;44:524–537. doi: 10.1093/nar/gkv1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fiszer A, Krzyzosiak WJ. RNA toxicity in polyglutamine disorders: concepts, models, and progress of research. J Mol Med (Berl) 2013;91:683–691. doi: 10.1007/s00109-013-1016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shieh SY, Bonini NM. Genes and pathways affected by CAG-repeat RNA-based toxicity in Drosophila. Hum Mol Genet. 2011;20:4810–4821. doi: 10.1093/hmg/ddr420. [DOI] [PMC free article] [PubMed] [Google Scholar]