Abstract
Objective
Dietary fiber may reduce knee pain in part by lowering body weight and inflammation. In this study, we assessed whether fiber intake was associated with knee pain development patterns.
Methods
In a prospective, multicenter cohort of 4,796 men and women aged 45-79 years with or at risk of knee osteoarthritis in Osteoarthritis Initiative, participants were followed up annually for 8 years. Dietary fiber was estimated using a validated food frequency questionnaire at baseline. Group-based trajectory modeling was used to identify WOMAC pain trajectories, which were assessed for the associations with dietary fiber intake using polytomous regression models.
Results
Of the 4,470 eligible participants (8,940 knees) [mean age: 61.3 (SD: 9.1) years, 58% women], 4.9% underwent knee replacement and were censored at the time of surgery. Four distinct knee pain patterns were identified: no pain (34.5%), mild pain (38.1%), moderate pain (21.2%) and severe pain (6.2%). Dietary total fiber was inversely related to membership in the moderate or severe pain group (both p for trend ≤0.006). Subjects in the highest versus lowest quartile of total fiber had lower risks of belonging to moderate pain (OR=0.76, 95% CI: 0.61, 0.93) and severe pain patterns (OR=0.56, 95% CI: 0.41, 0.78). Similar results were found for grain fiber with these two pain patters.
Conclusion
Our findings suggest that high dietary total or grain fiber, particularly in the recommended daily fiber average intake of 25g per day, was associated with lower risks of belonging to moderate and severe knee pain development patterns over time.
Introduction
Osteoarthritis (OA) is the most common form of arthritis accompanied by one or more characteristics such as synovial inflammation, destruction of cartilage and joint pain (1). Chronic pain and function loss are the primary causes of disability in OA patients. While nonsteroidal anti-inflammatory drugs (NSAIDs) are currently the widely used medication to relieve pain symptoms for OA, they are known to produce side effects including gastrointestinal symptoms and internal bleeding particularly in the elderly (2, 3). Hence, dietary approaches may provide safe alternative options for pain management in those with or at risk of painful knee OA.
Dietary fibers are carbohydrates that are indigestible or non-absorbable in the small intestine but partially or fully fermentable in the colon (4). The physiological properties of dietary fiber related to health benefits include reduced energy density (5) and lowered adiposity and inflammation through desirable microbes in the gut (6), both of which facilitate weight loss (7-10) and decrease pro-inflammatory markers (11-14). Epidemiologic studies have consistently reported that dietary total fiber and particular fiber from whole grain cereals are associated with lower risks of mortality (15, 16), cardiovascular diseases (CVD) (17), type 2 diabetes (18-21) and depression (22, 23) in part because dietary fiber reduces body weight and inflammation. Among these studies, fiber from cereal grains rather than that from fruits, vegetables or legumes and nuts was a prominent protective factor (15-17, 20, 21, 24, 25).
OA shares common risk factors with metabolic diseases including CVD and diabetes (26), where obesity and inflammation were strongly associated with pain symptoms related to OA. To our knowledge, no data to date has examined the relation of dietary fiber intake to knee pain in older adults. In this study, we examined the associations between dietary fiber and knee pain development patterns over 8 years.
Methods
Study population
We used data from the Osteoarthritis Initiative (OAI), a multi-center, longitudinal prospective cohort of 4,796 U.S. men (41.5%) and women aged 45-79 years with or at high risk of knee OA recruited from 2004 to 2006. Criteria for participation in the OAI included absence of rheumatoid arthritis or other forms of inflammatory arthritis at the screening of the study. Details of the study protocol can be found elsewhere (27). After enrollment, participants were followed annually up to 96 months. In this study, we further excluded those who had total or partial knee replacement (KR) at baseline (N=63). Institutional Review Board approval and study consent from each participant were obtained from all study sites.
Exposure measure: Baseline assessment of dietary fiber
At the baseline assessment, participants’ usual eating habits were recorded using the Block Brief 2000 food frequency questionnaire (FFQ). The Block Brief 2000 FFQ is a reduced version of 60 food items developed from the validated Block Full FFQ (28, 29) and has been further validated against multiple dietary records in different studies (29). Both Block FFQs showed similar correlation coefficients for major nutrients (28, 29). For each food item, participants were asked, on average, how often they consumed the food in the past year according to nine pre-determined categories with illustrated portion sizes. For example, for “dark bread like rye or whole wheat, including in sandwiches,” portion sizes ranged from 1/4 to 2 cups. Estimation of total fiber (sum of sub-category fibers) and fiber from major food groups (cereal grain, fruit and vegetables, and nuts and legumes) was calculated based on the food composition database for nutrients in the Second National Health and Nutrition Examination Survey (28). Quartiles of fiber intake were then defined among eligible participants separately by sex to account sex differences in food intake.
Other covariates
During the enrollment clinic visit, a self-administered questionnaire with standard instructions was used to collect information on demographics, tobacco and alcohol use, and depressive symptoms estimated by the Center for Epidemiologic Studies Depression Scale (CES-D). A clinic visit interview was further performed to record history of knee injury and surgery (including knee replacement), medication use, and physical activity assessed by the Physical Activity Scale for the Elderly (PASE). At baseline and each annual exam, each participant obtained a fixed flexion posterior-anterior radiograph evaluation for both knees, according to the Kellgren and Lawrence (KL) grading scale between 0 and 4. Radiographic OA was defined if KL grade was equal or over 2.
Outcome measure: Knee symptom assessment of pain
At each examination at baseline and annually up to 96 months, knee symptom assessment was conducted using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale of five activity items including “Walking,” “Stair climbing,” “Nocturnal, ” “Rest,” and “Weight bearing” scored from 0 (no difficulty) to 4 (extreme difficulty) for each item. Hence, the total WOMAC pain score ranged from 0 (no pain) to 20 (worst pain) points.
Statistical analysis
We further excluded participants who had missing dietary information (N=14) or had extreme calorie intake at baseline (<500 kcal or ≥4,200 kcal for men and ≥4,000 kcal for women, N=249) from the analyses. In addition, participants who had KR, died or for whom contact was lost at the time of the event during the 96-month follow-up were censored in the trajectory modeling analysis. Because improvement in WOMAC pain score was noted between baseline and month 12 for all patterns, we evaluated the WOMAC pain trajectory pattern starting at month 12, and conducted additional analysis that started from the baseline visit.
Group-based trajectories
Group-based trajectory modeling procedure (SAS PROC TRAJ) (30) was applied to identify distinct WOMAC pain trajectories over the 8-year study course. In this procedure, a multinomial modeling strategy was used to identify relatively homogenous clusters of developmental trajectories within a sample population, where the trajectory parameters are derived by latent class analysis using maximum likelihood estimation. The number of trajectories were determined by the patterns of change in WOMAC pain score and not forced to fit a particular model regarding number or shape of patterns. Among the eligible participants, the majority (95%) had at least three WOMAC scores. For each trajectory, we chose to estimate several possible combinations of WOMAC trajectory shapes (linear, quadratic, or cubic) to identify the model by maximizing the Bayesian Information Criteria while maintaining statistical significance of the model terms. The optimal number of WOMAC pain development patterns was assessed by model fit with the average posterior probabilities of group membership at least 0.7 (31), which implies the greatest likelihood of each person's assignment in one of the patterns generated.
We used the residual method (32) to estimate dietary fiber intake for men and women separately. The association of a higher relative to the lowest quartile of fiber with pain trajectories was examined using a multivariable polytomous regression model for nominal outcomes (pain patterns) after controlling for baseline risk factors and potential confounders including age (years), sex (men vs. women), race (white vs. non-white), education (below college vs. college or above), tobacco use (never, former, and current smokers), physical activity (PASE, continuous), total energy intake (kcal), prescribed or self-reported use of NSAIDs (yes vs. no), depressive symptoms (CESD), and baseline radiographic OA status [KL grade≥2 versus KL grade <2 ]. Adjustment for total energy intake in addition to the energy-adjusted fiber intake was based on the multivariate residual model developed by Willet for nutrients and disease outcomes in epidemiological studies (32) and includes adjustment for total energy intake because total energy intake not only affects dietary fiber consumption but is associated with disease outcomes due to its influence on body size, physical activity level and metabolic efficiency (32). For other covariates selected in the full model, we took into account previously published risk factors for OA, adjusting for age, sex, genetic/racial differences, tobacco use, physical activity, and use of NSAIDs (1, 33, 34). Linear trends were tested using the sex-specific median value of each quartile of dietary fiber as a continuous variable in the regression model. Because dietary fiber was previously suggested to lower body weight (7-10) and depression(22, 23), both of which have been shown to be linked to symptomatic OA (1, 34-38), BMI (kg/m2) and depression (CES-D<16 vs. ≥16 as a cut-off for clinical depression (39) were not adjusted for in the primary analysis. However, in secondary analysis, we adjusted for both covariates. No evidence suggested significant differences between men and women regarding pain trajectories or the association with dietary fiber, we therefore combined men and women in the analyses. In the secondary analyses, we assessed pain trajectories in participants with and without prevalent radiographic OA at baseline (KL grade ≥2) to assess whether dietary fiber had consistent impacts on pain patterns.
In addition, we carried out sensitivity analysis including one knee with maximum WOMAC or a random knee per individual if both knees had equal WOMAC pain scores, because Proc Traj does not account for the correlation between two knees for each person. All statistical analysis was conducted using SAS Version 9.3 (SAS Institute, Inc., Cary, North Carolina). A two-sided p-value < 0.05 was considered statistically significant.
Results
Baseline characteristics are described in Table 1 across quartiles of dietary total fiber among all eligible participants. Those who consumed more dietary fiber tended to be older and more educated, have lower BMI, and less likely to smoke tobacco. They were also more physically active, less likely used NSAIDs and had a lower prevalence of depression and a lower total caloric intake as compared to participants who consumed less dietary fiber. Decreased WOMAC pain score was observed as fiber intake increased at baseline and the 12-month exam.
Table 1.
Baseline characteristics of study participants by quartile intake of dietary total fiber
| Quartile (Q) of dietary total fiber | Q1 (Lowest) (N=2,239) | Q2 (N=2,231) | Q3 (N=2,236) | Q4 (Highest) (N=2,234) |
|---|---|---|---|---|
| Age (years) at baseline, mean (SD) | 59.7 (9.0) | 60.9 (9.1) | 61.8 (9.1) | 62.7 (9.1) |
| Caucasian, n (%) | 1753 (78.3) | 1863 (83.5) | 1827 (81.7) | 1794 (80.3) |
| Women, n (%) | 1301 (58.1) | 1296 (58.1) | 1286 (57.5) | 1296 (58.0) |
| BMI (kg/m2) | ||||
| At baseline | 29.4 (5.0) | 28.8 (4.7) | 28.6 (4.7) | 27.6 (4.4) |
| At 96-month exam | 29.7 (5.6) | 28.8 (5.1) | 28.5 (4.8) | 27.8 (4.7) |
| Tobacco use, n (%) | ||||
| Never | 1782 (79.6) | 1763 (79.0) | 1726 (77.2) | 1785 (79.9) |
| Former | 368 (16.5) | 384 (17.2) | 434 (19.4) | 389 (17.4) |
| Current | 89 (4.0) | 84 (3.8) | 76 (3.4) | 60 (2.7) |
| Education, n (%) | ||||
| less than college level | 1041 (46.5) | 895 (40.1) | 827 (37.0) | 753 (33.7) |
| college level or above | 1798 (53.5) | 1336 (59.9) | 1409 (63.0) | 1481 (66.3) |
| Physical Activity Scale for Elderly | 156.1 (80.9) | 160.0 (81.7) | 163.2 (79.9) | 166.4 (84.1) |
| Usage of NSAIDs (%) | 616 (27.5) | 580 (26.0) | 523 (23.4) | 500 (22.4) |
| Kellgren-Lawrence grade, n (%) | ||||
| 0-1 | 1187 (53.0) | 1248 (55.9) | 1263 (56.4) | 1300 (58.2) |
| 2-4 | 1052 (47.0) | 983 (44.1) | 973 (43.6) | 934 (41.8) |
| WOMAC pain score (range 0-20) | ||||
| Baseline | 2.75 (3.58) | 2.29 (3.02) | 2.26 (3.14) | 2.08 (3.02) |
| 12-month | 2.55 (3.65) | 2.06 (3.04) | 2.09 (3.06) | 1.78 (2.76) |
| Depression, n (%) | ||||
| CES-D <16 | 1917 (85.6) | 2021 (90.6) | 2046 (91.5) | 2069 (92.6) |
| CES-D ≥16 | 322 (14.4) | 210 (9.4) | 190 (8.5) | 165 (7.4) |
| Total energy, kcal/day, median (IQR) | 1333.5 (974.9,1728.5) | 1354.1 (1044.2, 1734.0) | 1314.0 (1008.3, 1691.9) | 1287.3 (978.3, 1640.1) |
| Total dietary fiber, g/day, median (IQR) | 8.6 (6.3, 11.3) | 12.5 (9.9, 15.6) | 15.2 (12.2, 19.0) | 20.6 (16.2, 26.5) |
| Grain fiber, g/day, median (IQR) | 3.7 (2.5, 5.2) | 5.0 (3.5, 6.8) | 5.7 (3.9, 8.0) | 6.8 (4.5, 9.8) |
| Fruit and vegetable fiber, g/day, median (IQR) | 3.8 (2.6, 5.4) | 6.1 (4.5, 8.1) | 7.8 (6.0, 10.0) | 10.5 (7.8, 14.0) |
| Nut and legume fiber, g/day, median (IQR) | 0.8 (0.4, 1.4) | 1.4 (0.8, 2.1) | 1.6 (1.0, 2.6) | 2.3 (1.2, 4.0) |
NSAIDs: nonsteroidal anti-inflammatory drug;
CES-D: Center for Epidemiologic Studies Depression Scale;
IQR: interquartile range.
All differences among quartiles of total fiber intake were statistically significant at p<0.01, except sex distribution (p=0.98) and tobacco use (p=0.056).
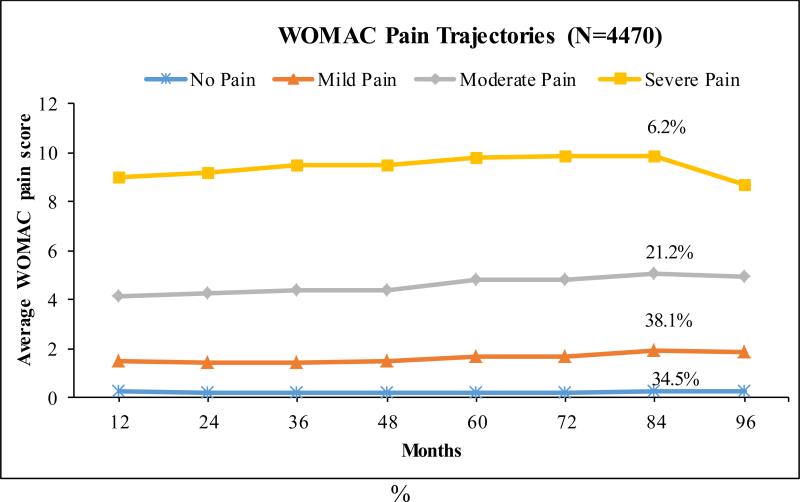
Among the originally enrolled 4,796 participants with exclusion of those who had KR at baseline (N=63), with missing dietary information (N=14) or had extreme caloric intake (N=249), by the 96-month exam, 540 (11%) were lost to follow up including 252 (5.3%) deaths. Four distinct pain trajectory patterns were identified among 4,470 eligible participants (8,940 knees) regardless radiographic OA status at baseline from month 12 to 96 months (Figure 1) after adjusting for the afore-mentioned covariates. These patterns include “no pain” (34.5%), “mild pain” (38.1%), “moderate pain” (21.2%) and “severe pain” (6.2%). The average posterior probability for each WOMAC pain trajectory group ranged from 0.87 to 0.91, indicating a high discrimination of the group assignment. In general, none of the trajectories suggested substantial worsening or improvement of pain over time among the four pain patterns. The “no pain” trajectory group showed a consistent pain score throughout the 8-years course with mean (95% confidence interval) WOMAC score as 0.28 (0.15, 0.24) for month 12 and 0.26 (0.15, 0.24) for month 96. A similar shape was found in the “mild pain” pattern with 1.50 (1.37, 1.54) for month 12 and 1.84 (1.75, 2.03) for month 96 and the “moderate pain” pattern with 4.13 (3.87, 4.39) for month 12 and 4.91 (4.77, 5.26) for month 96. In the “severe pain” pattern, the average WOMAC score was 9.02 (8.51, 9.20) for month 12 and 8.65 (8.63, 9.41) for month 96. In the “severe pain” pattern, there was a modest increase in WOMAC pain until month 84, followed by a decline at month 96, which is most likely due to the censoring of KR cases after month 72. The trajectory groups of knee pain starting at baseline were very close to those beginning with month 12, comprising 4 patterns as persistent “no pain” (33.9%), “mild pain” (37.8%), “moderate pain” (21.7%) and “severe pain” (6.6%) with the average posterior probability ranged from 0.88 to 0.92.
Figure 1.
WOMAC knee pain trajectory groups over 8 years of follow-up among all eligible participants starting from month 12
In Table 2, the distribution of each pain pattern in each dietary quartile intake of fiber showed that those who consumed the most total fiber (Q4) had the highest proportion in the no pain pattern (38.1%) and the lowest proportion in the severe pain pattern (4.3). Using ‘no pain’ as the reference group, we observed that a high intake of total fiber was associated with lower risk of membership in moderate or severe pain pattern (both p for trend <0.01). Compared to the lowest quartile fiber intake, participants who consumed the highest quartile had 24% lower likelihood (OR: 0.76; 95% CI: 0.61, 0.93) of belonging to the moderate pain pattern and a 44% lower risk (OR: 0.56; 95% CI: 0.41, 0.98) being in the “severe pain” pattern. No association was found between dietary total fiber and mild pain pattern. Similar results were observed for cereal grain fiber intake and membership of pain patterns, with a significant inverse relationship with the severe pain group and a marginally significant relationship with the moderate pain group. No apparent relationship was found between fiber from fruits and vegetables or legumes and nuts and pain patterns.
Table 2.
Odds ratio (95% Confidence Interval) between dietary fiber and WOMAC pain sub-groups among eligible participants regardless status of radiographic OA at baseline
| Dietary Fiber | Odds Ratio (95% CI)2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (lowest) (n=2239)1 | Q2 (n=2231) | Q3 (n=2236) | Q4 (highest) (n=2234) | Q2 vs. Q1 | Q3 vs. Q1 | Q4 vs. Q1 | P for trend | |
| Pain patterns | Total fiber (g/day) | |||||||
| No pain, n (%) | 719 (32.1) | 754 (33.8) | 807 (36.2) | 851 (38.1) | 1.0 | 1.0 | 1.0 | |
| Mild pain, n (%) | 846 (37.8) | 937 (42.0) | 863 (38.6) | 907 (40.6) | 1.16 (0.97,1.39) | 0.92 (0.77,1.10) | 1.05 (0.88,1.26) | 0.92 |
| Moderate pain, n (%) | 488 (21.8) | 433 (19.4) | 434 (19.4) | 380 (17.0) | 0.92 (0.75,1.13) | 0.85 (0.70,1.04) | 0.76 (0.61,0.93) | 0.006 |
| Severe pain, n (%) | 186 (8.3) | 107 (4.8) | 132 (5.9) | 96 (4.3) | 0.65 (0.48,0.89) | 0.79 (0.59,1.07) | 0.56 (0.41,0.78) | 0.002 |
| Cereal grain fiber (g/day) | ||||||||
| No pain, n (%) | 734 (32.8) | 745 (33.4) | 785 (35.1) | 867 (38.8) | 1.0 | 1.0 | 1.0 | |
| Mild pain, n (%) | 840 (37.5) | 910 (40.8) | 912 (40.8) | 889 (39.8) | 1.10 (0.92,1.31) | 1.00 (0.84,1.20) | 0.99 (0.83,1.18) | 0.49 |
| Moderate pain, n (%) | 481 (21.5) | 428 (19.2) | 436 (19.5) | 391 (17.5) | 0.89 (0.73,1.10) | 0.93 (0.76,1.13) | 0.85 (0.70,1.04) | 0.09 |
| Severe pain, n (%) | 184 (8.2) | 147 (6.6) | 103 (4.6) | 87 (3.9) | 0.95 (0.71,1.28) | 0.70 (0.51,0.97) | 0.55 (0.39,0.79) | 0.0002 |
| Fruit and vegetable fiber (g/day) | ||||||||
| No pain, n (%) | 750 (33.5) | 779 (34.9) | 783 (35.0) | 818 (36.6) | 1.0 | 1.0 | 1.0 | |
| Mild pain, n (%) | 873 (39.0) | 906 (40.6) | 901 (40.3) | 876 (39.2) | 1.10 (0.92,1.31) | 1.14 (0.95,1.36) | 1.01 (0.84,1.21) | 0.96 |
| Moderate pain, n (%) | 463 (20.7) | 431 (19.3) | 432 (19.3) | 411 (18.4) | 0.91 (0.75,1.12) | 0.98 (0.80,1.20) | 0.80 (0.66,0.99) | 0.05 |
| Severe pain, n (%) | 152 (6.8) | 116 (5.2) | 121 (5.4) | 130 (5.8) | 0.92 (0.67,1.27) | 1.02 (0.74,1.40) | 0.84 (0.61,1.15) | 0.30 |
| Legume and nut fiber (g/day) | ||||||||
| No pain, n (%) | 752 (33.6) | 796 (35.7) | 785 (35.1) | 800 (35.8) | 1.0 | 1.0 | 1.0 | |
| Mild pain, n (%) | 862 (38.5) | 890 (39.9) | 879 (39.3) | 923 (41.3) | 0.98 (0.82,1.17) | 1.02 (0.85,1.22) | 1.09 (0.92,1.30) | 0.21 |
| Moderate pain, n (%) | 448 (20.0) | 440 (19.7) | 443 (19.9) | 404 (18.1) | 0.92 (0.75,1.12) | 0.99 (0.81,1.22) | 0.87 (0.70,1.07) | 0.26 |
| Severe pain, n (%) | 177 (7.9) | 105 (4.7) | 130 (5.8) | 107 (4.8) | 0.63 (0.46,0.87) | 0.86 (0.63,1.16) | 0.75 (0.55,1.03) | 0.29 |
n for number of knees;
Model adjusted for age (years), sex (men vs. women), race (white vs. non-white), education level (below vs. college or above), tobacco use (never, former, current smokers), total calorie intake (kcal), physical activity (PASE, continuous), baseline radiographic OA status (yes vs. no), and NSAIDs use (yes vs. no).
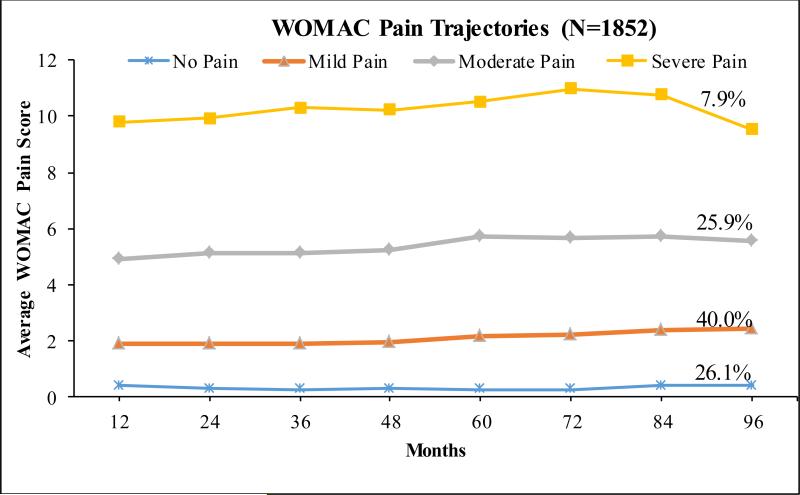
When we restricted to the participants with radiographic OA at baseline (3,703 knees), four distinct pain patterns were identified similarly as of all eligible participants shown in Figure 1, although a smaller proportion of 26.1% in the no pain pattern and a higher proportion of 7.9% in the severe pain sub-group (Figure 2). The average posterior probability for each pattern ranged from 0.85 to 0.90. Again, we found four similar pain patterns if we started with baseline WOMAC pain scores, with 26.8% in the no pain group, 40.5% in the mild pain group, 25.3% and 7.4% in the moderate and severe pain patterns, respectively.
Figure 2.
WOMAC knee pain trajectory groups over 8 years follow-up among participants with ROA at baseline starting from month 12
The relations between dietary fiber and pain patterns among participants with prevalent ROA (Table 3) were comparable in terms of the distributions of WOMAC pain patterns in each quartile of dietary fibers but with a greater effect magnitude for the association between dietary total fiber and moderate or severe pain pattern. A significant inverse relationship was also found between grain fiber and severe pain patterns (p for trend <0.01). For fiber from fruits and vegetables, we found an inverse relationship for moderate and severe pain groups (both p for trend ≤0.02). No significant associations were found for fiber from nuts and legumes.
Table 3.
Odds ratio (95% Confidence Interval) between dietary fiber and WOMAC pain trajectories among those with radiographic OA at baseline
| Dietary Fiber | Odds Ratio2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (lowest) (n=9761) | Q2 (n=929) | Q3 (n=920) | Q4 (highest) (n=878) | Q2 vs. Q1 | Q3 vs. Q1 | Q4 vs. Q1 | P for trend | |
| Pain sub-groups | Total fiber (g/day) | |||||||
| No pain, n (%) | 221 (22.6) | 248 (26.7) | 264 (28.7) | 268 (30.5) | 1.00 | 1.00 | 1.00 | |
| Mild pain, n (%) | 378 (38.7) | 374 (40.3) | 346 (37.6) | 369 (42.0) | 0.88 (0.66,1.17) | 0.76 (0.58,1.01) | 0.87 (0.65,1.16) | 0.28 |
| Moderate pain, n (%) | 269 (27.6) | 240 (25.8) | 240 (26.1) | 189 (21.5) | 0.76 (0.57,1.02) | 0.70 (0.52,0.94) | 0.57 (0.42,0.77) | 0.0004 |
| Severe pain, n (%) | 108 (11.1) | 67 (7.2) | 70 (7.6) | 53 (6.0) | 0.59 (0.38,0.90) | 0.61 (0.39,0.93) | 0.41 (0.24,0.68) | 0.0006 |
| Cereal grain fiber (g/day) | ||||||||
| No pain, n (%) | 252 (25.8) | 223 (24.0) | 259 (28.1) | 268 (30.5) | 1.00 | 1.00 | 1.00 | |
| Mild pain, n (%) | 366 (37.5) | 388 (41.8) | 376 (40.9) | 335 (38.2) | 1.28 (0.96,1.69) | 1.00 (0.76,1.31) | 1.01 (0.77,1.33) | 0.6 |
| Moderate pain, n (%) | 257 (26.3) | 234 (25.2) | 221 (24.0) | 227 (25.8) | 1.08 (0.80,1.45) | 0.91 (0.68,1.22) | 0.98 (0.73,1.32) | 0.59 |
| Severe pain, n (%) | 102 (10.4) | 84 (9.0) | 64 (7.0) | 48 (5.5) | 1.04 (0.69,1.57) | 0.65 (0.41,1.03) | 0.55 (0.33,0.91) | 0.006 |
| Fruit and vegetable fiber (g/day) | ||||||||
| No pain, n (%) | 247 (25.3) | 244 (26.3) | 239 (26.0) | 267 (30.4) | 1.00 | 1.00 | 1.00 | |
| Mild pain, n (%) | 367 (37.6) | 369 (39.7) | 384 (41.7) | 345 (39.3) | 1.13 (0.84,1.51) | 1.17 (0.88,1.56) | 0.90 (0.68,1.20) | 0.35 |
| Moderate pain, n (%) | 268 (27.5) | 243 (26.2) | 230 (25.0) | 199 (22.7) | 0.93 (0.69,1.25) | 0.85 (0.63,1.15) | 0.60 (0.45,0.81) | 0.0004 |
| Severe pain, n (%) | 94 (9.6) | 72 (7.8) | 67 (7.3) | 67 (7.6) | 0.89 (0.57,1.39) | 0.85 (0.55,1.33) | 0.61 (0.39,0.95) | 0.02 |
| Legume and nut fiber (g/day) | ||||||||
| No pain, n (%) | 247 (25.3) | 242 (26.0) | 269 (29.2) | 243 (27.7) | 1.00 | 1.00 | 1.00 | |
| Mild pain, n (%) | 380 (38.9) | 382 (41.1) | 334 (36.3) | 371 (42.2) | 1.03 (0.78,1.36) | 0.88 (0.67,1.17) | 1.09 (0.83,1.44) | 0.57 |
| Moderate pain, n (%) | 245 (25.1) | 242 (26.1) | 245 (26.6) | 208 (23.7) | 0.98 (0.73,1.32) | 0.92 (0.69,1.23) | 0.77 (0.57,1.05) | 0.09 |
| Severe pain, n (%) | 104 (10.7) | 63 (6.8) | 73 (7.9) | 56 (6.4) | 0.60 (0.38,0.94) | 0.70 (0.46,1.09) | 0.69 (0.44,1.09) | 0.23 |
n for number of knees;
Model adjusted for age (years), sex (men vs. women), race (white vs. non-white), education level (below vs. college or above), tobacco use (never, former, current smokers), total calorie intake (kcal), physical activity (PASE, continuous), and NSAIDs use (yes vs. no).
We found similar results in the sensitivity analyses including 1) further adjustment for baseline BMI and depression (C-ESD), 2) started from WOMAC pain score at baseline, 3) using only one knee per subject with maximum or equal WOMAC pain score, and 4) restricted to those without ROA at baseline. Although the results were attenuated with further adjustment for BMI and CES-D, the effect estimates remained statistically significant at the highest quartile of total or cereal grain fiber intake with severe pain pattern in all sensitivity analyses. For example, further adjustment for BMI and CES-D for both knees among all regardless of baseline radiographic OA, the OR (95% CI) was 0.71 (0.50 to 0.995) for the highest quartile of total fiber and 0.63 (0.44 to 0.90) for the highest quartile of grain fiber. If we followed WOMAC pain score from baseline, the associations were materially the same with 0.70 (0.50 to 0.96) at the highest quartile of total fiber and 0.62 (0.44 to 0.88) at the highest quartile of cereal grain fiber with p for trend < 0.01. Among those without ROA at baseline, a similar protective association was found with total fiber in the moderate (p for trend =0.01) and severe pain groups (p for trend =0.06). Again, no significant results were found for fiber from fruits and vegetable or from nuts and legumes.
Discussion
In the present study, we identified four distinct WOMAC knee pain trajectory patterns over an 8-year course and found that dietary total or cereal grain fiber intake was inversely associated with likelihood of belonging to the moderate and severe pain groups. At the highest quartile intake of total fiber, significantly lower odds were found for the membership in moderate and severe pain patterns as compared with the lowest quartile using ‘no pain’ as the reference group. Such associations were more apparent among persons with prevalent radiographic OA.
Consistent with Collins and colleagues (40) who identified five WOMAC knee pain trajectories among the participants who had radiographic OA and WOMAC pain score >0 at baseline followed for 6 years, we found similar patterns of WOMAC pain trajectories. We included eligible participants regardless their radiographic OA status at baseline and with WOMAC pain score ≥0 up to 8 years. Compared to the patterns Collins and colleagues identified (40), we had a slightly higher posterior probability ranged from 0.87 to 0.92 (versus 0.80 to 0.87). The proportion of the subjects in the severe pain pattern is similar between the two studies, and the slight divergence between our patterns and theirs could be due to the rescale of WOMAC score to >0-100 in addition to other criteria (status of radiographic OA and WOMAC score). Nonetheless, the pain trajectories in ours and Collins's (40) are consistent, showing that WOMAC pain score did not change substantially over time and was primarily determined by the baseline score.
To our knowledge, this is the first study examining dietary fiber and WOMAC pain trajectory patterns with a protective association seen for moderate and severe pain patterns. We noted that participants in these two sub-groups, in general, were heavier, less physically active and less educated; they also carried more risk profiles of OA such as depression and structural deterioration in the joint, factors we adjusted for in analyses. We also noted that subjects who developed knee pain worsening defined as change of WOMAC pain score by more than 14% between baseline and each annual exam (41) were primarily categorized in the moderate and severe pain patterns. In knee OA, obesity contributes increased loading in weight-bearing joints (1, 35) as well as inflammation (36), both of which were linked with joint pain (37, 38, 42, 43). And previous epidemiologic studies consistently showed that high intake of dietary total fiber particular cereal grain fiber was associated with lower risks of CVD and type 2 diabetes (20, 21) via reduced body weight (7, 8, 44, 45) and inflammation (11-14). Therefore, it is biologically plausible that older persons who consumed more fiber could experience less persistent moderate and severe knee pain related to OA. Plausibility that cereal grain fiber may be more healthful than fiber from other plant sources may include 1) whole grain wheat and bran cereals are the major source of dietary and cereal fiber (27, 28); and 2) as cereal fiber is consumed in the form of a whole food, a natural package of nutrients such as antioxidant vitamins, minerals and unique phytochemicals found in whole grain may exert higher antioxidant and anti-inflammatory effect compared to fruits and vegetables (24, 25, 46), when grain intake is the major energy source.
Restricted to participants with prevalent radiographic OA at baseline, we observed stronger inverse relationships between dietary total fiber and severe pain pattern than we did in the entire sample. This is in line with our hypothesis that dietary fiber may reduce the risk of painful knee OA, presuming those with prevalent radiographic OA could further develop pain symptoms. The results were consistent when we included only participants without ROA at baseline. Using one knee with maximum or equal WOMAC score also yielded materially the same results as using two knees per subject. Although we found consistent patterns and associations using WOMAC from baseline or month 12, we conducted our primary analysis started at month 12 as to minimize the bias in observed improvement of WOMAC scores in all patterns from baseline to month 12. Overall, these sensitivity analyses demonstrated consistent results and yielded high discrimination for the four distinct patterns generated in this study. Furthermore, consistent associations were found for dietary total and grain fiber with moderate and severe pain patterns.
Although we included all possible established risk factors and confounders for WOMAC knee pain trajectory and also controlled for BMI and CES-D in our secondary analysis, we could not rule out the possibility of residual confounding by factors such as diet quality (47). Additionally, while dietary fiber from fruits, vegetables, and legumes would be related to healthier diet and lifestyle, only total and cereal grain fibers were shown to be associated with lower risk of moderate or severe knee pain groups. Hence, residual confounding effect by other healthful food components or lifestyle factors that may account for the relationship we observed in the present study seems to be unlikely.
Strengths of this study included a prospective cohort design and the relatively large sample size. Our four distinct WOMAC pain patterns showed more than 0.87 in the average posterior probability for each pain group, indicating a very high discrimination and good model fit. During the 8 year follow-up to assess the prospective relationship between dietary fiber at baseline and pain patterns overtime, we carefully carried out censoring techniques to account for those who experienced knee replacement, had lost of follow up or died at the time of the event to ensure a complete inclusion of all eligible participants in the trajectory analysis.
Limitations in terms of dietary information included no follow-up data on dietary information, which precluded us assessing change of dietary fiber intake, although dietary intake of fiber has been reported rather stable. For example, the average fiber intake in U.S. adults was 15.6 g/day for 1999-2000 and increased to 15.9 g/day for 2007-2008 in the National Health and Nutrition Examination Survey (48), thus, a slight increment that could also have been present in our sample. The subtle change, if present, should not alter the effect estimates substantially and would have likely attenuated the observed associations. Another limitation is that each WOMAC pain development pattern is relatively steady over time, where participants’ pain scores were primarily determined by their baseline levels. Although this is consistent with the previous study in pain trajectory (40) and with other trajectory studies such as depression (49) and gait speed (50), this scenario could be due to the “horse-racing” effect where in a chronic state, when a prolonged condition has been ongoing before the baseline observation, such condition would in general maintain its baseline level over time, unless the important risk factors under investigation, for example, dietary intakes for WOAMC pain also change substantially during the study period. But, as mentioned above, dietary intake of fiber barely changes over time, thus, such a stable exposure limits our ability from examining the association between modified dietary fiber intake and WOMAC knee pain patterns over time. A well-designed intervention study would be helpful to answer such question. Finally, results from observational studies often cannot generate causality, as there may have been residual/unmeasured confounding factors.
Conclusion
Our findings suggest that greater dietary intake of total and cereal grain fiber, particularly in the recommended daily fiber average intake of 25 grams per day is related to lower likelihood being in moderate to severe pain patterns over 8 years.
Significance and Innovation.
Dietary approaches for knee pain management are lacking.
This study is the first to show that higher dietary fiber intake was associated with lower risk of moderate and severe knee pain patterns.
Such protective associations persist regardless prevalent status of radiographic knee osteoarthritis.
Acknowledgments
Funding: This study was supported by NIH grants T32 AR 7598-18, AR47785 and AR051568.
Footnotes
Disclosure: All authors have no conflicts of interest to disclose.
References
- 1.Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004;42(1):1–9. v. doi: 10.1016/S0033-8389(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 2.Smith SG. Dangers of NSAIDs in the elderly. Can Fam Physician. 1989;35:653–4. [PMC free article] [PubMed] [Google Scholar]
- 3.Buffum M, Buffum JC. Nonsteroidal anti-inflammatory drugs in the elderly. Pain Manag Nurs. 2000;1(2):40–50. doi: 10.1053/jpmn.2000.7779. [DOI] [PubMed] [Google Scholar]
- 4.The American Association of Cereal Chemists The definition of dietary fiber. Cereal Food World. 2001:112–26. [Google Scholar]
- 5.Slavin JL. Nutrition. 3. Vol. 21. Calif; Burbank, Los Angeles County: 2005. Dietary fiber and body weight. pp. 411–8. [DOI] [PubMed] [Google Scholar]
- 6.Lyon MR, Kacinik V. Is There a Place for Dietary Fiber Supplements in Weight Management? Curr Obes Rep. 2012;1(2):59–67. doi: 10.1007/s13679-012-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jull AB, Ni Mhurchu C, Bennett DA, Dunshea-Mooij CA, Rodgers A. Chitosan for overweight or obesity. The Cochrane database of systematic reviews. 2008;(3):CD003892. doi: 10.1002/14651858.CD003892.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Ma Y, Olendzki BC, Wang J, Persuitte GM, Li W, Fang H, et al. Single-component versus multicomponent dietary goals for the metabolic syndrome: a randomized trial. Annals of internal medicine. 2015;162(4):248–57. doi: 10.7326/M14-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. The American journal of clinical nutrition. 2003;78(5):920–7. doi: 10.1093/ajcn/78.5.920. [DOI] [PubMed] [Google Scholar]
- 10.Koh-Banerjee P, Franz M, Sampson L, Liu S, Jacobs DR, Jr., Spiegelman D, et al. Changes in whole-grain, bran, and cereal fiber consumption in relation to 8-y weight gain among men. The American journal of clinical nutrition. 2004;80(5):1237–45. doi: 10.1093/ajcn/80.5.1237. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ, 3rd, et al. Association between dietary fiber and serum C-reactive protein. The American journal of clinical nutrition. 2006;83(4):760–6. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King DE, Egan BM, Woolson RF, Mainous AG, 3rd, Al-Solaiman Y, Jesri A. Effect of a high-fiber diet vs a fiber-supplemented diet on C-reactive protein level. Archives of internal medicine. 2007;167(5):502–6. doi: 10.1001/archinte.167.5.502. [DOI] [PubMed] [Google Scholar]
- 13.Schiffrin EJ, Thomas DR, Kumar VB, Brown C, Hager C, Van't Hof MA, et al. Systemic inflammatory markers in older persons: the effect of oral nutritional supplementation with prebiotics. J Nutr Health Aging. 2007;11(6):475–9. [PubMed] [Google Scholar]
- 14.Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48(Pt 3):233–7. doi: 10.1258/acb.2010.010093. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y, Je Y. Dietary fiber intake and total mortality: a meta-analysis of prospective cohort studies. American journal of epidemiology. 2014;180(6):565–73. doi: 10.1093/aje/kwu174. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Zhao LG, Wu QJ, Ma X, Xiang YB. Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. American journal of epidemiology. 2015;181(2):83–91. doi: 10.1093/aje/kwu257. [DOI] [PubMed] [Google Scholar]
- 17.Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ (Clinical research ed) 2013;347:f6879. doi: 10.1136/bmj.f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. The American journal of clinical nutrition. 2003;77(3):622–9. doi: 10.1093/ajcn/77.3.622. [DOI] [PubMed] [Google Scholar]
- 19.AlEssa HB, Ley SH, Rosner B, Malik VS, Willett WC, Campos H, et al. High Fiber and Low Starch Intakes Are Associated with Circulating Intermediate Biomarkers of Type 2 Diabetes among Women. The Journal of nutrition. 2016;146(2):306–17. doi: 10.3945/jn.115.219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Archives of internal medicine. 2007;167(9):956–65. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 21.The InterAct Consortium Dietary fibre and incidence of type 2 diabetes in eight European countries: the EPIC-InterAct Study and a meta-analysis of prospective studies. Diabetologia. 2015;58(7):1394–408. doi: 10.1007/s00125-015-3585-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangwisch JE, Hale L, Garcia L, Malaspina D, Opler MG, Payne ME, et al. High glycemic index diet as a risk factor for depression: analyses from the Women's Health Initiative. The American journal of clinical nutrition. 2015;102(2):454–63. doi: 10.3945/ajcn.114.103846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miki T, Eguchi M, Kurotani K, Kochi T, Kuwahara K, Ito R, et al. Nutrition. 5. Vol. 32. Calif; Burbank, Los Angeles County: 2016. Dietary fiber intake and depressive symptoms in Japanese employees: The Furukawa Nutrition and Health Study. pp. 584–9. [DOI] [PubMed] [Google Scholar]
- 24.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutrition research reviews. 2010;23(1):65–134. doi: 10.1017/S0954422410000041. [DOI] [PubMed] [Google Scholar]
- 25.de Koning L, Hu FB. Do the health benefits of dietary fiber extend beyond cardiovascular disease? Archives of internal medicine. 2011;171(12):1069–70. doi: 10.1001/archinternmed.2011.19. [DOI] [PubMed] [Google Scholar]
- 26.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nature reviews Rheumatology. 2012;8(12):729–37. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 27.Nevitt M, Felson DT, Lester G. The Osteoarthritis Initiative - Protocol for the cohort study. 2005:74. [Google Scholar]
- 28.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data based approach to diet questionnaire design and testing. American journal of epidemiology. 1986;124(3):453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 29.Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1(1):58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Jones B. [2016 July 14];Traj - Group-based modeling of longitudinal data. 2016 Available from: http://www.andrew.cmu.edu/user/bjones/index.htm.
- 31.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 32.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. The American journal of clinical nutrition. 1997;65(4 Suppl):1220S–8S. doi: 10.1093/ajcn/65.4.1220S. discussion 9S-31S. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Niu J. Editorial: Shifting Gears in Osteoarthritis Research Toward Symptomatic Osteoarthritis. Arthritis & rheumatology (Hoboken, NJ) 2016;68(8):1797–800. doi: 10.1002/art.39704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vargas-Prada S, Coggon D. Psychological and psychosocial determinants of musculoskeletal pain and associated disability. Best practice & research Clinical rheumatology. 2015;29(3):374–90. doi: 10.1016/j.berh.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malfait AM. Osteoarthritis year in review 2015: biology. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2016;24(1):21–6. doi: 10.1016/j.joca.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thijssen E, van Caam A, van der Kraan PM. Obesity and osteoarthritis, more than just wear and tear: pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology (Oxford, England) 2015;54(4):588–600. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 37.Jin X, Beguerie JR, Zhang W, Blizzard L, Otahal P, Jones G, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2015;74(4):703–10. doi: 10.1136/annrheumdis-2013-204494. [DOI] [PubMed] [Google Scholar]
- 38.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Annals of the rheumatic diseases. 2013;72(4):535–40. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 39.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–87. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 40.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2014;22(5):622–30. doi: 10.1016/j.joca.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. The Journal of rheumatology. 2002;29(1):131–8. [PubMed] [Google Scholar]
- 42.Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis and rheumatism. 1998;41(8):1343–55. doi: 10.1002/1529-0131(199808)41:8<1343::AID-ART3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 43.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Annals of the rheumatic diseases. 2007;66(4):433–9. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grube B, Chong PW, Lau KZ, Orzechowski HD. A natural fiber complex reduces body weight in the overweight and obese: a double-blind, randomized, placebo-controlled study. Obesity (Silver Spring) 2013;21(1):58–64. doi: 10.1002/oby.20244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kristensen M, Toubro S, Jensen MG, Ross AB, Riboldi G, Petronio M, et al. Whole grain compared with refined wheat decreases the percentage of body fat following a 12-week, energy-restricted dietary intervention in postmenopausal women. The Journal of nutrition. 2012;142(4):710–6. doi: 10.3945/jn.111.142315. [DOI] [PubMed] [Google Scholar]
- 46.Liu RH. Whole grain phytochemicals and health. J Cereal Sci. 2007;46(3):207–19. [Google Scholar]
- 47.Kant AK, Schatzkin A, Graubard BI, Schairer C. A prospective study of diet quality and mortality in women. Jama. 2000;283(16):2109–15. doi: 10.1001/jama.283.16.2109. [DOI] [PubMed] [Google Scholar]
- 48.King DE, Mainous AG, 3rd, Lambourne CA. Trends in dietary fiber intake in the United States, 1999-2008. Journal of the Academy of Nutrition and Dietetics. 2012;112(5):642–8. doi: 10.1016/j.jand.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Marron MM, Anderson SJ, Garrity J, Reynolds CF, 3rd, Lotrich FE. Association of Baseline Sleep Quality With Trajectories of Depressive Symptoms in Patients Undergoing Interferon Treatment. Psychosom Med. 2015;77(8):911–20. doi: 10.1097/PSY.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White DK, Niu J, Zhang Y. Is symptomatic knee osteoarthritis a risk factor for a trajectory of fast decline in gait speed? Results from a longitudinal cohort study. Arthritis care & research. 2013;65(2):187–94. doi: 10.1002/acr.21816. [DOI] [PMC free article] [PubMed] [Google Scholar]