Abstract
Background & Aims
We compared the effectiveness and safety of infliximab and adalimumab in biologic-naïve patients with ulcerative colitis (UC), in a nationwide register-based propensity score-matched study of patients in Denmark.
Methods
We collected data from 1719 adults with UC, 15–75 years old, in Denmark who were treated with either infliximab or adalimumab as their first biologic agent. We compared rates of all-cause hospitalization, UC-related hospitalization, major abdominal surgery, and serious infections after a variable 2:1 propensity score matching, accounting for baseline clinical characteristics, disease severity, healthcare use, and use of UC-related medications.
Results
Compared to patients treated with infliximab, patients treated with adalimumab had a higher risk for all-cause hospitalization (hazard ratio [HR], 1.84; 95% CI, 1.18–2.85), with a trend towards higher risk of UC-related hospitalization (HR, 1.71; 95% CI, 0.95–3.07)—particularly for patients on concomitant immune modulator therapy. However, risk of abdominal surgery (HR, 1.35; 95% CI, 0.62–2.94) did not differ between patients given adalimumab vs infliximab. Risk of serious infection requiring hospitalization was significantly higher among patients treated with adalimumab (HR, 5.11; 95% CI, 1.20–21.80).
Conclusion
In a nationwide propensity score matched-cohort study of biologic-naïve adults with UC in Denmark, use of adalimumab as first-line biologic vs infliximab was associated with higher risk of hospitalization and serious infections, though risk of surgery was not different. In the absence of trials to directly compare these drugs, these findings could assist patients, healthcare providers, purchasers, and policy makers in making decisions that might improve care for patients with UC.
Keywords: Comparative effectiveness, ropensity matching, biologics, inflammatory bowel disease
Introduction
Biologic therapy with tumor necrosis factor-alpha (TNF-α) inhibitors agents such as infliximab and adalimumab, alone or in combination with immunomodulators, is one of the most effective treatments in inducing and maintaining clinical remission in patients with ulcerative colitis (UC), and has been shown to decrease risk of hospitalization and surgery.1-4 In the absence of head-to-head trials, there is a unmet need among patients and physicians to better understand the relative effectiveness and safety of different TNF-α inhibitors. Current decisions on the choice of TNF-α inhibitor are primarily driven by patient and clinician preferences, and in some countries, on insurance coverage; however, there are differences in the molecular construct, dosing and route of administration of these agents, and hence, there may be differences in effectiveness and safety.5
Indirect treatment comparison network meta-analysis have suggested that in a subset of biologic-naïve patients with UC, infliximab may be superior to adalimumab for induction of clinical response and mucosal healing, but comparable for maintenance of remission.6,7 However, these short-term trials had restrictive inclusion criteria, and hence, had limited generalizability. In contrast, observational comparative effectiveness studies using health claims administrative databases and retrospective chart review have observed no significant differences in rates of hospitalization or clinical remission, between infliximab- and adalimumab-treated patients.8,9 These data are limited by potential misclassification of prior TNF-α inhibitor exposure, low event rates with inability to comment on differences in risk of surgery or serious infections, and inadequate adjustment for disease severity and other confounders.
Therefore, to overcome these limitations, we studied the comparative effectiveness and safety of infliximab and adalimumab in biologic-naïve adults with UC, using a population-based, propensity-score matched cohort study from the Danish nationwide registry. Using patient-important outcomes of all-cause and UC-related hospitalizations, abdominal surgery, need for corticosteroids, and risk of serious infections, in a biologic-naïve population, the results of this study might assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care in IBD, both at the individual and the population level.
Methods
Data Sources
The source population consisted of all individuals 15 years or older and living in Denmark between 2005 and 2014 according to the Danish Civil Registration System.10 Using the unique personal identification number given to each Danish citizen at birth, the population was linked to the National Patient Registry which contains information on all hospitalizations in Denmark since 1977 and all outpatient visits and emergency department contacts since 1995.11 In the National Patient Registry, we identified patients with IBD using International Classification of Diseases (ICD) codes (ICD-8 codes 56300-02 and 56308-09 and ICD-10 code K50 for Crohn's disease; ICD-8 codes 56319 and 56904 and ICD-10 code K51 for UC). Using a pathology database as reference, an assessment of nearly 800 patients estimated the completeness of registration of inflammatory bowel disease in the National Patient Registry to be 94%, whereas the estimated validity, expressed as the proportion of confirmed diagnoses in the registry, was 97% for Crohn's disease and 90% for UC.12
Information on prescription of infliximab and adalimumab was obtained using procedure codes (BOHJ18A1, BOHJ18A3) from the Danish Ministry of Health and Danish Drug Prescription Registry (ATC code L04AB04). Although the treatment with TNF-α inhibitors for inflammatory bowel disease were introduced in Denmark in 1999, we started the study in 2005 and excluded people who used TNF-α inhibitors before the start of the study; in this way, early drug users who may have been treated in the first years after the introduction of TNF-α inhibitors (who are likely to be different from the drugs' eventual stable user population, in terms of factors such as disease severity and therefore may introduce bias) were excluded. We also excluded patients with a concomitant diagnosis of rheumatoid arthritis, ankylosing spondylitis, psoriasis or psoriatic arthritis, as competing causes for prescribing TNF-α inhibitors. In case a patient received diagnostic codes for both UC and Crohn's disease, then the patient was classified as having UC if the most recent diagnosis was UC. Golimumab was not approved for commercial use in moderate to severe UC until May 2013; therefore, we did not have sufficient data on the use of this TNF-α inhibitor in our cohort. Figure 1 shows the flow of patients for identification of the cohort.
Figure 1.
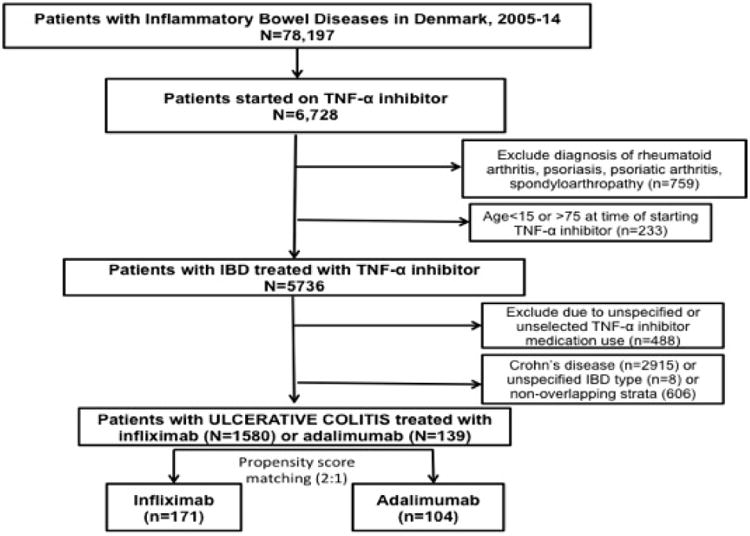
Flow of patients for identification of biologic-naïve patients with ulcerative colitis.
The study was approved by the Danish Data Protection Agency. Ethics approval is not required for registry-based research in Denmark.
Exposures and Outcomes of Interest
The primary exposures of interest were prescription of infliximab or adalimumab for UC. We considered patients as being continuously exposed from the index date (date of first registration of TNF-α inhibitor) for the duration of their prescription. Patients were followed until occurrence of the outcome of interest (see below), treatment discontinuation or switching (absence of new registration for a period of >4 months [for infliximab] or >3 months [for adalimumab], either discontinuing all TNF-α inhibitors or switching to another TNF-α inhibitor), death, or completion of the study (last date of follow-up, December 31, 2014).
The primary outcomes of interest were:
Effectiveness Outcomes
All-cause hospitalization
UC-related hospitalization, with UC either as the primary diagnosis, or as a secondary diagnosis if the primary diagnosis was related to a gastrointestinal symptom (abdominal pain, diarrhea, nausea, vomiting, constipation, gastrointestinal bleeding)
Colectomy (identified using established procedural codes)13
Corticosteroid prescription, occurring at least 60 days after the start of anti-TNF therapy (to minimize confounding by disease severity)
Safety Outcomes
If the index TNF-α inhibitor was started during an inpatient hospitalization, then that hospitalization was not counted as an outcome; only inpatient admissions occurring >23 hours after drug initiation were regarded as outcomes (to avoid misclassifying observation visits for infliximab infusions as outcome).
Covariates of Interest
Independent variables of interest included measures of healthcare utilization, surrogate markers of disease severity, and use of IBD-related medications, in the baseline period (prior to initiation of the index anti-TNF agent). We assessed baseline healthcare utilization based on the number of all-cause hospitalizations, emergency department visits, endoscopic procedures (upper endoscopy, colonoscopy, sigmoidoscopy, capsule endoscopy) identified via Current Procedural Terminology codes, and abdominal/pelvic radiologic procedures (magnetic resonance imaging, computed tomography, small bowel follow-through, barium enemas), in the preceding 12 months. While we did not have access to individual participant data medical records, endoscopy reports or biochemical parameters, we modified an existing score for ‘disabling’ Crohn's disease by Beaugerie et al.15 Patients were classified as ‘disabling’ UC if they had any two of following since UC diagnosis, prior to initiation of TNF-α inhibitor – need for >2 course of corticosteroids since UC diagnosis, need for abdominal surgery, UC-related hospitalization and/or use of immunosuppressive medications. We evaluated UC-related medication use in the baseline 3- and 12-months including use of 5-aminosalicylates (mesalamine, sulfasalazine, balsalazide, olsalazine), oral corticosteroids (prednisone, budesonide), immunomodulators (azathioprine, 6-mercaptopurine, methotrexate), other immunosuppressive medications (tacrolimus, cyclosporine, mycophenolate mofetil), and use of narcotic pain medications.
Patients were classified as being on concomitant therapy with immunomodulators (TNF-α inhibitor-based combination therapy) if they received immunomodulator prescriptions within 30 days before and/or after TNF-α inhibitor index start date.16 Based on corticosteroid use, patients were classified as: (a) remote users (if they received prescriptions for corticosteroids in the preceding 90 to 365 prior to index TNF-α inhibitor start date, but not within 90 days prior to start of TNF-α inhibitor), and (b) recent users (if they received prescriptions for corticosteroids in the preceding 90 days prior to index TNF-α inhibitor start date).
Statistical Analysis
To take into account potential confounders we used variable 2:1 propensity score matching using the nearest neighbor caliper matching with a caliper width of 0.2.17 We calculated the propensity scores as the predicted probability of starting treatment with TNF-α inhibitors conditional on variables thought to be either confounders or predictors for the outcome. These variables included demographic variables (age at initiation of TNF-α inhibitor, sex), disease-related characteristics (age at disease onset, disease duration, prior abdominal surgery), disease severity, measures of healthcare utilization (all-cause or UC-related outpatient visit, emergency department visit or hospitalization, endoscopy and/or radiology procedure within preceding 5 years), comorbidity burden (based on Charlson-Deyo index)18 and medication use (all and IBD-related) (eTable 2). We measured the standardized difference of each covariate in the propensity score model using logistic regression, and variables were considered to be different across treatment if after propensity score matching the standardized difference was greater than 10%.
We estimated hazard ratios (HR) with 95% confidence intervals (CI) for each outcome of interest separately using time since first TNF- α inhibitor treatment as underlying time-scale, and patients were censored at time of treatment discontinuation or switching (to another TNF-α inhibitor) or death or end of observation period. Analyses were carried out using Phreg procedure in SAS version 9.4 (Cary, NC). We considered differences to be statistically significant when the 95% confidence intervals did not overlap 1.0 or when the P value was <0.05 (all tests were two sided).
Sensitivity Analyses
We performed stratified analysis based on use of TNF-α inhibitor monotherapy or combination therapy (concomitant use of immunomodulators), and by sex. Due to potential for incomplete adjustment for confounders, we performed a sensitivity analysis using an array approach suggested by Schneeweiss et al.19 In this approach, if an incompletely measured (for example, disease severity based on clinical disease activity indices or endoscopic measures, not captured in our cohort) or unmeasured confounder (for example, smoking) may preferentially result in prescription of one agent over another, and may influence risk of outcomes, then we can infer the probable relationship between the two agents in different scenarios of confounder distribution. Additional post-hoc sensitivity analyses suggested during the peer review stage were performed, based on (a) restricting to patients who were prescribed TNF-α inhibitor in or after 2012 (since adalimumab was approved for UC only in 2012), and (b) restricting to patients classified as having ‘disabling’ disease based on surrogate measures captured in the modified Beaugerie index.
Results
Characteristics of Patients
We identified 1719 biologic-naïve patients with UC who started their first anti-TNF agent between 2005 and 2014, of whom 139 were treated with adalimumab and 1580 with infliximab. After propensity score matching the cohort was restricted to 104 patients treated with adalimumab and 171 patients with infliximab (c-statistic, 0.77), and their baseline demographic, clinical, treatment characteristics and healthcare utilization were comparable (Table 1, eTable 3). Median disease duration prior to starting TNF-α inhibitor was 3.3y for adalimumab- and 3.9y for infliximab-treated patients. About 24% patients were classified as having severe disease; 40% patients received recent steroids, and 48% patients were on concomitant immunomodulators at time of TNF-α inhibitor initiation.
Table 1. Baseline demographic characteristics, healthcare utilization, disease severity and UC-related medication use in patients with ulcerative colitis, in the propensity score matched cohort.
| Patient Characteristics | Infliximab (n=171) | Adalimumab (n=104) | p-value |
|---|---|---|---|
|
| |||
| Demographic variables | |||
|
| |||
| Mean age at initiation of TNF-α inhibitor ± SD, years | 37.8 ± 12.1 | 38.7 ± 12.8 | 0.54 |
|
| |||
| Sex (% males) | 46.2 | 46.2 | 1.00 |
|
| |||
| Disease duration prior to starting TNF-α inhibitor, median (IQR) in years | 3.9 (1.0-10.0) | 3.3 (1.1-10.5) | 0.65 |
|
| |||
| Follow-up after starting TNF-α inhibitor, median (IQR), in years | 2.3 (0.8-4.3) | 1.3 (0.3-3.2) | 0.23 |
|
| |||
| Healthcare utilization (in 12 months prior to starting TNF-α inhibitors) | |||
|
| |||
| Emergency room visits (% pts with ≥1) | 15.8 | 8.7 | 0.13 |
|
| |||
| Inpatient visits (% pts with ≥1) | 52.0 | 47.1 | 0.50 |
|
| |||
| Imaging (% pts with ≥1) | 18.7 | 19.2 | 1.00 |
|
| |||
| Endoscopic procedures (% pts with ≥1) | 86.0 | 80.8 | 0.33 |
|
| |||
| Disease Severity Assessment | |||
|
| |||
| Disabling disease (any 2 of the following 4: Need for >2 course of steroids since UC diagnosis, need for UC-related surgery, UC-related hospitalization, use of immunosuppressive medications) | 22.2 | 26.9 | 0.46 |
|
| |||
| Any prior abdominal surgery (% pts with ≥1) | 5.9 | 12.5 | 0.09 |
|
| |||
| UC-related medication use (in 12 months prior to starting TNF-α inhibitors) | |||
|
| |||
| Mesalamine, oral, % | 72.5 | 74.0 | 0.89 |
|
| |||
| Steroids | |||
| • Any prior use in preceding 12m, % | 66.1 | 60.6 | 0.43 |
| • Recent (in 90 days prior to anti-TNF), % | 40.9 | 38.5 | 0.78 |
|
| |||
| Immunomodulators | |||
| • Any prior use in preceding 12m, % | 41.5 | 46.2 | 0.53 |
| • Concurrent (index date − 30, +30 days), % | 28.7/19.9 | 26.9/19.2 | 0.86/1.00 |
|
| |||
| Narcotics, % | 11.7 | 9.6 | 0.74 |
[Abbreviations: IQR-interquartile range, n-number of patients, pts-patients, SD-standard deviation, TNF-tumor necrosis factor]
Comparative Effectiveness of Adalimumab vs. Infliximab
As compared to infliximab-treated patients, adalimumab-treated patients had higher risk of all-cause hospitalization (HR, 1.84; 95% CI, 1.18-2.85), and trend towards increased risk of UC-related hospitalization (HR, 1.71; 95% CI, 0.95-3.07) (Table 2). Twenty-eight patients underwent coloectomy after starting TNF-α inhibitor, and there was no significant difference in the risk of surgery (adalimumab vs. infliximab: HR, 1.35; 95% CI, 0.62-2.94). Similarly, there was no difference in the risk of new corticosteroid prescription between adalimumab-and infliximab-treated patients.
Table 2. Comparative effectiveness and safety of adalimumab vs. infliximab in biologic-naïve patients with ulcerative colitis, using propensity score-matched analysis.
| Adalimumab (N=104) | Infliximab (N=131-171)+ | Adalimumab vs. infliximab | ||||
|---|---|---|---|---|---|---|
| Number of events | Incidence rate, per 100-py (95% CI) | Number of events | Incidence rate, per 100-py (95% CI) | HR (95% CI) | p-value | |
| All-cause hospitalization | 36 | 91.5 (66.0-126.9) | 49 | 37.4 (28.3-49.5) | 1.84 (1.18-2.85) | 0.007 |
| UC-related hospitalization | 20 | 46.7 (30.1-72.3) | 28 | 19.3 (13.4-28.0) | 1.71 (0.95-3.07) | 0.07 |
| Major abdominal surgery | 10 | 20.7 (11.1-38.4) | 18 | 11.4 (7.2-18.1) | 1.35 (0.62-2.94) | 0.45 |
| New steroid use | 5 | 18.6 (7.7-44.6) | 20 | 22.6 (14.6-35.0) | 0.66 (0.24-1.79) | 0.41 |
| Serious infection | 5 | 10.3 (4.3-24.7) | 3 | 1.9 (0.6-5.8) | 5.11 (1.20-21.80) | 0.03 |
Depending on outcome, the number of matched infliximab-treated patients with UC were 131-171
[Abbreviations: CI-confidence interval, HR-hazard ratio, py-person-years, UC-ulcerative colitis]
On stratified analysis by TNF-α inhibitor monotherapy and concomitant immunomodulator therapy, significant differences in the risks of all-cause and UC-related hospitalization were observed between adalimumab- and infliximab-treated patients in a subset of patients on concomitant immunomodulator therapy, but not in patients on TNF-α inhibitor monotherapy (Table 3). On separate stratified analysis by sex, males were more likely to have higher risks of all-cause and UC-related hospitalization with adalimumab as compared to infliximab, but no significant differences were observed in females (eTable 4).
Table 3.
Comparative effectiveness and safety of adalimumab vs. infliximab in biologic-naïve patients with ulcerative colitis, stratified by (A) use of TNF-α inhibitor monotherapy and (B) concomitant immunomodulator therapy.
| Outcomes of interest | (A) TNF-α inhibitor monotherapy (n=144) | (B) TNF-α inhibitors + immunomodulator therapy (n=131) | ||
|---|---|---|---|---|
| HR (95% CI) (ADA vs. IFX) | p-value | HR (95% CI) (ADA vs. IFX) | p-value | |
| All-cause hospitalization | 1.42 (0.86-2.34) | 0.17 | 4.03 (1.56-10.40) | 0.004 |
| UC-related hospitalization | 1.22 (0.60-2.50) | 0.58 | 3.89 (1.32-11.50) | 0.01 |
| Major abdominal surgery | 0.97 (0.37-2.54) | 0.95 | 2.85 (0.70-11.60) | 0.14 |
| New steroid use | 0.64 (0.20-1.99) | 0.44 | 0.68 (0.08-5.65) | 0.72 |
| Serious Infections | 3.63 (0.80-16.50) | 0.09 | - | - |
[Abbreviations: ADA-adalimumab, CI-confidence interval, IFX-infliximab, TNF-tumor necrosis factor]
On sensitivity analyses restricted to patients prescribed adalimumab or infliximab in or after 2012, and on restricting to patients classified as having ‘disabling’ disease based on the modified Beaugerie index, similar results were performed – adalimumab-treated patients had higher risk of all-cause and UC-related hospitalization, as compared to infliximab-treated patients (Table 4). On sensitivity analyses using an array approach, assuming that patients with clinically severe disease (based on disease activity indices) were more likely to be prescribed infliximab as compared to adalimumab (considering it's use as rescue therapy in acute severe colitis),20, the observed point estimate for UC-related hospitalization (HR, 1.71) and surgery (HR, 1.35), would have been biased by 24% and 21%, respectively, and the confounder-adjusted estimate would be 2.1 and 1.7 for adalimumab vs. infliximab, respectively (eTable 5).
Table 4.
Sensitivity analyses. Comparative effectiveness and safety of adalimumab vs. infliximab in biologic-naïve patients with ulcerative colitis, based on (A) restriction to patients started on anti-TNF agents in or after 2012 (adalimumab was approved for UC in 2012), and (B) restriction to patients classified as having ‘disabling’ disease based on surrogate measures captured in modified Beaugeric index.
| (A). Adalimumab (n=36) vs. infliximab (n=43) | (B). Adalimumab (n=28) vs. infliximab (n=46) | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| All-cause hospitalization | 4.20 (1.56-11.30) | 0.005 | 4.20 (1.56-11.30) | 0.005 |
| UC-related hospitalization | 10.60 (1.25-90.1) | 0.03 | 10.60 (1.25-90.1) | 0.03 |
| Major abdominal surgery | 5.66 (0.57-55.8) | 0.14 | 5.66 (0.57-55.8) | 0.14 |
| New steroid use | 0.54 (0.05-5.30) | 0.59 | 0.54 (0.05-5.30) | 0.59 |
| Serious infection | 1.90 (0.12-30.40) | 0.65 | 1.90 (0.12-30.40) | 0.65 |
Comparative Safety of Adalimumab vs. Infliximab
In propensity score matched analysis, as compared to infliximab-treated patients, adalimumab-treated patients had a higher risk of serious infections (HR, 5.11; 95% CI, 1.20-21.80), though the number of events was small (n=8) (Table 2). No opportunistic infections were observed. On stratified analysis, all serious infections were observed in patients on TNF-α inhibitor monotherapy (Table 3). On specifically evaluating these 8 patients, 4 patients were on concomitant steroids at start of anti-TNF (3/5 adalimumab-treated patients, 1/3 infliximab-treated patients), and 4 patients were classified as having ‘disabling’ UC (2/5 adalimumab-treated patients, 2/3 infliximab-treated patients). Seven patients were on corticosteroids at time of serious infection (4/5 adalimumab-treated patients, 3/3 infliximab-treated patients).
Discussion
While TNF-α inhibitor-based therapy is one of the most effective treatments for UC, there are limited data on the comparative effectiveness and safety of different agents. In this Danish nationwide cohort study of 1719 biologic-naïve patients with UC (104 patients treated with adalimumab and 171 patients with infliximab, in propensity score matched cohort), we observed that adalimumab was associated with a higher risk of all-cause hospitalization and showed a trend towards higher risk of UC-related hospitalization, as compared to infliximab; the risks of surgery and new corticosteroid initiation were comparable. This difference in effectiveness was most pronounced in patients on concomitant immunomodulator monotherapy, and in males. These findings were persistent, with higher summary estimates, on sensitivity analyses restricted to patients treated with TNF-α inhibitors in or after 2012 (when adalimumab was approved for UC), and to patients classified as having ‘disabling’ disease based on modified Beaugerie index. We also observed that adalimumab use was associated with a higher risk of serious infections as compared to infliximab, but these results were based on small number of outcome events. Based on findings from this population-based, propensity score-matched contemporary cohort of UC patients, infliximab appears to be more effective and safer as compared to adalimumab for key patient-related effectiveness and safety outcomes for treatment of UC.
Our results are consistent with findings from indirect treatment comparison network meta-analyses, which have suggested that in a subset of biologic-naïve patients with UC, infliximab may be superior to adalimumab for induction of clinical response and mucosal healing, but comparable for maintenance of remission.6,7 However, network meta-analysis is limited by lack of head-to-head trials, limited long-term follow-up, restrictive inclusion criteria in registration trials, and lack of data on patient-important outcomes like hospitalization, surgery and serious infections.
There have been a limited number of observational studies on the comparative effectiveness of different TNF-α inhibitors in UC. In a United States administrative claims-based retrospective analysis of 1400 UC patients, we had previously observed no significant differences in the risk of hospitalization and serious infections between infliximab and adalimumab-treated patients; however, infliximab-treated patients had lower risk of corticosteroid use and were more likely to remain on index TNF-α inhibitor at 6- and 12-months.8 However, the study was limited by small number of surgical events, potential misclassification of biologic-naïve patients, and inability to account for unmeasured confounders particularly disease severity. In another retrospective multi-site cohort study in which 170 physicians reported on 6-month outcomes of biologic-naïve infliximab-(n=380) or adalimumab-treated (n=424) UC patients from their practices, there were no significant differences in clinical response (based on physician global assessment), healthcare utilization and partial Mayo score.9
This observed superiority of infliximab over adalimumab has also been observed in patients with Crohn's disease. In an administrative claims-based study on 3205 patients with Crohn's disease, we observed that infliximab-treated patients had a lower risk of Crohn's disease-related hospitalization (HR, 0.80; 95% CI, 0.66-0.98), abdominal surgery (HR, 0.76; 95% CI, 0.58-0.99) and corticosteroid use (HR, 0.85; 95% CI, 0.75-0.96).16 In the U.S. Medicare retrospective cohort study of patients with Crohn's disease, among patients younger than 65 years, Osterman and colleagues also observed that infliximab use was associated with a lower risk of surgery compared to adalimumab (adjusted odds ratio [OR], 0.66; 95% CI, 0.47–0.93).21
Despite both TNF-α inhibitors having a similar mechanism of action, there are subtle differences in pharmacokinetics, which may explain differences in effectiveness. Infliximab administered intravenously, is dosed based on body weight, whereas adalimumab is a fixed dose drug, administered subcutaneously, resulting in different bioavailability. Obesity has been shown to modify drug clearance and treatment response,22-24 and with 20-40% of patients with inflammatory bowel diseases now being obese, differential effectiveness of these agents in obese patients may have contributed to observed differences.25-27 In a retrospective cohort study, Bhalme et al observed higher need for dose escalation due to therapeutic failure in obese patients with Crohn's disease, treated with adalimumab, but not infliximab.28 The cause for observed differences in safety of infliximab and adalimumab is unclear. There were no differences between infliximab- and adalimumab-treated patients in known factors that may contribute to increased risk of infections, such as age, baseline disease severity, concomitant use of immunomodulators, corticosteroids and narcotics, etc.29 It is possible that by offering superior disease control and lower rates of hospitalization, infliximab-treated patients may have lower susceptibility to infections, particularly hospital-acquired infections.
Our findings, favoring infliximab over adalimumab, were significant in a stratum of patients on concomitant immunomodulator therapy. Generally, patients with more severe disease, or those who have failed immunomodulator monotherapy (and hence, inherently more treatment resistant) are treated with the combination of TNF-α inhibitors and immunomodulators. So, patients on concomitant immunomodulator therapy may represent more severe cases, where infliximab may be more effective than adalimumab; in patients with moderate disease, who may be treated with TNF-α inhibitor monotherapy, both medications were observed to have comparable effectiveness. Similarly, we observed that infliximab was superior to adalimumab in a stratum of male patients, but not in females. It is unclear why this was observed. Pharmacokinetic studies suggest more rapid drug clearance in males as compared to females, and this may preferentially impact clearance of fixed dose agent like adalimumab, as compared to weight-based medications like infliximab. The finding of all eight serious infections being observed in patients on TNF-α inhibitor monotherapy, with no event observed in the TNF-α inhibitor combination therapy group, was counter-intuitive. However, we observed that seven (out of 8) patients were on corticosteroids at time of serious infection, which may have increased risk of infections even in the absence of concomitant use of immunomodulators.
These findings must be interpreted with caution, given the limitations associated with our study design. First, this was an observational, not an interventional study, and hence, there may be unmeasured confounders across groups. The potential for unmeasured confounding by severity is of particular importance, since we were not able to objectively assess endoscopic or biochemical disease activity. However, the effect of this in our analysis is probably minimal. The overall prevalence of measures of ‘disabling’ UC was comparable between adalimumab- and infliximab-treated patients. Also, since patients with more severe disease, including those with acute severe colitis, may preferentially be recommended infliximab over adalimumab, the results are likely to be biased results against infliximab, unlike what was observed in our analysis. We tried to ascertain the magnitude of this impact through an array sensitivity analysis, which confirmed our findings. Second, we were unable to account for the impact of therapeutic drug monitoring or dose escalation, practices which have become more common recently, given inaccurate estimation using administrative databases. Third, both baseline covariates and outcomes were measured using administrative claims codes and may be subject to errors. Definitions for some covariates and outcomes, such as combination immunosuppressive therapy, treatment discontinuation, etc. were chosen using best investigator judgment, and have not been validated.
In conclusion, using a population-based, propensity-matched cohort study, we observed that infliximab may be more effective and safer than adalimumab in biologic-naïve patients with UC. With the advent of biosimilars, with similar effectiveness of their originator compound, this information may enable informed decision-making in choosing TNF-α inhibitors in biologic-naïve patients with UC.
Supplementary Material
eTable 1: All diagnoses of infections with related ICD 10-codes included as any serious infection with the subdivision into six site-specific groups.
eTable 2: Variables included in the propensity score and variables for UC-related medication with diagnostic, operation and ATC codes, categories, source of data and missing values.
eTable 3. Standardized differences between infliximab and adalimumab exposed patients with UC before and after matching on fixed factors and propensity scores.
eTable 4. Comparative effectiveness and safety of adalimumab vs. infliximab in biologic-naïve patients with ulcerative colitis in (A) males and (B) females
eTable 5. Sensitivity analysis for incompletely measured confounders (disease severity) or unmeasured confounders (smoking).
Acknowledgments
Disclosures: This study was made possible by support from (a) the American College of Gastroenterology Clinical Research Award 2014 to SS. Dr. Singh is supported by the NIH/NLM training grant T15LM011271, American College of Gastroenterology Junior Faculty Development Award and Crohn's and Colitis Foundation of America Career Development Award.
Dr. Singh has received a research grant from Pfizer. Dr. Andersen has received funding for travel and speakers fees from MSD and from Ferring. Dr. Loftus has consulted for and has received research support from Janssen Biotech, AbbVie, and UCB Pharma. Dr. Jess has received funding for travel and a speakers fee from AbbVie.
Role of Sponsor: The sponsor was NOT involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript
Footnotes
Conflicts of Interest: Mr. Andersson does not have any conflicts of interest.
- Study concept and design: SS, TJ
- Acquisition of data: NNA, MA
- Analysis and interpretation of data: SS, NNA, MA, TJ
- Drafting of the manuscript: SS
- Critical revision of the manuscript for important intellectual content: NNA, MA, EVL, TJ
- Approval of the final manuscript: SS, NNA, MA, EVL, TJ
- Study supervision: TJ
Guarantor of Article: Dr. Siddharth Singh had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369(8):754–62. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
- 2.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of G. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. The Am J Gastroenterol. 2010;105(3):501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Rutgeerts P, Feagan BG, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137(4):1250–60. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Feagan BG, Sandborn WJ, Lazar A, et al. Adalimumab therapy is associated with reduced risk of hospitalization in patients with ulcerative colitis. Gastroenterology. 2014;146(1):110–18. doi: 10.1053/j.gastro.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Nestorov I. Clinical pharmacokinetics of TNF antagonists: how do they differ? Semin Arthritis Rheum. 2005;34(5 Suppl1):12–8. doi: 10.1016/j.semarthrit.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Danese S, Fiorino G, Peyrin-Biroulet L, et al. Biological agents for moderately to severely active ulcerative colitis: a systematic review and network meta-analysis. Ann Intern Med. 2014;160(10):704–11. doi: 10.7326/M13-2403. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Garg SK, Wang Z, et al. Letter: comparative efficacy of biological therapy in patients with ulcerative colitis. Aliment Pharmacol Ther. 2014;39(12):1432–3. doi: 10.1111/apt.12760. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Heien HC, Sangaralingham LR, et al. Comparative effectiveness and safety of infliximab and adalimumab in patients with ulcerative colitis. Aliment Pharmacol Ther. 2016;43(9):994–1003. doi: 10.1111/apt.13580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandborn WJ, Sakuraba A, Wang A, et al. Comparison of real-world outcomes of adalimumab and infliximab for patients with ulcerative colitis in the United States. Current medical research and opinion. 2016;32(7):1233–41. doi: 10.1185/03007995.2016.1168290. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen CB, Gotzsche H, Moller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–9. [PubMed] [Google Scholar]
- 11.Andersen TF, Madsen M, Jorgensen J, et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–8. [PubMed] [Google Scholar]
- 12.Fonager K, Sorensen HT, Rasmussen SN, et al. Assessment of the diagnoses of Crohn's disease and ulcerative colitis in a Danish hospital information system. Scand J Gastroenterol. 1996;31(2):154–9. doi: 10.3109/00365529609031980. [DOI] [PubMed] [Google Scholar]
- 13.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979-2011. Gut. 2014;63(10):1607–16. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 14.Nyboe Andersen N, Pasternak B, Friis-Moller N, et al. Association between tumour necrosis factor-alpha inhibitors and risk of serious infections in people with inflammatory bowel disease: nationwide Danish cohort study. BMJ. 2015;350:h2809. doi: 10.1136/bmj.h2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn's disease. Gastroenterology. 2006;130(3):650–6. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Heien HC, Sangaralingham LR, et al. Comparative Effectiveness and Safety of Anti-Tumor Necrosis Factor Agents in Biologic-Naive Patients With Crohn's Disease. Clin Gastroenterol Hepatol. 2016;14(8):994–1003. doi: 10.1016/j.cgh.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rassen JA, Shelat AA, Myers J, et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(2):69–80. doi: 10.1002/pds.3263. [DOI] [PubMed] [Google Scholar]
- 18.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. doi: 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 20.Laharie D, Bourreille A, Branche J, et al. Ciclosporin versus infliximab in patients with severe ulcerative colitis refractory to intravenous steroids: a parallel, open-label randomised controlled trial. Lancet. 2012;380(9857):1909–15. doi: 10.1016/S0140-6736(12)61084-8. [DOI] [PubMed] [Google Scholar]
- 21.Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn's disease. Clin Gastroenterol Hepatol. 2014;12(5):811–17. doi: 10.1016/j.cgh.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20(12):2247–59. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 23.Sharma S, Eckert D, Hyams JS, et al. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn's disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis. 2015;21(4):783–92. doi: 10.1097/MIB.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 24.Bultman E, de Haar C, van Liere-Baron A, et al. Predictors of dose escalation of adalimumab in a prospective cohort of Crohn's disease patients. Aliment Pharmacol Ther. 2012;35(3):335–41. doi: 10.1111/j.1365-2036.2011.04946.x. [DOI] [PubMed] [Google Scholar]
- 25.Seminerio JL, Koutroubakis IE, Ramos-Rivers C, et al. Impact of Obesity on the Management and Clinical Course of Patients with Inflammatory Bowel Disease. Inflammatory bowel diseases. 2015;21(12):2857–63. doi: 10.1097/MIB.0000000000000560. [DOI] [PubMed] [Google Scholar]
- 26.Flores A, Burstein E, Cipher DJ, et al. Obesity in Inflammatory Bowel Disease: A Marker of Less Severe Disease. Dig Dis Sci. 2015;60(8):2436–45. doi: 10.1007/s10620-015-3629-5. [DOI] [PubMed] [Google Scholar]
- 27.Stabroth-Akil D, Leifeld L, Pfutzer R, et al. The effect of body weight on the severity and clinical course of ulcerative colitis. Int J Colorectal Dis. 2015;30(2):237–42. doi: 10.1007/s00384-014-2051-3. [DOI] [PubMed] [Google Scholar]
- 28.Bhalme M, Sharma A, Keld R, et al. Does weight-adjusted anti-tumour necrosis factor treatment favour obese patients with Crohn's disease? Eur J Gastroenterol Hepatol. 2013;25(5):543–9. doi: 10.1097/MEG.0b013e32835d1f15. [DOI] [PubMed] [Google Scholar]
- 29.Andersen NN, Jess T. Risk of infections associated with biological treatment in inflammatory bowel disease. World J Gastroenterol. 2014;20(43):16014–9. doi: 10.3748/wjg.v20.i43.16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1: All diagnoses of infections with related ICD 10-codes included as any serious infection with the subdivision into six site-specific groups.
eTable 2: Variables included in the propensity score and variables for UC-related medication with diagnostic, operation and ATC codes, categories, source of data and missing values.
eTable 3. Standardized differences between infliximab and adalimumab exposed patients with UC before and after matching on fixed factors and propensity scores.
eTable 4. Comparative effectiveness and safety of adalimumab vs. infliximab in biologic-naïve patients with ulcerative colitis in (A) males and (B) females
eTable 5. Sensitivity analysis for incompletely measured confounders (disease severity) or unmeasured confounders (smoking).


