Summary
Inhibitory circuits are essential for brain function. Our understanding of their synaptic organization has advanced extensively with the identification and classification of an impressive variety of neuron groups, receptor types, and patterns of connectivity. However, the conceptual discussion regarding the role of in neural circuits still revolves around the idea that its primary role is to regulate circuit excitability.
Here, I will focus on recent findings from cortical circuits and argue that inhibitory circuits are central to the integration of incoming inputs and can promote sophisticated fine-scale control of local circuits. I propose that inhibitory circuits should not be viewed so much as brakes on principal neurons activity, but as primary contributors to a variety of neural network functions.
Graphical Abstract
Inhibitory neurons: a central node of integration of long range input to neocortex
Inhibitory neurons are central to cortical processing as they are involved in shaping many aspects of cortical function such as gating incoming signals [1], gain modulation [2], spike timing and fidelity [3], network synchronization [4], feature discrimination [5], learning [6*], multisensory integration [7] just to mention a few. Not all types of inhibitory neurons equally contribute to these processes, as patterns of connectivity specify which inhibitory circuit becomes engaged. Thus, to understand how inhibitory neurons influence circuit computations it is crucial to learn about the inputs driving them. Many reviews focused on the identification of distinct groups of inhibitory neurons and their role in cortical function. The scope of this Opinion is not to provide an additional discussion on diversity, or of functional response properties of inhibitory neurons. Instead, I will report current knowledge about two prominent long range extracortical inputs, thalamic and limbic, driving neocortical inhibitory neurons. I will then discuss how engagement of inhibitory neurons by long range inputs and specificity of local connectivity endows inhibitory circuits with ability to promote fine-scale circuit refinement.
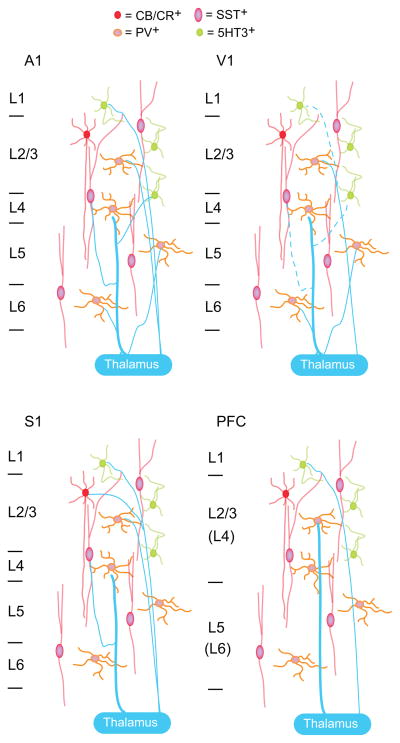
One of the best studied long range projection onto neocortical inhibitory neurons is the thalamocortical (TC) input. Many studies focused on TC projections from primary sensory thalamic nuclei that carry information from the sensory organs to the cortex; although a few recent ones investigated inputs from high order thalamic nuclei, that receive information from neocortex and project to other cortical regions. Thalamocortical afferents directly target fast spiking (FS) inhibitory neurons, often expressing the calcium binding protein parvabumin (PV), in the principal input layers of primary sensory [8,9], prefrontal (PFC) [10] and anterior cingulate cortices [11]. Another well studied population of inhibitory neurons, the one expressing somatostatin (SST), is not always directly engaged by thalamocortical afferents suggesting regional specificity for the engagement of inhibitory circuits. In the primary visual cortex (V1) there is only marginal evidence of TC inputs onto non-FS inhibitory neurons [12], and their monosynaptic nature is not clear [8]. In the somatosensory cortex (S1) [13] and in the auditory cortex (A1) [12] TC inputs drive SST neurons. In addition, TC afferents in S1 directly activate a group of calbindin expressing inhibitory neurons [14] and a subset of 5-HT3 expressing inhibitory neurons [15]. While the most prominent TC projections from sensory thalamic nuclei are found in the mid and deep layers of neocortex [16], projections from high order thalamic nuclei preferentially contact neurons in superficial and infragranular layers [17]. Inhibitory neurons in primary sensory and high order cortical regions receive information both from primary sensory and high order thalamic nuclei. These results strongly suggest that inhibitory circuits contribute to sensory processing and may also encode the affective dimensions of a sensory stimulus [18**].
Thalamocortical synaptic responses onto non-FS neurons show marked differences from those onto FS neurons in laminar specificity, amplitude, short term dynamics and latency from stimulus onset [12,13]. Furthermore, the short term dynamics of TC responses depend on the postsynaptic target [13]. Another marked difference regarding TC inputs relies on the composition of postsynaptic receptors. Depending on the target region, inputs onto FS neurons activate both AMPA and NMDA receptors [10], or drive primarily AMPA receptors [8]. The composition of postsynaptic receptors directly affects kinetics and short term dynamics of synaptic responses [8]. Thalamocortical inputs onto inhibitory neurons are plastic, and inputs onto distinct groups of inhibitory neurons can be differentially affected by changes in sensory experience [19]. All of these factors influence how inhibitory circuits are engaged by incoming activity and may provide specificity on how the distinct groups of neurons exert their function. Recent studies showed that in addition to TC inputs, inhibitory neurons are directly activated by amygdalocortical (Am) projections [20*,21]. Amygdalocortical inputs have been implicated in fear learning [22], coding of anticipatory activity [23] and enriching sensory stimuli with information regarding their hedonic value [24]. Direct Am projection onto inhibitory neurons suggests that they may be implicated in one or more of these processes. Recordings from awake behaving rats showed that neurons with fast spike-like waveforms show anticipatory responses when a rewarding stimulus (sucrose) is paired with a predictive cue [23], suggesting a role for inhibition in encoding anticipatory information.
Our understanding of the postsynaptic targets of Am circuits is rather limited. In the primary gustatory cortex (GC), a cortical region receiving a substantial direct projection from the amygdala, activation of amygdalar afferents in vivo evokes time-varying responses that contain an early inhibitory component, an excitatory component and a late inhibitory component [25], suggesting that amygdala-dependent recruitment of cortical inhibition can gate and sculpt the activity of GC excitatory neurons. A study in the PFC [21] and one in GC [20*] demonstrated that, in addition to pyramidal neurons, Am axons make direct synaptic connections with PV expressing, FS neurons. Furthermore, in GC there is a direct Am projection onto SST expressing inhibitory neurons [20*]. These projections have laminar-specific connectivity, indicating that, like TC inputs, Am projections can activate cortical circuits in a layer-specific fashion [20*]. While TC inputs onto FS neurons typically show larger amplitudes and different short term dynamics than onto excitatory neurons [8]; Am inputs have comparable properties onto all types of postsynaptic neurons [20*]. Much work needs to be done to establish whether Am axons project to other regions of neocortex and whether they recruit PV and SST expressing neurons in other areas. However, some studies suggest that Am connectivity may not be unique to PFC and GC. Tracing studies showed the presence of Am axons in primates and cats V1 [26,27]. There is also evidence that stimulation of the amygdala can evoke neural responses in V1 [28]. Neurons in V1 are involved in reward learning [29], a process that can involve the amygdala. Whether Am projections onto inhibitory neurons are involved in reward learning in V1 has not been investigated.
Cortical inhibition and local circuit activation
Afferent inputs can activate inhibitory neurons above threshold for action potentials leading to the release of GABA, the primary inhibitory neurotransmitter in the central nervous system. Many factors can determine how activation of GABAergic inhibition will affect local cortical circuits. Type, laminar location and pattern of connectivity of specific populations of inhibitory neuron determine the subcellular location targeted by the GABAergic axon [30]. Firing patterns of inhibitory neurons in response to incoming stimuli and the organization of presynaptic release sites of inhibitory synapses determine how much GABA will be released. Many other factors, including uptake and degradation mechanisms, and expression of membrane transporters will control the duration of the signal.
Once released, the effect of GABA on postsynaptic neurons depends on the properties of the receptor that binds it. GABA can modulate neuronal excitability through a variety of mechanisms depending on the nature whether receptors are ionotropic (GABAA), mediating the opening of an anion channel, or metabotropic (GABAB), activating a G-protein-dependent signaling cascade [31]. This distinction determines timing and duration of GABA signaling. Lastly, the location of GABA receptors - presynaptic, postsynaptic or extrasynaptic [32] - will influence the role of inhibition. A mechanistic review of all of these factors is beyond the scope of this Opinion. In this section, I will focus on recent evidence showing how inhibition may shape local circuit activation and plasticity.
The effect of inhibition on cortical circuits is often viewed as a shift neurons or circuit excitability, by modulation of action potentials generation. Indeed, GABAergic inhibition affects the capacity of a postsynaptic neuron to fire by hyperpolarizing the membrane potential [33], or by shunting its depolarization [34]. These factors likely contribute to shaping the tuning of neuronal response curves, to modulating input/output functions and can limit the propagation of signals in the circuit [35]. The targeting of GABAergic inputs to specific subcellular locations is thought to refine the modulation of such functions, adding complexity to the computational capacity of the circuit [35].
Inhibitory neurons are interconnected through synaptic contacts and gap junctions [36], and can exert control over large portions of cortical circuits thanks to their widespread connectivity onto excitatory neurons [37,38]. These features certainly make inhibitory circuits effective regulators of neurons and circuit excitability and network synchronization. Consistent with this, theoretical models of neural networks that include inhibitory circuits have primarily focused on the role of overall levels of inhibition on the activation of cortical circuits and on tuning of excitatory neurons’ response properties [39]. Whether these processes are mediated by overall modulations of inhibition or require activation of specific inhibitory circuits remains unclear.
The same properties that provide an advantage for the control of excitability and rhythms, make it difficult to think of GABAergic inhibition as a fine-tuner of local connectivity. However, there is strong evidence that GABAergic inhibition is involved in experience-dependent refinement of cortical activity [40,41], a process that is thought to require selectivity. Inhibitory synapses from distinct populations of inhibitory neurons are affected differently by experience [42], indicating that they may engage mechanisms that are specific for activity-dependent demands of the refinement process. While macroscopically, the changes in neuronal responsiveness induced by manipulations of experience may be evident as changes in firing rates [43], subtler modifications may be occurring subthreshold that do not necessarily influence spiking activity. Such modifications may depend on the state of maturation of specific elements of the circuit [44], activation signaling pathways that influence the ability of a neuron to respond to incoming activity [45*,46], or altered the capacity for plasticity of synapses in the circuit [47**]. The diversity of target location, type and signaling cascade activated by a specific pattern of activity becomes therefore central for the fine-scale regulation of a circuit.
Activation of FS inhibitory neurons may influence the activity of cortical excitatory neurons through a number of different pathways. As FS neurons contact the perisomatic region of pyramidal neurons [48], they can impair the ability of the postsynaptic neurons to fire action potentials. If inhibitory neuron activity is high enough to release a large amount of GABA, spillover onto extrasynaptic receptors may occur, leading to a further shunting effect on the postsynaptic neuron’s ability to generate an action potential. These effects can occur independently of the level of activity of the postsynaptic neuron, and rely on the amount of GABA and its diffusion within and outside of the synapse. The perisomatic location of FS inputs would make them highly effective in controlling firing rate. Differently, a similar process at dendritic targeting inhibitory inputs may modulate local computations [49], but may not necessarily result in changes in pyramidal neuron firing [50]. Thus, experience-induced changes in inhibitory drive onto different pyramidal neurons compartments can facilitate site-specific modulations.
Additional selectivity could be achieved if pairing of pre- and postsynaptic patterns of activity can differentiate inputs coming from the same presynaptic neuron. A recent study demonstrated that activity-dependent changes in inhibitory synaptic transmission can be connection specific and are determined by the level of postsynaptic activity paired with the activity of a FS neuron [47**]. This degree of connection specificity may be suitable for fine-scale refinement of specific inputs within a cortical circuit if it influences synaptic transmission or plasticity at converging inputs.
Recent data demonstrated that changes in the efficacy of GABAergic inhibitory synaptic transmission can affect the induction other forms of plasticity at inputs targeting the same postsynaptic neuron [47**]. The signaling mechanisms engaged by a connection-specific form of inhibitory plasticity alters the state of the postsynaptic neuron either by affecting its membrane potential [51] or by engaging signaling mechanisms that interfere with those involved in other forms of plasticity [47**,52]. The spatial and temporal resolution of such interactions depend on how inhibitory neurons and pyramidal neurons are activated by their afferent inputs, on the location of the converging excitatory and inhibitory inputs, on the type of receptor and on recruited signaling pathways.
Conclusions
The evidence presented here indicate as recipient of TC and limbic inputs, inhibitory neurons play a central role in sensory processing and in establishing the hedonic value of a stimulus. The diversity in neuron types and mechanisms of action, confers inhibitory neurons with a high degree of specificity in their effects on local circuits. These properties make them suitable not only for regulating circuit excitability, but also to contribute to events that require a high degree of selectivity such as experience-dependent refinement and learning.
Figure 1. Summary of current results regarding thalamocortical projections onto inhibitory neurons in four different cortical regions.
primary auditory cortex (A1), primary visual cortex (V1), primary somatosensory cortex (S1) and prefrontal cortex (PFC). The dashed lines indicate connections for which the evidence is controversial at the moment. The thickness of the lines indicates differences in reported synaptic strength. CB/CR+: calbindin/calretinin expressing inhibitory neurons; PV+: parvalbumin expressing inhibitory neurons; SST+: somatostating expressing inhibitory neurons; 5HT3+: type 3 serotonin receptor expressing inhibitory neurons. In the PFC panel L4 and L6 are in brackets as in this region these layers are not thought to be clearly identifiable.
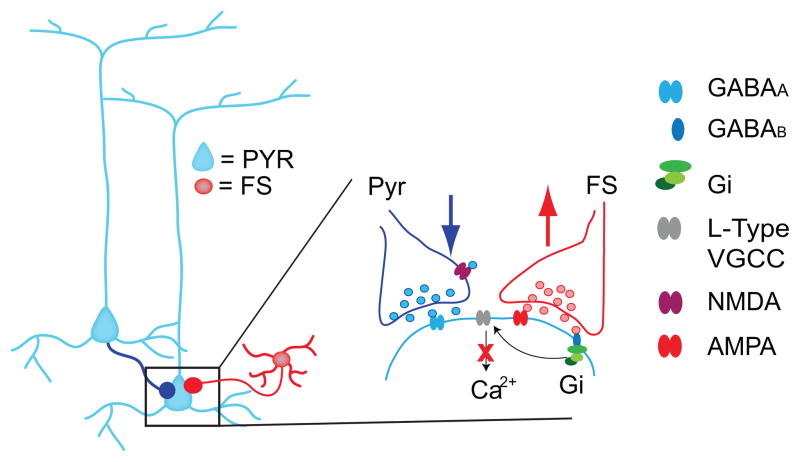
Figure 2. Crosstalk of excitatory and inhibitory forms of plasticity.
The leftmost diagram shows convergent excitatory and inhibitory inputs from a fast spiking neuron (FS) onto a pyramidal neuron (Pyr). The area in the black square is diagrammed in expanded form to show a summary of recent results regarding the interaction between GABAergic and glutamatergic long term potentiation (LTP) co-induced at converging inputs. The induction of GABAergic LTP prevents glutamatergic LTP and favors long term depression (LTD) by decreasing calcium (Ca2+) influx through L-type voltage gated calcium channels (L-type VGCC). GABAA: ionotropic GABA receptor; GABAB: metabotropic GABA receptor; Gi: inhibitory G protein; NMDA: NMDA receptor; AMPA: AMPA receptor. The red X indicates decreased Ca2+ inflow.
Visual summary.
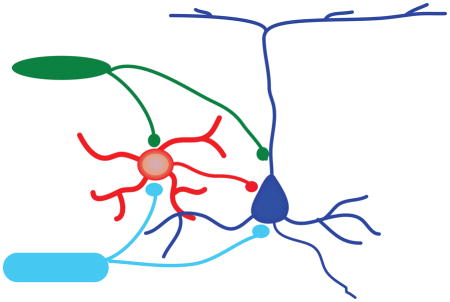
In this Opinion I discuss how cortical inhibitory neurons are central to the integration of sensory and limbic stimuli. I report recent findings regarding activation of inhibitory neurons by thalamocortical (TC) and amygdalocortical (Am) inputs and discuss the role of inhibition on excitability and plasticity of local circuits. The image shows that in cortex pyramidal neuron (Pyr) and GABAergic neurons (GABA) can be directly activated both by thalamic and amygdalar inputs.
Highlights.
Inhibitory neurons are nodes of integration for sensory and limbic inputs
The diversity of inhibitory neuron suggests they do not only act as brakes on activity
Fine-scale control of local circuit refinement may rely on distinct inhibitory circuits
Acknowledgments
Work for this Opinion was supported by the Whitehall Foundation, NIH-NIDCD grants R01-DC013770, R01-DC015234 and the Hartman Foundation
Footnotes
Conflict of Interest statement
The author has no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rozas C, Frank H, Heynen AJ, Morales B, Bear MF, Kirkwood A. Developmental inhibitory gate controls the relay of activity to the superficial layers of the visual cortex. J Neurosci. 2001;21:6791–6801. doi: 10.1523/JNEUROSCI.21-17-06791.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 4.Takada N, Pi HJ, Sousa VH, Waters J, Fishell G, Kepecs A, Osten P. A developmental cell-type switch in cortical interneurons leads to a selective defect in cortical oscillations. Nat Commun. 2014;5:5333. doi: 10.1038/ncomms6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SH, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Donato F, Rompani SB, Caroni P. Parvalbumin-expressing basket-cell network plasticity induced by experience regulates adult learning. Nature. 2013;504:272–276. doi: 10.1038/nature12866. This is a great study that shows the contribution of PV expressing inhibitory neurons to learning in the adult brain. [DOI] [PubMed] [Google Scholar]
- 7.Iurilli G, Ghezzi D, Olcese U, Lassi G, Nazzaro C, Tonini R, Tucci V, Benfenati F, Medini P. Sound-driven synaptic inhibition in primary visual cortex. Neuron. 2012;73:814–828. doi: 10.1016/j.neuron.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloc M, Maffei A. Target-specific properties of thalamocortical synapses onto layer 4 of mouse primary visual cortex. J Neurosci. 2014;34:15455–15465. doi: 10.1523/JNEUROSCI.2595-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takesian AE, Kotak VC, Sharma N, Sanes DH. Hearing loss differentially affects thalamic drive to two cortical interneuron subtypes. J Neurophysiol. 2013;110:999–1008. doi: 10.1152/jn.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol. 2010;588:2823–2838. doi: 10.1113/jphysiol.2010.187591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delevich K, Tucciarone J, Huang ZJ, Li B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci. 2015;35:5743–5753. doi: 10.1523/JNEUROSCI.4565-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji XY, Zingg B, Mesik L, Xiao Z, Zhang LI, Tao HW. Thalamocortical Innervation Pattern in Mouse Auditory and Visual Cortex: Laminar and Cell-Type Specificity. Cereb Cortex. 2016;26:2612–2625. doi: 10.1093/cercor/bhv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Z, Hu H, Huang ZJ, Agmon A. Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc Natl Acad Sci U S A. 2008;105:2187–2192. doi: 10.1073/pnas.0710628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Costa NM, Martin KA. The proportion of synapses formed by the axons of the lateral geniculate nucleus in layer 4 of area 17 of the cat. J Comp Neurol. 2009;516:264–276. doi: 10.1002/cne.22133. [DOI] [PubMed] [Google Scholar]
- 17.Roth MM, Dahmen JC, Muir DR, Imhof F, Martini FJ, Hofer SB. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat Neurosci. 2016;19:299–307. doi: 10.1038/nn.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. A great study that report the role of prefrontal cortex dishibition in fear behavior. [DOI] [PubMed] [Google Scholar]
- 19.Simons DJ, Carvell GE, Kyriazi HT. Alterations in functional thalamocortical connectivity following neonatal whisker trimming with adult regrowth. J Neurophysiol. 2015;114:1912–1922. doi: 10.1152/jn.00488.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Haley MS, Fontanini A, Maffei A. Laminar- and Target-Specific Amygdalar Inputs in Rat Primary Gustatory Cortex. J Neurosci. 2016;36:2623–2637. doi: 10.1523/JNEUROSCI.3224-15.2016. This is the first report of target-specific and laminar specific properties of basolateral amygdala inputs onto neurons in a sensory cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dilgen J, Tejeda HA, O’Donnell P. Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J Neurophysiol. 2013;110:221–229. doi: 10.1152/jn.00531.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popa D, Duvarci S, Popescu AT, Lena C, Pare D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci U S A. 2010;107:6516–6519. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuelsen CL, Gardner MP, Fontanini A. Effects of cue-triggered expectation on cortical processing of taste. Neuron. 2012;74:410–422. doi: 10.1016/j.neuron.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piette CE, Baez-Santiago MA, Reid EE, Katz DB, Moran A. Inactivation of basolateral amygdala specifically eliminates palatability-related information in cortical sensory responses. J Neurosci. 2012;32:9981–9991. doi: 10.1523/JNEUROSCI.0669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone ME, Maffei A, Fontanini A. Amygdala stimulation evokes time-varying synaptic responses in the gustatory cortex of anesthetized rats. Front Integr Neurosci. 2011;5:3. doi: 10.3389/fnint.2011.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Zhu B, Shou T. Anatomical evidence for the projections from the basal nucleus of the amygdala to the primary visual cortex in the cat. Neurosci Lett. 2009;453:126–130. doi: 10.1016/j.neulet.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Li H, Jin Z, Shou T, Yu H. Feedback of the amygdala globally modulates visual response of primary visual cortex in the cat. Neuroimage. 2014;84:775–785. doi: 10.1016/j.neuroimage.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Shuler MG, Bear MF. Reward timing in the primary visual cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- 30.Buzsaki G, Geisler C, Henze DA, Wang XJ. Interneuron Diversity series: Circuit complexity and axon wiring economy of cortical interneurons. Trends Neurosci. 2004;27:186–193. doi: 10.1016/j.tins.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 31.Bormann J. The ‘ABC’ of GABA receptors. Trends Pharmacol Sci. 2000;21:16–19. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 32.Kullmann DM, Ruiz A, Rusakov DM, Scott R, Semyanov A, Walker MC. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glickfeld LL, Roberts JD, Somogyi P, Scanziani M. Interneurons hyperpolarize pyramidal cells along their entire somatodendritic axis. Nat Neurosci. 2009;12:21–23. doi: 10.1038/nn.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tseng GF, Haberly LB. Characterization of synaptically mediated fast and slow inhibitory processes in piriform cortex in an in vitro slice preparation. J Neurophysiol. 1988;59:1352–1376. doi: 10.1152/jn.1988.59.5.1352. [DOI] [PubMed] [Google Scholar]
- 35.Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 37.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, Mrsic-Flogel TD. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci. 2011;14:1045–1052. doi: 10.1038/nn.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, Miller KD. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron. 2013;80:51–63. doi: 10.1016/j.neuron.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- 41.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- 43.Kuhlman SJ, Olivas ND, Tring E, Ikrar T, Xu X, Trachtenberg JT. A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature. 2013;501:543–546. doi: 10.1038/nature12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis MF, Figueroa Velez DX, Guevarra RP, Yang MC, Habeeb M, Carathedathu MC, Gandhi SP. Inhibitory Neuron Transplantation into Adult Visual Cortex Creates a New Critical Period that Rescues Impaired Vision. Neuron. 2015;86:1055–1066. doi: 10.1016/j.neuron.2015.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Urban-Ciecko J, Fanselow EE, Barth AL. Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Curr Biol. 2015;25:722–731. doi: 10.1016/j.cub.2015.01.035. The results reported in this study show how inhibition can modulate the strength and dynamics of glutamatergic synapses depending on the state of excitability of the circuit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Cristo G, Chattopadhyaya B, Kuhlman SJ, Fu Y, Belanger MC, Wu CZ, Rutishauser U, Maffei L, Huang ZJ. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat Neurosci. 2007;10:1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- 47**.Wang L, Maffei A. Inhibitory plasticity dictates the sign of plasticity at excitatory synapses. J Neurosci. 2014;34:1083–1093. doi: 10.1523/JNEUROSCI.4711-13.2014. This is the first direct demonstration that excitatory and inhibitory forms of plasticity are not independent events, but interact cooperatively to sculpt local circuits. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubota Y, Karube F, Nomura M, Kawaguchi Y. The Diversity of Cortical Inhibitory Synapses. Front Neural Circuits. 2016;10:27. doi: 10.3389/fncir.2016.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bloss EB, Cembrowski MS, Karsh B, Colonell J, Fetter RD, Spruston N. Structured Dendritic Inhibition Supports Branch-Selective Integration in CA1 Pyramidal Cells. Neuron. 2016;89:1016–1030. doi: 10.1016/j.neuron.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 50.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 51.Balena T, Woodin MA. Coincident pre- and postsynaptic activity downregulates NKCC1 to hyperpolarize E(Cl) during development. Eur J Neurosci. 2008;27:2402–2412. doi: 10.1111/j.1460-9568.2008.06194.x. [DOI] [PubMed] [Google Scholar]
- 52.Balena T, Acton BA, Woodin MA. GABAergic synaptic transmission regulates calcium influx during spike-timing dependent plasticity. Front Synaptic Neurosci. 2010;2:16. doi: 10.3389/fnsyn.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]