Abstract
Migraine is a common neurological disease with a high prevalence and unsatisfactory treatment options. The specific pathophysiological mechanisms of migraine remain unclear, which restricts the development of effective treatments for this prevalent disorder. The aims of this study were to 1) compare the spontaneous brain activity differences between Migraine without Aura (MwoA) patients and healthy controls (HCs), using amplitude of low-frequency fluctuations (ALFF) calculation method, and 2) explore how an effective treatment (verum acupuncture) could modulate the ALFF of MwoA patients. One hundred MwoA patients and forty-six matched HCs were recruited. Patients were randomized to four weeks' verum acupuncture, sham acupuncture, and waiting list groups. Patients had resting state BOLD-fMRI scan before and after treatment, while HCs only had resting state BOLD-fMRI scan at baseline. Headache intensity, headache frequency, self-rating anxiety and self-rating depression were used for clinical efficacy evaluation. Compared with HCs, MwoA patients showed increased ALFF in posterior insula and putamen/caudate, and reduced ALFF in rostral ventromedial medulla (RVM)/trigeminocervical complex (TCC). After longitudinal verum acupuncture treatment, the decreased ALFF of the RVM/TCC was normalized in migraine patients. Verum acupuncture and sham acupuncture have different modulation effects on ALFF of RVM/TCC in migraine patients. Our results suggest that impairment of the homeostasis of the trigeminovascular nociceptive pathway is involved in the neural pathophysiology of migraines. Effective treatments, such as verum acupuncture, could help to restore this imbalance.
Keywords: Migraine, Amplitude of low-frequency fluctuations, Brainsterm, Trigeminocervical complex, Acupuncture, Resting state fMRI
Highlights
-
•
Impairment of the homeostasis of the brainstem activity is involved in the neural pathophysiology of migraines.
-
•
Effective treatments, such as verum acupuncture, could help to normalise the abnormal brainstem activity in migraines.
-
•
Verum and sham acupuncture have different modulation effects on the abnormal brainstem activity in migraines.
1. Introduction
Migraine is a common neurological disease with a high prevalence, a heavy social and economic burden (Stovner and Hagen, 2006), disabling effects (Leonardi et al., 2005) and unsatisfactory treatment options (Diener et al., 2015). About 64% of migraines are classified as Migraine without Aura type (MwoA) (Rasmussen and Olesen, 1992). However, the pathophysiological mechanisms of MwoA remain unclear, which restricts the development of effective treatments for this prevalent disorder.
Current models of migraine pathophysiology suggest that the disturbed homeostasis of the trigeminovascular nociceptive pathway, especially in the brainstem, is a key factor for susceptibility to migraine headaches. Specifically, sensory afferents from the head and neck either travel as trigeminal afferents through the trigeminal ganglion or as afferents from the greater occipital nerve through the cervical ganglion, and synapse on second-order neurons in the trigeminocervical complex (TCC) (DaSilva et al., 2002). In the brainstem, the descending modulation of the TCC nociceptive signals originate mainly from the periaqueductal gray (PAG) and the rostral ventromedial medulla (RVM) (Akerman et al., 2011). The TCC/RVM (ascending input/descending modulation) imbalance hypothesis, based on animal models, is increasingly supported by human brain imaging studies in migraine patients (Weiller et al., 1995, Stankewitz and May, 2011, Stankewitz et al., 2011, Kröger and May, 2015). For instance, in a positron emission tomography study (Weiller et al., 1995), Weiller and colleagues found that only the cerebral blood flow increase in the brainstem (including PAG, RVM, etc.) was observed after an injection of sumatriptan, which induced complete relief from migraine attack. In another study, Stankewitz and colleagues (Stankewitz et al., 2011) compared the fMRI signal changes between migraine patients and controls during trigeminal nerve stimulation. The only difference between the two groups was detected in the lower brainstem (TCC area). These results suggest that an imbalance in activity between brainstem nuclei regulating antinociception and vascular control may play an important role in the pathogenesis of migraines.
Recently, resting state fMRI has drawn the attention of investigators. One method to characterize the resting state regional spontaneous neuronal activity is the amplitude of low frequency fluctuations (ALFF) calculation (Zang et al., 2007). As a reliable and reproducible data-driven approach measuring low frequency BOLD signals, ALFF can be defined as the total power within a certain frequency range, which reflects the intensity of regional spontaneous brain activity (Zou et al., 2009, Zuo et al., 2010). The alternations in ALFF have also been observed in several disorders including chronic pain (Xue et al., 2013, Qi et al., 2015, Wang et al., 2016), amnestic mild cognitive impairment (Han et al., 2011), schizophrenia (Hoptman et al., 2010) and hepatic encephalopathy (Qi et al., 2012).
In this study, we first compared the ALFF differences between MwoA patients and healthy controls (HCs), then explored how an effective treatment could modulate the ALFF of patients with migraine. We hypothesized that 1) MwoA patients had dysfunction in the ascending input/descending modulation system, especially in the brainstem regions, and 2) effective treatment might regulate the dysfunction of the ascending input/descending modulation system.
We chose acupuncture as an effective treatment in this study, as many studies have demonstrated that acupuncture treatments are effective and safe for migraine prophylaxis (Linde et al., 2005, Diener et al., 2006, Facco et al., 2008, Wang et al., 2011, Vickers and Linde, 2014, Wang et al., 2015). And verum acupuncture treatment is more effective in reducing headache intensity for MwoA patients, compared with sham acupuncture treatment (Li et al., 2012, Zhao et al., 2017); thus, this study focuses on how the ascending input and descending modulation system can be modified after an effective treatment (verum acupuncture treatment) and the different modulation effects between verum and sham acupuncture treatment.
2. Methods and materials
The experimental procedures are briefly described below. Please also see a previously published study for more details on the experimental procedure (Li et al., 2016). In this study, we investigated the ALFF difference between the migraineurs and controls as well as the modulation effect of repeated acupuncture treatments on ALFF.
2.1. Participants
2.1.1. Migraine without aura patients
MwoA patients were recruited at the outpatient department of the 3rd Teaching Hospital and the campus of Chengdu University of Traditional Chinese Medicine. Diagnosis of MwoA was based on the International Classification of Headache Disorders, 2nd Edition ICHD-II MwoA criteria (Headache Classification Subcommittee of the International Headache, 2004). Inclusion criteria required that all patients (1) were aged between 17 and 45 years old and were right-handed, (2) had not taken any prophylactic headache medicine nor had acupuncture treatment during the last three months, (3) had at least six months migraine duration, and (4) had at least once headache attack per month during the last three months. Patients were excluded if they (1) were alcohol or drug abusers, (2) were pregnant or lactating, (3) suffered from psychiatric, neurological, cardiovascular, respiratory, or renal illnesses, (4) suffered from any other chronic pain conditions or had a history of head trauma with loss of consciousness, (5) had MRI contraindications, such as claustrophobia, and (6) had acupuncture contraindications, such as a bleeding tendency.
2.1.2. Healthy controls
Aged-matched, right-handed HCs were recruited for this study by advertisement. Each subject underwent a medical history evaluation, physical examination, hepatic and renal function tests, and routine analysis of blood, urine, and stool to exclude organic disease carriers. Individuals were excluded for any chronic pain conditions, abnormal test results, history of head trauma with loss of consciousness, or for pregnancy/lactation.
The study protocol was approved by the Ethics Committee of the 1st Teaching Hospital of Chengdu University of Traditional Chinese Medicine, and registered on www.clinicaltrial.gov (NCT01152632, June 27, 2010). Informed consent was obtained from all participants.
2.2. Study design
The total observation period for MwoA patients was eight weeks. After screening, all MwoA patients were randomized into five groups, including verum acupuncture groups 1, 2, and 3 (VA1, VA2, VA3), sham acupuncture group (SA), and waiting-list (WT) group. In this study, we included three verum acupuncture prescriptions to better represent different acupoint selection strategies (Li et al., 2012). Weeks 1 to 4 served as a baseline phase used to record baseline headache diaries. Weeks 5 to 8 served as an intervention phase, during which patients in treatment groups received either verum or sham acupuncture treatment. All patients continued recording headache diaries during this period. In addition, MRI scans were administered at the ends of the fourth and eighth weeks for migraine patients. All MwoA patients were migraine-free for at least 72 h at the time of the MRI scans. HCs received only the baseline MRI scans.
2.3. Interventions
The treatment for the acupuncture groups consisted of 20 sessions of acupuncture treatment with a duration of 30 min per session, each administered over a period of 4 weeks (5 sessions per week). No verum acupuncture nor sham acupuncture treatments were applied on HC and MwoA patients in the waiting-list group.
Acupoint and non-acupoint selections were similar to those in our previous RCT studies (Li et al., 2009, Li et al., 2012). Acupoints selected in VA1 were Yanglingquan (GB34), Qiuxu (GB40) and Waiguan (SJ5). VA2 acupoints were Xiyangguan (GB33), Diwuhui (GB42) and Sanyangluo (SJ8). VA3 acupoints were Zusanli (ST36), Chongyang (ST42) and Pianli (L16). SA acupoints included NAP1, NAP2 and NAP3.
Two licensed acupuncturists administrated all acupuncture treatments. All acupoints and non-acupoints were punctured bilaterally. The needles were inserted perpendicularly at a penetration of 5 to 15 mm and were gently manipulated to achieve deqi sensation (a complex feeling including soreness, numbness, heaviness, distention or dull pain at the site of needle placement) in all treatment groups (Kong et al., 2007).
Patients agreed not to take any regular medications for the treatment of migraines for the duration of the study. In case of severe pain, ibuprofen (300 mg capsule with sustained release) was allowed as a rescue medication.
2.4. Outcome measures
Migraine intensity and frequency of migraine attacks were chosen as outcome measures to assess the clinical efficacy of acupuncture treatments. Headache intensity was evaluated with a 0–10 visual analogue scale (VAS), with 0 indicating no pain, and 10 indicating the worst pain imaginable. The frequency of migraine attacks is defined as the number of migraines separated by pain free intervals of at least 48 h, and is based on patients' headache diaries according to the guidelines of the IHS for Clinical Trials in Migraine (Tfelt-Hansen et al., 2000). In addition, the self-rating anxiety scale (SAS) and the self-rating depression scale (SDS) were administered to assess the MwoA patients' anxiety and depression status (Seidel et al., 2009, Usai et al., 2009).
2.5. MRI data acquisition
MRI data was acquired with a 3.0T magnetic resonance scanner (Siemens 3.0T Trio Tim, Munich, Germany) with an 8-channel phase-array head coil at the West China Hospital MRI center. Subjects were asked to stay awake and to keep their heads still during the scan, with their eyes closed and ears plugged. Prior to the functional run, a high-resolution structural image for each subject was acquired using a three-dimensional MRI sequence with a voxel size of 1 mm3 employing an axial fast spoiled gradient recalled sequence (TR = 1900 ms; TE = 2.26 ms; data matrix: 256 × 256; field of view: 256 × 256 mm2). The BOLD resting-state functional images were obtained with echo-planar imaging (30 contiguous slices with a slice thickness of 5 mm; TR = 2000 ms; TE = 30 ms; flip angle: 90°; field of view: 240 × 240 mm2; data matrix: 64 × 64; total volumes: 180).
2.6. Data analysis
2.6.1. Clinical data analysis
The clinical outcomes were analyzed using SPSS 16.0 (SPSS Inc., Chicago, IL). Within and between-group comparisons were performed using paired or unpaired t-tests or χ2, as appropriate. The significance level used for the statistical analysis with 2-tailed testing was P < 0.05.
2.6.2. ALFF analysis
The fMRI data preprocessing was performed using Data Processing Assistant for Resting-State fMRI (DPARSF) software (available at: http://rfmri.org/DPARSF) in MATLAB (Mathworks, Inc., Natick, Massachusetts). The software is based on Statistical Parametric Mapping (SPM12) (http://www.fil.ion.ucl.ac.uk/spm) and a Resting-State fMRI Data Analysis Toolkit (http://www.restfmri.net) (Song et al., 2011).
2.6.2.1. Data processing
The first 10 volumes of functional data for each subject were discarded to allow for signal equilibration effects. The remaining volumes were slice timing corrected, within-subject spatially realigned, coregistered to the respective structural images for each subject, segmented. Then, white matter and CSF signals were regressed out (global signal not included (Saad et al., 2012)). To perform subject-level correction of head motion, the Friston 24-parameter model (6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items) (Friston et al., 1996, Yan et al., 2013) were used. Images were normalized by using structural image unified segmentation and then re-sampled to 3-mm cubic voxels. Subjects with head movements exceeding 2 mm on any axis or with head rotation > 2° were excluded. After smoothing with a 6 mm full-width at half maximum (FWHM) Gaussian kernel, the linear and quadric trends of the time courses were removed. Finally, imaging data were temporally filtered (band pass, 0.01–0.1 Hz) to remove the affects of very low-frequency drift and high-frequency noise (e.g., respiratory and cardiac rhythms).
Group analysis was performed with a random effect model using SPM12. We first compared the ALFF difference between MwoA patients and HCs using two sample t-tests. Then, we compared the changes of ALFF difference (post-treatment minus pre-treatment) between verum acupuncture groups (VA, VA1 + VA2 + VA3) and waiting-list group in factorial design module in SPM12. To explore the association between the clinical outcomes and the ALFF, we also applied regression analyses between baseline ALFF and the corresponding migraine symptom (VAS). For all group analyses, age, gender, disease duration, SAS, and SDS were included as non-interest covariates. A threshold of a voxel-wise P < 0.001 uncorrected and P < 0.05 family wise error (FWE) correction at cluster level were applied for all analyses.
3. Results
150 MwoA patients were screened and 100 of them were recruited for this study. 46 age- and gender-matched healthy controls were recruited in this study, and 4 HCs were excluded due to excessive head movements (head movements exceeding 2 mm on any axis or head rotation > 2°). 88 patients participated in the first fMRI scan, and 81 patients participated in the second fMRI scan; 7 patients did not participate in the second fMRI scan due to scheduling conflicts (2 in VA1, 2 in VA2, 1 in VA3, and 2 in SA groups). Of the 81 patients who participated in the two fMRI scans, 9 patients were excluded from data analysis due to incomplete scans (lack of resting state fMRI or T1 anatomy; 3 in V1, 1 in V2, 2 in V3, 2 in SA, and 1 in WT), and 10 patients were excluded due to excessive head movements (head movements exceeding 2 mm on any axis or head rotation > 2°; 1 in V1, 3 in V2, 2 in V3, 2 in SA and 2 in WT).
3.1. Baseline characteristics
We found no statistical differences among VA1, VA2, VA3, SA and WT groups in age, gender, weight, height, duration of disease, headache intensity (VAS score), headache frequency, SAS and SDS (P > 0.05). We also found no statistical differences in age, gender, weight and height between all MwoA patients and healthy controls (P > 0.05) (Table 1).
Table 1.
Baseline characteristics of MwoA patients (subjects completed two MRI scans with completed MRI data) in different groups and healthy controls.
HC, healthy controls; MwoA, migraine without aura; VA, verum acupuncture; SA, sham acupuncture group; SAS, self-rating anxiety scale; SDS, self-rating depression scale; WT, waiting-list. *, one-way ANOVA was applied for the comparisons among VA1, VA2, VA3, SA and WT groups; **, two-sample t-test was applied for the comparisons between HC and MwoA groups. A P value < 0.05 was considered statistically significant.
| Characteristics | VA1, n = 11 | VA2, n = 11 | VA3, n = 13 | SA, n = 11 | WT, n = 16 | P value* | HC, n = 42 | MwoA, n = 62 | P value** |
|---|---|---|---|---|---|---|---|---|---|
| Female n (%) | 9 (81.8%) | 8 (72.7%) | 10 (77.2%) | 9 (81.8%) | 12 (77.5%) | 0.979 | 34 (81.0%) | 48 (77.4%) | 0.808 |
| Age (y) Mean (95%CI) |
21.73 (20.56; 22.89) |
21.18 (19.69; 22.67) |
21.00 (19.98; 22.02) |
21.18 (20.52; 21.84) |
21.38 (20.33; 22.42) |
0.889 | 21.21 (20.93; 21.49) |
21.29 (20.85; 21.73) |
0.771 |
| Height (cm) Mean (95%CI) |
158.00 (153.65; 162.35) |
163.45 (158.25; 168.67) |
159.69 (155.35; 164.04) |
157.00 (154.00; 160.00) |
162.63 (157.60; 167.65) |
0.163 | 161.00 (159.23; 162.77) |
160.34 (158.40; 162.28) |
0.633 |
| Weight (kg) Mean (95%CI) |
51.64 (47.22; 56.05) |
56.27 (48.61; 63.93) |
50.73 (47.94; 53.52) |
48.27 (45.91; 50.64) |
53.94 (48.58; 59.29) |
0.164 | 50.98 (49.12; 52.84) |
52.27 (50.19; 54.34) |
0.382 |
| Duration (mo) Mean (95%CI) |
62.91 (43.91; 81.84) |
68.91 (39.88; 97.93) |
67.38 (45.44; 89.33) |
58.00 (39.94; 76.06) |
73.31 (53.15; 93.47) |
0.843 | – | – | – |
| Headache intensity Mean (95%CI) |
5.45 (4.54; 6.37) |
5.32 (4.68; 5.96) |
5.69 (5.12; 6.26) |
5.18 (4.19; 6.17) |
5.66 (5.16; 6.15) |
0.765 | – | – | – |
| Headache frequency Mean (95%CI) |
5.91 (3.88; 7.93) |
7.45 (5.03; 9.88) |
5.54 (3.35; 7.72) |
6.45 (4.34; 8.57) |
4.31 (2.91; 5.71) |
0.152 | – | – | – |
| SAS score Mean (95%CI) |
45.05 (39.72; 50.37) |
45.27 (39.71; 50.83) |
46.75 (40.50; 53.00) |
45.68 (40.35; 51.01) |
46.81 (41.52; 52.11) |
0.980 | – | – | – |
| SDS score Mean (95%CI) |
42.41 (34.55; 50.27) |
50.86 (45.14; 56.59) |
43.90 (36.43; 51.38) |
44.27 (37.41; 51.14) |
47.34 (42.48; 52.21) |
0.326 | – | – | – |
3.2. Clinical outcomes
After acupuncture treatment, VA1, VA2, and VA3 groups showed significant improvement in VAS score (P < 0.05) (Table 2). VA1 and VA3 groups showed significant improvement in headache frequency (P < 0.05), while VA2 group showed a tendency to improve headache frequency (P = 0.111). SA showed insignificant improvement in VAS score and headache frequency (P > 0.05). However, insignificant differences were found in the changes of VAS score, changes in headache frequency, the SAS and SDS improvement (P > 0.05) (Table 3) among VA1, VA2, VA3 and SA groups, and there were no significant differences between VA (VA1 + VA2 + VA3) and SA groups, due to small sample size. We observed that VA showed significant therapeutic effects compared to the waiting-list group in VAS score and headache frequency improvement (P < 0.05) (Table 3).
Table 2.
Clinical outcomes at the baseline and end of the study in different groups.
VA, verum acupuncture (VA1 + VA2 + VA3); SA, sham acupuncture group; SAS, self-rating anxiety scale; SDS, self-rating depression scale; WT, waiting list. Paired-t-test was applied for comparisons in each group. A P value < 0.05 was considered statistically significant.
| Outcome measures | VA1, n = 11 | VA2, n = 11 | VA3, n = 13 | SA, n = 11 | VA, n = 35 | WT, n = 16 |
|---|---|---|---|---|---|---|
| Headache intensity Mean (95%CI) | ||||||
| Baseline | 5.45 (4.54; 6.37) |
5.32 (4.68; 5.96) |
5.69 (5.12; 6.26) |
5.18 (4.19; 6.17) |
5.50 (5.13; 5.87) |
5.66 (5.16; 6.15) |
| End of treatment | 3.18 (2.24; 4.12) |
3.73 (2.82; 4.63) |
3.15 (2.31; 3.99) |
4.04 (3.09; 5.00) |
3.34 (2.87;3.81) |
5.72 (4.84; 6.59) |
| P value | 0.003 | 0.002 | 0.000 | 0.158 | 0.000 | 0.861 |
| Headache frequency Mean (95%CI) | ||||||
| Baseline | 5.91 (3.88; 7.93) |
7.45 (5.03; 9.88) |
5.54 (3.35; 7.72) |
6.45 (4.34; 8.57) |
6.26 (5.08; 7.44) |
4.31 (2.91; 5.71) |
| End of treatment | 4.18 (2.60; 5.77) |
6.18 (4.13; 8.03) |
4.08 (2.40; 5.76) |
6.45 (3.97; 8.94) |
4.77 (3.83; 5.71) |
8.63 (6.14; 11.11) |
| P value | 0.029 | 0.111 | 0.038 | 0.999 | 0.009 | 0.000 |
| SAS score Mean (95%CI) | ||||||
| Baseline | 45.05 (39.72; 50.37) |
45.27 (39.71; 50.83) |
46.75 (40.50; 53.00) |
45.68 (40.35; 51.01) |
45.75 (42.74; 48.76) |
46.81 (41.52; 52.11) |
| End of treatment | 39.20 (31.93; 46.48) |
41.77 (36.43; 47.11) |
37.96 (31.68; 44.25) |
38.02 (32.97; 43.08) |
39.55 (36.22; 42.88) |
41.64 (37.76; 45.52) |
| P value | 0.101 | 0.180 | 0.015 | 0.081 | 0.001 | 0.040 |
| SDS score Mean (95%CI) | ||||||
| Baseline | 42.41 (34.55; 50.27) |
50.86 (45.14; 56.59) |
43.90 (36.43; 51.38) |
44.27 (37.41; 51.14) |
45.37 (41.53; 49.21) |
47.34 (42.48; 52.21) |
| End of treatment | 41.93 (33.11; 50.75) |
43.86 (36.67; 51.05) |
38.92 (32.31; 45.53) |
37.34 (30.69; 43.98) |
41.03 (37.17; 44.89) |
41.25 (35.99; 46.51) |
| P value | 0.832 | 0.071 | 0.070 | 0.118 | 0.011 | 0.065 |
Table 3.
Comparisons of the therapeutic effects between different groups.
HC, healthy controls; VA, verum acupuncture (VA1 + VA2 + VA3); SA, sham acupuncture; SAS, self-rating anxiety scale; SDS, self-rating depression scale; WT, waiting list; *, one-way ANOVA was applied for the comparisons among VA1, VA2, VA3 and SA groups; **, two-sample t-test was applied for the comparisons between VA and WT groups. A P value < 0.05 was considered statistically significant.
| Outcome measures | VA1, n = 11 | VA2, n = 11 | VA3, n = 13 | SA, n = 11 | P value* | VA, n = 35 | WT, n = 16 | P value** |
|---|---|---|---|---|---|---|---|---|
| Headache intensity Mean (95%CI) | ||||||||
| End of treatment | 3.18 (2.24; 4.12) |
3.73 (2.82; 4.63) |
3.15 (2.31; 3.99) |
4.04 (3.09; 5.00) |
0.351 | 3.34 (2.87;3.81) |
5.72 (4.84; 6.59) |
0.000 |
| End - baseline | − 2.27 (− 3.55; − 0.99) |
− 1.59 (− 2.42; − 0.75) |
− 2.54 (− 3.41; − 1.66) |
− 1.14 (− 2.80; 0.52) |
0.242 | − 2.16 (− 2.69; − 1.62) |
0.06 (− 0.68; 0.81) |
0.000 |
| Headache frequency Mean (95%CI) | ||||||||
| End of treatment | 4.18 (2.60; 5.77) |
6.18 (4.13; 8.03) |
4.08 (2.40; 5.76) |
6.45 (3.97; 8.94) |
0.107 | 4.77 (3.83; 5.71) |
8.63 (6.14; 11.11) |
0.001 |
| End - baseline | − 1.73 (− 3.23; − 0.22) |
− 1.27 (− 2.89; 0.35) |
− 1.46 (− 2.83; − 0.10) |
0.00 (− 2.75; 2.75) |
0.493 | − 1.49 (− 2.26; − 0.72) |
4.31 (2.26; 6.37) |
0.000 |
| SAS score Mean (95%CI) | ||||||||
| End of treatment | 39.20 (31.93; 46.48) |
41.77 (36.43; 47.11) |
37.96 (31.68; 44.25) |
38.02 (32.97; 43.08) |
0.745 | 39.55 (36.22; 42.88) |
41.64 (37.76; 45.52) |
0.399 |
| End - baseline | − 5.84 (− 13.05; 1.37) |
− 3.50 (− 8.91; 1.91) |
− 8.79 (− 15.54; − 2.04) |
− 7.66 (− 16.46; 1.14) |
0.670 | − 6.20 (− 9.67; − 2.73) |
− 5.17 (− 10.07; − 0.27) |
0.722 |
| SDS score Mean (95%CI) | ||||||||
| End of treatment | 41.93 (33.11; 50.75) |
43.86 (36.67; 51.05) |
38.92 (32.31; 45.53) |
37.34 (30.69; 43.98) |
0.520 | 41.03 (37.17; 44.89) |
41.25 (35.99; 46.51) |
0.944 |
| End - baseline | − 0.48 (− 5.35; 4.40) |
− 7.00 (− 14.72; 0.72) |
− 4.98 (− 10.44; 0.48) |
− 6.93 (− 15.95; 2.09) |
0.432 | − 4.34 (− 7.62; − 1.07) |
− 6.09 (− 12.61; 0.42) |
0.617 |
3.3. ALFF results
3.3.1. MwoA patients vs. HCs
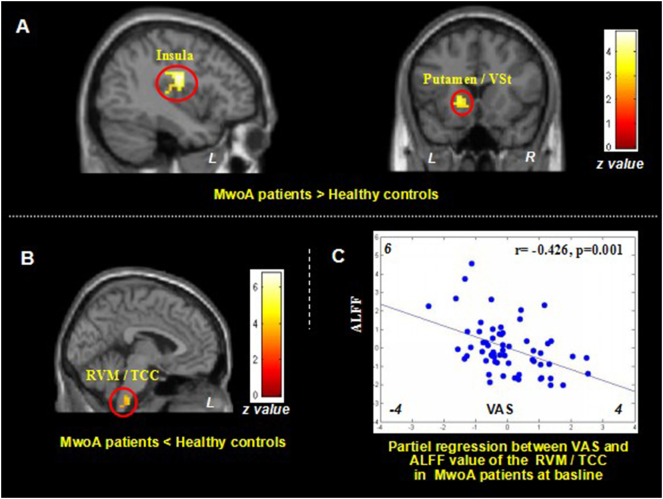
Compared with healthy controls, MwoA patients showed significant ALFF increases at the left posterior insula and left putamen/caudate, and ALFF decreases in the bilateral middle occipital cortex/cuneus and bilateral rostral ventromedial medulla (RVM)/trigeminocervical complex (TCC) (Fig. 1A and B, Table 4A). To explore the association between the ALFF value and baseline clinical outcomes, we extracted the ALFF value of the peak MNI coordinate (sphere, 2 mm radius) at each of the above regions, applied partial regressions with clinical variables (VAS and headache frequency), including age, gender, disease duration, SAS and SDS as non-interest variables. Partial regression showed that only ALFF value in RVM/TCC (x = − 3, y = − 33, z = 57, sphere, 2 mm radius) was negatively associated with VAS (r = − 0.426, p = 0.001, significant after Bonferroni correction) (Fig. 1C).
Fig. 1.
Altered resting state ALFF in MwoA patients.
1A. Brain regions showed increased resting state ALFF in MwoA patients compared to healthy controls; 1B. Brain regions showed reduced resting state ALFF in MwoA patients, compared to healthy controls; 1C. Reduced ALFF value in RVM/TCC is negatively associated with increased headache intensity as indicated by VAS scores in MwoA patients at baseline, controlled for age, gender, disease duration, SAS and SDS. ALFF, amplitude of low frequency fluctuation; L, left side; MwoA, migraine without aura; R, right side; RVM, rostral ventromedial medulla; TCC, trigeminocervical complex; VAS, visual analogue scale; Vst, ventral striatum.
Table 4.
The ALFF in MwoA patients and the changes in different groups.
4A. ALFF comparison between MwoA and HCs; 4B. ALFF changes in MwoA patients in acupuncture group; 4C. ALFF changes in MwoA patients in waiting-list group; 4D. ALFF modulation difference in MwoA patients between acupuncture group and waiting-list group. ALFF, amplitude of low frequency fluctuation; B, bilateral; L, left side; HCs, healthy controls; MwoA, migraine without aura; OFC, orbitofrontal cortex; PCC, posterior cingulate cortex; R, right side; rACC, rostral anterior cingulate cortex; RVM, rostral ventromedial medulla; TCC, trigeminocervical complex; WT, waiting-list. A threshold of a voxel-wise P < 0.001 uncorrected and P < 0.05 family wise error (FWE) correction at cluster level were applied.
| A. ALFF difference between MwoA and HCs |
B. ALFF changes in verum acupuncture group |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | Voxels | Brain region | MNI (x, y, z) | Z | Contrast | Voxels | Brain region | MNI (x, y, z) | Z | ||||
| MwoA > HC | 132 | L posterior insula | − 39 | − 12 | 24 | 5.05 | Pre > Post | 178 | L middle occip/cuneues | − 30 | − 72 | 30 | 5.21 |
| 128 | L putamen/caudate | − 15 | 18 | 0 | 4.03 | ||||||||
| MwoA < HC | 287 | R middle occip/cuneues | 30 | − 75 | 24 | 6.16 | Pre < Post | 88 | B RVM/TCC | − 12 | − 39 | − 39 | 5.40 |
| 153 | L middle occip/cuneues | − 27 | − 69 | 24 | 5.35 | 83 | B OFC | − 6 | 27 | − 30 | 5.15 | ||
| 59 | B RVM/TCC | − 3 | − 33 | − 57 | 3.93 | 41 | B rotral midbrain | 0 | − 24 | − 25 | 4.33 | ||
| C. ALFF changes in waiting-list group |
D. ALFF difference between verum acupuncture group and waiting-list group |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contrast | Voxels | Brain region | MNI (x, y, z) | Z | Contrast | Voxels | Brain region | MNI (x, y, z) | Z | ||||
| Pre > Post | None | VA > WT (Post-Pre) |
55 | B OFC | − 6 | 27 | − 30 | 4.78 | |||||
| 60 | B RVM/TCC | − 12 | − 39 | − 39 | 4.70 | ||||||||
| Pre < Post | 35 | R rACC | 9 | 24 | − 3 | 4.02 | VA < WT (Post-Pre) |
124 | L middle occip/cuneues | − 30 | − 72 | 30 | 4.95 |
| 91 | B PCC | − 6 | − 45 | 27 | 4.58 | ||||||||
3.3.2. Modulation effects of treatment (acupuncture)
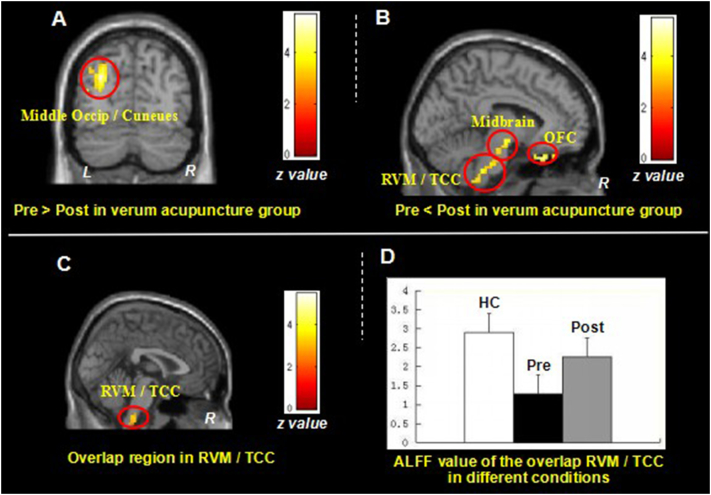
We then compared post- and pre-treatment differences in all treatment groups. Results revealed that after longitudinal verum acupuncture treatments, MwoA patients showed significant ALFF increases in the bilateral OFC, bilateral RVM/TCC(Stankewitz et al., 2011, Schulte et al., 2016) and bilateral rostral midbrain, and ALFF decreases in left middle occipital cortex/cuneus (Fig. 2A and B, Table 4B). Interestingly, this finding partially overlapped with the ALFF differences between healthy controls and MwoA patients at RVM/TCC (Fig. 2C). To further explore the ALFF value changes at different conditions, we extracted the ALFF value in the overlapping RVM/TCC region and found that effective treatment can normalize the decreased RVM/TCC ALFF value in MwoA patients (Fig. 2D).
Fig. 2.
Resting state ALFF changes of MwoA patients in verum acupuncture group.
2A. Brain regions showed decreased ALFF value in MowA patients after verum acupuncture treatment; 2B. Brain regions showed increased ALFF value in MowA patients after verum acupuncture treatment; 2C. Brain regions (RVM/TCC) showed overlap between Fig. 1B and Fig. 2B; 2D. The Fisher-z value of the overlap RVM/TCC in healthy controls and MwoA patients before and after verum acupuncture treatment respectively (mean ± SE). ALFF, amplitude of low frequency fluctuation; L, left side; MwoA, migraine without aura; OFC, orbital frontal cortex; R, right side; RVM, rostral ventromedial medulla; TCC, trigeminocervical complex; VAS, visual analogue scale.
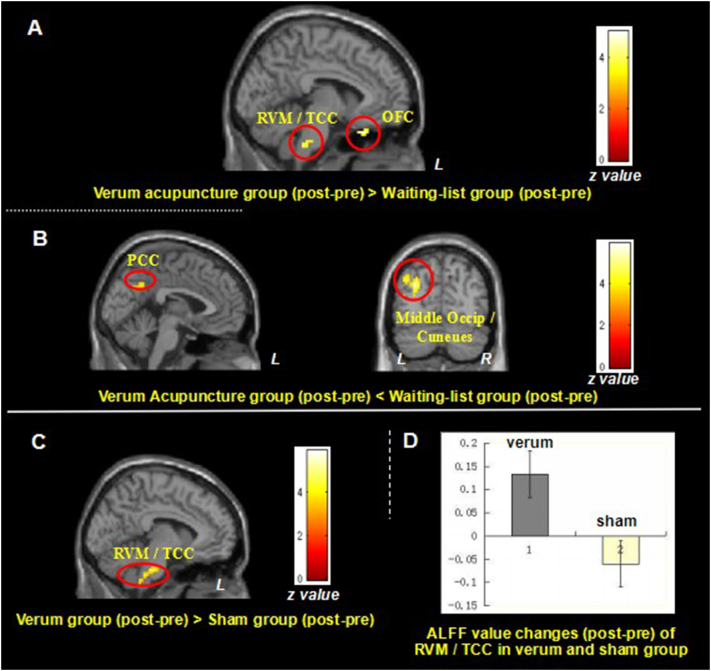
In the waiting-list group, comparison between the first and second fMRI scan showed increased ALFF only in the right rostral anterior cingulate cortex (Table 4C). Compared with the waiting-list group, the verum acupuncture treatment group (VA1 + VA2 + VA3) showed greater ALFF increases in the bilateral OFC and bilateral RVM/TCC after verum acupuncture treatments (Fig. 3A and Table 4D). The opposite comparison found the waiting-list group showed greater ALFF increases in the bilateral PCC and left middle occipital cortex/cuneus (Fig. 3B and Table 4D).
Fig. 3.
The Difference of resting state ALFF changes of MwoA patients in verum acupuncture group VS. waiting-list group and verum acupuncture group VS. sham acupuncture group.
3A. Brain region showed greater ALFF value increase (post- minus pre-treatment) in the verum acupuncture treatment group compared to waiting-list control; 3B. Brain region showed greater ALFF value increase (post- minus pre-treatment) in the waiting-list control compared to verum acupuncture treatment group; 3C. Brain regions showed greater ALFF value increase (post- minus pre-treatment) in the verum acupuncture group compared to sham acupuncture group; 3D. ALFF value changes of RVM/TCC in verum and sham group. L, left side; MwoA, migraine without aura; OFC, orbital frontal cortex; PCC, posterior cingulate cortex; R, right side; TCC, trigeminocervical complex.
We also compared the differences between verum acupuncture group and sham acupuncture group. The results indicated that, compared with the sham acupuncture group, the verum acupuncture group (VA1 + VA2 + VA3) showed greater ALFF increases in the bilateral RVM/TCC (peak MNI, x = − 12, y = − 36, z = − 36, z = 4.72, cluster size = 92) (Fig. 3C). We extracted the ALFF value of the RVM/TCC (peak MNI, x = − 12, y = − 36, z = − 36, sphere, 2 mm) in each group, and found that verum acupuncture could increase the ALFF value of RVM/TCC in MwoA patients while sham acupuncture decreases the ALFF value (Fig. 3D).
4. Discussion
In this study, we investigated the ALFF changes between migraine patients and healthy controls, the modulation effects of an effective treatment (verum acupuncture treatment) as well as the different modulation effects between verum and sham acupuncture. We found that migraine symptoms are associated with reduced ALFF in the RVM/TCC as compared with healthy controls, and longitudinal verum acupuncture treatment can normalize the decreased ALFF of the RVM/TCC in migraine patients. And verum acupuncture and sham acupuncture have different modulation effects on ALFF of RVM/TCC in migraine patients.
In this study, we found an increased ALFF at the insula, putamen and caudate in MwoA patients, compared with HCs. Previous studies suggested that these regions are involved in pain information processing in the brain (Kong et al., 2006, Kong et al., 2010, Schwedt et al., 2015). We thus speculate these changes may represent an adaptive response to repeated migraine attacks in these patients.
We also found decreased ALFF in RVM and TCC. The decreased ALFF value of RVM/TCC was associated with increased headache intensity. RVM is a key region of the descending pain modulatory system (Millan, 2002, Fields, 2004, Kong et al., 2010), while TCC comprises the second-order neurons of the trigeminovascular nociceptive pathway. Animal studies show that the descending modulation of the TCC, through the PAG and RVM, could cause the activation of ‘on’ cells and the inhibition of ‘off’ cells in the RVM, which is critical for activation of TCC and development of migraine headaches (Akerman et al., 2011). Our results provide direct evidence in patient population in favor of findings from animal studies.
Our findings of the decreased ALFF in RVM are also in line with many human brain imaging studies reporting that migraine is associated with an impaired descending pain modulatory system (May, 2008, Mainero et al., 2011, Schwedt et al., 2014). In a previous study, investigators also found that migraine patients in the interictal phase revealed lower BOLD-fMRI signals in the TCC compared with healthy controls (Stankewitz et al., 2011).
Recent studies suggested that the neuropeptide calcitonin gene-related peptide (CGRP) may play a critical role in the central and peripheral pathways leading to a migraine attack (Wrobel Goldberg and Silberstein, 2015). CGRP and its receptor components were found in different subregions of TCC in both humans and rats (Unger and Lange, 1991, Van Rossum et al., 1997). A more recent study showed an inhibitory effect of CGRP on mechanically evoked activity in the spinal trigeminal nuclei after pretreatment with glyceryl trinitrate in rats (Covasala et al., 2012). Taken together, these results support the crucial role of TCC in the pathology of migraine, and provide direct support for our findings indicating reduced ALFF value in TCC.
In this study, we also found that verum acupuncture treatments can normalize the decreased ALFF of the RVM and TCC in migraine patients, compared with the waiting-list group. This finding is in line with many brain imaging studies reporting that acupuncture treatment could normalize the impaired descending pain modulatory system in migraine (Li et al., 2016) and other chronic pain conditions (Chen et al., 2014, Chen et al., 2015, Egorova et al., 2015). It is well accepted that the endogenous opioid system is involved in mediating acupuncture analgesia (Han, 2011, Zhang et al., 2014, Dougherty et al., 2008). Specifically, in the RVM level, ‘on’ cells facilitate the firing of neurons that receive nociceptive inputs and are inhibited by opioids, whereas ‘off’ cells are tonically active, inhibit nociceptive responses, and are activated by opioids (Fields and Heinricher, 1985). This might be the one of the underlying mechanisms of verum acupuncture treatment for migraine (Vickers and Linde, 2014, Zhao et al., 2014, Yang et al., 2012, Li et al., 2015).
Recently, an neuroimaging study found that triptans (a class of migraine-specific medication) could significant increase the BOLD signal in the trigeminal nuclei in migraine patients compared with placebo or a nonsteroidal anti-inflammatory drug (broad spectrum pain killer) (Kröger and May, 2015). The study further illuminated that the increase in BOLD signal changes after triptan administration could be attributable to an inhibition of the inhibitory action of CGRP on trigeminal neurons in the brainstem, and concluded that a specific functional inhibition of trigemino-cortical projections might be one of the reasons that triptans, unlike pain killers, act specifically on migraine but not pain (Kröger and May, 2015). Therefore, we speculate that verum acupuncture might also work through the CGRP system to achieve treatment effects. Future studies are needed to elucidate the details of this process.
Although, due to small sample size in each group, insignificant differences were found in the changes of VAS score, changes in headache frequency, the SAS and SDS improvement between verum acupuncture (VA1 + VA2 + VA3) and sham acupuncture group in this study. Our previous RCTs reporting that puncturing at true acupoints, compared with puncturing at non-acupoints, showed better clinical improvement for migraine prevention (Li et al., 2012, Zhao et al., 2017). Thus, we compared verum acupuncture with sham acupuncture, and found that verum acupuncture showed an increase, but sham acupuncture showed a decrease in the RVM/TCC in migraine patients, which suggested that verum and sham acupuncture may have different modulation effects at RVM/TCC. Although the superiority of verum acupuncture over sham control remains a global controversy, accumulating neuroimaging studies suggest that verum acupuncture works in a more targeted and special manner on migraine patients, compared with sham acupuncture (Yang et al., 2012, Zhao et al., 2014, Yang et al., 2014). Further studies with larger sample sizes are needed to validate this finding.
There are several potential limitations in this study. 1) Sample size in each group was relatively small, which prevented us from testing the clinical outcome differences between different treatment groups. 2) Dropout rate in each group was relatively high; however, we would like to emphasize that the reasons for dropout do not seem to be associated with treatment response. Also, the aim of this study was to explore if an effective treatment could modulate the ALFF in migraine patients rather than to test the efficacy of acupuncture treatment. 3) We did not quantitatively record intensity of ‘deqi’ sensation, which is thought to be an important contributor to acupuncture effect (Kong et al., 2007). Studies with larger sample size and quantitative ‘deqi’ records are needed in the future.
5. Conclusion
Our results demonstrate that impairment of the descending pain modulatory system and ascending nociceptive pathway at RVM/TCC are associated with neural pathophysiology of migraine during the interictal period. Verum acupuncture treatment can normalize (increase) the reduced ALFF of RVM/TCC in migraine patients. Verum acupuncture and sham acupuncture have different modulation effects on ALFF of RVM/TCC in migraine patients.
Conflicts of interest
None of the authors have any conflict of interest.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (No. 81590950, No. 81603708, No. 81471737), Sichuan Youth Science and Technology Innovation Research Team on Acupoint Effect Mechanism (NO. 2015TD0010) and Collabrative Innovation Center of Acupoint Effect of Acupuncture and Moxibustion (Sichuan 2011 Collabrative Innovation Center). Jian Kong is supported by R01AT006364 (NCCIH/NIH), R01AT008563 (NCCIH/NIH), R21AT008707 (NCCIH/NIH), and P01 AT006663 (NCCIH/NIH).
References
- Akerman S., Holland P.R., Goadsby P.J. Diencephalic and brainstem mechanisms in migraine. Nat. Rev. Neurosci. 2011;12:570–584. doi: 10.1038/nrn3057. [DOI] [PubMed] [Google Scholar]
- Chen X., Spaeth R.B., Retzepi K., Ott D., Kong J. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci. Rep. 2014;4:6482. doi: 10.1038/srep06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Spaeth R.B., Freeman S.G., Scarborough D.M., Hashmi J.A., Wey H.-Y., Egorova N., Vangel M., Mao J., Wasan A.D., Edwards R.R., Gollub R.L., Kong J. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol. Pain. 2015;11:67. doi: 10.1186/s12990-015-0071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covasala O., Stirn S.L., Albrecht S., De Col R., Messlinger K. Calcitonin gene-related peptide receptors in rat trigeminal ganglion do not control spinal trigeminal activity. J. Neurophysiol. 2012;108:431–440. doi: 10.1152/jn.00167.2011. [DOI] [PubMed] [Google Scholar]
- DaSilva A.F.M., Becerra L., Makris N., Strassman A.M., Gonzalez R.G., Geatrakis N., Borsook D. Somatotopic activation in the human trigeminal pain pathway. J. Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. (doi:22/18/8183 [pii]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener H.C., Kronfeld K., Boewing G., Lungenhausen M., Maier C., Molsberger A., Tegenthoff M., Trampisch H.J., Zenz M., Meinert R. Efficacy of acupuncture for the prophylaxis of migraine: a multicentre randomised controlled clinical trial. Lancet Neurol. 2006;5:310–316. doi: 10.1016/S1474-4422(06)70382-9. [DOI] [PubMed] [Google Scholar]
- Diener H.-C., Charles A., Goadsby P.J., Holle D. New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol. 2015;14:1010–1022. doi: 10.1016/S1474-4422(15)00198-2. [DOI] [PubMed] [Google Scholar]
- Dougherty D.D., Kong J., Webb M., Bonab A.A., Fischman A.J., Gollub R.L. A combined [11C]diprenorphine PET study and fMRI study of acupuncture analgesia. Behav. Brain Res. 2008;193:63–68. doi: 10.1016/j.bbr.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova N., Gollub R.L., Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin. 2015;9:430–435. doi: 10.1016/j.nicl.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facco E., Liguori A., Petti F., Zanette G., Coluzzi F., De Nardin M., Mattia C. Traditional acupuncture in migraine: a controlled, randomized study. Headache. 2008;48:398–407. doi: 10.1111/j.1526-4610.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- Fields H.L. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields H.L., Heinricher M.M. Anatomy and physiology of a nociceptive modulatory system. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Han J.S. Acupuncture analgesia: areas of consensus and controversy. Pain. 2011;152:S41–S48. doi: 10.1016/j.pain.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Han Y., Wang J., Zhao Z., Min B., Lu J., Li K., He Y., Jia J. NeuroImage frequency-dependent changes in the amplitude of low-frequency fl uctuations in amnestic mild cognitive impairment : a resting-state fMRI study. NeuroImage. 2011;55:287–295. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- Headache Classification Subcommittee of the International Headache, S The International Classification of headache disorders: 2nd edition. Cephalalgia. 2004;24(Suppl. 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- Hoptman M.J., Zuo X.N., Butler P.D., Javitt D.C., D'Angelo D., Mauro C.J., Milham M.P. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr. Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., White N.S., Kwong K.K., Vangel M.G., Rosman I.S., Gracely R.H., Gollub R.L. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum. Brain Mapp. 2006;27:715–721. doi: 10.1002/hbm.20213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Gollub R., Huang T., Polich G., Napadow V., Hui K., Vangel M., Rosen B., Kaptchuk T.J. Acupuncture de qi, from qualitative history to quantitative measurement. J. Altern. Complement. Med. 2007;13:1059–1070. doi: 10.1089/acm.2007.0524. [DOI] [PubMed] [Google Scholar]
- Kong J., Loggia M.L., Zyloney C., Tu P., Laviolette P., Gollub R.L. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148:257–267. doi: 10.1016/j.pain.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Tu P.C., Zyloney C., Su T.P. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behav. Brain Res. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger I.L., May A. Triptan-induced disruption of trigemino-cortical connectivity. Neurology. 2015;84:2124–2131. doi: 10.1212/WNL.0000000000001610. [DOI] [PubMed] [Google Scholar]
- Leonardi M., Steiner T.J., Scher A.T., Lipton R.B. The global burden of migraine: measuring disability in headache disorders with WHO's classification of functioning, disability and health (ICF) J. Headache Pain. 2005;6:429–440. doi: 10.1007/s10194-005-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liang F., Yang X., Tian X., Yan J., Sun G., Chang X., Tang Y., Ma T., Zhou L., Lan L., Yao W., Zou R. Acupuncture for treating acute attacks of migraine: a randomized controlled trial. Headache. 2009;49:805–816. doi: 10.1111/j.1526-4610.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- Li Y., Zheng H., Witt C.M., Roll S., Yu S.G., Yan J., Sun G.J., Zhao L., Huang W.J., Chang X.R., Zhang H.X., Wang D.J., Lan L., Zou R., Liang F.R. Acupuncture for migraine prophylaxis: a randomized controlled trial. CMAJ. 2012;184:401–410. doi: 10.1503/cmaj.110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Zhang Y., Ning Y., Zhang H., Liu H., Fu C., Ren Y., Zou Y. The effects of acupuncture treatment on the right frontoparietal network in migraine without aura patients. J. Headache Pain. 2015;16:1–10. doi: 10.1186/s10194-015-0518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liu M., Lan L., Zeng F., Makris N., Liang Y., Guo T., Wu F., Gao Y., Dong M., Yang J., Li Y., Gong Q., Liang F., Kong J. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci. Rep. 2016;6:20298. doi: 10.1038/srep20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde K., Streng A., Jürgens S., Hoppe A., Brinkhaus B., Witt C., Wagenpfeil S., Pfaffenrath V., Hammes M.G., Weidenhammer W., Willich S.N., Melchart D. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. 2005 doi: 10.1001/jama.293.17.2118. [DOI] [PubMed] [Google Scholar]
- Mainero C., Boshyan J., Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann. Neurol. 2011;70:838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Millan M.J. Descending control of pain. Prog. Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Qi R., Zhang L., Wu S., Zhong J., Zhang Z., Zhong Y., Ni L., Li K., Jiao Q., Wu X., Fan X., Liu Y., Lu G. Altered resting-state brain activity at functional MR imaging during the progression of hepatic encephalopathy. Radiology. 2012;264:187–195. doi: 10.1148/radiol.12111429. [DOI] [PubMed] [Google Scholar]
- Qi R., Liu C., Ke J., Xu Q., Zhong J., Wang F., Zhang L.J., Lu G.M. Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9478-1. [DOI] [PubMed] [Google Scholar]
- Rasmussen B.K., Olesen J. Migraine with aura and migraine without aura: an epidemiological study. Cephalalgia. 1992;12:221–228. doi: 10.1046/j.1468-2982.1992.1204221.x. (discussion 186) [DOI] [PubMed] [Google Scholar]
- Saad Z.S., Gotts S.J., Murphy K., Chen G., Jo H.J., Martin A., Cox R.W. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. doi: 10.1089/brain.2012.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte L.H., Sprenger C., May A. Physiological brainstem mechanisms of trigeminal nociception: an fMRI study at 3T. NeuroImage. 2016;124:518–525. doi: 10.1016/j.neuroimage.2015.09.023. [DOI] [PubMed] [Google Scholar]
- Schwedt T.J., Larson-Prior L., Coalson R.S., Nolan T., Mar S., Ances B.M., Benzinger T., Schlaggar B.L. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med. 2014;15:154–165. doi: 10.1111/pme.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwedt T.J., Chiang C.-C., Chong C.D., Dodick D.W. Functional MRI of migraine. Lancet Neurol. 2015;14:81–91. doi: 10.1016/S1474-4422(14)70193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S., Hartl T., Weber M., Matterey S., Paul A., Riederer F., Gharabaghi M., Wöber-Bingöl Ç., Wöber C. Quality of sleep, fatigue and daytime sleepiness in migraine - a controlled study. Cephalalgia. 2009;29:662–669. doi: 10.1111/j.1468-2982.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z., He Y., Yan C.-G., Zang Y.-F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011 doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankewitz A., May A. Increased limbic and brainstem activity during migraine attacks following olfactory stimulation. Neurology. 2011;77:476–482. doi: 10.1212/WNL.0b013e318227e4a8. [DOI] [PubMed] [Google Scholar]
- Stankewitz A., Aderjan D., Eippert F., May A. Trigeminal nociceptive transmission in migraineurs predicts migraine attacks. J. Neurosci. 2011;31:1937–1943. doi: 10.1523/JNEUROSCI.4496-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovner L.J., Hagen K. Prevalence, burden, and cost of headache disorders. Curr. Opin. Neurol. 2006;19:281–285. doi: 10.1097/01.wco.0000227039.16071.92. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P., Block G., Dahlof C., Diener H.C., Ferrari M.D., Goadsby P.J., Guidetti V., Jones B., Lipton R.B., Massiou H., Meinert C., Sandrini G., Steiner T., Winter P.B., International Headache Society Clinical Trials, S Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20:765–786. doi: 10.1046/j.1468-2982.2000.00117.x. [DOI] [PubMed] [Google Scholar]
- Unger J.W., Lange W. Immunohistochemical mapping of neurophysins and calcitonin gene-related peptide in the human brainstem and cervical spinal cord. J. Chem. Neuroanat. 1991;4:299–309. doi: 10.1016/0891-0618(91)90020-d. (doi:0891-0618(91)90020-D [pii]) [DOI] [PubMed] [Google Scholar]
- Usai S., Grazzi L., D'Amico D., Andrasik F., Bussone G. Psychological variables in chronic migraine with medication overuse before and after inpatient withdrawal: results at 1-year follow-up. Neurol. Sci. 2009;30 doi: 10.1007/s10072-009-0066-2. [DOI] [PubMed] [Google Scholar]
- Van Rossum D., Hanisch U.K., Quirion R. Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- Vickers A.J., Linde K. Acupuncture for chronic pain. JAMA. 2014;311:955–956. doi: 10.1001/jama.2013.285478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.P., Zhang X.Z., Guo J., Liu H.L., Zhang Y., Liu C.Z., Yi J.H., Wang L.P., Zhao J.P., Li S.S. Efficacy of acupuncture for migraine prophylaxis: a single-blinded, double-dummy, randomized controlled trial. Pain. 2011;152:1864–1871. doi: 10.1016/j.pain.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xue C.C., Helme R., Da Costa C., Zheng Z. Acupuncture for frequent migraine: a randomized, patient/assessor blinded, controlled trial with one-year follow-up. Evid. Based Complement. Alternat. Med. 2015;2015:920353. doi: 10.1155/2015/920353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-J., Chen X., Sah S.K., Zeng C., Li Y.-M., Li N., Liu M.-Q., Du S.-L. Amplitude of low-frequency fluctuation (ALFF) and fractional ALFF in migraine patients: a resting-state functional MRI study. Clin. Radiol. 2016;71:558–564. doi: 10.1016/j.crad.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Weiller C., May A., Limmroth V., Juptner M., Kaube H., Schayck R.V., Coenen H.H., Diener H.C. Brain stem activation in spontaneous human migraine attacks. Nat. Med. 1995;1:658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- Wrobel Goldberg S., Silberstein S.D. Targeting CGRP: a new era for migraine treatment. CNS Drugs. 2015;29:443–452. doi: 10.1007/s40263-015-0253-z. [DOI] [PubMed] [Google Scholar]
- Xue T., Yuan K., Cheng P., Zhao L., Zhao L., Yu D., Dong T., von Deneen K.M., Gong Q., Qin W., Tian J. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013;26:1051–1058. doi: 10.1002/nbm.2917. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., Colcombe S., Craddock R.C., Di Martino A., Li Q., Zuo X.N., Castellanos F.X., Milham M.P. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zeng F., Feng Y., Fang L., Qin W., Liu X., Song W., Xie H., Chen J., Liang F. A PET-CT study on the specificity of acupoints through acupuncture treatment in migraine patients. BMC Complement. Altern. Med. 2012;12:123. doi: 10.1186/1472-6882-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Yang J., Zeng F., Liu P., Lai Z., Deng S., Fang L., Song W., Xie H., Liang F. Electroacupuncture stimulation at sub-specific acupoint and non-acupoint induced distinct brain glucose metabolism change in migraineurs: a PET-CT study. J. Transl. Med. 2014;12:351. doi: 10.1186/s12967-014-0351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y.-F., He Y., Zhu C.-Z., Cao Q.-J., Sui M.-Q., Liang M., Tian L.-X., Jiang T.-Z., Wang Y.-F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang R., Lao L., Ren K., Berman B.M. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503. doi: 10.1097/ALN.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Liu J., Zhang F., Dong X., Peng Y., Qin W., Wu F., Li Y., Yuan K., von Deneen K.M., Gong Q., Tang Z., Liang F. Effects of long-term acupuncture treatment on resting-state brain activity in migraine patients: a randomized controlled trial on active acupoints and inactive acupoints. PLoS One. 2014;9 doi: 10.1371/journal.pone.0099538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Liu J., Yan X., Dun W., Yang J., Huang L., Kai Y., Yu D., Qin W., Jie T., Liang F. Abnormal brain activity changes in patients with migraine: a short-term longitudinal study. J. Clin. Neurol. 2014;10:229–235. doi: 10.3988/jcn.2014.10.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Chen J., Li Y., Sun X., Chang X., Zheng H., Gong B., Huang Y., Yang M., Wu X., Li X., Liang F. The long-term effect of acupuncture for migraine prophylaxis. JAMA Intern. Med. 2017 doi: 10.1001/jamainternmed.2016.9378. [DOI] [PubMed] [Google Scholar]
- Zou Q., Wu C.W., Stein E.A., Zang Y., Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48:515–524. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Di Martino A., Kelly C., Shehzad Z.E., Gee D.G., Klein D.F., Castellanos F.X., Biswal B.B., Milham M.P. The oscillating brain: complex and reliable. NeuroImage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]