Abstract
The question of whether there are biological differences between male and female brains is a fraught one, and political positions and prior expectations seem to have a strong influence on the interpretation of scientific data in this field. This question is relevant to issues of gender differences in the prevalence of psychiatric conditions, including autism, attention deficit hyperactivity disorder (ADHD), Tourette's syndrome, schizophrenia, dyslexia, depression, and eating disorders. Understanding how gender influences vulnerability to these conditions is significant. Diffusion magnetic resonance imaging (dMRI) provides a non-invasive method to investigate brain microstructure and the integrity of anatomical connectivity. Generalized q-sampling imaging (GQI) has been proposed to characterize complicated fiber patterns and distinguish fiber orientations, providing an opportunity for more accurate, higher-order descriptions through the water diffusion process. Therefore, we aimed to investigate differences in the brain's structural network between teenage males and females using GQI. This study included 59 (i.e., 33 males and 26 females) age- and education-matched subjects (age range: 13 to 14 years). The structural connectome was obtained by graph theoretical and network-based statistical (NBS) analyses. Our findings show that teenage male brains exhibit better intrahemispheric communication, and teenage female brains exhibit better interhemispheric communication. Our results also suggest that the network organization of teenage male brains is more local, more segregated, and more similar to small-world networks than teenage female brains. We conclude that the use of an MRI study with a GQI-based structural connectomic approach like ours presents novel insights into network-based systems of the brain and provides a new piece of the puzzle regarding gender differences.
Keywords: Gender difference, Generalized q-sampling imaging (GQI), Structural connectome, Graph theoretical analysis, Network-based statistical (NBS) analysis
Highlights
-
•
The GQI-based structural connectomic study provides a new piece of the puzzle regarding gender differences.
-
•
Male brains exhibit better intrahemispheric communication, and female exhibit better interhemispheric communication.
-
•
The network organization of teenage male brains is more local and more segregated than teenage female brains.
1. Introduction
It is commonly thought that males and females exhibit different behaviors. Common stereotypes include that females can do many things at the same time, but they have a poor sense of direction when driving, whereas males can coordinate and cooperate easily but they are not good at expressing emotions. That is, males tend to have better motor ability and spatial cognition, while females tend to have superior memories, facial recognition, and social skills (Gur et al., 2012, Halpern et al., 2007). The question of whether there are biological differences between male and female brains is a fraught one, and political positions and prior expectations seem to have a strong influence on the interpretation of scientific data in this field (Abramov et al., 2012, Fairchild et al., 2016, Ingalhalikar et al., 2014, Joel, 2011, Joel et al., 2015, Xu et al., 2015).
Gender differences in human brains is an important topic because the prevalence of psychiatric conditions varies between the genders; such differences have been observed in autism (much more common in males) (Baron-Cohen, 2009, Baron-Cohen et al., 2005), attention deficit hyperactivity disorder (ADHD, much more common in males) (Arnett et al., 2015), Tourette's syndrome (much more common in males) (Yang et al., 2016), schizophrenia and dyslexia (more common in males) (Arnett et al., 2017, McGrath et al., 2008), depression (more common in females) (Goldstein et al., 2014, Schuch et al., 2014, Shansky, 2009), and eating disorders (much more common in females) (Lipson and Sonneville, 2017). Understanding how gender influences vulnerability to these conditions is, therefore, a significant question.
Previous studies found strong group differences between male and female brains; despite comparable findings, the authors of these studies interpreted the results in almost polar opposite fashions (Ingalhalikar et al., 2014, Joel et al., 2015, Szalkai et al., 2015). One structural connectivity study interpreted these group differences as the basis for gender differences in cognition (Ingalhalikar et al., 2014). They defined the structural connectivity networks across the brains of 428 males and 521 females using diffusion tensor imaging (DTI). They subsequently analyzed these networks using a variety of statistical measures of regional and global connectivity and compared the results between males and females. They found that on average, females had greater connectivity between hemispheres than males, while males had greater connectivity within each hemisphere. Males also showed, on average, greater local connectivity and concomitantly increased modularity in the network.
These authors extrapolated their findings to explain a variety of group differences in cognition between men and women. The participants in the structural connectivity analysis were part of a larger sample for which cognitive data had already been obtained, showing gender differences in a variety of domains. Such differences have been widely documented and range from very small to fairly large. The results revealed stark differences between the groups and suggested complementarity in the architecture of the human brain, which helps provide a potential neural basis for why men excel at certain tasks and women at others. For instance, men are usually better at learning and performing a single task at hand, such as cycling or navigating directions, whereas women have superior memory and social cognition skills, making them more equipped for multitasking and creating solutions that work for a group (Ingalhalikar et al., 2014).
A different study on the volume of brain regions downplayed these differences entirely and instead emphasized the inherent variability within genders, concluding that there was no such thing as a “male brain” or a “female brain” (Joel et al., 2015). They analyzed the MRI scans of 169 females and 112 males and segmented them into 116 regions using a standard brain atlas. By analyzing how much warping was required to map each brain onto a reference template, it was possible to compare the relative gray matter volume of all these regions across the two genders. From this group comparison, the 10 regions showing the largest gender differences were chosen for subsequent analyses. The researchers found statistically significant group differences between males and females in gray matter volume across many brain regions. A recent meta-analysis of 167 studies confirms consistent gender differences in many brain areas between men and women (Ruigrok et al., 2014).
Joel et al. (2015) went on to ask a more interesting question: across those ten regions, how “male” or “female” were the structures of individual brains? This is where subjectivity comes in – there are many ways to analyze these data, and the authors chose arguably the most simplistic and extreme one, which enabled them to draw the conclusion that male and female brains are not categorically different. They reported that 35% of brains showed substantial variability, and only 6% of brains were internally consistent. Importantly, they chose to classify only those subjects showing extreme male or female values for all ten regions as internally consistent.
The fact that most individuals show this pattern does not mean that each of us has a “mosaic brain” that is partly male and partly female, as claimed by the authors. It is exactly what is expected given that gender is only one of the factors affecting the size of each of these regions. We can only deduce from the group average effects that there would likely have been some effect. The conclusion that male and female brains are not that different is not well supported by these findings. The group differences are clear and highly significant. Even if very few of the males or females are at the extreme end of the distribution for all ten of these regions, the overall pattern suggests that you could build a highly accurate classifier from the volumes of these ten regions taken together that would be successful at predicting whether a given brain scan came from a male or a female. Indeed, this would have been a far more objective test of whether MRI volumetric differences between male and female brains are categorical or dimensional.
Another group published several studies that showed striking sex differences among human connectomes using graph theoretical parameters; they revealed clear differences and suggested the superiority of female brains (Szalkai et al., 2015). They also accounted for possible artifacts caused by statistical size differences between male and female brains. The graph theoretical parameters of 36 large-brained females and 36 small-brained males were compared, and the differences remained statistically significant.
Diffusion MRI is increasingly applied in the study of many white matter disorders (Blood et al., 2010, Cheng et al., 2014, Ota et al., 2015, Roine et al., 2015b, Thomason and Thompson, 2011, van den Heuvel and Fornito, 2014). It can provide a non-invasive method to investigate brain microstructure and the integrity of anatomical connectivity; this is not possible with other imaging modalities. Fractional anisotropy (FA), the most commonly used index of DTI, provides a measure of white matter tract integrity (Beaulieu, 2002, Srivastava et al., 2016, Wise et al., 2016). However, DTI is unable to detect the crossing or branching patterns of complex regions, and DTI which based on Gaussian diffusion and monoexponential b-value dependence reflects the weighted average of all compartments even though the partial volumes of different diffusion compartments may vary (Roine et al., 2014, Roine et al., 2015a, Vos et al., 2011). To better characterize the complicated fiber patterns and distinguish fiber orientations, several novel diffusion-based methods have been proposed, providing an opportunity for more accurate, higher-order descriptions when compared to DTI (Descoteaux et al., 2009, Jensen et al., 2005, Tournier et al., 2007, Tournier et al., 2004, Tuch, 2004, Wang et al., 2014, Wang et al., 2011, Wedeen et al., 2005, Yeh et al., 2010, Zhang et al., 2012). Generalized q-sampling imaging (GQI) is a novel q-space reconstruction method derived from q-space imaging. Compared with DTI, GQI can be applied to a wider range of q-space datasets for a more accurate and sophisticated diffusion MR approach (Yeh et al., 2010). For example, GQI can extract additional information about the altered diffusion environments by including several indices, such as generalized fractional anisotropy (GFA), normalized quantitative anisotropy (NQA), and the isotropic value of the orientation distribution function (ISO) (Shen et al., 2015, Yeh et al., 2010, Zhang et al., 2013).
Recently, the concept of the connectome has been proposed as a conceptual framework for brain research. This model assumes that the structural and functional organization of the human brain is organized into complex networks, allowing for the measurement of network properties such as the segregation or integration of the information processing within the network. Based on topology, graph theoretical analysis quantitatively provides novel insight into the connectome by using nodes, edges and several additional topological parameters, such as the clustering coefficient, characteristic path length and small-worldness (Bullmore and Sporns, 2009, Hosseini et al., 2012, Sporns, 2013). In structural connection analysis, nodes are usually derived by parcellating cortical and subcortical gray matter regions according to anatomical landmarks, or by defining a random parcellation into evenly sized voxel clusters, and edges are usually referred to as the white matter projections linking cortical and subcortical regions (Sporns, 2013). Available graph-theoretical studies have broadly aimed to assess the organization of structural and functional brain networks using MRI during normal development, aging, and organic and neuropsychiatric brain disorders; the results suggest that brain networks are correlated with behavioral and cognitive functions (Bullmore and Bassett, 2011, Gong and He, 2015, Lo et al., 2011, Zhang et al., 2011).
A recent MRI study has identified the areas of the brain that change the most during the teenage years and are associated with complex thought processes (Whitaker et al., 2016). Furthermore, a link between teenage brain development and mental illness, such as schizophrenia, has also been discovered (Whitaker et al., 2016). Because the developmental trajectories of males and females diverge at a young age, resulting in wide differences during adolescence and adulthood, we would like to focus on gender differences in the brain during the developmental period. Therefore, the main aim of this study was to use GQI-based analysis to assess differences in the teenage brain structural connectome between males and females using graph theoretical and network-based statistical analyses.
2. Materials and methods
2.1. MRI data acquisition
A total of 59 age- and education-matched teenagers (33 males and 26 females) between 12 and 14 years of age (male: 13.9 ± 0.58, female: 14 ± 0.28) were recruited for this study. Informed consent was obtained from all participants; the study was approved by the Institutional Review Board of Chung Shan Medical University Hospital. All participants were right-handed. No participant had a history of psychiatric or neurological illness or substance abuse, and none were currently taking any prescription or psychotropic medications. The exclusion criteria for the study included metallic implants or other contraindications to MRI.
All diffusion images were acquired using a 3-Tesla MRI (Skyra, Siemens, Germany) with a 20 channel head neck coil. The diffusion images were acquired using a multi-shell scheme with repetition time (TR) = 4800 ms, echo time (TE) = 97 ms, voxel size = 2 × 2 × 4 mm3, 35 axial contiguous slices, signal average = 1, 64 × 3 non-collinear diffusion weighting gradient direction with b = 1000, 1500, 2000 s/mm2, and 12 additional null images without diffusion weighting (b = 0 s/mm2). The scan time was approximately 16.5 min.
2.2. GQI analysis
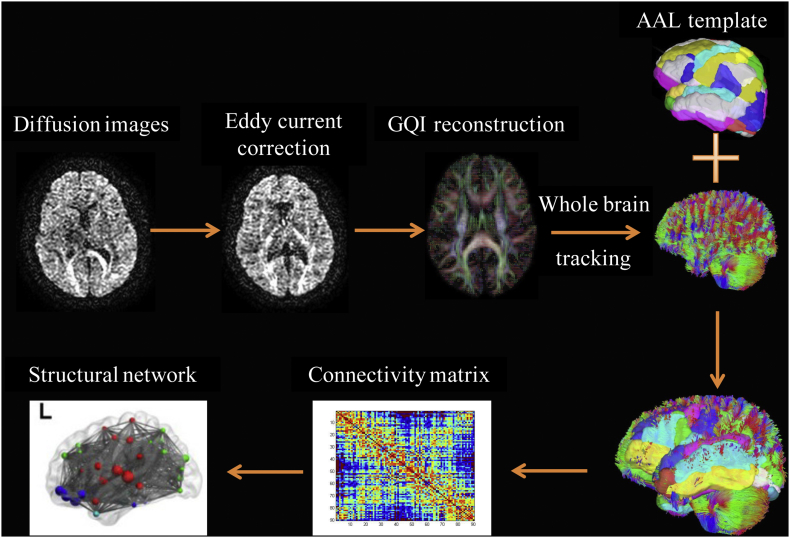
After using FSL (FMRIB Software Library, Oxford, UK) for both eddy currents and subject movements (including b-vectors rotation) correction (Leemans and Jones, 2009), each participant's echo planar image was spatially normalized to the Montreal Neurological Institute (MNI) T2 template using parameters determined from the normalization of the diffusion null image to the T2 template using Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK). The DSI Studio (National Taiwan University, Taipei, Taiwan) was employed for whole brain GQI tractography (seeds were placed in the whole brain) with a normalized quantitative anisotropy (NQA) threshold of 0.15 and a maximum angle of 70° (Yeh et al., 2010). Next, the individual structural connectivity matrix (fiber number × NQA / mean fiber length) of each participant (size of 90 × 90) could be outputted and the ROIs inputted based on a standard parcellation template contained in the Automated Anatomical Labeling (AAL) software package. The schematic of the pipeline for creating the structural connectivity matrix and network are shown in Fig. 1.
Fig. 1.
Schematic of the pipeline for creating the brain structural connectivity matrix and network using GQI.
2.3. Graph theoretical analysis
Graph theoretical analysis (GTA) was performed on the interregional connectivity matrix using the Graph Analysis Toolbox (GAT, Stanford University School of Medicine, Stanford, CA, USA), a Matlab-based package with graphical user interface that integrates the Brain Connectivity Toolbox (BCT) (Hosseini et al., 2012, Rubinov and Sporns, 2010). The density was calculated by connection with a threshold divided by the connection of random network. The topological measures of structural brain network densities were calculated with different correlation thresholds (0.05–0.3 in 0.01 increments), including the clustering coefficient (C), normalized clustering coefficient (γ), characteristic path length (L), normalized characteristic path length (λ), local efficiency (Elocal), global efficiency (Eglobal), small-worldness index (σ), transitivity, and modularity (Bassett and Bullmore, 2006, Bassett and Gazzaniga, 2011, Bullmore and Sporns, 2009, Hosseini et al., 2012, Onnela et al., 2005, Saramaki et al., 2007, Watts and Strogatz, 1998). C and Elocal reflect local segregation; C quantifies the extent of local interconnectivity in the network, while Elocal indicates how well the sub-graphs exchange information with each other. High scores on the two measures correspond to highly segregated neural processing. L and Eglobal reflect the global integration; L measures the capability for information transfer between brain regions, while Eglobal is a measure of the overall capacity for parallel information transfer and integrated processing. A lower L score or higher Eglobal score indicates more rapid integration of specialized information from distributed brain regions. The γ and λ values are normalized relative to the C and L of 100 random networks. Small-worldness (σ) is calculated by dividing γ by λ. The transitivity is the ratio of triangles to triplets in the network and is an alternative to the clustering coefficient. The modularity is a statistic that quantifies the degree to which the network may be subdivided into clearly delineated groups.
2.4. Network-based statistical analysis
Using network-based statistical analysis (NBS) (Zalesky et al., 2010a), differences in network topology and regional networks between groups were evaluated using two-sample t-tests calculated from the areas under the curve of topological measures with multiple thresholds and non-parametric permutation tests (1000 repetitions; NBS, Melbourne Neuropsychiatry Centre, The University of Melbourne and Melbourne Health, Australia). p-Values < 0.05 were considered statistically significant for both the permutation test in the graph theoretical analysis (implemented in GAT toolbox) and the two-sample Student's t-test in NBS analysis. The BrainNet viewer (The MathWorks Inc., Natick, MA, US) was applied to visualize the significant subnetworks revealed by NBS.
3. Results
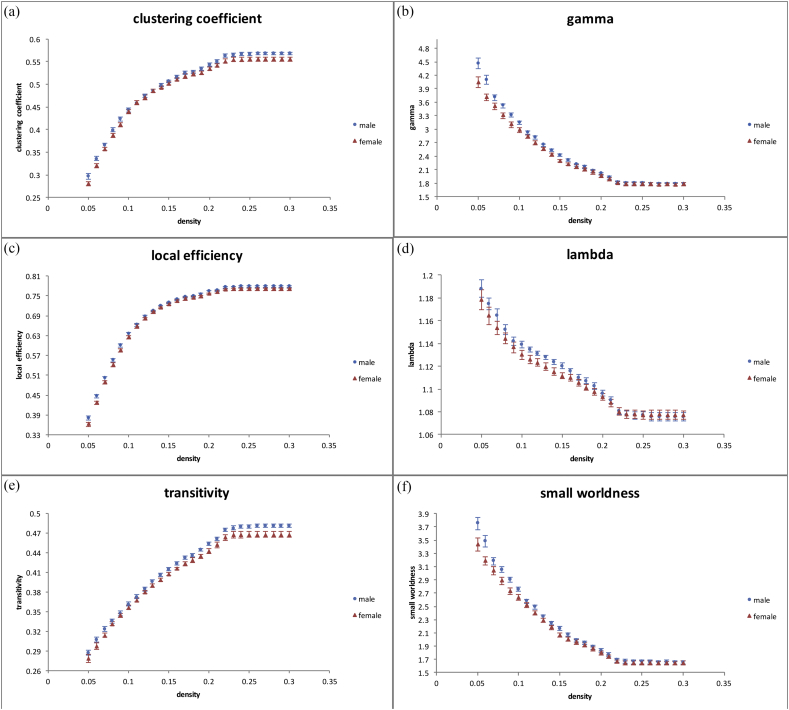
The GTA revealed a significantly higher clustering coefficient (Fig. 2a), normalized clustering coefficient (γ, Fig. 2b), local efficiency (Fig. 2c), and transitivity (Fig. 2e) in teenage males than in teenage females (p < 0.05), and a significantly lower normalized characteristic path length (λ, Fig. 2d) was observed in teenage females than in teenage males (p < 0.05). In addition, a higher degree of small-worldness (σ, Fig. 2f) was found in teenage males compared with teenage females (p < 0.05). All results were expressed as the mean ± standard error.
Fig. 2.
Higher topological measures were found in teenage male brain networks with GQI, including (a) clustering coefficient, (b) normalized clustering coefficient, (c) local efficiency, (d) normalized characteristic path length, (e) transitivity, and (f) small-worldness index (p < 0.05).
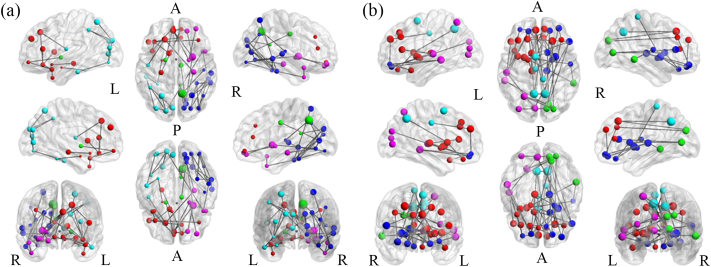
Based on NBS, we found that teenage males had better intrahemispheric, local, short-range, within-lobe connectivity, especially within bilateral frontal lobes and parieto-occipital lobes (Fig. 3a), while teenage females had better interhemispheric, long-range connectivity, especially between bilateral frontal lobes and between bilateral frontal to contralateral parieto-occipital lobes (Fig. 3b) (p < 0.05). Node size represents the degree of information, with bigger nodes indicating a higher degree. Modularity information is represented by the colors of the nodes. Modularity is a more complex measure of network segregation; it breaks the network into non-overlapping modules of nodes in a way that maximizes the number of within-module edges and minimizes the number of between-module edges. The community structure with the highest maximized modularity value was used as the representative modular structure.
Fig. 3.
In the results of network-based statistical analysis, (a) more intrahemispheric, local, short-range, within-lobe connectivity was found in teenage males, especially within bilateral frontal lobes and parieto-occipital lobes, (b) while more interhemispheric, long-range connectivity was found in teenage females, especially between bilateral frontal lobes and between bilateral frontal to contralateral parieto-occipital lobes (p < 0.05). The nodes with the same color represent the same module. Node size represents the degree of information, with bigger nodes indicating a higher degree.
4. Discussion
In this study, we examined gender differences in a group of 59 teenagers by comprehensively analyzing the GQI-based structural connectome of the brain. Our findings confirmed earlier hypotheses and contributed to the literature on the novel utility of GQI in the analysis of gender differences. Previous structural imaging showed a higher proportion of cortical white matter in males except in the corpus callosum (Schmithorst et al., 2008); this result may suggest that male brains are better for communicating within hemispheres, whereas female brains are better for interhemispheric communication (Cherney et al., 2008, Dubb et al., 2003, Gur et al., 1999, Steinmetz et al., 1995). Based on NBS, our results supported and consolidated this hypothesis at a global and regional level and revealed gender differences in the brain architecture. Significantly, these differences occurred in early adolescence, suggesting that teenage male brains are indeed structured to facilitate intrahemispheric cortical connectivity; in contrast, teenage female brains displayed higher interhemispheric connectivity (Ingalhalikar et al., 2014).
In addition to NBS, we investigated a complementary network measurement using GTA that revealed significantly higher clustering coefficients, normalized clustering coefficients, local efficiency and transitivity in teenage male brains, which indicated higher local segregation of brain networks in teenage males. Additionally, we found a significantly lower normalized characteristic path length in teenage female brains, which indicated higher global integration of brain networks in teenage females. Furthermore, a higher small-worldness was found in teenage males, which indicated that the neural connections of teenage male brains were closer to small-world networks. These topographical measurements delineated and quantified the distribution of the connectome, that is, how the connectome can be divided into subnetworks. Higher (normalized) clustering coefficients, local efficiency and transitivity in teenage males pointed to a region's neighbors being more strongly connected to each other within each lobe of the brain, resulting in increased global modularity. This is also indicative of the enhanced local, short-range, within-lobe connectivity among teenage males. In contrast, the lower normalized characteristic path lengths of the teenage females indicated that teenage females begin to develop higher long-range connectivity, which is mainly interhemispheric.
During developmental periods, male brains tend to be structured to facilitate within-lobe and within-hemisphere connectivity, involving links between perception and action and building more efficient systems for coordinated actions. In contrast, female brains tend to have better interhemispheric connectivity and better cross-hemispheric participation, which increases integration of bilateral hemispheric functional performance (Ingalhalikar et al., 2014). Several recent behavioral studies demonstrated distinct gender differences, with females performing better on attention, word and face memory, and social cognition tests, and males performing better on spatial processing and motor and sensorimotor speed. Most gender differences were apparent by early adolescence (Cherney et al., 2008, Gur et al., 2012).
In addition to being consistent with behavioral studies, our results are also consistent with several advanced MRI studies. In previous DTI studies, a higher FA and lower mean diffusivity (MD) in the corpus callosum were found in females during mid-adolescence (Asato et al., 2010, Bava et al., 2011, Schmithorst and Yuan, 2010). In previous functional MRI studies, better interhemispheric activation in females on language tasks (Shaywitz et al., 1995) and better focal intra-hemispheric activation in males on spatial tasks have been revealed (Gur et al., 2000). Taken together, all of the above-mentioned MRI studies with either structural or functional approaches support the concept of behavioral complementarity between male and female brains.
Several limitations in the present study need to be considered. First, the age-matched cross-sectional design did not allow us to observe the effects of development or aging in the participants, hence, longitudinal studies are needed to examine such effects. Second, puberty status and sex steroids level can influence brain development and may cause possible bias for gender differences analysis (Neufang et al., 2009), but both were not considered in our study. Third, the limitation of our MR scanner forced us to use less slides number for whole brain coverage and resulted in anisotropic voxel size for GQI analysis. Fourth, we performed eddy currents correction but no other echo planar imaging distortions correction, which may affect the alignment of the diffusion and anatomical data for normalization. Last, in the connectome analysis, the parcellation scheme we used was based on the widely-used AAL template, which divided and limited the whole brain into 90 regions and may cause risks of the results to be driven by bigger regions such as those of the frontal lobe. Several studies have reported that different schemes could result in distinct topological patterns and suggested a reasonable trade-off between increased spatial resolution and attenuated signal-to-noise ratio should be carefully considered (Fornito et al., 2010, Salvador et al., 2008, Zalesky et al., 2010b). Therefore, further studies should combine more parcellation strategies to explore the effects on network topology.
5. Conclusions
Our results establish that teenage male brains exhibit better intrahemispheric communication and teenage female brains exhibit interhemispheric communication. Our results also suggest that the network organization of teenage male brains is more local, more segregated, and more similar to small-world networks than teenage female brains. We conclude that MRI studies using a GQI-based structural connectomic approach like ours can offer novel insights into the network-based systems of the brain and provide a new piece of the puzzle regarding gender differences.
Acknowledgements
This study was supported in part by the inter-institutional research program NCHU-CSMU-10512 from the National Chung Hsing University and Chung Shan Medical University, Taichung, Taiwan, and the research programs MOST104-2314-B-040-001 and NSC103-2420-H-040-002, which were sponsored by the Ministry of Science and Technology, Taiwan.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2017.05.014.
Appendix A. Supplementary data
The following is the supplementary data related to this article.
Supplementary tables
References
- Abramov I., Gordon J., Feldman O., Chavarga A. Sex & vision I: spatio-temporal resolution. Biol. Sex Differ. 2012;3:20. doi: 10.1186/2042-6410-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett A.B., Pennington B.F., Willcutt E.G., DeFries J.C., Olson R.K. Sex differences in ADHD symptom severity. J. Child Psychol. Psychiatry. 2015;56:632–639. doi: 10.1111/jcpp.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett A.B., Pennington B.F., Peterson R.L., Willcutt E.G., DeFries J.C., Olson R.K. Explaining the sex difference in dyslexia. J. Child Psychol. Psychiatry. 2017;58:719–727. doi: 10.1111/jcpp.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescence: a DTI study. Cereb. Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Autism: the empathizing-systemizing (E-S) theory. Ann. N. Y. Acad. Sci. 2009;1156:68–80. doi: 10.1111/j.1749-6632.2009.04467.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Knickmeyer R.C., Belmonte M.K. Sex differences in the brain: implications for explaining autism. Science. 2005;310:819–823. doi: 10.1126/science.1115455. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett D.S., Gazzaniga M.S. Understanding complexity in the human brain. Trends Cogn. Sci. 2011;15:200–209. doi: 10.1016/j.tics.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S., Boucquey V., Goldenberg D., Thayer R.E., Ward M., Jacobus J., Tapert S.F. Sex differences in adolescent white matter architecture. Brain Res. 2011;1375:41–48. doi: 10.1016/j.brainres.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blood A.J., Iosifescu D.V., Makris N., Perlis R.H., Kennedy D.N., Dougherty D.D., Kim B.W., Lee M.J., Wu S., Lee S., Calhoun J., Hodge S.M., Fava M., Rosen B.R., Smoller J.W., Gasic G.P., Breiter H.C., Phenotype Genotype Project on Addiction and Mood Disorders Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS One. 2010;5:e13945. doi: 10.1371/journal.pone.0013945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E.T., Bassett D.S. Brain graphs: graphical models of the human brain connectome. Annu. Rev. Clin. Psychol. 2011;7:113–140. doi: 10.1146/annurev-clinpsy-040510-143934. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Xu J., Yu H., Nie B., Li N., Luo C., Li H., Liu F., Bai Y., Shan B., Xu L., Xu X. Delineation of early and later adult onset depression by diffusion tensor imaging. PLoS One. 2014;9:e112307. doi: 10.1371/journal.pone.0112307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney I.D., Brabec C.M., Runco D.V. Mapping out spatial ability: sex differences in way-finding navigation. Percept. Mot. Skills. 2008;107:747–760. doi: 10.2466/pms.107.3.747-760. [DOI] [PubMed] [Google Scholar]
- Descoteaux M., Deriche R., Knosche T.R., Anwander A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans. Med. Imaging. 2009;28:269–286. doi: 10.1109/TMI.2008.2004424. [DOI] [PubMed] [Google Scholar]
- Dubb A., Gur R., Avants B., Gee J. Characterization of sexual dimorphism in the human corpus callosum. NeuroImage. 2003;20:512–519. doi: 10.1016/s1053-8119(03)00313-6. [DOI] [PubMed] [Google Scholar]
- Fairchild G., Toschi N., Sully K., Sonuga-Barke E.J., Hagan C.C., Diciotti S., Goodyer I.M., Calder A.J., Passamonti L. Mapping the structural organization of the brain in conduct disorder: replication of findings in two independent samples. J. Child Psychol. Psychiatry. 2016;57:1018–1026. doi: 10.1111/jcpp.12581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Zalesky A., Bullmore E.T. Network scaling effects in graph analytic studies of human resting-state FMRI data. Front. Syst. Neurosci. 2010;4:22. doi: 10.3389/fnsys.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J.M., Holsen L., Handa R., Tobet S. Fetal hormonal programming of sex differences in depression: linking women's mental health with sex differences in the brain across the lifespan. Front. Neurosci. 2014;8:247. doi: 10.3389/fnins.2014.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q., He Y. Depression, neuroimaging and connectomics: a selective overview. Biol. Psychiatry. 2015;77:223–235. doi: 10.1016/j.biopsych.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Gur R.C., Turetsky B.I., Matsui M., Yan M., Bilker W., Hughett P., Gur R.E. Sex differences in brain gray and white matter in healthy young adults: correlations with cognitive performance. J. Neurosci. 1999;19:4065–4072. doi: 10.1523/JNEUROSCI.19-10-04065.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R.C., Alsop D., Glahn D., Petty R., Swanson C.L., Maldjian J.A., Turetsky B.I., Detre J.A., Gee J., Gur R.E. An fMRI study of sex differences in regional activation to a verbal and a spatial task. Brain Lang. 2000;74:157–170. doi: 10.1006/brln.2000.2325. [DOI] [PubMed] [Google Scholar]
- Gur R.C., Richard J., Calkins M.E., Chiavacci R., Hansen J.A., Bilker W.B., Loughead J., Connolly J.J., Qiu H., Mentch F.D., Abou-Sleiman P.M., Hakonarson H., Gur R.E. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern D.F., Benbow C.P., Geary D.C., Gur R.C., Hyde J.S., Gernsbacher M.A. The science of sex differences in science and mathematics. Psychol. Sci. Public Interest. 2007;8:1–51. doi: 10.1111/j.1529-1006.2007.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M.P., Fornito A. Brain networks in schizophrenia. Neuropsychol. Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- Hosseini S.M., Hoeft F., Kesler S.R. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012;7:e40709. doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalhalikar M., Smith A., Parker D., Satterthwaite T.D., Elliott M.A., Ruparel K., Hakonarson H., Gur R.E., Gur R.C., Verma R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. U. S. A. 2014;111:823–828. doi: 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H., Helpern J.A., Ramani A., Lu H., Kaczynski K. Diffusional kurtosis imaging: the quantification of non-Gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Joel D. Male or female? Brains are intersex. Front. Integr. Neurosci. 2011;5:57. doi: 10.3389/fnint.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel D., Berman Z., Tavor I., Wexler N., Gaber O., Stein Y., Shefi N., Pool J., Urchs S., Margulies D.S., Liem F., Hanggi J., Jancke L., Assaf Y. Sex beyond the genitalia: the human brain mosaic. Proc. Natl. Acad. Sci. U. S. A. 2015;112:15468–15473. doi: 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemans A., Jones D.K. The B-matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 2009;61:1336–1349. doi: 10.1002/mrm.21890. [DOI] [PubMed] [Google Scholar]
- Lipson S.K., Sonneville K.R. Eating disorder symptoms among undergraduate and graduate students at 12 U.S. colleges and universities. Eat. Behav. 2017;24:81–88. doi: 10.1016/j.eatbeh.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Lo C.Y., He Y., Lin C.P. Graph theoretical analysis of human brain structural networks. Rev. Neurosci. 2011;22:551–563. doi: 10.1515/RNS.2011.039. [DOI] [PubMed] [Google Scholar]
- McGrath J., Saha S., Chant D., Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Neufang S., Specht K., Hausmann M., Gunturkun O., Herpertz-Dahlmann B., Fink G.R., Konrad K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex. 2009;19:464–473. doi: 10.1093/cercor/bhn100. [DOI] [PubMed] [Google Scholar]
- Onnela J.P., Saramaki J., Kertesz J., Kaski K. Intensity and coherence of motifs in weighted complex networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005;71:065103. doi: 10.1103/PhysRevE.71.065103. [DOI] [PubMed] [Google Scholar]
- Ota M., Noda T., Sato N., Hattori K., Hori H., Sasayama D., Teraishi T., Nagashima A., Obu S., Higuchi T., Kunugi H. White matter abnormalities in major depressive disorder with melancholic and atypical features: a diffusion tensor imaging study. Psychiatry Clin. Neurosci. 2015;69:360–368. doi: 10.1111/pcn.12255. [DOI] [PubMed] [Google Scholar]
- Roine T., Jeurissen B., Perrone D., Aelterman J., Leemans A., Philips W., Sijbers J. Isotropic non-white matter partial volume effects in constrained spherical deconvolution. Front. Neuroinform. 2014;8:28. doi: 10.3389/fninf.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roine T., Jeurissen B., Perrone D., Aelterman J., Philips W., Leemans A., Sijbers J. Informed constrained spherical deconvolution (iCSD) Med. Image Anal. 2015;24:269–281. doi: 10.1016/j.media.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Roine U., Roine T., Salmi J., Nieminen-von Wendt T., Tani P., Leppamaki S., Rintahaka P., Caeyenberghs K., Leemans A., Sams M. Abnormal wiring of the connectome in adults with high-functioning autism spectrum disorder. Mol. Autism. 2015;6:65. doi: 10.1186/s13229-015-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ruigrok A.N., Salimi-Khorshidi G., Lai M.C., Baron-Cohen S., Lombardo M.V., Tait R.J., Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R., Martinez A., Pomarol-Clotet E., Gomar J., Vila F., Sarro S., Capdevila A., Bullmore E. A simple view of the brain through a frequency-specific functional connectivity measure. NeuroImage. 2008;39:279–289. doi: 10.1016/j.neuroimage.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Saramaki J., Kivela M., Onnela J.P., Kaski K., Kertesz J. Generalizations of the clustering coefficient to weighted complex networks. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2007;75:027105. doi: 10.1103/PhysRevE.75.027105. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn. 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Schmithorst V.J., Holland S.K., Dardzinski B.J. Developmental differences in white matter architecture between boys and girls. Hum. Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch J.J., Roest A.M., Nolen W.A., Penninx B.W., de Jonge P. Gender differences in major depressive disorder: results from the Netherlands study of depression and anxiety. J. Affect. Disord. 2014;156:156–163. doi: 10.1016/j.jad.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Shansky R.M. Estrogen, stress and the brain: progress toward unraveling gender discrepancies in major depressive disorder. Expert. Rev. Neurother. 2009;9:967–973. doi: 10.1586/ern.09.46. [DOI] [PubMed] [Google Scholar]
- Shaywitz B.A., Shaywitz S.E., Pugh K.R., Constable R.T., Skudlarski P., Fulbright R.K., Bronen R.A., Fletcher J.M., Shankweiler D.P., Katz L. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Shen C.Y., Tyan Y.S., Kuo L.W., Wu C.W., Weng J.C. Quantitative evaluation of rabbit brain injury after cerebral hemisphere radiation exposure using generalized q-sampling imaging. PLoS One. 2015;10:e0133001. doi: 10.1371/journal.pone.0133001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Structure and function of complex brain networks. Dialogues Clin. Neurosci. 2013;15:247–262. doi: 10.31887/DCNS.2013.15.3/osporns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Bhatia M.S., Bhargava S.K., Kumari R., Chandra S. A diffusion tensor imaging study using a voxel-based analysis, region-of-interest method to analyze white matter abnormalities in first-episode, treatment-naive major depressive disorder. J. Neuropsychiatry Clin. Neurosci. 2016;28:131–137. doi: 10.1176/appi.neuropsych.15050120. (appineuropsych15050120) [DOI] [PubMed] [Google Scholar]
- Steinmetz H., Staiger J.F., Schlaug G., Huang Y., Jancke L. Corpus callosum and brain volume in women and men. Neuroreport. 1995;6:1002–1004. doi: 10.1097/00001756-199505090-00013. [DOI] [PubMed] [Google Scholar]
- Szalkai B., Varga B., Grolmusz V. Graph theoretical analysis reveals: women's brains are better connected than men's. PLoS One. 2015;10:e0130045. doi: 10.1371/journal.pone.0130045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Thompson P.M. Diffusion imaging, white matter, and psychopathology. Annu. Rev. Clin. Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Gadian D.G., Connelly A. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. NeuroImage. 2004;23:1176–1185. doi: 10.1016/j.neuroimage.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Tournier J.D., Calamante F., Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. NeuroImage. 2007;35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tuch D.S. Q-ball imaging. Magn. Reson. Med. 2004;52:1358–1372. doi: 10.1002/mrm.20279. [DOI] [PubMed] [Google Scholar]
- Vos S.B., Jones D.K., Viergever M.A., Leemans A. Partial volume effect as a hidden covariate in DTI analyses. NeuroImage. 2011;55:1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Q., Haldar J.P., Yeh F.C., Xie M., Sun P., Tu T.W., Trinkaus K., Klein R.S., Cross A.H., Song S.K. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Cusick M.F., Wang Y., Sun P., Libbey J.E., Trinkaus K., Fujinami R.S., Song S.K. Diffusion basis spectrum imaging detects and distinguishes coexisting subclinical inflammation, demyelination and axonal injury in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2014;27:843–852. doi: 10.1002/nbm.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wedeen V.J., Hagmann P., Tseng W.Y., Reese T.G., Weisskoff R.M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn. Reson. Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Whitaker K.J., Vertes P.E., Romero-Garcia R., Vasa F., Moutoussis M., Prabhu G., Weiskopf N., Callaghan M.F., Wagstyl K., Rittman T., Tait R., Ooi C., Suckling J., Inkster B., Fonagy P., Dolan R.J., Jones P.B., Goodyer I.M., Consortium N., Bullmore E.T. Adolescence is associated with genomically patterned consolidation of the hubs of the human brain connectome. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9105–9110. doi: 10.1073/pnas.1601745113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise T., Radua J., Nortje G., Cleare A.J., Young A.H., Arnone D. Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol. Psychiatry. 2016;79:293–302. doi: 10.1016/j.biopsych.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Xu C., Li C., Wu H., Wu Y., Hu S., Zhu Y., Zhang W., Wang L., Zhu S., Liu J., Zhang Q., Yang J., Zhang X. Gender differences in cerebral regional homogeneity of adult healthy volunteers: a resting-state FMRI study. Biomed. Res. Int. 2015;2015:183074. doi: 10.1155/2015/183074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hirsch L., Martino D., Jette N., Roberts J., Pringsheim T. The prevalence of diagnosed tourette syndrome in Canada: a national population-based study. Mov. Disord. 2016;31:1658–1663. doi: 10.1002/mds.26766. [DOI] [PubMed] [Google Scholar]
- Yeh F.C., Wedeen V.J., Tseng W.Y. Generalized q-sampling imaging. IEEE Trans. Med. Imaging. 2010;29:1626–1635. doi: 10.1109/TMI.2010.2045126. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Harding I.H., Cocchi L., Yucel M., Pantelis C., Bullmore E.T. Whole-brain anatomical networks: does the choice of nodes matter? NeuroImage. 2010;50:970–983. doi: 10.1016/j.neuroimage.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang J., Wu Q., Kuang W., Huang X., He Y., Gong Q. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol. Psychiatry. 2011;70:334–342. doi: 10.1016/j.biopsych.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang H., Schneider T., Wheeler-Kingshott C.A., Alexander D.C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 2012;61:1000–1016. doi: 10.1016/j.neuroimage.2012.03.072. [DOI] [PubMed] [Google Scholar]
- Zhang H., Wang Y., Lu T., Qiu B., Tang Y., Ou S., Tie X., Sun C., Xu K. Differences between generalized q-sampling imaging and diffusion tensor imaging in the preoperative visualization of the nerve fiber tracts within peritumoral edema in brain. Neurosurgery. 2013;73:1044–1053. doi: 10.1227/NEU.0000000000000146. (discussion 1053) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables